Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2241
Peer-review started: January 2, 2024
First decision: January 25, 2024
Revised: February 3, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: May 15, 2024
Processing time: 128 Days and 12.2 Hours
Hepatocellular carcinoma (HCC) is a primary liver tumor generally diagnosed based on radiographic findings. Metastatic disease is typically associated with increased tumor diameter, multifocality, and vascular invasion. We report a case of a patient who presented with extrahepatic HCC metastasis to a portocaval lymph node with occult hepatic primary on computed tomography (CT). We re
A 67-year-old male with remotely treated hepatis C was referred for evaluation of an enlarging portocaval, mixed cystic-solid mass. Serial CT evaluations demon
Hepatocellular carcinoma can seldomly present with extrahepatic metastasis in the setting of occult primary. In patients with risk factors for HCC and lesions suspicious for metastatic disease, MRI may be integral to identifying small hepatic lesions and differentiating from ectopic HCC. Tumor markers may also have utility in establishing the diagnosis.
Core Tip: Hepatocellular carcinoma (HCC) metastases are typically associated with increased tumor diameter, multifocality, and vascular invasion. We report a rare case of HCC metastasis to a portocaval lymph node with initially occult hepatic primary. Our case illustrates that multimodal evaluation including alpha fetoprotein and contrast-enhanced magnetic resonance imaging may improve the sensitivity of identification of primary HCC lesions. We also reviewed the literature for HCC presenting as extrahepatic masses (18 cases: Metastatic lesions; 30 cases: Primary ectopic HCC), and discuss that, in patients with extrahepatic sites of HCC, thorough assessment for intrahepatic lesions is critical to classifying disease as metastatic HCC or ectopic HCC.
- Citation: Wu WK, Patel K, Padmanabhan C, Idrees K. Hepatocellular carcinoma presenting as an extrahepatic mass: A case report and review of literature. World J Gastrointest Oncol 2024; 16(5): 2241-2252
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2241.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2241
Hepatocellular carcinoma (HCC) is the fastest growing cause of cancer-related deaths in the United States[1]. The majority of HCC cases in the western hemisphere occur in patients with chronic liver disease, and surveillance in at-risk patients with abdominal ultrasound every six months is recommended[2,3]. Implementation of surveillance strategies has enabled early-stage detection of HCC, and thus, many patients are asymptomatic at the time of diagnosis, or have symptoms and findings associated with underlying cirrhosis rather than the tumor itself.
HCC diagnosed in patients outside of surveillance protocols are associated with metastatic disease at the time of diagnosis in 10%-15% of cases[4-6]. Metastases are typically found in patients with advanced local disease (size > 5 cm, macrovascular invasion)[7], with the most common sites of metastasis being lung, peritoneal lymph nodes, bone, and adrenal gland. Median survival in patients with metastatic disease is between 7 and 15 months[8].
HCC presenting as an extrahepatic lesion without known intrahepatic disease is extremely rare. Metastases from occult hepatic lesions and HCC arising from ectopic liver tissue have both been described[9,10]. We report the case of a patient who presented with an enlarging portocaval mass in the absence of known hepatic lesions, who was found to have metastatic HCC upon surgical resection and follow-up imaging. We review other cases reported in the literature of extrahepatic HCC presentation without evidence of primary hepatic lesion and discuss strategies for diagnostic differentiation between metastatic and ectopic HCC.
A 67-year-old male was referred to the surgical oncology clinic for abdominal pain and a growing portocaval mass.
He reported a one-year history of aching abdominal discomfort, nausea, and 10-pound weight loss. His review of systems was unremarkable other than abdominal pain and weight loss.
His medical history was notable for hepatitis C treated with pegylated interferon and ribavirin two decades prior and poorly controlled hypertension. He did not carry a diagnosis of cirrhosis and had never had pancreatitis. He had previously undergone an exploratory laparotomy and appendectomy four decades prior under circumstances the patient could not recall, as well as a remote laparoscopic cholecystectomy. He was a one pack per week smoker. He had no prior family history of gastrointestinal malignancy.
On physical exam, he was a well-appearing, overweight (body mass index 28.6) male with well-healed midline lapa
His complete blood count was notable for a hemoglobin of 10.4 g/dL, metabolic panel was notable for a creatinine of 1.36 mg/dL (estimated glomerular filtration rate of 57 mL/min), aspartate aminotransferase of 61 U/L (but normal alanine aminotransferase, alkaline phosphatase, and bilirubin levels), and tumor markers were notable for a carcinoembryonic antigen level of 2.9 ng/mL and carbohydrate antigen 19-9 level of 52 U/mL.
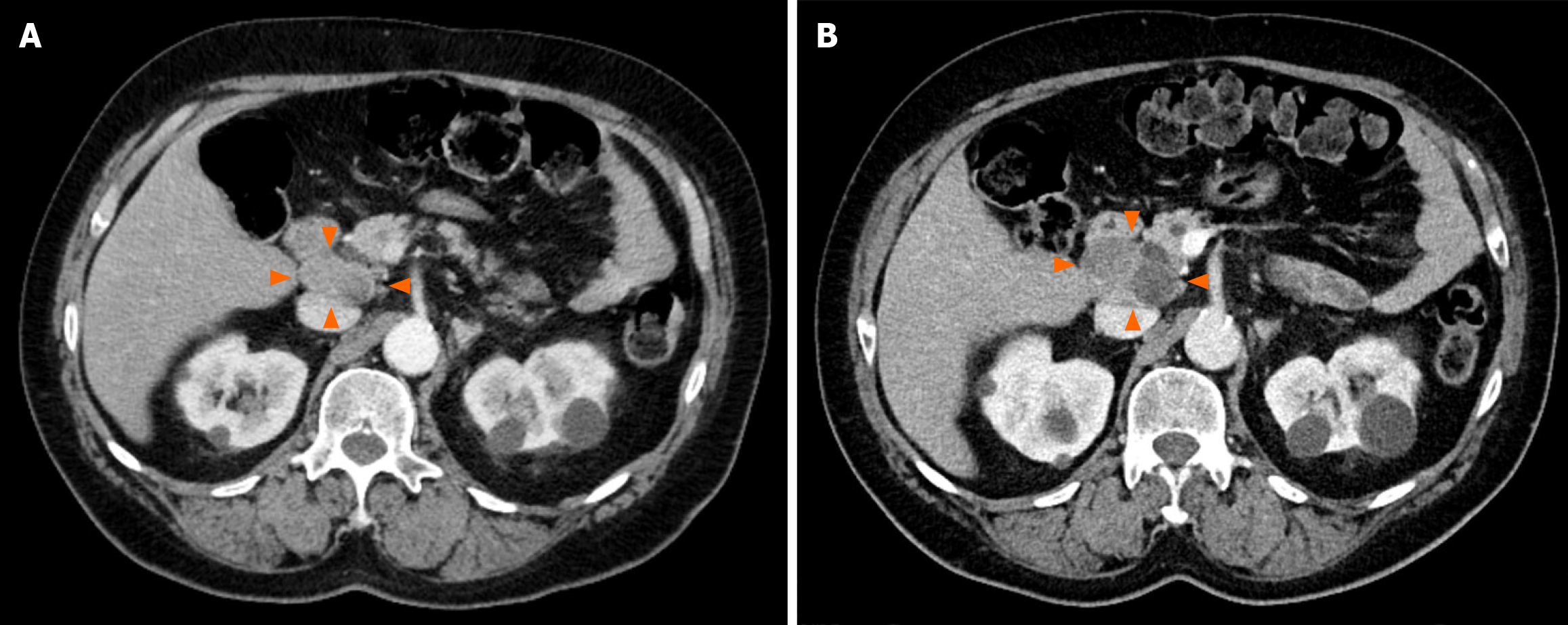
He had undergone serial intravenous-contrasted computed tomography (CT) scans of the abdomen and pelvis leading up to surgical referral, which demonstrated an enlarging portocaval lesion from 3.6 cm × 2 cm eight months prior (Figure 1A) to 5.2 cm × 3.2 cm (Figure 1B). CT also demonstrated hepatic steatosis, but no abnormal pancreatic, biliary, hepatic, or duodenal lesions.
He had undergone an upper endoscopy that demonstrated duodenal erythema, ulceration, and a possible submucosal mass. An endoscopic ultrasound demonstrated a mixed cystic-solid mass between the pancreas and inferior vena cava, as well as a normal-appearing pancreas, biliary system, and liver. Fine needle aspiration demonstrated rare fragments of cytokeratin 8/18 and arginase-1 positive atypical cells with hepatoid differentiation, negative for CD34, SOX10, synaptophysin and chromogranin immunohistochemical stains. Although suspicious, the scant nature of the specimen precluded a definitive diagnosis of malignancy.
Differential diagnosis for a portocaval, retroduodenal, peripancreatic, mixed cystic-solid lesion includes a duodenal or pancreatic cyst, lymphoproliferative cyst, stromal or mesenchymal lesions, nodal involvement from gastrointestinal or hematologic malignancy, or duodenal gastro-intestinal stromal tumor. Given an enlarging lesion with symptomatic mass effect and histologic features of atypia concerning for malignancy, surgical resection was recommended after review at our multidisciplinary tumor board.
The patient underwent open surgical resection of a 5.2 cm × 5.5 cm encapsulated retroperitoneal mass via a right sub
He re-presented on POD 9 with bilious emesis and was found to have a leukocytosis of 15.9 × 103/µL with a 5.1 cm × 5.0 cm × 8.4 cm rim-enhancing collection in the resection bed associated with compressive mass effect on the duodenum. CT-guided aspiration of the fluid yielded hematoma with no organisms on gram stain or culture. The patient had marked symptomatic improvement and was discharged home on hospital day 4 of his readmission.
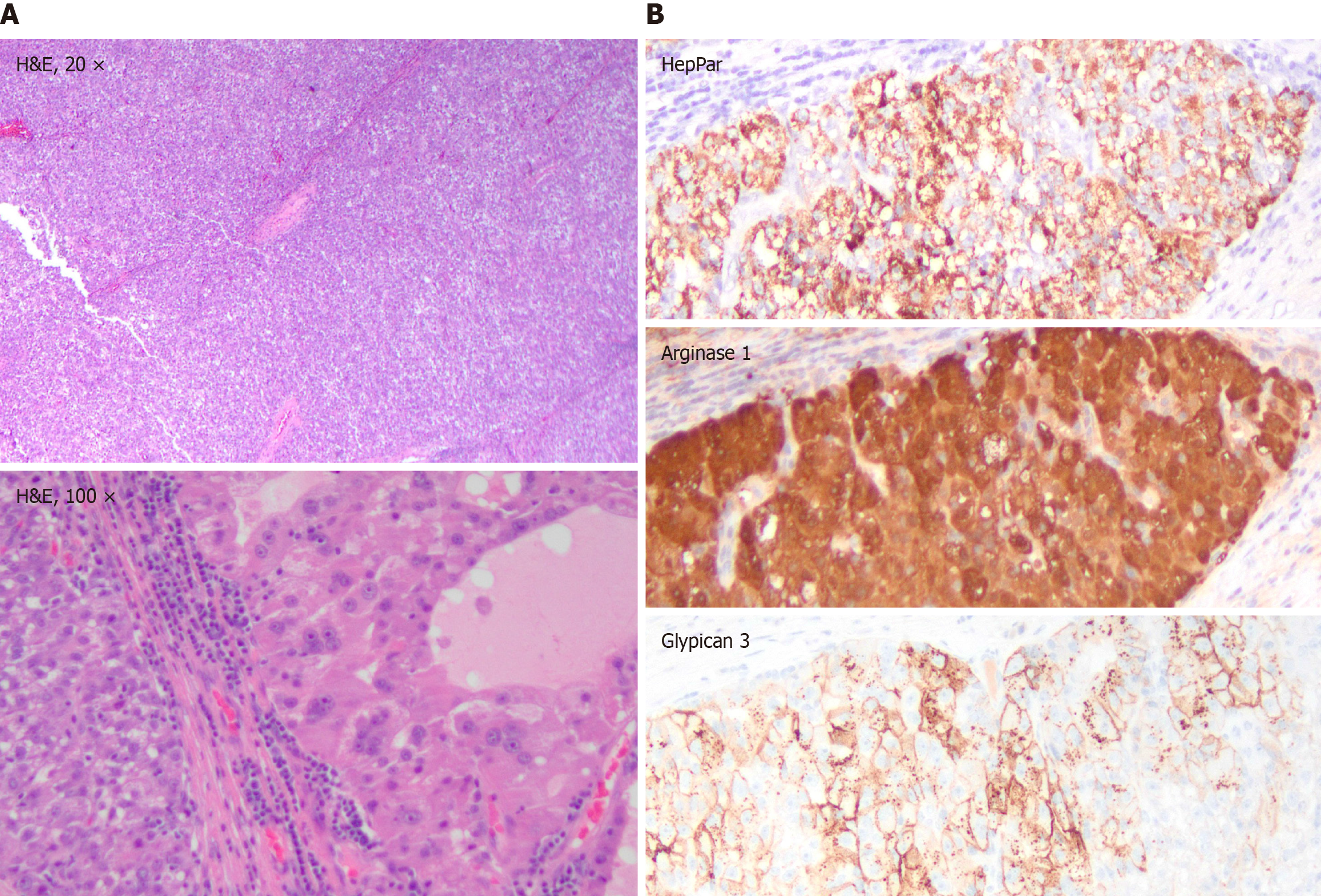
Pathologic evaluation revealed vague nodules of large polygonal cells with eosinophilic granular to clear vacuolated cytoplasm, steatotic and clear-cell changes, increased mitosis, necrosis and rare hyaline bodies (Figure 2A), positive for cytokeratin 8/18, pancytokeratin, HepPar1, arginase-1, glypican-3 (Figure 2B), and CA-9, and negative for cytokeratin 7/20, synaptophysin, chromogranin, DOG-1, CD117, PAX-8, SF-1, SOX-10, and Melan-A. Morphology and immuno
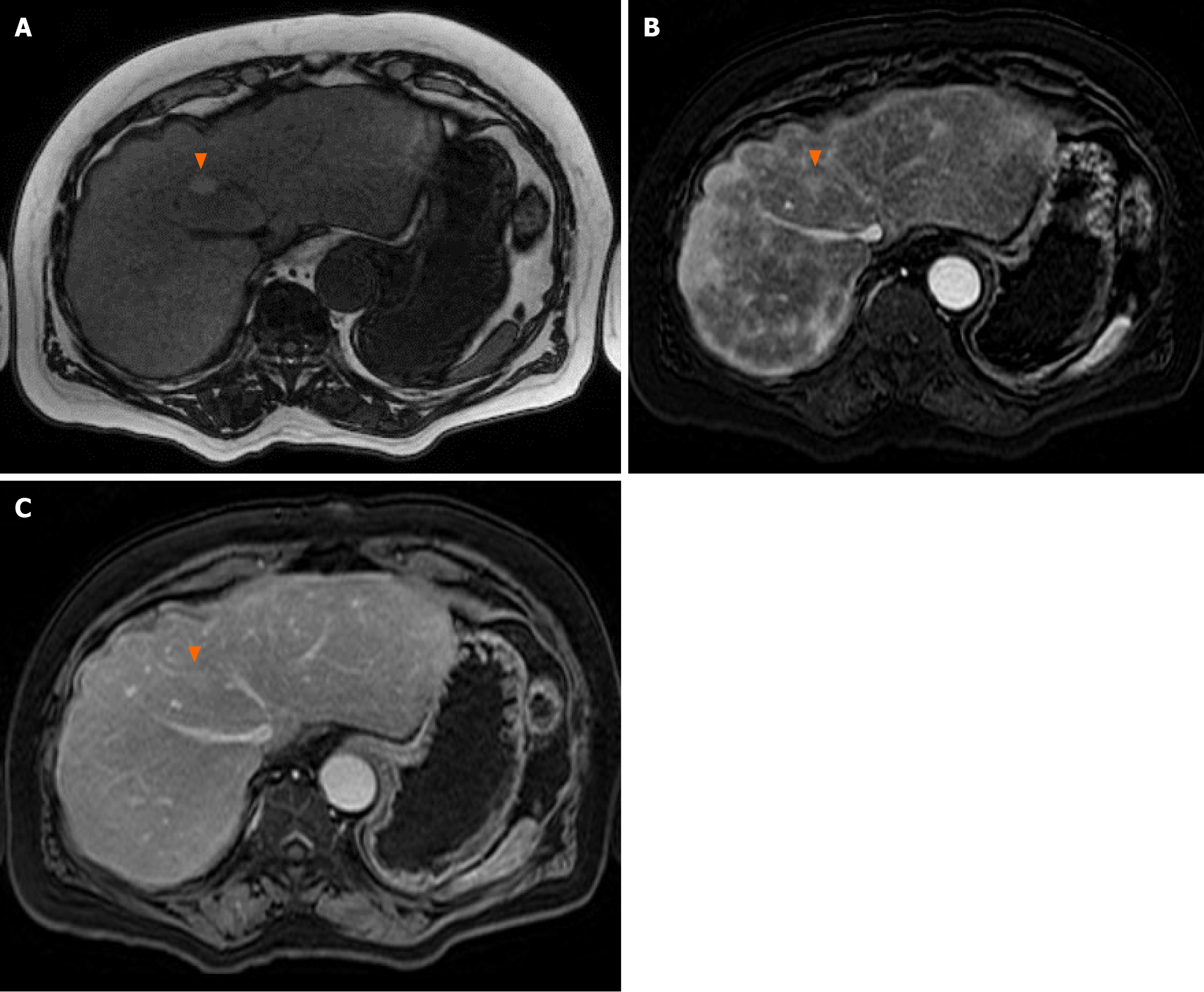
A gadobutrol-contrasted magnetic resonance imaging (MRI) abdomen was obtained, which demonstrated a 1.2 cm lesion in segment VIII with late arterial enhancement, fatty sparing, and intrinsic T1 hyperintensity, but no washout on delayed images (Figure 3), and two 1.2 cm lesions in segments II and VII with late arterial enhancement and no washout or pseudocapsule. Alpha fetoprotein (AFP) was 23.3 ng/mL. The patient was diagnosed with HCC with portocaval nodal involvement. He was referred to medical oncology for consideration of systemic therapy for his advanced HCC and was initiated on durvalumab and tremelimumab. At 8-month follow-up after surgical resection, he remained clinically well with minimal enlargement of his hepatic lesions (segment VIII, 1.9 cm; segment II, 1.5 cm; segment VII, 1.3 cm).
We report a case of a patient who presented with extrahepatic nodal metastasis of HCC with occult hepatic primary. Diagnosis was achieved upon resection of the mass and histopathologic evaluation, which was consistent with HCC invasion of a portocaval lymph node. Subsequent MRI evaluation demonstrated several small intrahepatic lesions consistent with HCC and an elevated AFP. This case report includes longitudinal workup, evaluation, treatment, and follow-up of this patient. Our limitations, mostly inherent to the nature of case reports, include the single-subject data used and thus the generalizability of our findings. We thus also include a literature review of relevant cases to corro
HCC is the most common primary liver tumor that typically arises in the setting of chronic liver disease. HCC pre
Ectopic HCC, a distinct entity where malignant degeneration occurs in normal hepatic parenchyma found outside the liver, has also been reported in gallbladder, perihepatic ligaments, omentum, retroperitoneum, and thoracic locations–and can be difficult to distinguish from foci of metastatic HCC[14]. Ectopic liver tissue is postulated to have increased neoplastic potential over the orthotopic liver due to compromised vascular supply or biliary drainage[15,16]. In cases of ectopic HCC, the orthotopic liver is typically uninvolved, and resection of the ectopic focus can be curative and associated with good prognosis[17]. A list of previously reported sites of extrahepatic HCC presentation (1) as metastases from occult hepatic lesion; and (2) deriving from ectopic hepatic parenchyma is shown in Table 1.
| Center (number of cases) | Country | Report year | Site of extrahepatic HCC presentation | Largest dimension (cm) | AFP (ng/mL) | Diagnosis achieved by | Liver imaging at time of resection or biopsy | Follow-up duration (months) | Status at follow-up | Ref. |
| Metastatic HCC | ||||||||||
| Brigham and Women’s Hospital, Deaconess Hospital, Cardinal Cushing Hospital (7) | United States | 1986 | Retrogastric (1), ovarian (1), adrenal (1), omental (1), peripancreatic (3) | Not reported | Not reported | Resection (6), autopsy (1) | Not reported | Not reported | Not reported | Longmaid et al[9] |
| Centre Régional de Lutte Contre le Cancer | France | 1995 | Skull | 8 | 66000 | Resection | CT | 35 | Alive, recurrence-free | Raoul et al[29] |
| Universita Degli Studi Di Parma | Italy | 1998 | Left iliac bone | 10 | “Normal” | Resection | CT | 45 | Alive, recurrence-free | Iosca et al[30] |
| Ankara University Medical School | Turkey | 2004 | Left chest wall | 7 | 60000 | Biopsy | CT, ultrasound | < 1 | Palliative, deceased | Coban et al[31] |
| Tata Memorial Hospital | India | 2005 | Umbilical, lung | 6.5 | 63235 | Biopsy | CT | Not reported | Not reported | Shah et al[32] |
| Tata Memorial Hospital | India | 2005 | Sternum | 9 | 18303 | Biopsy | CT | 5 | Disease progression, deceased | Qureshi et al[33] |
| Aristotle University of Thessaloniki | Greece | 2005 | Adrenal | 10 | 75.6 | Resection | CT, Ultrasound | 10 | Disease progression, deceased | Tsalis et al[34] |
| Mayo Clinic | United States | 2005 | Mesentery | Not reported | “Normal” | Biopsy | CT, Ultrasound | 8 | Disease progression, deceased | Batsis et al[35] |
| Hanyang Medical Center | South Korea | 2006 | Left chest wall | 12 | 308 | Resection | CT, Ultrasound, MRI | 12 | Vertebral metastasis, no hepatic lesions | Hyun et al[36] |
| University of Pittsburgh | United States | 2007 | Left chest wall | 15 | 16125 | Resection | CT, MRI | Not reported | Not reported | Khalbuss et al[12] |
| Nara Medical University | Japan | 2010 | Left iliac bone | 20 | 115 | Biopsy | CT, MRI, angiography | 120 | Alive, recurrence-free after TACE | Takahama et al[37] |
| Yonsei University College of Medicine | South Korea | 2012 | Right iliac bone | 13 | 15 | Biopsy | CT, MRI, angiography, PET | 12 | Disease progression, alive | Jung et al[38] |
| University of Malaya | Malaysia | 2014 | Mediastinum | Not reported | 709 | Biopsy | CT | 1 | Deceased | Koh et al[13] |
| College of Medicine, the Catholic University of Korea | South Korea | 2015 | Cervical vertebrae, right iliac bone | 7.6 | 5013 | Debulking | CT, MRI | 6 | Lung metastases, alive | Hwang et al[39] |
| Changi General Hospital | Singapore | 2015 | Adrenal | 14 | 33127 | Biopsy | CT | 2 | Disease progression, deceased | Mundada et al[40] |
| Khyber Teaching Hospital | Pakistan | 2015 | Right ilium, vertebrae | 20 | 108795 | Biopsy | CT, MRI | Not reported | Not reported | Abbas et al[41] |
| KMCT Medical College | India | 2016 | Adrenal | 7.6 | “Normal” | Resection | CT | 7 | Disease progression, alive | Pradeep et al[11] |
| Saiseikai Kanazawa Hospital | Japan | 2022 | Thoracic vertebrae | Not reported | 3.1 | Biopsy | CT | 8 | Disease progression, deceased | Shirota et al[42] |
| HCC arising from ectopic liver tissue2 | ||||||||||
| Center for Adult Diseases, Osaka | Japan | 1973 | Lesser sac | 24 | Not reported | Autopsy | Autopsy | N/A | N/A | Horiuchi |
| Kanazawa University | Japan | 1988 | Multifocal peritoneum, diaphragm | Not reported | 117000 | Resection | CT, ultrasound, angiography | 39 | Multifocal metastases in liver, deceased | Kawahara et al[43] |
| National Cancer Center Institute | Japan | 1994 | Left diaphragm | 3 | 2207 | Resection | CT, angiography | 96 | Alive, recurrence-free | Takayasu et al[44] |
| Università di Udine | Italy | 1994 | Left subphrenic | 6.5 | 2325 | Resection | CT, angiography | 12 | Alive, recurrence-free | Basile et al[16] |
| Ohmuta Municipal Hospital, Chiba University School of Medicine | Japan | 1999 | Gastric | 4 | 4900 | Resection | CT, ultrasound, angiography | 12 | Multifocal hepatic and pulmonary metastases, deceased | Arakawa et al[10] |
| Université Bordeaux | France | 1999 | Left subphrenic | Not reported | Not reported | Resection | MRI | Not reported | Not reported | Le Bail et al[45] |
| Hôpital Beaujon | France | 2000 | Chest wall | 11 | “Normal range” | Resection | CT, ultrasound, MRI, angiography | 36 | Alive, recurrence-free | Asselah et al[46] |
| Korea University College of Medicine | South Korea | 2003 | Left subphrenic | 9 | Not measured | Resection | CT | 23 | Multifocal hepatic metastases, alive | Kim et al[47] |
| Molinette Hospital (3) | Italy | 2003 | Gallbladder (1), perisplenic (2), | 9 (2), 10 (1) | 4000 in one | Resection | CT | 48-alive, recurrence-free; 4-multifocal hepatic recurrence; 48-alive, recurrence-free | Leone et al[48] | |
| Otsu Red Cross Hospital | Japan | 2006 | Lower abdomen / small bowel | 7 | 99100 | Resection | CT, MRI, angiography | 18 | Alive, single hepatic recurrence | Shigemori et al[49] |
| National Defense Medical Center | Taiwan | 2007 | Left diaphragm | 10 | 45000 | Resection | CT, ultrasound | 8 | Alive, recurrence-free | Huang et al[50] |
| University of Florida College of Medicine | United States | 2007 | Pancreatic | 3.7 | Not reported | Resection | CT | 15 | Alive, recurrence-free | Cardona et al[51] |
| National Taiwan University College of Medicine | Taiwan | 2007 | Lower abdomen / small bowel | 15 | 87500 | Resection | CT, ultrasound, MRI | Not provided | Not provided | Liu et al[52] |
| Dokkyo University Hospital | Japan | 2007 | Pancreatic | 6.5 | Not measured | Resection | CT, MRI, angiography | 36 | Alive, recurrence-free | Kubota et al[53] |
| Korea University College of Medicine | South Korea | 2008 | Left subphrenic | 4.1 | “Within normal limits” | Resection | CT | Not reported | Not reported | Seo et al[17] |
| Chhatrapati Shahuji Maharaj Medical University | India | 2010 | Left suprarenal | 8 | “Strongly positive” | Resection | CT | 6 | Multifocal metastasis, deceased | Singh et al[54] |
| Oita University Faculty of Medicine | Japan | 2011 | Left subphrenic | Not provided | 84865 | Biopsy | CT | Not provided | Not provided | Nishikawa et al[55] |
| Hekinan Municipal Hospital | Japan | 2011 | Perisplenic | 6 | Not reported | Autopsy | CT | 33 | Deceased | Matsuyama et al[56] |
| Fukuoka University | Japan | 2012 | Diffuse peritoneal | 1 | 241 | Biopsy | CT, PET | Not provided | Systemic treatment for disseminated disease | Miyake et al[57] |
| Recep Tayyip Erdogan University Training and Research Hospital | Turkey | 2014 | Para-aortic / adrenal | 6.4 | 20000 | Biopsy | CT, Ultrasound | Not provided | Not provided | Yuce et al[58] |
| University of Oslo | Norway | 2015 | Left subphrenic | 3.5 | 200 | Resection | CT, ultrasound, MRI | 48 | Alive, 3 focal recurrences resected | Aarås et al[59] |
| Inje University College of Medicine | South Korea | 2015 | Left subphrenic | 3.8 | Not provided | Resection | CT | 17 | Alive, recurrence-free | Lee et al[60] |
| Third Military Medical University, Chongqing | China | 2016 | Multifocal (perigastric, paraortic, pulmonary) | 6.4 | 24793 | Biopsy | CT, MRI, PET | 15 | Alive, on systemic therapy | Cui et al[61] |
| Medical University of Lodz | Poland | 2017 | Pancreatic | 2.5 | “Not elevated” | Resection | CT | 24 | Alive, recurrence-free | Braun et al[62] |
| West China Hospital, Sichuan University | China | 2017 | Pancreatic | 5 | 1200 | Resection | CT, ultrasound, MRI, PET | 21 | Alive, recurrence-free | Li et al[63] |
| Zhejiang University School of Medicine | China | 2017 | Multifocal (gastrohepatic ligament, perisplenic, pelvic) | 30 | 8.0 | Resection | CT, MRI | 22 | Multifocal recurrences, deceased | Jin et al[64] |
| University of Arkansas for Medical Sciences | United States | 2017 | Within choledochal cyst | Not provided | 2.9 | Biopsy | CT | Not provided | Not provided | George et al[65] |
| Complejo Hospitalario de Navarra | Spain | 2019 | Mesentery | 8 | 651 | Resection | CT, ultrasound | 24 | Alive, recurrence-free | Martínez-Acitore et al[66] |
| Kumamoto University | Japan | 2020 | Peripancreatic | 7.5 | 1.7 | Resection | CT, MRI, PET | 8 | Alive, recurrence-free | Adachi et al[14] |
| New York Medical College | United States | 2021 | Adrenal | 9.1 | 1.9 | Resection | CT, MRI | 10 | Alive, multifocal recurrence | Wei et al[67] |
These aforementioned cases represent a minority of patients with HCC, as most patients with extrahepatic disease also have locally advanced primary tumors[7]. Several staging systems have been created to predict the prognosis for patients with HCC. Primary tumors < 2 cm in diameter are considered early-stage local disease under the American Joint Committee on Cancer[18], Barcelona Clinic Liver Cancer[19], and Hong Kong Liver Cancer staging systems[20]. Macro
Additionally, hepatic lesions were not noted on serial contrast enhanced CTs prior to surgical resection of the portocaval mass, and gadobutrol-contrasted MRI was ultimately used post-operatively to detect the primary hepatic lesions. Historically, studies evaluating the sensitivity of CT and MRI in diagnosing HCC have been equivocal[24,25], likely reflecting the unique features and advantages of each modality; CT has greater spatial resolution and is less prone to artifact, while MRI has superior soft tissue contrast[26]. However, several recent studies have reported higher sensitivity of MRI over CT in the detection of HCC lesions under 2 cm[2,27,28]. Use of hepatobiliary agent gadoxetate with MRI additionally increases sensitivity and positive predictive value over CT and MRI with other contrast agents[26,27]. In our patient, MRI identification of hepatic lesions after resection of his extrahepatic HCC was critical in classifying his disease as metastatic HCC[9,12,13,29-42], which requires adjuvant treatment, rather than ectopic HCC for which resection would be curative[10,14,16,17,43-67].
Our case illustrates that, although uncommon, early-stage HCC can seldomly present with extrahepatic metastasis in a patient without prior diagnosis of HCC. Contrast-enhanced CT may not identify small hepatic lesions. In the workup of undifferentiated masses in patients with risk factors for HCC (such as hepatitis C in our patient), multimodal evaluation that includes AFP and contrast-enhanced MRI may improve the sensitivity of the diagnostic algorithm in identifying primary HCC lesions. In patients found to have extrahepatic HCC without apparent hepatic lesion on CT, additional evaluation with MRI should be considered to exclude primary intrahepatic HCC and distinguish metastatic disease from ectopic HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Surgical Oncology; Association of Academic Surgery; Society of University Surgeons; American Hepato-Pancreato-Biliary Association.
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ullah K, Pakistan; Wang Z, China; Yarmahmoodi F, Iran S-Editor: Qu XL L-Editor: A P-Editor: Zheng XM
| 1. | Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 2. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3237] [Article Influence: 462.4] [Reference Citation Analysis (1)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6048] [Article Influence: 864.0] [Reference Citation Analysis (3)] |
| 4. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 5. | Yi J, Gwak GY, Sinn DH, Kim YJ, Kim HN, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Screening for extrahepatic metastases by additional staging modalities is required for hepatocellular carcinoma patients beyond modified UICC stage T1. Hepatogastroenterology. 2013;60:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Jin YJ, Lee HC, Lee D, Shim JH, Kim KM, Lim YS, Do KH, Ryu JS. Role of the routine use of chest computed tomography and bone scan in staging workup of hepatocellular carcinoma. J Hepatol. 2012;56:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer. 1990;66:2174-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Schütte K, Schinner R, Fabritius MP, Möller M, Kuhl C, Iezzi R, Öcal O, Pech M, Peynircioglu B, Seidensticker M, Sharma R, Palmer D, Bronowicki JP, Reimer P, Malfertheiner P, Ricke J. Impact of Extrahepatic Metastases on Overall Survival in Patients with Advanced Liver Dominant Hepatocellular Carcinoma: A Subanalysis of the SORAMIC Trial. Liver Cancer. 2020;9:771-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Longmaid HE 3rd, Seltzer SE, Costello P, Gordon P. Hepatocellular carcinoma presenting as primary extrahepatic mass on CT. AJR Am J Roentgenol. 1986;146:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Arakawa M, Kimura Y, Sakata K, Kubo Y, Fukushima T, Okuda K. Propensity of ectopic liver to hepatocarcinogenesis: case reports and a review of the literature. Hepatology. 1999;29:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Pradeep P, Mampilly N, Prabhakaran P. Isolated unilateral adrenal metastasis as a presenting feature of occult hepatocellular carcinoma. Saudi Surg J. 2016;4:87. [DOI] [Full Text] |
| 12. | Khalbuss WE, Bajestani S, D'Agostino HJ. Cytomorphology of a solitary left chest wall mass: an unusual presentation from unknown primary hepatocellular carcinoma. Diagn Cytopathol. 2007;35:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Koh PS, Yusof MM, Yoong BK, Rajadurai P. Mediastinal hepatocellular carcinoma with unknown primary: an unusual and rare presentation. J Gastrointest Cancer. 2014;45 Suppl 1:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Adachi Y, Hayashi H, Yusa T, Takematsu T, Matsumura K, Higashi T, Yamamura K, Yamao T, Imai K, Yamashita YI, Baba H. Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor: A case report. World J Gastroenterol. 2020;26:2268-2275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Caygill CP, Gatenby PA. Ectopic liver and hepatocarcinogenesis. Eur J Gastroenterol Hepatol. 2004;16:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Basile A, Croatto T, Gregoris A, Tavĉar I, Zavaroni C, Costa B, Li Volsi P, Moretti C, Pizzolitto S. An unusual case of primary liver cancer. Hepatocellular carcinoma in an accessory liver. Cancer. 1994;73:1332-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Seo UH, Lee HJ, Ryu WS, Kwak JM, Shin BK, Kim WB, Lee SI, Park SS, Choi JW, Kim SH, Choi SY, Mok YJ. Laparoscopic resection of a hepatocellular carcinoma arising from an ectopic liver. Surg Laparosc Endosc Percutan Tech. 2008;18:508-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847. [RCA] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 546] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 19. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2593] [Article Influence: 864.3] [Reference Citation Analysis (59)] |
| 20. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 21. | Lee CH, Chang CJ, Lin YJ, Yen CL, Shen CH, Cheng YT, Lin CC, Hsieh SY. Nomogram predicting extrahepatic metastasis of hepatocellular carcinoma based on commonly available clinical data. JGH Open. 2019;3:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Zhou L, Ren L, Yu W, Qi M, Yuan J, Wang W, Su X, Yin F, Deng M, Wang H, Long H, Zeng J, Yu J, Fan H, Wang Z. Construction and validation of a prediction model of extrahepatic metastasis for hepatocellular carcinoma based on common clinically available data. Front Oncol. 2022;12:961194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Yoon JH, Lee WJ, Kim SM, Kim KT, Cho SB, Kim HJ, Ko YS, Kook HY, Jun CH, Choi SK, Kim BS, Cho SY, You HS, Lee Y, Son S. Simple parameters predicting extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Sci Rep. 2021;11:12984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Floriani I, D'Onofrio M, Rulli E, Chen MH, Li R, Musicco L. Performance of imaging modalities in the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ultraschall Med. 2013;34:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Fung KT, Li FT, Raimondo ML, Maudgil D, Mancuso A, Tibballs JM, Watkinson AA, Patch D, Burroughs AK. Systematic review of radiological imaging for hepatocellular carcinoma in cirrhotic patients. Br J Radiol. 2004;77:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, Santillan CS, Wolfson T, Gamst A, Sirlin CB. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY). 2016;41:71-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 27. | Semaan S, Vietti Violi N, Lewis S, Chatterji M, Song C, Besa C, Babb JS, Fiel MI, Schwartz M, Thung S, Sirlin CB, Taouli B. Hepatocellular carcinoma detection in liver cirrhosis: diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. Eur Radiol. 2020;30:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Aubé C, Oberti F, Lonjon J, Pageaux G, Seror O, N'Kontchou G, Rode A, Radenne S, Cassinotto C, Vergniol J, Bricault I, Leroy V, Ronot M, Castera L, Michalak S, Esvan M, Vilgrain V; CHIC Group. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017;37:1515-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Raoul JL, Le Simple T, Le Prisé E, Meunier B, Ben Hassel M, Bretagne JF. Bone metastasis revealing hepatocellular carcinoma: a report of three cases with a long clinical course. Am J Gastroenterol. 1995;90:1162-1164. [PubMed] |
| 30. | Iosca A, Spaggiari L, Salcuni P. A bone hepatocellular carcinoma metastasis without hepatic tumor: a long-term follow-up. Am J Gastroenterol. 1998;93:663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Coban S, Yüksel O, Köklü S, Ceyhan K, Baykara M, Dökmeci A. Atypical presentation of hepatocellular carcinoma: a mass on the left thoracic wall. BMC Cancer. 2004;4:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Shah S, Gupta S, Shet T, Maheshwari A, Wuntkal R, Mohandas KM. Metastatic clear cell variant of hepatocellular carcinoma with an occult hepatic primary. Hepatobiliary Pancreat Dis Int. 2005;4:306-307. [PubMed] |
| 33. | Qureshi SS, Shrikhande SV, Borges AM, Shukla PJ. Chest wall metastases from unknown primary hepatocellular carcinoma. J Postgrad Med. 2005;51:41-42. [PubMed] |
| 34. | Tsalis K, Zacharakis E, Sapidis N, Lambrou I, Betsis D. Adrenal metastasis as first presentation of hepatocellular carcinoma. World J Surg Oncol. 2005;3:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Batsis JA, Halfdanarson TR, Pitot H. Extra-hepatic hepatocellular carcinoma presenting as obstructive jaundice. Dig Liver Dis. 2006;38:768-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Hyun YS, Choi HS, Bae JH, Jun DW, Lee HL, Lee OY, Yoon BC, Lee MH, Lee DH, Kee CS, Kang JH, Park MH. Chest wall metastasis from unknown primary site of hepatocellular carcinoma. World J Gastroenterol. 2006;12:2139-2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Takahama J, Taoka T, Marugami N, Anai H, Kitano S, Kichikawa K, Nonomura A. Hepatocellular carcinoma of the iliac bone with unknown primary. Skeletal Radiol. 2010;39:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Jung KS, Park KH, Chon YE, Lee SR, Park YN, Lee DY, Seong JS, Park JY. A case of isolated metastatic hepatocellular carcinoma arising from the pelvic bone. Korean J Hepatol. 2012;18:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Hwang SW, Lee JE, Lee JM, Hong SH, Lee MA, Chun HG, Chun HJ, Lee SH, Jung ES. Hepatocellular Carcinoma with Cervical Spine and Pelvic Bone Metastases Presenting as Unknown Primary Neoplasm. Korean J Gastroenterol. 2015;66:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Mundada P, Tan ML, Soh AW. Radiologically occult hepatocellular carcinoma in a cirrhotic liver presenting with bilateral adrenal metastases. Med J Malaysia. 2015;70:256-258. [PubMed] |
| 41. | Abbas SH, Khan MZ, Ijaz M, Hussain SJ. Metastatic hepatocellular carcinoma to the pelvis and vertebrae in a patient with chronic hepatitis 'C' with unknown primary. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Shirota Y, Ueda Y, Sato K, Takeda Y, Hodo Y, Wakabayashi T. Solitary extrahepatic hepatocellular carcinoma in vertebrae without a primary lesion in the liver might originate from bone marrow: a case report and new hypothesis based on a review of the literature and the latest findings. Clin J Gastroenterol. 2022;15:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Kawahara E, Kitamura T, Ueda H, Ogino T, Mai M, Ooi A, Nakanishi I. Hepatocellular carcinoma arising in the abdominal cavity. An autopsy case of ectopic liver origin. Acta Pathol Jpn. 1988;38:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Takayasu K, Itabashi M, Moriyama N. Case report: ectopic hepatocellular carcinoma arising from the left diaphragm. Clin Radiol. 1994;49:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Le Bail B, Carles J, Saric J, Balabaud C, Bioulac-Sage P. Ectopic liver and hepatocarcinogenesis. Hepatology. 1999;30:585-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Asselah T, Condat B, Cazals-Hatem D, Hassani Z, Bernuau J, Groussard O, Mussot S, Lesèche G, Marcellin P, Erlinger S, Valla D. Ectopic hepatocellular carcinoma arising in the left chest wall: a long-term follow-up. Eur J Gastroenterol Hepatol. 2001;13:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Kim KA, Park CM, Kim CH, Choi SY, Park SW, Hong SJ, Seol HY, Cha IH. Hepatocellular carcinoma in an ectopic liver: CT findings. Eur Radiol. 2003;13 Suppl 4:L45-L47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Leone N, De Paolis P, Carrera M, Carucci P, Musso A, David E, Brunello F, Fronda GR, Rizzetto M. Ectopic liver and hepatocarcinogenesis: report of three cases with four years' follow-up. Eur J Gastroenterol Hepatol. 2004;16:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Shigemori M, Kondo M, Azechi H, Inoue F, Tamura J, Kobayashi H, Saiga T. A case of ectopic hepatocellular carcinoma in the jejunum. J Gastroenterol. 2006;41:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Huang TW, Chan DC, Lee HS, Yao NS, Lee SC, Cheng YL. Ectopic hepatocellular carcinoma of the diaphragm. Dig Dis Sci. 2007;52:1118-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Cardona D, Grobmyer S, Crawford JM, Liu C. Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch. 2007;450:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Liu KL, Ho MC, Chen PJ. Ectopic liver with hepatocellular carcinoma in the peritoneum. AJR Am J Roentgenol. 2007;188:W206-W207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Kubota K, Kita J, Rokkaku K, Iwasaki Y, Sawada T, Imura J, Fujimori T. Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol. 2007;13:4270-4273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Singh V, Sinha RJ, Sankhwar SN, Kumar S, Mehrotra B, Puri M, Sengottayan VK. Primary hepatocellular carcinoma in ectopic liver masquerading as left adrenal carcinoma: a rare occurrence. Rare Tumors. 2010;2:e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Nishikawa K, Watanabe K, Hisamatsu Y, Shirao K. Ectopic hepatocellular carcinoma of the left sub-diaphragm with metastasis. Intern Med. 2011;50:1505-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Matsuyama M, Sugiura S, Kakita A, Sato Y, Kuroda M. Hepatocellular carcinoma arising from ectopic liver tissue in the spleen producing insulin-like growth factor II. Pathol Res Pract. 2011;207:124-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Miyake T, Hoshino S, Yoshida Y, Aisu N, Tanimura S, Hisano S, Kuno N, Sohda T, Sakisaka S, Yamashita Y. Multiple ectopic hepatocellular carcinomas arising in the abdominal cavity. Case Rep Gastroenterol. 2012;6:629-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Yuce S, Sahin O, Bedir R, Ayaz T, Durakoglugil T. A case report of retroperitoneal extrahepatic hepatocellular carcinoma presented with elevated level of Alpha fetoprotein. Hippokratia. 2014;18:80-82. [PubMed] |
| 59. | Aarås AM, Reitan-Gjersøe TA, Waage A, Mala T, Edwin B, Løberg EM, Abildgaard A, Røsok BI. Laparoscopic resection of recurrent ectopic hepatocellular carcinoma: A case report with review of the literature and guidelines for follow-up. Int J Surg Case Rep. 2015;17:92-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Lee JY, Kim KH, Kang MS. Ectopic Hepatocellular Carcinoma Arising from the Peritoneum in a Patient with a History of Oropharyngeal Cancer: A Case Report. Case Rep Oncol. 2015;8:456-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Cui T, Diao X, Chen X, Huang S, Sun J. A case report: delayed high fever and maculopapules during Sorafenib treatment of ectopic hepatocellular carcinoma. BMC Cancer. 2016;16:543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Braun M, Kuncman W, Teresiński L, Kupnicki P, Jesionek-Kupnicka D, Kordek R. Pure hepatocellular carcinoma originates from an ectopic liver nodule located in the pancreas. Contemp Oncol (Pozn). 2017;21:311-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Li Z, Wu X, Wen T, Li C, Peng W. Multiple ectopic hepatocellular carcinomas in the pancreas: A case report. Medicine (Baltimore). 2017;96:e6747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Jin R, Yu Q, Liang X. Ectopic hepatocellular carcinoma manifesting multiple abdominal masses: A case report. Medicine (Baltimore). 2017;96:e8968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | George NE, Raghavapuram S, Banerjee D, Al-Shoha M, Fedda F, Tharian B. Ectopic Hepatocellular Carcinoma within a Choledochal Cyst Diagnosed Using Single-Operator Digital Cholangioscopy. Am J Gastroenterol. 2017;112:1347-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Martínez-Acitores D, Hernández Ainsa M, Cortés García L, Bengochea Martínez ML, Palacios Fanlo MJ. Ectopic hepatocellular carcinoma arising from the peritoneum. Rev Esp Enferm Dig. 2019;111:809-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Wei N, Wong V, Matz A, Vemulakonda LA, Wang X, Phillips J. Ectopic hepatocellular carcinoma presenting as a right adrenal mass with IVC thrombus: Case report and review of the literature. Urol Case Rep. 2022;40:101900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |