Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2449
Revised: February 20, 2024
Accepted: April 7, 2024
Published online: June 15, 2024
Processing time: 178 Days and 0.5 Hours
Regorafenib (R) and fruquintinib (F) are the standard third-line regimens for colorectal cancer (CRC) according to the National Comprehensive Cancer Network guidelines, but both have limited efficacy. Several phase 2 trials have indicated that R or F combined with immune checkpoint inhibitors can reverse immunosuppression and achieve promising efficacy for microsatellite stable or proficient mismatch repair (MSS/pMMR) CRC. Due to the lack of studies com
To provide critical evidence for selecting the appropriate drugs for MSS/pMMR metastatic CRC (mCRC) patients in clinical practice.
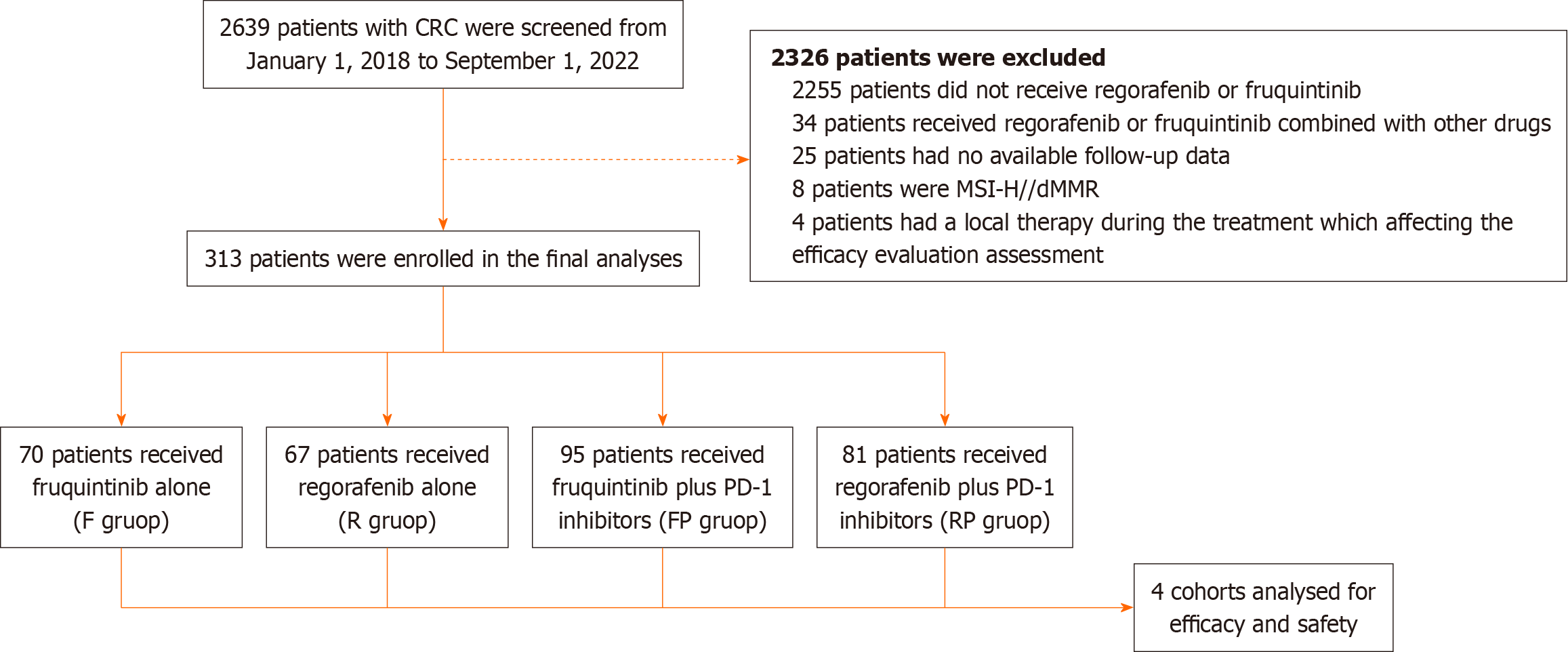
A total of 2639 CRC patients were enrolled from January 2018 to September 2022 in our hospital, and 313 MSS/pMMR mCRC patients were finally included.
A total of 313 eligible patients were divided into F (n = 70), R (n = 67), F plus PD-1 inhibitor (FP) (n = 95) and RP (n = 81) groups. The key clinical characteristics were well balanced among the groups. The median progression-free survival (PFS) of the F, R, FP, and RP groups was 3.5 months, 3.6 months, 4.9 months, and 3.0 months, respectively. The median overall survival (OS) was 14.6 months, 15.7 months, 16.7 months, and 14.1 months. The FP regimen had an improved disease control rate (DCR) (P = 0.044) and 6-month PFS (P = 0.014) and exhibited a better trend in PFS (P = 0.057) compared with F, and it was also significantly better in PFS than RP (P = 0.030). RP did not confer a significant survival benefit; instead, the R group had a trend toward greater benefit with OS (P = 0.080) compared with RP. No significant differences were observed between the R and F groups in PFS or OS (P > 0.05).
FP is superior to F in achieving 6-month PFS and DCR, while RP is not better than R. FP has an improved PFS and 6-month PFS compared with RP, but F and R had similar clinical efficacy. Therefore, FP may be a highly promising strategy in the treatment of MSS/pMMR mCRC.
Core Tip: Fruquintinib (F) and regorafenib (R) monotherapy or in combination with programmed death-1 (PD-1) inhibitors, are commonly used treatment options for microsatellite stable or proficient mismatch repair (MSS/pMMR) colorectal cancer (CRC). Nowadays, there is limited research data comparing the efficacy of F plus PD-1 inhibitors (FP) and R plus PD-1 inhibitors (RP) to F and R monotherapy. And there is also no consensus on whether combination therapy is more effective than monotherapy. We included a total of 313 patients with MSS/pMMR metastatic CRC (mCRC) who received at least third-line treatment with F, R, FP, or RP at our hospital, and then conducted statistical analysis on their clinical data and prognosis. And then found that the FP regimen has the potential to yield favorable survival benefits for MSS/pMMR mCRC, making it worthy of further research and investigation.
- Citation: An TQ, Qiu H, Zhou QB, Zong H, Hu S, Lian YG, Zhao RH. Efficacy comparison of fruquintinib, regorafenib monotherapy or plus programmed death-1 inhibitors for microsatellite stable metastatic colorectal cancer. World J Gastrointest Oncol 2024; 16(6): 2449-2462
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2449.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2449
Colorectal cancer (CRC) is one of the most common cancers and the second leading cause of death from cancers globally[1]. Although early cancer screening techniques have improved significantly over the past decade, more than 20% of CRC patients are initially diagnosed at the metastatic stage, which brings great challenges to their treatment[2,3]. For refractory metastatic CRC (mCRC), the treatment options remain constrained. In China, regorafenib (R), fruquintinib (F), and TAS-102 have been approved as standard third-line therapies for mCRC patients. However, their efficacy is not entirely satisfactory. The objective response rate (ORR) is only 1%-5%, and the median progression-free survival (PFS) is usually less than 4 months, so there is an urgent need to explore new treatment strategies to improve patient survival[4-6].
Some studies have shown that immune checkpoint inhibitors (ICIs), particularly programmed death-1 (PD-1) and programmed cell death 1 Ligand 1 inhibitors, are highly effective in patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) CRC[7,8]. However, the population with MSI-H/dMMR is very low, accounting for only 5% of mCRC cases. Most of the remaining microsatellite stable or proficient mismatch repair (MSS/pMMR) CRC patients appeared not to benefit from single-agent PD-1 inhibitors[9]. Therefore, how to turn a 'cold' tumor of MSS/pMMR into a 'hot' tumor of MSI-H/dMMR and then break through the therapeutic bottleneck of MSS/pMMR CRC has become a research hotspot in the last few years.
Some studies have shown that R or F combined with ICIs can reverse immunosuppression and achieve promising efficacy for MSS/pMMR mCRC. Doleschel et al[10] and Wang et al[11] separately indicated that R or F in combination with PD-1 inhibitors could significantly suppress tumor growth and improve the tumor immune microenvironment. In the phase Ib REGONIVO trial, although most patients had MSS/pMMR mCRC (24/25, 96%), R plus nivolumab still showed encouraging antitumor activity in CRC patients, with PFS, a one-year PFS rate and a one-year overall survival (OS) rate of 7.9 months, 41.8% and 68.0%, respectively[12]. Since then, clinical exploration of combining immunotherapy with antiangiogenic therapy in the salvage-line treatment of MSS/pMMR CRC has been initiated, and an increasing number of patients have begun to receive the regimen of F plus PD-1 inhibitors (FP) or R plus PD-1 inhibitors (RP) in recent years. However, due to the lack of research on comparisons, whether PD-1 inhibitors plus F or R are safer and more effective than F or R monotherapies remains unknown. The regimen with the best efficacy among the four regimens is still uncertain.
Therefore, we designed this retrospective study in the First Affiliated Hospital of Zhengzhou University to compare the efficacy and safety of F, R, and their combination with PD-1 inhibitors in the treatment of MSS/pMMR mCRC. Our results presented in this study will provide critical evidence for drug selection for MSS/pMMR mCRC patients in clinical treatment.
This was a retrospective observational study conducted at the First Affiliated Hospital of Zhengzhou University. Approval was obtained from the Ethics Committee of the First Hospital of Zhengzhou University (No. 2022-KY-0910-001). Patient informed consent was not needed, as our study was retrospective.
We enrolled patients with MSS/pMMR mCRC who received at least third-line treatment with F, R, FP, or RP at our hospital from January 1, 2018, to September 1, 2022.
The inclusion criteria of our study included the following. (1) Patients were pathologically diagnosed with MSS/pMMR CRC; (2) The tumors were classified histopathologically as adenocarcinomas; (3) ≥ 18 years old; (4) Disease progressed after receiving two or more different prior regimens; (5) The Eastern Cooperative Oncology Group (ECOG) score was 0, 1 or 2; (6) At least one measurable lesion could be evaluated by radiographic imaging; and (7) Eligible patients had adequate hematologic, renal, hepatic, and cardiac function.
The exclusion criteria were as follows. (1) MSI-H/dMMR CRC; (2) Patients received local therapy such as cryosurgery or radiofrequency ablation during the medication, which affects efficacy assessment; and (3) Familial CRC.
A starting dose of 5 mg F or 120 mg R per day was used for the first 21 d of each 28-d cycle, and then the dose could be adjusted according to the patient's response and tolerance. Of note, the dose of R should not be less than 80 mg per day, and F should not be less than 3 mg per day. PD-1 inhibitors were administered as follows: Camrelizumab (200 mg), sintilimab (200 mg), tislelizumab (200 mg), toripalimab (240 mg) or pembrolizumab (200 mg) every 21 d or nivolumab (200 mg) every 14 d. Patients continued treatment until disease progression, intolerable toxicity, or death.
Tumor response was assessed by computed tomography and magnetic resonance imaging after at least two cycles of treatment according to Response Evaluation Criteria in Solid Tumors version 1.1. The ORR was the percentage of patients with complete response (CR) and partial response (PR). The disease control rate (DCR) was the percentage of CR, PR, and stable disease (SD). PFS was defined as the time from first treatment to disease progression or death as a result of any reason, and OS was defined as the time to death. Safety was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0 (NCI CTCAE V5.0). The main endpoints were PFS and OS, and other key outcomes, including ORR, DCR, and safety, were also analyzed and compared.
Statistical analyses were performed with SPSS version 26.0. Comparisons between the four groups for baseline characteristics, ORR, and DCR were assessed by the Pearson chi-square test and Fisher exact test. Survival analysis was performed using the Kaplan-Meier method. The median follow-up time was calculated by the reverse Kaplan-Meier method. Cox regression analysis was used in univariate and multivariate analyses. A significant P value was defined as below 0.05 (two-sided).
In total, 2639 patients with CRC were enrolled from January 1, 2018, to September 1, 2022 in our hospital, and 313 patients were finally included according to the inclusion and exclusion criteria. Among them, 70 patients received F, 67 patients received R, 95 patients received FP, and 81 patients received RP (Figure 1). The median follow-up time was 18.8 months (95%CI: 16.9-20.7), with the last follow-up date of January 3, 2023.
Of the 313 eligible patients, 251 patients (80.2%) were younger than 65 years old, and 52 patients (16.6%) had early-onset disease (≤ 45 years). The median age was 55 years (range: 21-88), with basically equal numbers of men and women. The primary tumor of the left-to-right ratio was 3.2:1. All these patients were pathologically diagnosed with adenocarcinoma, and all of them were MSS/pMMR. RAS mutations occurred in 144 patients (46.0%), while only 20 patients (6.4%) had a BRAF mutation.
All patients had received at least 2 prior regimens before enrollment, with 270 patients (86.3%) having received anti-VEGF antibody and 56 patients (17.9%) having received anti-EGFR antibody. Most patients (117/148, 79.1%) received final daily doses of R of 80 or 120 mg, and only 23 patients (15.5%) received 160 mg. In the F and FP groups, 150 patients (90.9%) received 5 mg F, and only 15 patients (9.1%) received 3 or 4 mg F (Supplementary Table 1). Furthermore, a total of six kinds of PD-1 inhibitors were used in the FP and RP groups (Table 1). The key clinical characteristics were well balanced between groups, and they are summarized in Table 2.
| PD-1 inhibitors | FP | RP |
| Camrelizumab | 28 (29.5) | 53 (65.5) |
| Sintilimab | 45 (47.4) | 18 (22.2) |
| Tislelizumab | 9 (9.5) | 1 (1.2) |
| Nivolumab | 1 (1.0) | 7 (8.6) |
| Toripalimab | 10 (10.5) | 2 (2.5) |
| Pembrolizumab | 2 (2.1) | 0 (0.0) |
| Characteristic | Total | F | R | FP | RP | P value |
| Patients | 313 | 70 | 67 | 95 | 81 | |
| Median age (range) | 55 (21-88) | 55 (29-88) | 57 (29-79) | 54 (22-81) | 54 (21-83) | |
| Age group | 0.895 | |||||
| < 65 yr | 251 (80.2) | 54 (77.1) | 55 (82.1) | 77 (81.1) | 65 (80.2) | |
| ≥ 65 yr | 62 (19.8) | 16 (22.9) | 12 (17.9) | 18 (18.9) | 16 (19.8) | |
| Sex | 0.476 | |||||
| Male | 171 (54.6) | 37 (52.9) | 32 (47.8) | 57 (60.0) | 45 (55.6) | |
| Female | 142 (45.4) | 33 (47.1) | 35 (52.2) | 38 (40.0) | 36 (44.4) | |
| ECOG | 0.189 | |||||
| 0-1 | 281 (89.8) | 59 (84.3) | 60 (89.6) | 85 (89.5) | 77 (95.1) | |
| 2 | 32 (10.2) | 11 (15.7) | 7 (10.4) | 10 (10.5) | 4 (4.9) | |
| Primary tumor location | 0.335 | |||||
| Left | 238 (76.0) | 51 (72.9) | 56 (83.6) | 73 (76.8) | 58 (71.6) | |
| Right | 75 (24.0) | 19 (27.1) | 11 (16.4) | 22 (23.2) | 23 (28.4) | |
| Status of the primary tumor | 0.289 | |||||
| Resected | 248 (79.2) | 53 (75.7) | 53 (79.1) | 72 (75.8) | 70 (86.4) | |
| Unresected | 65 (20.8) | 17 (24.3) | 14 (20.9) | 23 (24.2) | 11 (13.6) | |
| RAS mutation status1 | 0.934 | |||||
| Wild-type | 98 (31.3) | 23 (32.9) | 22 (32.8) | 30 (31.6) | 23 (28.4) | |
| Mutation | 144 (46.0) | 30 (42.9) | 35 (52.2) | 42 (44.2) | 37 (45.7) | |
| Unknown | 71 (22.7) | 17 (24.2) | 10 (15.0) | 23 (24.2) | 21 (25.9) | |
| BRAF mutation status1 | 0.233 | |||||
| Wild-type | 222 (70.9) | 50 (71.4) | 55 (82.1) | 65 (68.4) | 52 (64.2) | |
| Mutation | 20 (6.4) | 3 (4.3) | 2 (3.0) | 7 (7.4) | 8 (9.9) | |
| Unknown | 71 (22.7) | 17 (24.3) | 10 (14.9) | 23 (24.2) | 21 (25.9) | |
| Site of metastases | ||||||
| Liver | 178 (56.9) | 40 (57.1) | 35 (52.2) | 53 (55.8) | 50 (61.7) | 0.703 |
| Lung | 174 (55.6) | 43 (61.4) | 40 (59.7) | 45 (47.4) | 46 (56.8) | 0.254 |
| Number of metastatic sites | 0.060 | |||||
| < 3 | 206 (65.8) | 48 (68.6) | 50 (74.6) | 64 (67.4) | 44 (54.3) | |
| ≥ 3 | 107 (34.2) | 22 (31.4) | 17 (25.4) | 31 (32.6) | 37 (45.7) | |
| Prior treatment lines | ||||||
| 2 | 188 (60.1) | 40 (57.1) | 40 (59.7) | 53 (55.8) | 55 (67.9) | 0.384 |
| > 2 | 125 (39.9) | 30 (42.9) | 27 (40.3) | 42 (44.2) | 26 (32.1) | |
| Previous treatment agents | ||||||
| Fluoropyrimidine | 313 (100.0) | 70 (100.0) | 67 (100.0) | 95 (100.0) | 81 (100.0) | > 0.999 |
| Irinotecan | 280 (89.5) | 61 (87.1) | 61 (91.0) | 82 (86.3) | 76 (93.8) | 0.360 |
| Oxaliplatin | 297 (94.9) | 68 (97.1) | 62 (92.5) | 88 (92.6) | 79 (97.5) | 0.304 |
| Anti-VEGF antibody | 270 (86.3) | 65 (92.9) | 52 (77.6) | 83 (87.4) | 70 (86.4) | 0.075 |
| Anti-EGFR antibody | 56 (17.9) | 10 (14.3) | 15 (22.4) | 15 (15.8) | 16 (19.8) | 0.569 |
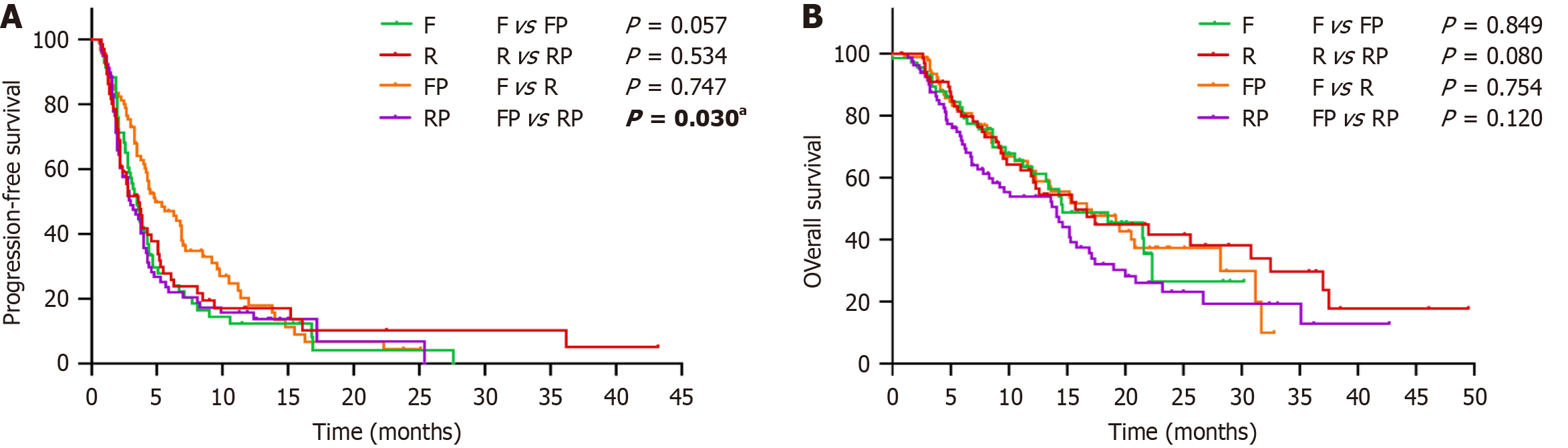
The median PFS of the F, R, FP, and RP groups was 3.5 (95%CI: 2.9-4.1) months, 3.6 (95%CI: 2.5-4.7) months, 4.9 (95%CI: 3.0-6.8) months, and 3.0 (95%CI: 2.0-4.1) months, respectively. The median OS was 14.6 (95%CI: 7.3-21.9) months, 15.7 (95%CI: 9.8-21.6) months, 16.7 (95%CI: 11.1-22.3) months, and 14.1 (95%CI: 8.9-19.3) months (Figure 2). The 6-month PFS rates of the F, R, FP, and RP groups were 27.8%, 27.8%, 47.1%, and 22.0%, respectively. The 1-year OS rates were 61.3%, 60.4%, 62.2% and 53.9%, respectively. The 3-year OS rates were 26.6%, 29.7%, 10.0%, and 12.9% (Table 3).
| Efficacy | F | R | FP | RP | P(F vs FP) | P(R vs RP) | P(F vs R) | P(FP vs RP) |
| ORR | 4.3% | 6.0% | 11.6% | 4.9% | 0.097 | > 0.999 | 0.714 | 0.116 |
| DCR | 60.0% | 61.2% | 74.7% | 70.4% | 0.044a | 0.240 | 0.886 | 0.517 |
| 6-month PFS, month | 27.8% | 27.8% | 47.1% | 22.0% | 0.014a | 0.521 | 0.862 | 0.001a |
| 1-yr OS | 61.3% | 60.4% | 62.2% | 53.9% | 0.922 | 0.259 | 0.945 | 0.157 |
| 3-yr OS | 26.6% | 29.7% | 10.0% | 12.9% | 0.849 | 0.066 | 0.754 | 0.120 |
| PFS, month | 3.5 | 3.6 | 4.9 | 3.0 | 0.057 | 0.534 | 0.747 | 0.030a |
| OS, month | 14.6 | 15.7 | 16.7 | 14.1 | 0.849 | 0.080 | 0.754 | 0.120 |
The evaluation of the response is summarized in Tables 3 and 4. None of the patients in the four groups achieved CR, and 22 patients achieved PR. The ORR of the F, R, FP, and RP groups were 4.3%, 6.0%, 11.6%, and 4.9%, respectively. The DCR were 60.0%, 61.2%, 74.7%, and 70.4%, respectively.
| Response | Total (n = 313) | F (n = 70) | R (n = 67) | FP (n = 95) | RP (n = 81) | |||||
| n | % | n | % | n | % | n | % | n | % | |
| PR | 22 | 7.0 | 3 | 4.3 | 4 | 6.0 | 11 | 11.6 | 4 | 4.9 |
| SD | 189 | 60.4 | 39 | 55.7 | 37 | 55.2 | 60 | 63.1 | 53 | 65.5 |
| PD | 102 | 32.6 | 28 | 40.0 | 26 | 38.8 | 24 | 25.3 | 24 | 29.6 |
| ORR | 22 | 7.0 | 3 | 4.3 | 4 | 6.0 | 11 | 11.6 | 4 | 4.9 |
| DCR | 211 | 67.4 | 42 | 60.0 | 41 | 61.2 | 71 | 74.7 | 57 | 70.4 |
Among patients treated with different PD-1 inhibitors in FP, there was no significant difference in ORR (P = 0.104), DCR (P = 0.663), PFS (P = 0.151), or OS (P = 0.073). Similarly, in the RP group using different PD-1 inhibitors, there was also no significant difference in ORR (P = 0.444), DCR (P = 0.850), PFS (P = 0.651) and OS (P = 0.591), which suggested that there was no influence of using different PD-1 inhibitors on the efficacy evaluation in the FP and RP groups.
Efficacy comparison between FP and F: The FP regimen exhibited a better trend in PFS than the F regimen (P = 0.057). The results of the 6-month PFS also suggested that the FP group was superior to the F group (P = 0.014). The DCR in the FP group was also significantly better than that in the F group (P = 0.044). However, for ORR and OS, there was no significant difference between them (P > 0.05).
Efficacy comparison between RP and R: Astonishingly, although RP had a higher DCR than R (70.4% vs 61.2%), it did not confer a significant survival benefit over R in ORR, DCR, PFS, or OS (P > 0.05), and the R group instead had a trend toward greater benefit with 3-year OS (P = 0.066) and OS (P = 0.080) compared with RP.
Efficacy comparison between F and R: The ORR, DCR, PFS, and OS between the F and R groups were similar, and the differences between the groups were not statistically significant (P > 0.05). Similarly, these numerical differences in the 6-month PFS, 1-year OS, and 3-year OS also failed to reach statistical significance (P > 0.05).
Efficacy comparison between FP and RP: PFS was significantly better in the FP group than in the RP group (P = 0.030). The results of the 6-month PFS also suggested that the FP group was superior to the RP group (P = 0.001). However, no significant differences were observed in ORR, DCR and OS between FP and RP.
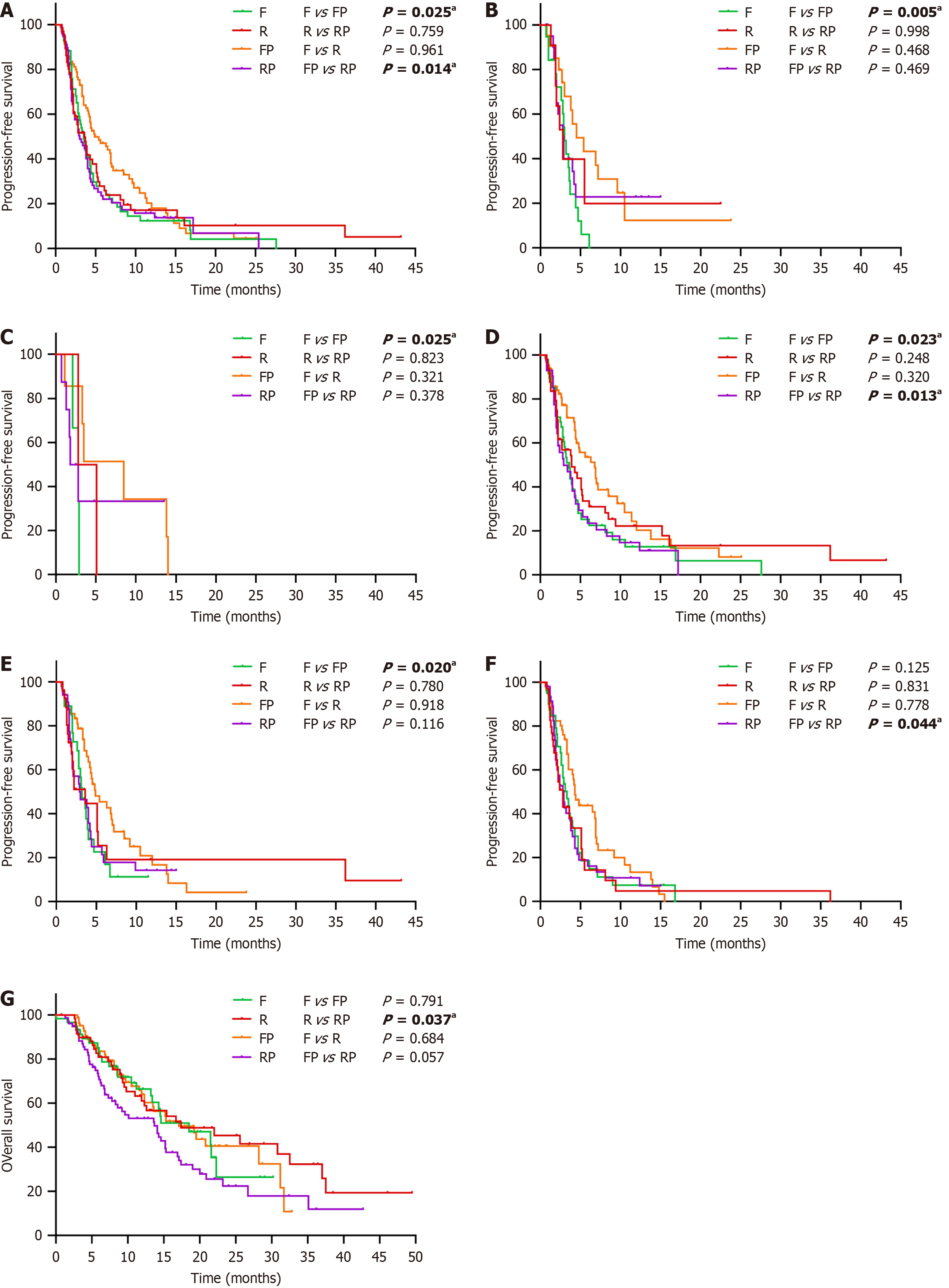
To determine a suitable regimen for patients with different clinicopathological characteristics, we also performed a detailed subgroup analysis with the Kaplan-Meier (log-rank) test among the four groups. The results suggested that PFS was significantly better in the FP group than in the F group in patients less than 65 years old, with primary lesions of the right-sided colon, with BRAF mutations, with fewer than three metastatic sites, and without lung metastases (P < 0.05; Figure 3A-E). However, compared with RP, patients with liver metastases and fewer than three metastatic sites were more likely to benefit from FP in terms of PFS (P < 0.05; Figure 3D and F). In the comparison between the R and RP groups, the OS of the R group was significantly better than that of the RP group in the ECOG of 0-1 (P = 0.037; Figure 3G).
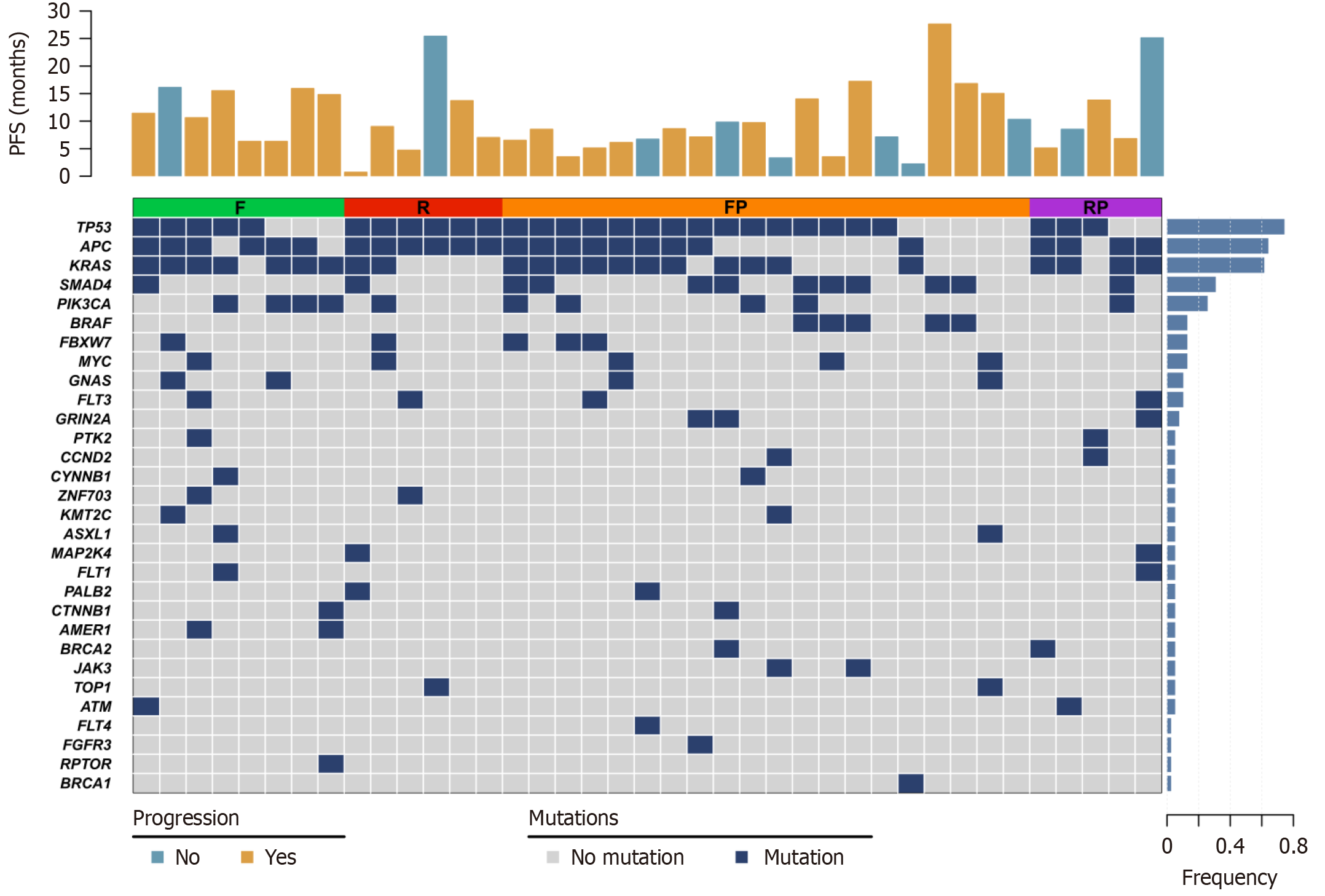
We analyzed the next-generation sequencing data of 39 patients who achieved excellent clinical response (with a PFS of more than 6 months or obtained PR) and found that the most frequently mutated genes in their patients were TP53 (74.4%), APC (64.1%), KRAS (61.5%), SMAD4 (30.8%) and PIK3CA (25.6%). TP53 and APC (F: 62.5%, 75.0%; R: 100%, 100%; FP: 75.0%, 45.0%; RP: 60.0%, 80.0%) mutations occurred in all patients with R, while KRAS was more enriched in F and RP (F: 87.5%, R: 33.3%, FP: 55.0%, RP: 80.0%; Figure 4).
To identify independent prognostic factors, all significant variables on univariate Cox regression analysis (P < 0.05) and indicators that may have an impact on prognosis (status of primary tumor and regimen) were subjected to multivariate Cox regression analysis (Table 5). The results showed that the RP regimen was inferior to FP in PFS (HR = 1.470, 95%CI: 1.030-2.097, P = 0.034), and the F regimen also extended a worse trend than FP (HR = 1.399, 95%CI: 0.974-2.010, P = 0.070), which was consistent with the results of Kaplan–Meier analysis. In addition, we also found that female sex, liver metastases, and prior treatment with anti-VEGF antibody were risk factors for PFS (P < 0.05), while liver metastases, without lung metastases, greater than or equal to three sites of metastatic disease and prior treatment with anti-VEGF antibody were risk factors for OS (P < 0.05).
| All patients | Progression-free survival | Overall survival | ||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (< 65/≥ 65 yr) | 0.764 | 0.533-1.095 | 0.143 | 1.054 | 0.713-1.558 | 0.791 | ||||||
| Sex (female/male) | 0.713 | 0.552-0.923 | 0.010 | 0.587 | 0.447-0.771 | < 0.001a | 0.755 | 0.556-1.024 | 0.071 | |||
| Primary tumor location (left/right) | 1.123 | 0.826-1.527 | 0.460 | 1.162 | 0.815-1.657 | 0.407 | ||||||
| Baseline ECOG (0/1) | 1.434 | 0.931-2.207 | 0.102 | 1.426 | 0.893-2.278 | 0.137 | ||||||
| RAS mutation status (wild/mutant) | 1.058 | 0.886-1.262 | 0.536 | 1.001 | 0.808-1.240 | 0.992 | ||||||
| BRAF mutation status (wild/mutant) | 1.034 | 0.888-1.204 | 0.664 | 1.127 | 0.946-1.342 | 0.180 | ||||||
| Status of primary tumor (unresected/resected) | 0.728 | 0.529-1.002 | 0.051 | 0.815 | 0.583-1.141 | 0.233 | 0.696 | 0.478-1.012 | 0.058 | 0.882 | 0.591-1.318 | 0.540 |
| Liver metastasis (no/yes) | 1.591 | 1.221-2.071 | 0.001a | 1.796 | 1.353-2.383 | < 0.001a | 1.843 | 1.344-2.527 | < 0.001a | 1.645 | 1.184-2.285 | 0.003a |
| Lung metastasis (no/yes) | 0.994 | 0.766-1.290 | 0.963 | 0.729 | 0.538-0.988 | 0.042a | 0.705 | 0.511-0.973 | 0.033a | |||
| Number of metastatic sites (</≥ 3) | 1.350 | 1.035-1.762 | 0.027a | 1.181 | 0.893-1.562 | 0.244 | 1.726 | 1.267-2.350 | 0.001a | 1.714 | 1.235-2.378 | 0.001a |
| Prior treatment lines (2/> 2) | 1.270 | 0.976-1.653 | 0.075 | 1.094 | 0.800-1.494 | 0.575 | ||||||
| Irinotecan (no/yes) | 1.833 | 1.157-2.905 | 0.010a | 1.625 | 0.976-2.707 | 0.062 | 1.586 | 0.900-2.794 | 0.111 | |||
| Oxaliplatin (no/yes) | 0.656 | 0.388-1.108 | 0.115 | 0.859 | 0.438-1.683 | 0.657 | ||||||
| Prior treatment with VEGF (no/yes) | 1.640 | 1.122-2.398 | 0.011a | 1.747 | 1.133-2.692 | 0.011a | 1.617 | 1.018-2.568 | 0.042a | 1.709 | 1.068-2.734 | 0.026a |
| Prior treatment with EGFR (no/yes) | 1.281 | 0.921-1.781 | 0.141 | 1.264 | 0.866-1.844 | 0.225 | ||||||
| Regimen (The F group as the reference) | ||||||||||||
| R | 0.928 | 0.632-1.363 | 0.702 | 0.917 | 0.564-1.491 | 0.727 | ||||||
| Regimen (the R group as the reference) | ||||||||||||
| RP | 1.145 | 0.787-1.666 | 0.479 | 1.474 | 0.966-2.250 | 0.072 | 1.517 | 0.744-1.801 | 0.518 | |||
| Regimen (the FP group as the reference) | ||||||||||||
| F | 1.400 | 0.979-2.001 | 0.065 | 1.399 | 0.974-2.010 | 0.070 | 1.012 | 0.640-1.600 | 0.959 | |||
| RP | 1.487 | 1.052-2.103 | 0.025a | 1.470 | 1.030-2.097 | 0.034a | 1.369 | 0.920-2.035 | 0.121 | |||
Each regimen has advantages, so we performed detailed subgroup analyses for each group. The results showed that in the F group, patients with the primary tumor location left were more likely to benefit from PFS than those with the primary tumor location on the right side, and patients with an ECOG score of 0-1 also had better PFS than those with an ECOG score of 2 (P < 0.05). In the FP and R groups, OS was significantly prolonged in patients without liver metastases compared with patients with liver metastases, and patients with < 3 metastatic sites also had a better OS than those with ≥ 3 metastatic sites (P < 0.05). In the patients who received RP, the PFS was longer in the primary tumor resected subgroup than in the unresected subgroup (P < 0.05; Supplementary Figure 1).
Overall, 257 patients (82.1%) had at least one treatment-related adverse event (Table 6). The most common adverse reactions we observed in this study were increased alanine aminotransferase (ALT)/aspartate aminotransferase (AST) (80/313, 25.6%), nausea/vomiting (77/313, 24.6%), and hand-foot skin reaction (67/313, 21.4%). A higher incidence of nausea/vomiting, hypothyroidism, and myocardial enzyme elevation could be observed in the combination group than in the monotherapy group. Some adverse events, including hand-foot skin reaction and ALT/AST elevation, were more frequent following R and RP, while hypertension occurred more frequently in patients who received F and FP. Although most of the adverse events were mild and could be relieved by drug dose reduction or discontinuation, seven patients experienced serious adverse events in this study: Four patients had gastrointestinal hemorrhage (one patient in the F group, two patients in the R group and one patient in the RP group), two patients had heart failure in the FP and RP groups, and one patient in the FP group suffered from rectovaginal fistulae, but all of them were well managed by long-term symptomatic treatment.
| Adverse events | All | Grade ≥ 3 | ||||||
| F | R | FP | RP | F | R | FP | RP | |
| Hand-foot skin reaction | 9 (12.9) | 18 (26.9) | 14 (14.7) | 26 (32.1) | 2 (2.9) | 5 (7.5) | 1 (1.1) | 3 (3.7) |
| Hypertension | 14 (20.0) | 8 (11.9) | 27 (28.4) | 11 (13.6) | 5 (7.1) | 2 (3.0) | 5 (5.3) | 1 (1.2) |
| Fatigue | 2 (2.9) | 3 (4.5) | 5 (5.3) | 10 (12.3) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bleeding | 2 (2.9) | 2 (3.0) | 1 (1.1) | 3 (3.7) | 1 (1.4) | 2 (3.0) | 0 (0.0) | 1 (1.2) |
| Weight loss | 1 (1.4) | 3 (4.5) | 5 (5.3) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fever | 7 (10.0) | 4 (6.0) | 8 (8.4) | 13 (16.0) | 2 (2.9) | 1 (1.5) | 1 (1.1) | 1 (1.2) |
| Oral mucositis | 5 (7.1) | 3 (4.5) | 4 (4.2) | 4 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Hoarseness | 2 (2.9) | 1 (1.5) | 3 (3.2) | 2 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hematuria | 9 (12.9) | 6 (9.0) | 15 (15.8) | 12 (14.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Proteinuria | 10 (14.3) | 9 (13.4) | 21 (22.1) | 10 (12.3) | 1 (1.4) | 0 (0.0) | 2 (2.1) | 0 (0.0) |
| Diarrhea | 6 (8.6) | 9 (13.4) | 10 (10.5) | 10 (12.3) | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (1.2) |
| Constipation | 5 (7.1) | 7 (10.4) | 12 (12.6) | 8 (9.9) | 1 (1.4) | 1 (1.5) | 0 (0.0) | 0 (0.0) |
| Nausea/Vomiting | 14 (20.0) | 15 (22.4) | 27 (28.4) | 21 (25.9) | 1 (1.4) | 3 (4.5) | 1 (1.1) | 1 (1.2) |
| Hyperthyroidism | 2 (2.9) | 1 (1.5) | 6 (6.3) | 3 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypothyroidism | 3 (4.3) | 0 (0.0) | 25 (26.3) | 13 (16.0) | 0 (0.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) |
| ALT/AST elevation | 14 (20.0) | 19 (28.4) | 19 (20.0) | 28 (34.6) | 2 (2.9) | 5 (7.5) | 4 (4.2) | 8 (9.9) |
| Myocardial enzyme elevation | 2 (2.9) | 3 (4.5) | 12 (12.6) | 14 (17.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) |
F is an oral small-molecule antiangiogenic inhibitor that is highly and selectively targeted to VEGFR1-3[13]. R mainly targets signaling pathways, including VEGFR1–3, TIE2, KIT, RET, PDGFR, and FGFR[14]. Both F and R were approved as standard third-line treatments for CRC in China. However, while the curative effects of F and R in prolonging OS were acceptable, the tumor-shrinking effect was not satisfactory; the ORR was only 1%-5%, and the PFS was usually less than 4 months[4,5,15-17].
In recent years, some investigators have found that F and R can not only inhibit tumor growth and neoangiogenesis but also modulate the tumor microenvironment[10,11]. In addition, based on the promising results of REGONIVO, an increasing number of studies on antiangiogenic therapy combined with immunotherapy for MSS/pMMR CRC have been carried out. However, it is worth noting that some studies of antiangiogenic therapy combined with immunotherapy were not as satisfactory as REGONIVO. In the multicenter, phase II REGOMUNE trial, the ORR and PFS of R combined with avelumab were only 0% (0/40) and 3.5 months, respectively[18]. For MSS/pMMR patients who received F combined with PD-1 inhibitors, the effects of different treatments were also not the same. In a phase 1b/2 trial, Guo et al[19] found that pMMR mCRC patients who received F plus sintilimab showed promising efficacy, with an ORR, median PFS, and OS of 20.0%, 6.9 months and 20.0 months, respectively. In a retrospective study, Zhang et al[20] also found that F combined with PD-1 inhibitors had antitumor activity and manageable safety in treating patients with MSS/pMMR advanced CRC, with an ORR, DCR, and PFS of 11.8%, 70.0% and 5.4 months, respectively. In Yang et al's study[21], the mOS and mPFS were 19.48 and 5.5 months respectively. However, in a real-world study of F plus sintilimab, the median PFS was only 3.8 months, which was much lower than the results in single-agent F[22]. At present, due to the lack of randomized phase 3 clinical studies to compare the efficacy between F and FP, R and RP, respectively, it is still unclear whether the combination therapy is more effective than monotherapy, and which of the four regimens has the best efficacy remains uncertain. Therefore, in this study, we compared the efficacy and safety among the four regimens.
The results of our study showed that the FP regimen had an improved DCR and exhibited a better trend in PFS compared with F. The results of 6-month PFS similarly suggested that the FP group was superior to the F group. Further subgroup analysis suggested that patients with specific characteristics could significantly benefit from FP than F, including BRAF mutations and fewer than three metastatic sites. BRAF is one of the most frequently mutated genes in CRC, accounting for approximately 10%, and often predicts a poorer prognosis[23]. In the FIRE-3 and PETACC-3 studies, researchers also found that patients with BRAF mutations have lower OS and a higher risk of death than wild-type BRAF patients[24,25]. However, in our study, we found that patients with BRAF mutation could obtain survival benefits from FP over F, which may provide a new idea for the treatment of this population. In addition, to the best of our knowledge, this is the first study with a larger sample size to evaluate the difference in efficacy between F and FP, and we believe that our study will provide critical evidence for drug selection in CRC treatment.
In the comparison of RP and R, we found that RP did not confer a significant survival benefit, and the R group had a trend toward greater benefit with OS and 3-year OS. In patients with an ECOG score of 0-1, the OS of the RP group was even worse than that of the R group. In a retrospective study, He et al[26] indicated that there was no significant difference between the combination therapy group (RP) and the R group in the median PFS (2.4 months vs 1.9 months, P > 0.05). In female patients with liver metastasis who were administered R combined with a PD-1 antibody even had a shorter PFS than those administered R monotherapy (1.8 vs 2.0 months, P = 0.030)[26]. In our study, there was also no evidence that the RP regimen could bring a significant survival benefit to patients compared with R. RP even increased the incidence of some adverse reactions, leading to discontinuation of medication or a decline in physical status in some patients, which may affect patients survival. Thus, the regimen of RP should not be routinely recommended for all MSS/pMMR mCRC patients.
In addition, the results of our study also suggested that PFS was significantly better in the FP group than in the RP group. The 6-month PFS results similarly suggested that the FP group was superior to the RP group. The same was also true for the multivariate Cox regression analysis. In a small-sample retrospective study, Sun et al[27] indicated that FP had a better PFS time of patients with advanced mCRC than RP (6.4 vs 3.9 months, P = 0.0209), which was consistent with the results of our study.
Data on the better option between R and F are limited. Some researchers suggest that the efficacy of the two drugs is similar, but in other studies, there may be slight differences between furquanib and R[28-30]. However, due to the lack of randomized phase III clinical studies and direct comparison research, it was still controversial to make conclusions. In our study, we found that R had similar efficacy to F in terms of ORR, DCR, PFS, and OS and even in terms of 6-month PFS, 1-year OS, and 3-year OS, which was largely consistent with the most above studies and again confirms the similar efficacy between F and R. In terms of safety, hypertension and hematuria were more common in the F group, whereas patients in the R group were more likely to have a hand-foot skin reaction and ALT/AST elevation.
The results of Deng et al[31] suggested that FP had a better PFS (5.9 vs 3.0 months, P = 0.009) and OS (17.5 vs 11.3 months, P = 0.008) than F in the treatment of mCRC. FP also had a longer PFS (5.9 vs 3.8 months, P = 0.018) and OS (17.5 vs 14.8 months, P = 0.044) than RP. This conclusion was consistent with ours; that is, FP may have better clinical efficacy than F and RP. There may be three reasons for this. First, compared with F monotherapy, FP could synergistically suppress CRC progression and alter the tumor microenvironment in favor of antitumor immune responses[11]. Second, compared with RP, F is highly selective for VEGFR 1-3 but has no obvious inhibitory effect on other kinase activities, which means that the antivascular effects of F are more specialized and that the interference of other targets on efficacy is much lower. In contrast, R is a multitarget kinase inhibitor with less inhibitory effect on the VEGFR pathway than F, as a result, the immunosuppressive effect of R in reversing VEGF may not be as strong as that of F. Third, FP was more tolerable than RP, which makes the majority of patients able to sustain FP treatment and achieve decent therapeutic effects[27]. Therefore, FP might be a good choice for patients with advanced MSS/pMMR CRC.
Notably, Deng et al[31] found that although there was no significant difference in median PFS between RP and R (3.8 vs 2.4 months, P = 0.262), RP had a longer OS than R (14.8 vs 10.0 months, P = 0.045), which was inconsistent with our findings [median OS (RP: 14.1 months, R: 15.7 months, P = 0.08)]. In a real-world study, Jiang et al[32] also reported that RP did not prolong the median OS compared with R alone (11.0 vs 11.2 months, P = 0.814). In addition, as has been mentioned previously, in some studies, the efficacy of RP is not satisfactory. In the REGOMUNE study, the ORR of R combined with avelumab was 0% (0/40)[18]. In another retrospective study, R combined with PD-1 inhibitors also had no objective response in 23 MSS/pMMR CRC patients[33]. Thus, R combined with PD-1 inhibitors does not seem to be available to all CRC patients. Currently, since there is still a lack of phase 3 randomized controlled studies to compare the efficacy between RP and R, the conclusion still needs to be further verified.
Of course, there were certain limitations in our study. First, it was a retrospective observational study, which is prone to bias. Second, PD-1 inhibitors were not the same in this study. Although this represented clinical practice and we found that there was no influence of different PD-1 inhibitors on clinical efficacy, it may inevitably affect the consistency of the treatment process. Therefore, in the future, more high-quality, large-scale prospective randomized controlled trials will be required to validate our results.
In conclusion, FP is superior to F in achieving 6-month PFS and DCR, while RP is not better than R. FP has an improved PFS and 6-month PFS compared with RP, but F and R had similar clinical efficacy. Although both F and R are anti-vascular multitarget small molecule drugs, the efficacy of the two in combination with immunotherapy may be different. Therefore, for patients with advanced MSS/pMMR CRC, the combination therapy regimen should be carefully selected.
FP is superior to F in achieving 6-month PFS and DCR, while RP is not better than R. FP has an improved PFS and 6-month PFS compared with RP, but F and R had similar clinical efficacy. Although both F and R are anti-vascular multitarget small molecule drugs, the efficacy of the two in combination with immunotherapy may be different. Therefore, for patients with advanced MSS/pMMR CRC, the combination therapy regimen should be carefully selected.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64722] [Article Influence: 16180.5] [Reference Citation Analysis (177)] |
| 2. | Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1122] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11943] [Article Influence: 2985.8] [Reference Citation Analysis (4)] |
| 4. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2120] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 5. | Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 6. | Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 1011] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 7. | Grothey A. Pembrolizumab in MSI-H-dMMR Advanced Colorectal Cancer - A New Standard of Care. N Engl J Med. 2020;383:2283-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Oliveira AF, Bretes L, Furtado I. Review of PD-1/PD-L1 Inhibitors in Metastatic dMMR/MSI-H Colorectal Cancer. Front Oncol. 2019;9:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 1018] [Article Influence: 169.7] [Reference Citation Analysis (0)] |
| 10. | Doleschel D, Hoff S, Koletnik S, Rix A, Zopf D, Kiessling F, Lederle W. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res. 2021;40:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Wei B, Gao J, Cai X, Xu L, Zhong H, Wang B, Sun Y, Guo W, Xu Q, Gu Y. Combination of Fruquintinib and Anti-PD-1 for the Treatment of Colorectal Cancer. J Immunol. 2020;205:2905-2915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 534] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 13. | Cao J, Zhang J, Peng W, Chen Z, Fan S, Su W, Li K, Li J. A Phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, -2, and -3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;78:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1022] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 15. | Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW; CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 570] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 16. | Song Y, Qu T, Zhang H, Sun Y, Cui C, Chi Y, Zhang W, Wang X, Yang L. The Real-World Practice of Fruquintinib for Chinese Patients with Metastatic Colorectal Cancer. Cancer Manag Res. 2021;13:6199-6205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Yeh KH, Yang TS, Hsu TC, Tzu-Liang Chen W, Chen HH, Teng HW, Lin BW, Kuan FC, Chiang FF, Duann CW, Li YS, Lin MT, Fiala-Buskies S, Ducreux M, Wang JY. Real-world evidence of the safety and effectiveness of regorafenib in Taiwanese patients with metastatic colorectal cancer: CORRELATE Taiwan. J Formos Med Assoc. 2021;120:2023-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, Pernot S, Bellera C, Kind M, Auzanneau C, Le Loarer F, Soubeyran I, Bessede A, Italiano A. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial. Clin Cancer Res. 2021;27:2139-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 19. | Guo Y, Zhang W, Ying J, Zhang Y, Pan Y, Qiu W, Fan Q, Xu Q, Ma Y, Wang G, Guo J, Su W, Fan S, Tan P, Wang Y, Luo Y, Zhou H, Li J. Phase 1b/2 trial of fruquintinib plus sintilimab in treating advanced solid tumours: The dose-escalation and metastatic colorectal cancer cohort in the dose-expansion phases. Eur J Cancer. 2023;181:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Zhang W, Zhang Z, Lou S, Li D, Ma Z, Xue L. Efficacy, safety and predictors of combined fruquintinib with programmed death-1 inhibitors for advanced microsatellite-stable colorectal cancer: A retrospective study. Front Oncol. 2022;12:929342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 21. | Yang X, Yin X, Qu X, Guo G, Zeng Y, Liu W, Jagielski M, Liu Z, Zhou H. Efficacy, safety, and predictors of fruquintinib plus anti-programmed death receptor-1 (PD-1) antibody in refractory microsatellite stable metastatic colorectal cancer in a real-world setting: a retrospective cohort study. J Gastrointest Oncol. 2023;14:2425-2435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Gou M, Qian N, Zhang Y, Yan H, Si H, Wang Z, Dai G. Fruquintinib in Combination With PD-1 Inhibitors in Patients With Refractory Non-MSI-H/pMMR Metastatic Colorectal Cancer: A Real-World Study in China. Front Oncol. 2022;12:851756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 23. | Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 24. | Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, Topham C, Tabernero J, André T, Sobrero AF, Mini E, Greil R, Di Costanzo F, Collette L, Cisar L, Zhang X, Khayat D, Bokemeyer C, Roth AD, Cunningham D. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 25. | Stahler A, Stintzing S, von Einem JC, Westphalen CB, Heinrich K, Krämer N, Michl M, Modest DP, von Weikersthal LF, Decker T, Kiani A, Heintges T, Kahl C, Kullmann F, Scheithauer W, Moehler M, Kaiser F, Kirchner T, Jung A, Heinemann V. Single-nucleotide variants, tumour mutational burden and microsatellite instability in patients with metastatic colorectal cancer: Next-generation sequencing results of the FIRE-3 trial. Eur J Cancer. 2020;137:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | He WZ, Wang L, Yin CX, Yi JH, Jin YN, Jiang C, Guo GF, Xia LP. Regorafenib with or without a programmed cell death protein 1 antibody as third-line treatment for microsatellite stable metastatic colorectal cancer. Cancer Med. 2023;12:6488-6498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Sun L, Huang S, Li D, Mao Y, Wang Y, Wu J. Efficacy and Safety of Fruquintinib Plus PD-1 Inhibitors Versus Regorafenib Plus PD-1 Inhibitors in Refractory Microsatellite Stable Metastatic Colorectal Cancer. Front Oncol. 2021;11:754881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Zhang Q, Wang Q, Wang X, Li J, Shen L, Peng Z. Regorafenib, TAS-102, or fruquintinib for metastatic colorectal cancer: any difference in randomized trials? Int J Colorectal Dis. 2020;35:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Jing Z, Rui Z, Binglan Z. A comparison of regorafenib and fruquintinib for metastatic colorectal cancer: a systematic review and network meta-analysis. J Cancer Res Clin Oncol. 2019;145:2313-2323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Wu Y, Fan Y, Dong D, Dong X, Hu Y, Shi Y, Jing J, Li E. Efficacy and safety of regorafenib as beyond second-line therapy in patients with metastatic colorectal cancer: an adjusted indirect meta-analysis and systematic review. Ther Adv Med Oncol. 2020;12:1758835920940932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Deng YY, Zhang XY, Zhu PF, Lu HR, Liu Q, Pan SY, Chen ZL, Yang L. Comparison of the efficacy and safety of fruquintinib and regorafenib in the treatment of metastatic colorectal cancer: A real-world study. Front Oncol. 2023;13:1097911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 32. | Jiang ZC, Sun YK, Zhang W, Yang L, Cui CX, Wang HY, Zhang HG, Yihebali C, Zhou AP. [Analysis of metastatic colorectal cancer patients treated with regorafenib in real-world practice]. Zhonghua Yi Xue Za Zhi. 2020;100:2018-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Li J, Cong L, Liu J, Peng L, Wang J, Feng A, Yue J, Li L, Wang X. The Efficacy and Safety of Regorafenib in Combination With Anti-PD-1 Antibody in Refractory Microsatellite Stable Metastatic Colorectal Cancer: A Retrospective Study. Front Oncol. 2020;10:594125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |