Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2394
Revised: March 2, 2024
Accepted: April 11, 2024
Published online: June 15, 2024
Processing time: 171 Days and 23.3 Hours
Colorectal cancer (CRC) is one of the most common cancers diagnosed in the world. Although environmental and genetic factors play a major role in the pathogenesis of CRC, extensive research has suggested that vitamin D may play a pivotal role in the development of CRC. Vitamin D, primarily obtained through sunlight exposure, dietary sources, and supplements, has long been recognized for its essential functions in maintaining health, including immune regulation. This article delves into the intricate relationship between vitamin D, the immune system, gut flora, and the prevention of CRC. It presents a synthesis of epidemiological data, experimental studies, and clinical trials, highlighting the mech
Core Tip: Our study explores the intricate connections between vitamin D, the immune system, and gut flora in the context of colorectal cancer (CRC) prevention. We uncover how vitamin D influences these interrelated factors and its potential role in reducing CRC risk. This research sheds light on novel avenues for preventive strategies and underscores the importance of a holistic approach to CRC prevention.
- Citation: Zhan ZS, Zheng ZS, Shi J, Chen J, Wu SY, Zhang SY. Unraveling colorectal cancer prevention: The vitamin D - gut flora - immune system nexus. World J Gastrointest Oncol 2024; 16(6): 2394-2403
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2394.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2394
Colorectal cancer (CRC) is one of the most common malignancies in the world, showing high incidence and mortality rates in both China and the United States[1,2]. In the United States, the American Cancer Society periodically releases updates on CRC occurrence, utilizing incidence data up to 2016 from population-based cancer registries and mortality data up to 2017 from the National Center for Health Statistics[2]. In 2020, it was estimated that around 147950 individuals would receive a CRC diagnosis, with 53200 succumbing to the disease[2]. This included 17930 cases and 3640 fatalities among individuals under the age of 50[2]. Annually in China, it is estimated that there are over 376000 new cases of CRC and approximately 191000 deaths associated with the disease[3]. With its distressingly high incidence and mortality rates, it imposes an enormous burden on societies worldwide[4,5].
CRC is a disease with a multifaceted origin influenced by both genetic predisposition and environmental factors[6]. Given its prevalence and the significant impact it has on public health, preventing CRC has become a central focus in clinical practice and medical research. Importantly, screening for CRC plays a critical role in its prevention by identifying and removing colon polyps before they can develop into cancer[7,8]. Historically, preventive measures primarily involved lifestyle modifications such as quitting smoking, reducing alcohol intake, minimizing high-salt and high-fat foods, and adopting healthier diet and lifestyle habits to mitigate the risk of CRC. In recent years, relevant researchers have divided the prevention of CRC into two categories: Molecular prevention and chemoprevention. Molecular pre
To gain a comprehensive understanding of the potential for preventing CRC, it is essential to delve into the intricate web of associations involving vitamin D, the immune system, gut flora, and CRC itself[10]. This journey takes us on a thorough exploration of the multifaceted relationships among these elements, each contributing to the complex landscape of CRC development.
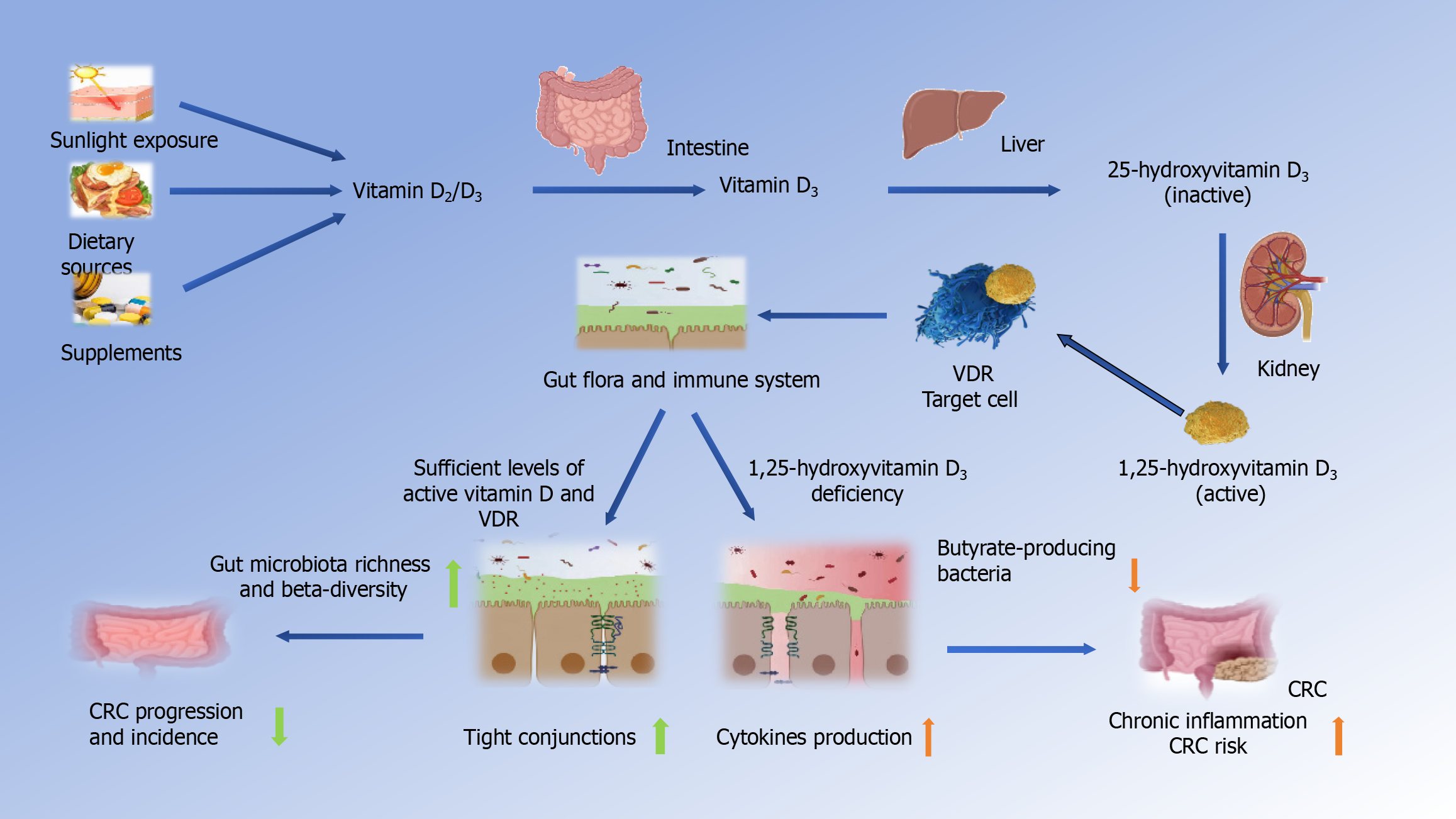
Vitamin D, often referred to as the “sunshine vitamin”, is a fat-soluble vitamin that plays a pivotal role in human health[11]. One of the primary sources of vitamin D is sunlight exposure. When the skin is exposed to ultraviolet B rays from the sunlight, it can synthesize vitamin D from cholesterol derivatives[12]. This natural synthesis process in the skin is a crucial means by which individuals obtain this essential nutrient[13]. In addition to sunlight, dietary sources and vitamin D-rich supplements are significant avenues through which people acquire vitamin D[14]. It’s important to note that there are two primary forms of vitamin D: Vitamin D2 and vitamin D3. Both of these forms have essential roles in maintaining human health[15].
The major form of vitamin D metabolite found in the serum is 25-hydroxyvitamin D (25-OH-D)[16]. In the body, both vitamin D2 and D3 are converted into 25-OH-D in the liver. Subsequently, this form of vitamin D travels through the bloodstream to the kidneys, where it undergoes further metabolism, transforming into 1,25-dihydroxyvitamin D (1,25-D) or osteotriol (25-(OH) D3)[17]. Among these metabolites, 1,25-dihydroxyvitamin D is the active form of vitamin D in the body, while 25-(OH) D3 serves as the stored form primarily found in the liver[18].
Throughout this metabolic process in the human body, active vitamin D binds to transporter proteins and is transported to various organs via the bloodstream. At the same time, the concentration of the stored form, 25-(OH) D3, is maintained at a relatively constant level[12,19]. These intricate processes underscore the significance of vitamin D in maintaining overall health and its potential role in various physiological functions[20].
The human microbiota encompasses a diverse array of microorganisms, comprising bacteria, archaea, fungi, viruses, and parasites. These microorganisms inhabit and colonize various niches within human tissues and biofluids, as well as on their surfaces[21,22]. Extensive study has established the crucial relationship between a healthy gut microbiome and its impact on the host’s immune response, energy metabolism, and pathogen colonization resistance[23]. The gut microbiota is a highly intricate and diverse community of microorganisms residing in the human gastrointestinal tract. In the digestive system of healthy individuals, this microbial ecosystem is composed of an astonishingly diverse array of over 100 trillion microorganisms[14,24], representing a complex interplay of various bacterial species.
This microbial community is a dynamic and ever-changing system influenced by a myriad of factors, including diet, genetics, age, and environmental exposures[25]. It plays a pivotal role in shaping the overall health and well-being of an individual. Understanding the intricate composition and dynamics of the gut microbiota is fundamental to comprehending its impact on health and diseases like CRC[26].
The main functions of the gut microbiota extend beyond traditional classifications, encompassing nutritional, defensive, metabolic, and additional key roles[27]. Nutritional functions: The gut microbiota plays a vital role in nutrient metabolism. It assists in the breakdown of indigestible food components, such as dietary fiber, through a process of fermentation. This fermentation process results in the production of short-chain fatty acids (SCFAs), including butyrate[28]. Butyrate, in particular, is crucial for maintaining intestinal homeostasis, as it promotes the development of colon cells while repressing the growth of cancerous cells[29]. SCFAs also serve as an energy source for colonocytes and contribute to the secretion of mucin, which is essential for maintaining the integrity of the intestinal barrier[30].
Defensive functions: The gut microbiota has a defensive role in protecting against harmful pathogens and bolstering the immune system[31]. It helps prevent inflammation and reduce the risk of cancer by generating regulatory T cells, which are involved in maintaining immune balance. Additionally, a diverse and balanced gut microbiota can compete with pathogenic bacteria for resources and inhibit their growth, further enhancing the defensive functions of the gut[32].
Metabolic functions: Beyond nutritional support, the gut microbiota plays a significant role in metabolic processes[33]. It is involved in the synthesis of essential vitamins and amino acids necessary for human growth and development. These bacteria also contribute to sugar and protein metabolism and aid in the absorption of essential minerals. The gut microbiota’s metabolic functions are critical for overall health, and imbalances in this ecosystem can give rise to various diseases[34].
Regulation of gas composition and redox potential: The microbiota regulates intestinal gas mixtures, including hydrogen, methane, and carbon dioxide production, playing a key role in physiological processes and maintaining gut redox balance[35].
Production of digestive enzymes: It produces a broad spectrum of enzymes for breaking down proteins, carbohydrates, and lipids, aiding the digestion and absorption of nutrients beyond the capacity of human enzymes[36].
Participation in water-salt metabolism: Microbial activity regulates intestinal movement, facilitating food transit and preventing disorders like constipation and irritable bowel syndrome[37].
Enhancement of intestinal motility: Furthermore, the microbiota contributes to the body’s water-salt equilibrium, influencing electrolyte and water absorption and secretion in the intestines, essential for hydration and blood pressure regulation[38].
Vitamin D is not just essential for bone health; it also plays a crucial role in regulating the immune system[39]. This multifaceted vitamin is known to modulate various aspects of immune function. Vitamin D does this by interacting with immune cells and signaling pathways, ultimately contributing to the body’s ability to defend against infections and maintain immune homeostasis[40].
Vitamin D exerts a significant impact on the immune system by reducing the activity of T helper 1 (Th1) and Th17 CD4 T cells, while promoting the activity of regulatory T cells (Tregs)[39]. It inhibits the production of Th1 cytokines, including interleukin (IL)-2 and interferon-γ, as well as Th17 cytokines like IL-17 and IL-21, and the Th9 cytokine IL-9[39,41,42]. This shift in T cell activity has profound implications for immune balance, inflammation, and autoimmunity. Moreover, vitamin D has been shown to inhibit dendritic cell differentiation in the intestinal lamina propria, further contributing to immune regulation[39] (Figure 1).
Moreover, vitamin D enhances the production of antimicrobial peptides (AMPs) and cytokines like β-defensin[43]. These AMPs are critical components of innate host defense against infections. Vitamin D also has a direct role in reducing the production of pro-inflammatory molecules by downregulating nuclear factor κB (NF-κB) activation, an essential pathway in inflammatory responses[44]. These immune-regulatory effects of vitamin D are particularly relevant in the context of CRC prevention, as they contribute to maintaining a balanced and controlled immune response in the gut[45].
Vitamin D’s influence extends beyond immune regulation to impact gut function. In the gut, vitamin D and its receptor (VDR) are involved in various processes that contribute to gut health[46]. One notable aspect is the improvement of the intestinal barrier. Vitamin D helps strengthen the intestinal barrier, which is essential for preventing the infiltration of harmful pathogens and maintaining gut integrity[47].
Additionally, vitamin D affects antigen presentation and adaptive T cells in the gut[48]. This means that it can influence how the immune system recognizes and responds to potential threats, including cancer cells. The role of vitamin D in the regulation of the gut microbiota is also of significance. It can shape the composition of the gut microbiome, promoting a balanced microbial ecosystem that contributes to overall gut health[49].
The emergence of inflammatory bowel disease (IBD) frequently coincides with shifts in microbial communities, a condition known as dysbiosis, within the gut[50]. The interplay between genetic predisposition and environmental factors can set the stage for chronic inflammation in the intestinal tract[51]. In line with the “common ground hypothesis”, it is proposed that microbial dysbiosis, coupled with compromised intestinal barrier function leading to increased permeability (leaky gut), forms the crux of the chronic inflammatory process that underlies IBD-CRC[52]. A body of research, encompassing investigations with patient cohorts and gnotobiotic mouse models[53,54], has lent substantial support to this hypothesis[55].
Dysbiosis has emerged as a significant factor in the development and progression of CRC[56]. In individuals with CRC, there is often a notable shift in the gut bacterial composition compared to healthy individuals[34]. This shift, characterized by reduced levels of beneficial bacteria and an increase in opportunistic pathogens, is indicative of intestinal dysbiosis[57].
One critical aspect of dysbiosis in CRC is the reduction in butyrate-producing bacteria. Butyrate promotes the growth and differentiation of colon cells while inhibiting the proliferation of cancerous cells[58]. Lower levels of butyrate are associated with increased intestinal inflammation and a higher risk of CRC development[59].
Additionally, dysbiosis can lead to the overgrowth of pro-inflammatory bacteria in the gut of CRC patients[60]. This shift in bacterial composition contributes to a pro-inflammatory environment, which is a known factor in the development of cancer[60]. Furthermore, invasive pathogens, such as Escherichia coli (E. coli), can inhibit the host’s epithelial colonocytes’ antimicrobial responses, potentially exacerbating the inflammatory milieu in the gut[61].
Research has identified specific bacterial species that are associated with CRC. Comparative analysis of stool samples from CRC patients and healthy individuals reveals notable differences in gut bacterial composition. CRC patients often exhibit an enrichment of pro-inflammatory species, including Micrococcus microti, Clostridium nucleatum, and Bacteroides fragilis, compared to control samples[58]. Conversely, control samples tend to have a higher abundance of beneficial bacteria such as Bacteroides and Bifidobacterium species[62]. This distinction in bacterial profiles underscores the potential role of these specific bacterial species in either promoting or inhibiting CRC development[63]. Notably, patients with IBD face an increased risk of CRC, and this risk is correlated with the duration and severity of colitis. The connection between IBD, gut inflammation, and CRC highlights the critical link between chronic intestinal inflammation and cancer development[64].
Mounting evidence suggests that vitamin D plays a vital role as a protective factor against CRC. Studies have provided compelling evidence supporting the notion that increased levels of vitamin D, defined quantitatively as higher serum concentrations of 25(OH)D, are associated with a reduced risk of CRC development[65]. These elevated serum 25(OH)D levels, which are indicative of sufficient vitamin D intake and effective bodily absorption, are crucial for the activation of various protective mechanisms within the body. Such mechanisms include modulation of cell growth, reduction of infla
One such study, conducted by Tsounis et al[67], revealed a robust protective effect of vitamin D against CRC. This effect extended not only to the development of CRC but also to colon polyps, which are often precursors to cancer[67]. The findings of this study, and others like it, highlight the potential of vitamin D as a crucial factor in preventing CRC[68,69]. The protective effect of vitamin D in CRC is not merely coincidental. Instead, it arises from a complex interplay of molecular mechanisms that influence various aspects of cancer biology and immune regulation[70].
Vitamin D’s anti-tumor effect is underpinned by a multitude of molecular mechanisms that impact cell growth, differentiation, and apoptosis[71]. When vitamin D binds to its receptor VDR, it initiates a cascade of intracellular and nuclear pathways that collectively act as a formidable barrier against the onset and progression of CRC[72].
VDRs, which belong to the nuclear receptor superfamily, are expressed in a wide range of tissues, including the intes
Furthermore, VDR is directly involved in the regulation of NF-κB activation in activated B cells, an essential pathway in inflammatory responses[74]. VDR deficiency leads to reduced levels of an endogenous inhibitor of NF-κB, contributing to heightened inflammation[75]. Vitamin D’s ability to inhibit NF-κB activation serves as a mechanism to mitigate inflammation in the gut[76].
Vitamin D also enhances immune homeostasis by modulating various immune cell activities. It decreases the activity of Th1 and Th17 CD4 T cells while increasing the activity of Tregs[77]. Additionally, it downregulates T cell-driven immunoglobulin G production and inhibits dendritic cell differentiation in the intestinal lamina propria[78]. These effects collectively contribute to the maintenance of a balanced and controlled immune response in the gut.
In summary, the anti-tumor effect of vitamin D in CRC is rooted in its ability to modulate molecular pathways in
Vitamin D’s influence on gut flora is a multifaceted interplay that extends beyond its role in immune regulation[79]. This intricate relationship involves vitamin D, immune cells, and the gut microbiota, collectively contributing to gut health and, potentially, CRC prevention[80]. One of the key aspects of this interplay is the role of immune cells in the gut. VDRs are expressed in various immune cell lineages, including CD4 T cells, CD8 T cells, B cells, neutrophils, macrophages, and dendritic cells[48]. When these immune cells are exposed to vitamin D, it triggers a series of responses that have a profound impact on gut health[81].
Studies have shown that vitamin D supplementation significantly alters the composition of the gut microbiota. It leads to a reduction in opportunistic pathogens and an increase in bacterial richness[46]. Notably, there is a decrease in gamma-proteobacteria, including bacteria like Pseudomonas and E. coli/Shigella, among individuals who adhere to vitamin D supplementation. This shift in microbial composition is mediated through mucosal CD8 and T cells, which exhibit high VDR expression[21]. CD8 and T cells, influenced by vitamin D supplementation, reduce the inflammatory environment in the gut[79]. This change in the immune response allows beneficial bacteria, such as bacilli, to outcompete opportunistic pathogens. These alterations in the gut microbiota can contribute to slowing the development and progression of CRC[82].
On the contrary, vitamin D deficiency can lead to dysbiosis in the gut microbiota, resulting in an imbalance in microbial composition[33]. This disruption in the gut ecosystem can have far-reaching consequences. This dysbiosis is characterized by reduced bacterial production of butyrate, a critical SCFA involved in maintaining intestinal homeostasis. SCFAs, including butyrate, propionate, and acetate, are the end-products of microbial fermentation and play crucial roles in nu
In individuals with vitamin D deficiency, the gut microbial profile may shift towards an enrichment of pro-inflammatory species and a decrease in beneficial bacteria[90]. This microbial imbalance can create a pro-inflammatory environment in the gut, which is conducive to the development and progression of CRC[91]. Notably, epidemiological investigations indicate that vitamin D deficiency is associated with a heightened risk of colon cancer incidence and has adverse implications for the survival rates of colon cancer patients[92].
In conclusion, vitamin D’s influence on gut flora involves a complex interplay with immune cells and the gut microbiota. Vitamin D supplementation can promote a balanced and diverse gut microbiota, which is associated with reduced inflammation and a lower risk of CRC. Conversely, vitamin D deficiency can lead to dysbiosis, creating an inflammatory milieu that may contribute to CRC development. Understanding these interactions sheds light on the potential preventive role of vitamin D in the context of CRC.
Inflammation has long been recognized as a significant driver in the development and progression of CRC[93]. Chronic inflammation in the gastrointestinal tract is associated with an increased risk of CRC, and conditions such as IBD, including Crohn’s disease and ulcerative colitis, are well-established risk factors for CRC[94]. During chronic inflammation, immune cells are continually activated, releasing pro-inflammatory cytokines and reactive oxygen species. This sustained inflammatory environment can lead to several detrimental effects, including DNA damage, oxidative stress, and the promotion of cell proliferation-factors that contribute to the initiation and growth of cancerous cells[95]. Moreover, the gut microbiota plays a crucial role in modulating inflammation in the intestinal tract. Dysbiosis, or an imbalance in the gut microbial composition, can contribute to heightened inflammation in the gut, further increasing the risk of CRC.
The insights gained from the intricate interplay between vitamin D, the immune system, gut flora, inflammation, and CRC hold significant implications for potential strategies in CRC prevention. One promising avenue is the utilization of vitamin D supplementation as a preventive measure. The evidence pointing to the protective effect of vitamin D against CRC is compelling. Clinical studies have demonstrated that increased levels of vitamin D are associated with a reduced risk of CRC development. Therefore, optimizing vitamin D levels through dietary sources, sunlight exposure, and supplements may be a viable strategy for reducing CRC risk, particularly in individuals with vitamin D deficiency.
Additionally, strategies aimed at maintaining a healthy and diverse gut microbiota are of paramount importance. Dysbiosis, characterized by an imbalance in gut microbial composition, is closely linked to CRC development. Encouraging dietary habits that promote the growth of beneficial bacteria, such as those that ferment dietary fiber to produce SCFAs like butyrate, could play a pivotal role in CRC prevention. Moreover, interventions like probiotics and microbiological agents may help rectify dysbiosis and promote gut health.
The control of chronic inflammation in the gut is another critical aspect of CRC prevention. Given the well-established association between inflammation and CRC, strategies that target inflammation, such as the use of anti-inflammatory agents or lifestyle modifications, could hold promise. Maintaining an anti-inflammatory diet, rich in antioxidants and anti-inflammatory foods, may contribute to reducing the risk of CRC.
While the existing body of research provides valuable insights into the potential preventive role of vitamin D, the immune system, and gut flora in CRC, further investigations are imperative. Rigorous clinical studies are needed to solidify the complex associations observed in experimental and epidemiological research. To establish vitamin D as a credible preventive agent against CRC, large-scale clinical trials with diverse populations are essential. These trials should focus on optimizing vitamin D levels and assessing its impact on CRC incidence. Randomized controlled trials can provide robust evidence regarding the effectiveness of vitamin D supplementation in reducing CRC risk.
Moreover, comprehensive studies that delve into the intricate interplay between vitamin D, the immune system, gut microbiota, and inflammation are warranted. These studies should encompass larger sample sizes and employ randomized designs to ensure the reliability of their findings. In conclusion, the potential strategies for CRC prevention lie in optimizing vitamin D levels, promoting gut health, and controlling inflammation. However, to translate these possibilities into practical recommendations, rigorous clinical studies are required. These studies will not only enhance our understanding of CRC prevention but also pave the way for innovative approaches to enhance public health and well-being in the fight against this devastating disease.
The intricate web of interactions among vitamin D, the immune system, gut flora, inflammation, and CRC reveals a promising avenue for CRC prevention. With CRC ranking among the most common and deadly malignancies worldwide, understanding these connections holds immense potential for improving public health.
| 1. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1339] [Article Influence: 334.8] [Reference Citation Analysis (5)] |
| 2. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3264] [Article Influence: 652.8] [Reference Citation Analysis (2)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13206] [Article Influence: 1467.3] [Reference Citation Analysis (3)] |
| 4. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64450] [Article Influence: 16112.5] [Reference Citation Analysis (176)] |
| 5. | Pourhoseingholi MA. Increased burden of colorectal cancer in Asia. World J Gastrointest Oncol. 2012;4:68-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, Sanders JG, Valsta L, Brożyńska M, Zhu Q, Tripathi A, Vázquez-Baeza Y, Loomba R, Cheng S, Jain M, Niiranen T, Lahti L, Knight R, Salomaa V, Inouye M, Méric G. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 280] [Article Influence: 93.3] [Reference Citation Analysis (1)] |
| 7. | Dekker E, Rex DK. Advances in CRC Prevention: Screening and Surveillance. Gastroenterology. 2018;154:1970-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. BMJ. 2021;374:n1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 9. | Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244-60.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 466] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 11. | Nair R, Maseeh A. Vitamin D: The "sunshine" vitamin. J Pharmacol Pharmacother. 2012;3:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 235] [Reference Citation Analysis (0)] |
| 12. | Pludowski P, Takacs I, Boyanov M, Belaya Z, Diaconu CC, Mokhort T, Zherdova N, Rasa I, Payer J, Pilz S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 13. | Srivastava SB. Vitamin D: Do We Need More Than Sunshine? Am J Lifestyle Med. 2021;15:397-401. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Rinninella E, Mele MC, Raoul P, Cintoni M, Gasbarrini A. Vitamin D and colorectal cancer: Chemopreventive perspectives through the gut microbiota and the immune system. Biofactors. 2022;48:285-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Alayed Albarri EM, Sameer Alnuaimi A, Abdelghani D. Effectiveness of vitamin D2 compared with vitamin D3 replacement therapy in a primary healthcare setting: a retrospective cohort study. Qatar Med J. 2022;2022:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Tuckey RC, Cheng CYS, Slominski AT. The serum vitamin D metabolome: What we know and what is still to discover. J Steroid Biochem Mol Biol. 2019;186:4-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | Saponaro F, Saba A, Zucchi R. An Update on Vitamin D Metabolism. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Ramasamy I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin Biochem Rev. 2020;41:103-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Bikle DD, Schwartz J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front Endocrinol (Lausanne). 2019;10:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 20. | Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1206] [Cited by in RCA: 1208] [Article Influence: 134.2] [Reference Citation Analysis (0)] |
| 21. | Fakharian F, Asgari B, Nabavi-Rad A, Sadeghi A, Soleimani N, Yadegar A, Zali MR. The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front Cell Infect Microbiol. 2022;12:953718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Berg G, Rybakova D, Fischer D, Cernava T, Vergès MC, Charles T, Chen X, Cocolin L, Eversole K, Corral GH, Kazou M, Kinkel L, Lange L, Lima N, Loy A, Macklin JA, Maguin E, Mauchline T, McClure R, Mitter B, Ryan M, Sarand I, Smidt H, Schelkle B, Roume H, Kiran GS, Selvin J, Souza RSC, van Overbeek L, Singh BK, Wagner M, Walsh A, Sessitsch A, Schloter M. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 716] [Cited by in RCA: 971] [Article Influence: 194.2] [Reference Citation Analysis (0)] |
| 23. | Pascale A, Marchesi N, Govoni S, Coppola A, Gazzaruso C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: new insights into old diseases. Curr Opin Pharmacol. 2019;49:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 24. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3641] [Article Influence: 280.1] [Reference Citation Analysis (0)] |
| 25. | Romano-Keeler J, Zhang J, Sun J. The Life-Long Role of Nutrition on the Gut Microbiome and Gastrointestinal Disease. Gastroenterol Clin North Am. 2021;50:77-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39:4925-4943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 27. | Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Front Cell Infect Microbiol. 2020;10:572912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 464] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 28. | Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 1604] [Article Influence: 200.5] [Reference Citation Analysis (0)] |
| 29. | Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 970] [Cited by in RCA: 2173] [Article Influence: 362.2] [Reference Citation Analysis (0)] |
| 30. | Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, Khalil M, Wang DQ, Sperandio M, Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 459] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 31. | Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut Microbiota and Immune System Interactions. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 32. | Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 2221] [Article Influence: 444.2] [Reference Citation Analysis (1)] |
| 33. | Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 720] [Article Influence: 180.0] [Reference Citation Analysis (0)] |
| 34. | Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 1323] [Article Influence: 441.0] [Reference Citation Analysis (0)] |
| 35. | Reese AT, Cho EH, Klitzman B, Nichols SP, Wisniewski NA, Villa MM, Durand HK, Jiang S, Midani FS, Nimmagadda SN, O'Connell TM, Wright JP, Deshusses MA, David LA. Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 36. | Zhao J, Zhang X, Liu H, Brown MA, Qiao S. Dietary Protein and Gut Microbiota Composition and Function. Curr Protein Pept Sci. 2019;20:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 37. | Fayfman M, Flint K, Srinivasan S. Obesity, Motility, Diet, and Intestinal Microbiota-Connecting the Dots. Curr Gastroenterol Rep. 2019;21:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Zhang T, Zhu T, Wen J, Chen Y, Wang L, Lv X, Yang W, Jia Y, Qu C, Li H, Wang H, Qu L, Ning Z. Gut microbiota and transcriptome analysis reveals a genetic component to dropping moisture in chickens. Poult Sci. 2023;102:102242. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D's Effect on Immune Function. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 40. | Ismailova A, White JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord. 2022;23:265-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 41. | Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 42. | Takiishi T, Van Belle T, Gysemans C, Mathieu C. Effects of vitamin D on antigen-specific and non-antigen-specific immune modulation: relevance for type 1 diabetes. Pediatr Diabetes. 2013;14:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | White JH. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 44. | Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 1398] [Article Influence: 349.5] [Reference Citation Analysis (2)] |
| 45. | Charoenngam N, Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 46. | Yamamoto EA, Jørgensen TN. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front Immunol. 2019;10:3141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 47. | Sun J, Zhang YG. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 48. | L Bishop E, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus. 2021;5:e10405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 49. | Singh P, Rawat A, Alwakeel M, Sharif E, Al Khodor S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep. 2020;10:21641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 50. | Majumder S, Shivaji UN, Kasturi R, Sigamani A, Ghosh S, Iacucci M. Inflammatory bowel disease-related colorectal cancer: Past, present and future perspectives. World J Gastrointest Oncol. 2022;14:547-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (3)] |
| 51. | Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2318] [Article Influence: 257.6] [Reference Citation Analysis (0)] |
| 52. | Yu LC. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 277] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 53. | Rogala AR, Oka A, Sartor RB. Strategies to Dissect Host-Microbial Immune Interactions That Determine Mucosal Homeostasis vs. Intestinal Inflammation in Gnotobiotic Mice. Front Immunol. 2020;11:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Matson V, Chervin CS, Gajewski TF. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology. 2021;160:600-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 55. | Kåhrström CT. Host response: Phagocytosis runs like clockwork. Nat Rev Microbiol. 2012;10:162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Artemev A, Naik S, Pougno A, Honnavar P, Shanbhag NM. The Association of Microbiome Dysbiosis With Colorectal Cancer. Cureus. 2022;14:e22156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Perry WB, Lindsay E, Payne CJ, Brodie C, Kazlauskaite R. The role of the gut microbiome in sustainable teleost aquaculture. Proc Biol Sci. 2020;287:20200184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 58. | Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J, Queipo-Ortuño MI. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 59. | Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, Khani Ali Akbari S, Yousefimashouf R, Karampoor S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139:111619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 60. | Voskarides K. An evolutionary explanation for antibiotics' association with increased colon cancer risk. Evol Med Public Health. 2022;10:214-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 61. | Kayama H, Okumura R, Takeda K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu Rev Immunol. 2020;38:23-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 439] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 62. | Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 63. | Shi JL, Lv YH, Huang J, Huang X, Liu Y. Patients with inflammatory bowel disease and post-inflammatory polyps have an increased risk of colorectal neoplasia: A meta-analysis. World J Clin Cases. 2022;10:966-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 64. | Keller DS, Windsor A, Cohen R, Chand M. Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech Coloproctol. 2019;23:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 65. | Grant WB. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer Res. 2020;40:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR, Eliassen AH, Zeleniuch-Jacquotte A, Agnoli C, Albanes D, Barnett MJ, Buring JE, Campbell PT, Clendenen TV, Freedman ND, Gapstur SM, Giovannucci EL, Goodman GG, Haiman CA, Ho GYF, Horst RL, Hou T, Huang WY, Jenab M, Jones ME, Joshu CE, Krogh V, Lee IM, Lee JE, Männistö S, Le Marchand L, Mondul AM, Neuhouser ML, Platz EA, Purdue MP, Riboli E, Robsahm TE, Rohan TE, Sasazuki S, Schoemaker MJ, Sieri S, Stampfer MJ, Swerdlow AJ, Thomson CA, Tretli S, Tsugane S, Ursin G, Visvanathan K, White KK, Wu K, Yaun SS, Zhang X, Willett WC, Gail MH, Ziegler RG, Smith-Warner SA. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst. 2019;111:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 67. | Tsounis D, Villiotou V, Melpidou A, Pantsiou C, Argyrou A, Giannopoulou C, Grigoratou A, Rontogianni D, Mantzaris GJ, Papatheodoridis G. Oxidative imbalance increases the risk for colonic polyp and colorectal cancer development. World J Gastrointest Oncol. 2022;14:2208-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 68. | Muñoz A, Grant WB. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 147] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 69. | Vernia F, Longo S, Stefanelli G, Viscido A, Latella G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 70. | Ma Y, Deng L, Huangfu Y, Zhou Y, Wang P, Shen L. Adequate vitamin D level associated with reduced risk of sporadic colorectal cancer. Front Nutr. 2023;10:1024849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 71. | Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, Li J. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7:391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 632] [Article Influence: 210.7] [Reference Citation Analysis (0)] |
| 72. | Yu J, Sun Q, Hui Y, Xu J, Shi P, Chen Y. Vitamin D receptor prevents tumour development by regulating the Wnt/β-catenin signalling pathway in human colorectal cancer. BMC Cancer. 2023;23:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 73. | Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J. 2020;91:e13357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 413] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 74. | Dorrington MG, Fraser IDC. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front Immunol. 2019;10:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 495] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 75. | Huang D, Guo Y, Li X, Pan M, Liu J, Zhang W, Mai K. Vitamin D(3)/VDR inhibits inflammation through NF-κB pathway accompanied by resisting apoptosis and inducing autophagy in abalone Haliotis discus hannai. Cell Biol Toxicol. 2023;39:885-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 76. | Huang Z, Zhang Y, Li H, Zhou Y, Zhang Q, Chen R, Jin T, Hu K, Li S, Wang Y, Chen W, Huang Z. Vitamin D promotes the cisplatin sensitivity of oral squamous cell carcinoma by inhibiting LCN2-modulated NF-κB pathway activation through RPS3. Cell Death Dis. 2019;10:936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 77. | Harrison SR, Li D, Jeffery LE, Raza K, Hewison M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif Tissue Int. 2020;106:58-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 78. | Tindemans I, Joosse ME, Samsom JN. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 79. | Fakhoury HMA, Kvietys PR, AlKattan W, Anouti FA, Elahi MA, Karras SN, Grant WB. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020;200:105663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 80. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 811] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 81. | Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. 2020;151:56-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 82. | Loke YL, Chew MT, Ngeow YF, Lim WWD, Peh SC. Colon Carcinogenesis: The Interplay Between Diet and Gut Microbiota. Front Cell Infect Microbiol. 2020;10:603086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 83. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2364] [Article Influence: 262.7] [Reference Citation Analysis (0)] |
| 84. | Kim CH. Microbiota or short-chain fatty acids: which regulates diabetes? Cell Mol Immunol. 2018;15:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 85. | Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 86. | Hu J, Lin S, Zheng B, Cheung PCK. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr. 2018;58:1243-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 87. | Ratajczak W, Rył A, Mizerski A, Walczakiewicz K, Sipak O, Laszczyńska M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim Pol. 2019;66:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 88. | Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr Physiol. 2018;8:1091-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 89. | Kang J, Sun M, Chang Y, Chen H, Zhang J, Liang X, Xiao T. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anticancer Drugs. 2023;34:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 90. | Battistini C, Ballan R, Herkenhoff ME, Saad SMI, Sun J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 91. | Cheng Y, Ling Z, Li L. The Intestinal Microbiota and Colorectal Cancer. Front Immunol. 2020;11:615056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 92. | Klampfer L. Vitamin D and colon cancer. World J Gastrointest Oncol. 2014;6:430-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 93. | Shah SC, Itzkowitz SH. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology. 2022;162:715-730.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 438] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 94. | Nagao-Kitamoto H, Kitamoto S, Kamada N. Inflammatory bowel disease and carcinogenesis. Cancer Metastasis Rev. 2022;41:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 95. | Fiorilla I, Martinotti S, Todesco AM, Bonsignore G, Cavaletto M, Patrone M, Ranzato E, Audrito V. Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |