Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2439
Revised: February 28, 2024
Accepted: April 11, 2024
Published online: June 15, 2024
Processing time: 186 Days and 17.1 Hours
The liver imaging reporting and data system (LI-RADS) diagnostic table has 15 cells and is too complex. The diagnostic performance of LI-RADS for hepatocellular carcinoma (HCC) is not satisfactory on gadoxetic acid-enhanced magnetic resonance imaging (EOB-MRI).
To evaluate the ability of the simplified LI-RADS (sLI-RADS) to diagnose HCC on EOB-MRI.
A total of 331 patients with 356 hepatic observations were retrospectively analysed. The diagnostic performance of sLI-RADS A-D using a single threshold was evaluated and compared with LI-RADS v2018 to determine the optimal sLI-RADS. The algorithms of sLI-RADS A-D are as follows: The single threshold for sLI-RADS A and B was 10 mm, that is, classified observations ≥ 10mm using an algorithm of 10-19 mm observations (sLI-RADS A) and ≥ 20 mm observations (sLI-RADS B) in the diagnosis table of LI-RADS v2018, respectively, while the classification algorithm remained unchanged for observations < 10 mm; the single threshold for sLI-RADS C and D was 20 mm, that is, for < 20 mm observations, the algorithms for < 10 mm observations (sLI-RADS C)and 10-19 mm observa
The optimal sLI-RADS was sLI-RADS D (with a single threshold of 20 mm), because its sensitivity was greater than that of LI-RADS v2018 (89.8% vs 87.0%, P = 0.031), and its specificity was not lower (89.4% vs 90.1%, P > 0.999). With HBP hypointensity as an MF, the sensitivity of F-sLI-RADS was greater than that of LI-RADS v2018 (93.0% vs 87.0%, P < 0.001) and sLI-RADS D (93.0% vs 89.8%, P = 0.016), without a lower specificity (86.5% vs 90.1%, P = 0.062; 86.5% vs 89.4%, P = 0.125). Compared with that of LI-RADS v2018, the time to classify lesions according to F-sLI-RADS was shorter (51 ± 21 s vs 73 ± 24 s, P < 0.001).
The use of sLI-RADS with HBP hypointensity as an MF may improve the sensitivity of HCC diagnosis and reduce lesion classification time.
Core Tip: This retrospective study included 356 hepatic observations. A single threshold (observation size) was used to simplify the liver imaging reporting and data system (LI-RADS) diagnostic table, and hepatobiliary phase hypointensity was added as a major feature to improve the sensitivity of LI-RADS for the diagnosis of hepatocellular carcinoma and shorten the observation classification time.
- Citation: Lyu R, Hu WJ, Wang D, Wang J, Ye YB, Jia KF. Simplified liver imaging reporting and data system for the diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced magnetic resonance imaging. World J Gastrointest Oncol 2024; 16(6): 2439-2448
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2439.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2439
The most common malignant tumour in the liver is hepatocellular carcinoma (HCC), which has attracted global medical attention due to its high mortality rate[1,2]. Unlike other malignancies, an accurate diagnosis of HCC can be made by imaging features, even without the pathological confirmation[3]. The prognosis of early HCC patients is better than that of advanced-stage HCC patients, and these patients can choose various curative treatment methods, such as local ablation, surgical resection and liver transplantation, to improve long-term survival[4]. Therefore, the early diagnosis of HCC is important, and imaging examination plays a key role in this process.
To standardize the imaging diagnosis and reporting terminology, the American College of Radiology published the Liver Imaging Reporting and Data System (LI-RADS) to categorize hepatic observations in high-risk populations for HCC and to indicate the likelihood of benign or malignant lesions and HCC[5]. The computed tomography (CT)/magnetic resonance imaging (MRI) LI-RADS has been updated to four versions since its release, with the latest version being the LI-RADS version 2018 (LI-RADS v2018)[6].
In LI-RADS, LR-3 (intermediate probability of malignancy), LR-4 (probably HCC), and LR-5 (definitely HCC) are assigned to observations according to the CT/MRI diagnostic table. This diagnosis is assigned a category to the observation by combining the major features (MFs) of HCC, including the observation size, arterial phase hyperenhancement (APHE), “washout”, enhancing “capsule”, and the threshold growth. The diagnostic table with 15 cells is rather complex[7].
Gadoxetate disodium (EOB) is a liver-specific contrast agent used in MRI, and it can provide important information for the evaluation of liver cell function. In 2014, EOB was added to the updated LI-RADS version[6]. EOB-MRI can display all the ancillary features (AFs) involved in LI-RADS, but its sensitivity for HCC diagnosis is lower than that of extracellular contrast agent (ECA)-enhanced MRI (55% vs 73%)[2], which is also a factor restricting its combination with LI-RADS.
Although there have been many studies on improving the diagnostic performance of LI-RADS, few have focused on simplifying the diagnostic table. Therefore, this study investigated whether it was possible to simplify the diagnostic algorithm for hepatic focal observations with APHE in the diagnostic table and improve the diagnosis of LI-RADS on EOB-MRI.
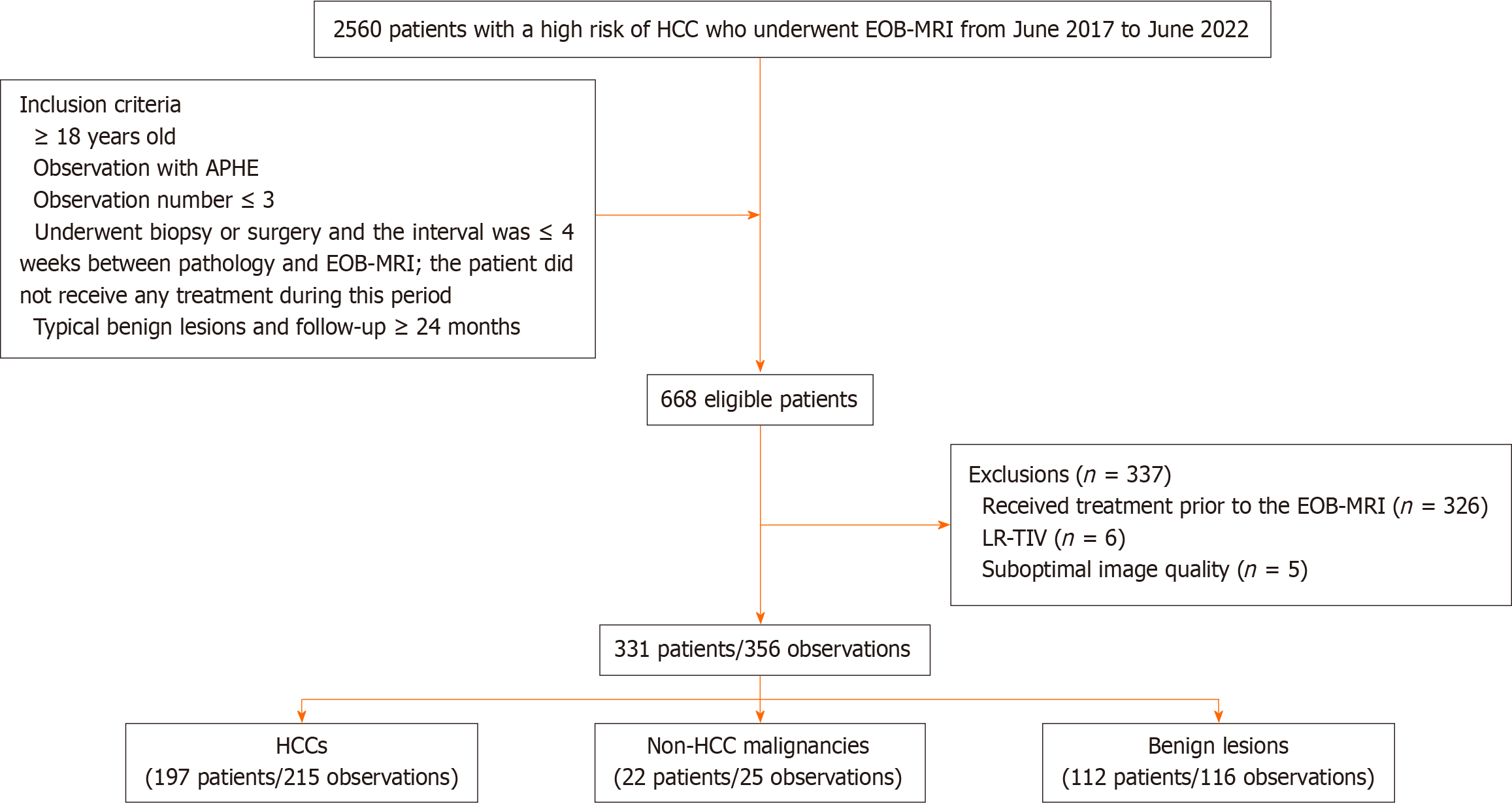
The Ethics Committee of Tianjin Third Central Hospital approved this study, and informed consent was waived due to the retrospective study design. A total of 2560 patients in a single centre with a high risk of HCC who underwent EOB-MRI from June 2017 to June 2022 were consecutively enrolled. The inclusion criteria were as follows: (1) ≥ 18 years old; (2) focal hepatic observation with APHE; (3) observation number ≤ 3; (4) biopsy or surgery and an interval ≤ 4 wk between pathology and EOB-MRI, with the patient not receiving any treatment during this period; and (5) typical benign lesions and follow-up ≥ 24 months. Patients who met the following criteria were excluded: (1) Treatment was performed prior to EOB-MRI; (2) LR-TIV (tumour-in-vein); or (3) suboptimal image quality: Missing or poor image quality did not meet diagnostic needs (Figure 1).
All HCCs, non-HCC malignant tumours and some benign lesions were pathologically confirmed, while the remaining benign lesions were diagnosed based on the presence of typical benign imaging features and the lesions remained stable or reduced for at least 24 months of follow-up. The pathological diagnosis was based on the official pathological report of our hospital. The LR-5 category was used for the diagnosis of HCC. The above confirmed diagnostic criteria were used as the gold standard to calculate the performance of LI-RADS in diagnosing HCC.
Liver MRI was performed with a 3.0-T magnetic resonance scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany). The MRI sequences used were as follows: A breath-hold in and opposed-phase T1-weighted dual-echo gradient recalled echo sequence, a respiratory-triggered fat-saturated T2-weighted turbo spin echo sequence, and diffusion-weighted imaging (DWI) at b values of 0, 50 and 1000 s/mm2. For dynamic phase imaging, a weight-based dose of 0.025 mmol/kg gadoxetic acid (Primovist, Bayer Healthcare, Leverkusen, Germany) was intravenously administered at a rate of 1.0 mL/s using a power injector, and this was immediately followed by a 25 mL saline injection at the same rate. Images in the late arterial phase (AP, 30-35 s), portal venous phase (PVP, 60-75 s), transitional phase (TP, 150-180 s), and hepatobiliary phase (HBP, 20 min) were obtained after intravenous contrast material was administered.
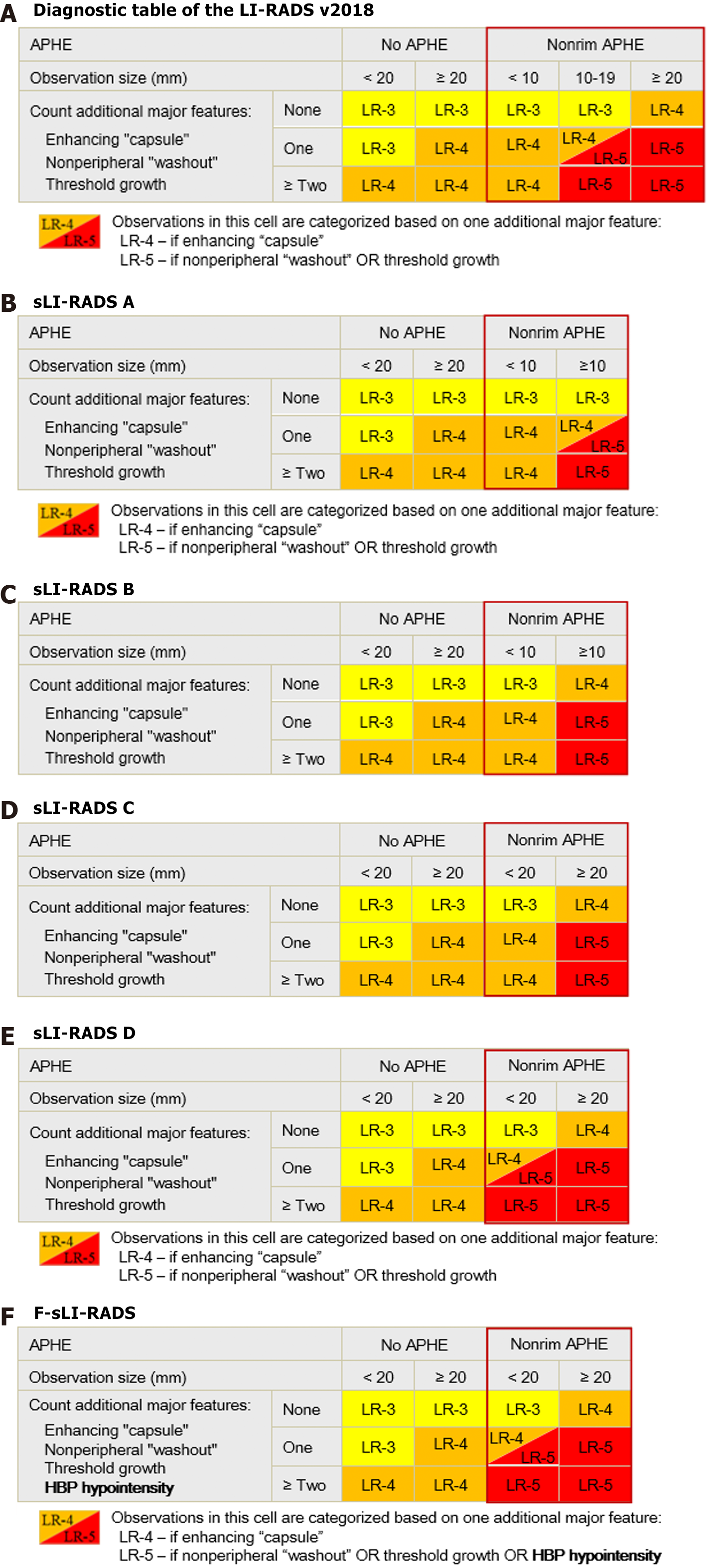
The detailed classification method is shown in Figure 2. The four strategies of simplified LI-RADS (sLI-RADS) are as follows: In sLI-RADS A, the classification criteria of 10-19 mm lesions in the diagnostic table were used for all lesions ≥ 10 mm. sLI-RADS B: For all ≥ 10 mm lesions, the classification criteria for lesions ≥ 20 mm were adopted. In sLI-RADS C, the classification criteria of < 10 mm lesions in the diagnostic table were used for all < 20 mm lesions. sLI-RADS D: For all < 20 mm lesions, the classification criteria for 10-19 mm nodules were adopted. All the above criteria were changed for lesions with APHE, and the classification criteria for other lesions remained unchanged. The diagnostic performance of the different strategies of sLI-RADS were compared with that of LI-RADS v2018, and the optimal sLI-RADS was selected.
According to previous studies[8], HBP hypointensity could improve the diagnostic performance of LI-RADS for HCC. Therefore, HBP hypointensity was added as an additional MF of HCC and combined with the optimal sLI-RADS to form the final sLI-RADS (F-sLI-RADS) (Figure 2).
Two radiologists with 8 years (Wei-Juan Hu) and 6 years (Di Wang) of experience in liver imaging reviewed all MRIs independently. Disagreements between the two physicians were resolved by a third, higher-ranking radiologist (Rong Lyu with 17 years of experience in liver imaging diagnosis) to achieve a final consistent reading. All readers were unaware of the pathological results. All imaging features (MFs and AFs) of hepatic observations were reviewed according to LI-RADS v2018. We chose images that could clearly show the boundary of the observation except AP and DWI to measure the observation size, such as TP and HBP images[9]. Threshold growth was defined as a diameter increase of a lesion of at least 50% in ≤ 6 months.
First, a LI-RADS category was assigned to each hepatic observation according to LI-RADS v2018 (the MFs were first used to assign the category and then adjusted by applying AFs). Second, the lesions were assigned to the LI-RADS category according to sLI-RADS A-D, respectively, and HBP hypointensity was used as an additional MF of HCC. The categories were reassigned to lesions according to F-sLI-RADS. These classifications were performed 4 wk after the first classification to avoid recall bias. The time to classify the lesions according to LI-RADS v2018 and F-sLI-RADS was measured and recorded by each reader on his own. The time was defined as the time needed from the beginning of evaluation (each imaging feature) and measurement (lesion diameter) to the final determination of the LI-RADS category.
Categorical variables are summarized as counts and percentages. The normally distributed continuous variables are summarized as the mean ± SD. Diagnostic performance is reported as the sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and Youden index. The sensitivity, specificity and accuracy were compared using McNemar’s test. The times needed for the LI-RADS category assignment were compared between the two systems using a paired t test. Unless otherwise indicated, all statistical tests were conducted at the 0.05 significance level using 2-tailed tests, and P values are reported. Statistical analyses were performed using SPSS software, version 25.0 (SPSS Inc.).
The study population included 331 patients (mean age, 57.5 ± 10.9 years, 236 men) with 356 observations. Hepatitis B virus was the most common cause (73.7%), followed by hepatitis C (15.4%). A total of 309 patients had one lesion, and 22 had multiple lesions. Among the 356 observations, there were 215 HCCs, 25 non-HCC malignancies and 116 benign lesions. A total of 286 (80.3%) lesions were confirmed by pathology (119 surgical and 167 biopsy), and 70 (19.7%) benign lesions were confirmed by typical benign imaging features and remained stable or reduced with a follow-up of ≥ 24 months. The clinicopathologic characteristics are shown in Table 1.
| Characteristic | Value |
| Patient (n = 331) | |
| Mean age (yr)1 | 57.5 ± 10.9 |
| Sex | |
| Male | 236 (71.3) |
| Female | 95 (28.7) |
| Cause of liver disease | |
| Hepatitis B virus | 244 (73.7) |
| Hepatitis C virus | 51 (15.4) |
| Hepatitis B and C virus | 9 (2.7) |
| Alcohol | 10 (3.0) |
| Autoimmune | 4 (1.2) |
| NASH | 3 (0.9) |
| Cirrhosis of unknown cause | 10 (3.0) |
| Number of observations per patient | |
| 1 | 309 (93.4) |
| 2 | 19 (5.7) |
| 3 | 3 (0.9) |
| Observation (n = 356) | |
| Size (mm)1 | |
| Overall | 31.5 ± 25.9 |
| HCC | 35.8 ± 27.1 |
| Non-HCC malignancy | 43.2 ± 27.8 |
| Benign lesion | 21.0 ± 19.2 |
| Final diagnosis | |
| HCC | 215 (60.4) |
| Non-HCC malignancy | 25 (7.0) |
| ICC | 7 (2.0) |
| CHC | 8 (2.2) |
| Metastasis | 5 (1.4) |
| Sarcomatoid carcinoma | 4 (1.1) |
| Neuroendocrine carcinoma | 1 (0.3) |
| Benign lesion | 116 (32.6) |
| Haemangioma | 14 (3.9) |
| DN | 24 (6.7) |
| RN | 42 (11.8) |
| FNH-like | 17 (4.8) |
| Adenoma or adenomatoid hyperplasia | 5 (1.4) |
| Abscess | 2 (0.6) |
| Angiomyolipoma | 2 (0.6) |
| Epithelioid angiomyolipoma | 3 (0.8) |
| Abnormal perfusion | 7 (2.0) |
| Standard reference of diagnosis | |
| Pathologic diagnosis | 286 (80.3) |
| Typical imaging features and follow-up | 70 (19.7) |
| The time of follow-up (months)1 | 32.3 ± 13.5 |
Compared with LI-RADS v2018, five LR-4 HCCs and 15 LR-4 non-HCC observations were reassigned as LR-3, 11 LR-5 HCCs were reassigned as LR-4 using sLI-RADS A, ten LR-3 HCCs and 11 LR-3 non-HCC observations were reassigned as LR-4, 3 LR-4 HCCs and 4 LR-4 non-HCC lesions were reassigned as LR-5 using sLI-RADS B, forty-nine LR-5 HCCs and 6 LR-5 non-HCC lesions were reassigned as LR-4 using sLI-RADS C, and six LR-4 HCCs and one LR-4 non-HCC lesion were reassigned as LR-5 using sLI-RADS D (Table 2).
| LI-RADS category | LI-RADS v2018 | sLI-RADS A | sLI-RADS B | sLI-RADS C | sLI-RADS D | F-sLI-RADS | ||||||
| HCC | non-HCC | HCC | non-HCC | HCC | non-HCC | HCC | non-HCC | HCC | non-HCC | HCC | non-HCC | |
| 1 | 0 | 42 | 0 | 42 | 0 | 42 | 0 | 42 | 0 | 42 | 0 | 42 |
| 2 | 1 | 38 | 1 | 38 | 1 | 38 | 1 | 38 | 1 | 38 | 1 | 38 |
| 3 | 11 | 13 | 16 | 28 | 1 | 2 | 11 | 13 | 11 | 13 | 7 | 13 |
| 4 | 15 | 19 | 21 | 4 | 22 | 26 | 64 | 25 | 9 | 18 | 6 | 14 |
| 5 | 187 | 14 | 176 | 14 | 190 | 18 | 138 | 8 | 193 | 15 | 200 | 19 |
| M | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 |
| Total | 215 | 141 | 215 | 141 | 215 | 141 | 215 | 141 | 215 | 141 | 215 | 141 |
The diagnostic performance of LI-RADS is shown in Table 3. The sensitivity and specificity of LI-RADS v2018 were 87.0% and 90.1%, respectively. Compared with those of LI-RADS v2018, the sensitivity and specificity of sLI-RADS B were not significantly different (all P > 0.05); sLI-RADS A and C had lower sensitivities (all P < 0.05); and the sensitivity of sLI-RADS D was significantly greater (89.8% vs 87.0%, P = 0.031), without lower specificity (89.4% vs 90.1%, P > 0.999).
| Sensitivity | Specificity | PPV | NPV | Accuracy | Youden | |
| LI-RADS v2018 | 87.0 | 90.1 | 93.0 | 81.9 | 88.2 | 0.770 |
| sLI-RADS A | 81.9 | 90.1 | 92.6 | 76.5 | 85.1 | 0.719 |
| sLI-RADS B | 88.4 | 87.2 | 91.3 | 83.1 | 87.9 | 0.756 |
| sLI-RADS C | 64.2 | 93.6 | 93.9 | 63.2 | 75.8 | 0.578 |
| sLI-RADS D | 89.8 | 89.4 | 92.8 | 85.1 | 89.6 | 0.791 |
| F-sLI-RADS | 93.0 | 86.5 | 91.3 | 89.1 | 90.4 | 0.795 |
| aPvalue | 0.001 | > 0.999 | — | — | 0.001 | — |
| bPvalue | 0.250 | 0.125 | — | — | > 0.999 | — |
| cPvalue | < 0.001 | 0.031 | — | — | < 0.001 | — |
| dPvalue | 0.031 | > 0.999 | — | — | 0.062 | — |
| ePvalue | < 0.001 | 0.062 | — | — | 0.008 | — |
| fPvalue | 0.016 | 0.125 | — | — | 0.250 | — |
F-sLI-RADS was formed in combination with HBP hypointensity as an MF of HCC with sLI-RADS D (Figure 3). Compared with sLI-RADS D, seven LR-4 HCCs and 4 LR-4 non-HCC lesions were reassigned as LR-5, and 4 LR-3 HCCs were reassigned as LR-4 (Table 2). Compared with LI-RADS v2018 or sLI-RADS D, F-sLI-RADS showed a significant increase in sensitivity (93.0% vs 87.0%, P < 0.001; 93.0% vs 89.8%, P = 0.016), without decreasing specificity (86.5% vs 90.1%, P = 0.062; 86.5% vs 89.4%, P = 0.125; Table 3).
The time taken to assign the category to the observation using F-sLI-RADS was shorter than that of LI-RADS v2018 (51 ± 21s vs 73 ± 24 s, P < 0.001).
In our study, compared to that of LI-RADS v2018, the sensitivity of F-sLI-RADS (for which the single threshold is 20 mm, for < 20 mm observations, the diagnostic algorithms for 10-19 mm observations in the diagnostic table were used and HBP hypointensity was added as an additional MF for HCC) was greater (93.0% vs 87.0%, P < 0.001) with EOB-MRI, and its specificity was not reduced (86.5% vs 90.1%, P = 0.062). In addition, the time needed to classify lesions using the F-sLI-RADS algorithm was shorter than that needed for classifying lesions using the LI-RADS v2018 algorithm (51 ± 21 s vs 73 ± 24 s, P < 0.001).
In our study, the sensitivity of sLI-RADS A (the classification algorithms of 10-19 mm observations in the diagnostic table were used for all ≥ 10 mm observations with APHE) was lower than that of LI-RADS v2018 (81.9% vs 87.0%, P = 0.001), which differed from the results of a previous study[10]. In this previous study, only one LR-5 HCC lesion was reassigned as LR-4 using sLI-RADS A, and the diagnostic performance was not different from that of LI-RADS v2018. However, that study focused on ≤ 30 mm nodules, while our study did not limit the lesion size. The sizes of the 11 LR-5 HCCs that were reassigned as LR-4 using sLI-RADS A in our study were all > 30 mm. Therefore, the conclusions of our study have more general applicability. The diagnostic performance of sLI-RADS B (the classification criteria of ≥ 20 mm lesions were adopted for all ≥ 10 mm lesions with APHE) was not different from that of LI-RADS v2018 (all P > 0.05), which was similar to the findings of a previous study[10]. Because there were more false negatives (49 LR-5 HCCs were reassigned as LR-4) using sLI-RADS C (the classification criteria of < 10 mm lesions were used for all < 20 mm lesions with APHE), the decrease in sensitivity was not acceptable (64.2% vs 87.0%, P < 0.001). Generally, 10-19 mm nodules with typical APHE and washout should also be diagnosed as HCC. However, these lesions were assigned to the LR-4 category based on sLI-RADS C. Because six < 10 mm LR-4 HCCs and only one LR-4 non-HCC lesion were reassigned as LR-5 according to sLI-RADS D (the classification criteria of 10-19 mm nodules were adopted for all < 20 mm lesions with APHE), the sensitivity of sLI-RADS D was greater than that of LI-RADS v2018 (89.8% vs 87.0%, P = 0.031), and there was no difference in specificity (89.4% vs 90.1%, P > 0.999). Based on the above conclusions, we chose sLI-RADS D as the optimal sLI-RADS. One previous study reported that simplified diagnostic tables using single threshold (20 mm) were similar to those of sLI-RADS D[11], but the specific classification principle was slightly different from that used in our study.
The sensitivity and specificity of LI-RADS for HCC were reported to be 21%-86% and 77%-100%, respectively[12]. In our study, these values were 87.0% and 90.1%, respectively. In Western countries, liver transplantation is the main treatment for HCC; to some extent, the suboptimal sensitivity of LI-RADS compromises its ability to achieve high specificity and PPV.
However, in other regions of East Asia, radiofrequency ablation and hepatectomy are far more common treatment options as more cases of early HCC have been discovered[13], and more emphasis should be placed on improving diagnostic sensitivity. In particular for EOB-MRI, the efficiency of EOB in the AP and PVP was lower than that in the ECA, and HBP hypointensity can only be used as an indicator of AF and cannot upgrade the lesions to the LR-5 category according to LI-RADS v2018[7]; thus the sensitivity of LI-RADS for diagnosing HCC on EOB-MRI is less than that on ECA-MRI[2]. Therefore, many previous studies have attempted to improve the diagnostic sensitivity of various methods, including regrouping the current MFs[3] or enhancing the diagnostic status of certain AFs[14,15]. In our study, the sensitivity of F-sLI-RADS (the lesion reclassification algorithm that combines sLI-RADS D and HBP hypointensity as an MF of HCC) improved, while the specificity did not decrease. Because HBP hypointensity indicates that there are no normal hepatocytes in the tumour and most of them are malignant lesions and because of the application of LI-RADS in patients at high risk for HCC, most of these malignancies are suggestive of HCC; in addition, some benign lesions (such as haemangioma) with HBP hypointensity can be differentiated by early enhancement imaging features. Therefore, we believe that adding HBP hypointensity as an MF of HCC is beneficial for improving the diagnostic performance of LI-RADS.
Because we simplified the diagnostic table, the classification of lesions with APHE using only a single threshold (20 mm), and the number of cells in the diagnostic table were reduced from 15 to 12, the time used to classify the observations according to the F-sLI-RADS was shorter than that of LI-RADS v2018 (51 ± 21 s vs 73 ± 24 s, P < 0.001). This is one of the advantages of promoting F-sLI-RADS.
The limitations of our study are as follows: (1) Selection bias may have occurred due to the retrospective single-centre nature of the study; (2) the number of included observations was low due to the exclusion of HCCs that were confirmed only by typical imaging features. However, our study may be less biased than other studies using compound conditions as a reference standard[12]; and (3) Due to the lack of prior imaging examinations, threshold growth could not be evaluated in any of the included observations.
Compared to LI-RADS v2018, the F-sLI-RADS in our study, which used a single threshold (20 mm) for observations with APHE and HBP hypointensity as an MF of HCC showed a greater sensitivity of LI-RADS for the diagnosis of HCC, without a lower specificity. This conclusion can improve the complexity of the diagnostic table and the suboptimal sensitivity of LI-RADS for identifying HCC lesions on EOB-MRI. F-sLI-RADS can also save time for lesion classification.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Moldovan CA, Romania S-Editor: Li LL-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Kim YY, Lee S, Shin J, Son WJ, Roh YH, Hwang JA, Lee JE. Diagnostic performance of CT versus MRI Liver Imaging Reporting and Data System category 5 for hepatocellular carcinoma: a systematic review and meta-analysis of comparative studies. Eur Radiol. 2022;32:6723-6729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Chen J, Kuang S, Zhang Y, Tang W, Xie S, Zhang L, Rong D, He B, Deng Y, Xiao Y, Shi W, Fowler K, Wang J, Sirlin CB. Increasing the sensitivity of LI-RADS v2018 for diagnosis of small (10-19 mm) HCC on extracellular contrast-enhanced MRI. Abdom Radiol (NY). 2021;46:1530-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3165] [Article Influence: 527.5] [Reference Citation Analysis (37)] |
| 5. | Moura Cunha G, Chernyak V, Fowler KJ, Sirlin CB. Up-to-Date Role of CT/MRI LI-RADS in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:513-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Elmohr M, Elsayes KM, Chernyak V. LI-RADS: Review and updates. Clin Liver Dis (Hoboken). 2021;17:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | American College of Radiology. CT/MRI LI-RADS® v2018 core. 2018. [cited 3 April 2024]. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf. |
| 8. | Pan J, Tao Y, Chi X, Yang L, Zhao Y, Chen F. Do transition and hepatobiliary phase hypointensity improve LI-RADS categorization as an alternative washout: a systematic review and meta-analysis. Eur Radiol. 2022;32:5134-5143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Choi JY, Ha J, Choi SH, Kang HJ, Kim SY, Kim KW. Comparison of gadoxetate disodium-enhanced MRI sequences for measuring hepatic observation size and its implication of LI-RADS classification. Abdom Radiol (NY). 2022;47:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kwag M, Choi SH, Choi SJ, Byun JH, Won HJ, Shin YM. Simplified LI-RADS for Hepatocellular Carcinoma Diagnosis at Gadoxetic Acid-enhanced MRI. Radiology. 2022;305:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Jiang H, Song B, Qin Y, Wei Y, Konanur M, Wu Y, Zaki IH, McInnes MDF, Lafata KJ, Bashir MR. Data-Driven Modification of the LI-RADS Major Feature System on Gadoxetate Disodium-Enhanced MRI: Toward Better Sensitivity and Simplicity. J Magn Reson Imaging. 2022;55:493-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kim DH, Choi SH, Park SH, Kim KW, Byun JH, Kim SY, Lee SS, Shin YM, Won HJ, Kim PN. Meta-analysis of the accuracy of Liver Imaging Reporting and Data System category 4 or 5 for diagnosing hepatocellular carcinoma. Gut. 2019;68:1719-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 14. | Lee S, Kim SS, Bae H, Shin J, Yoon JK, Kim MJ. Application of Liver Imaging Reporting and Data System version 2018 ancillary features to upgrade from LR-4 to LR-5 on gadoxetic acid-enhanced MRI. Eur Radiol. 2021;31:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Cha DI, Choi GS, Kim YK, Kim JM, Kang TW, Song KD, Ahn SH. Extracellular contrast-enhanced MRI with diffusion-weighted imaging for HCC diagnosis: prospective comparison with gadoxetic acid using LI-RADS. Eur Radiol. 2020;30:3723-3734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |