Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2233
Peer-review started: December 31, 2023
First decision: January 27, 2024
Revised: February 9, 2024
Accepted: March 13, 2024
Article in press: March 13, 2024
Published online: May 15, 2024
Processing time: 129 Days and 20 Hours
Metastatic pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy with dispiriting survival data. Immunotherapy is a promising approach to many cancer types, but achieves poor outcomes in advanced PDAC due to its immunosuppressive tumor microenvironment. We describe a case of metastatic PDAC effectively treated with pembrolizumab.
We report the case of a 67-year-old woman with unresectable locally advanced PDAC, treated with gemcitabine plus nab-paclitaxel followed by radiotherapy plus capecitabine. At nine months, pancreatic tumor progression was observed at the level of the hepatic hilum with the appearance of a new pulmonary nodule suggestive of a second primary, confirmed by left lung biopsy. Systemic immunotherapy was then initiated with pembrolizumab, an immune checkpoint inhibitor targeting programmed cell death protein-1 that covers the two tumor types. The patient showed a complete metabolic response that was maintained throughout the treatment. The patient continues to be disease-free at 5.6 years since the start of immunotherapy.
These results suggest that the administration of pembrolizumab after chemoradiotherapy has a beneficial effect in patients with metastatic PDAC. To our knowledge, this is the first reported case of a patient with metastatic PDAC and metastatic lung cancer showing such a long-lasting complete response after pembrolizumab treatment without curative surgery. Further studies are required to determine biomarkers that identify PDAC patients most likely to benefit from this immunotherapy.
Core Tip: The lack of specific symptoms in patients and the absence of a reliable and well-stablished diagnostic method, makes advanced pancreatic ductal adenocarcinoma (PDAC) the most common clinical appearance of pancreatic cancer cases. In these circumstances, surgical resection, the only curative approach for this malignancy, is unaffordable, leading to very low survival outcomes. Immunotherapy is a recent approach that has been studied in many cancer types showing encouraging results, but it has not been sufficiently investigated in PDAC. We report the case of a patient with unresectable PDAC that progressed to metastasis after treatment with chemoradiation, who showed an outstanding response to immunotherapy with pembrolizumab, achieving a complete remission within a few months, which was maintained until the end of treatment. Currently, the patient continues in disease-free state.
- Citation: Martínez-Galán J, Jiménez-Luna C, Rodriguez I, Maza E, García-Collado C, Rodríguez-Fernández A, López-Hidalgo JL, Caba O. Metastatic pancreatic and lung cancer patient in complete remission following immunotherapy: A case report and review of literature. World J Gastrointest Oncol 2024; 16(5): 2233-2240
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2233.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2233
Pancreatic cancer (PC) is the fourth leading cause of cancer death worldwide and has one of the worst 5-year relative survival rates (12%)[1]. The main reason is that more than 90% of PC cases are pancreatic ductal adenocarcinoma (PDAC)[2], and most of them are not usually candidates for curative surgical resection at the time of diagnosis, due to local invasion or distant metastasis[3].
Cancer immunotherapy modulates the immune system to enhance the antitumor immune response. One of the most widely applied therapies is the administration of immune checkpoint inhibitors which have demonstrated major potential in substantial subsets of patients with certain solid tumors[4]; however, a strong resistance has been observed in cases of PDAC due to its various immune escape mechanisms, which are favored by the dense stroma surrounding the tumor[5-7]. Pembrolizumab is a humanized monoclonal antibody that blocks programmed cell death protein-1 (PD-1), thereby avoiding immunosuppressive action through this immune checkpoint[8]. However, scant data are available on the usefulness of pembrolizumab against PDAC, considered a cancer with a poor immune response[9,10].
This report presents the case of a patient initially diagnosed with unresectable locally advanced PDAC that became metastatic after standard treatment and was then successfully treated with pembrolizumab. The patient showed a complete response and remains tumor free.
A 67-year-old woman with unresectable locally advanced PDAC treated with gemcitabine (GEM) plus nab-paclitaxel (nab-P) followed by radiotherapy plus capecitabine.
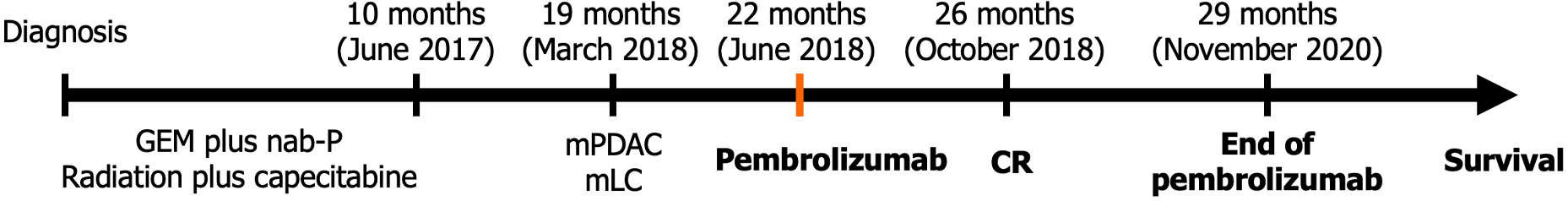
The patient diagnosed with unresectable locally advanced PDAC received neoadjuvant treatment with GEM plus nab-P that was completed without conversion to resectability, hence she continued with capecitabine-based chemoradiation therapy which ended 10 months after diagnosis, in June 2017.
Approximately nine months earlier, the patient presented with a feeling of heaviness and satiety with regurgitation. The patient had no other medical history of interest.
Her sister was diagnosed with breast cancer at the age of 52 years, two maternal cousins with breast cancer at the ages of 65 and 27 years, and one maternal cousin with colon cancer at the age of 70 years.
At physical examination, she had a depressed mood, she was slim, there was no dyspnea, but there was abdominal pain on deep palpation and residual neurotoxicity, mainly in hands.
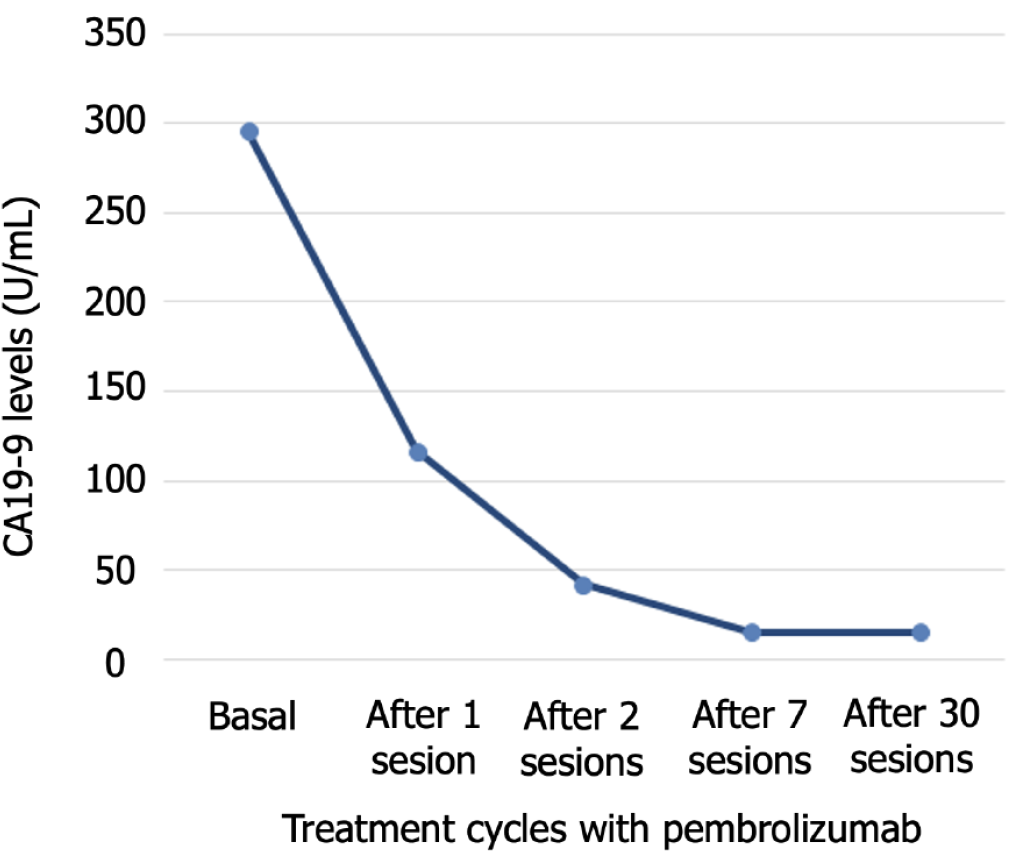
Serum tumor marker levels were abnormal: 6.1 ng/mL (normal value 0.00-5.00 ng/mL) for carcinoembryonic antigen (CEA) and 295.50 U/mL (normal value 0.0-39.0 U/mL) for carbohydrate antigen 19-9 (CA19-9). Other altered determinations were 235 mg/dL for cholesterol and 149 U/L for alkaline phosphatase. No other abnormalities were detected in routine blood or urine analyses.
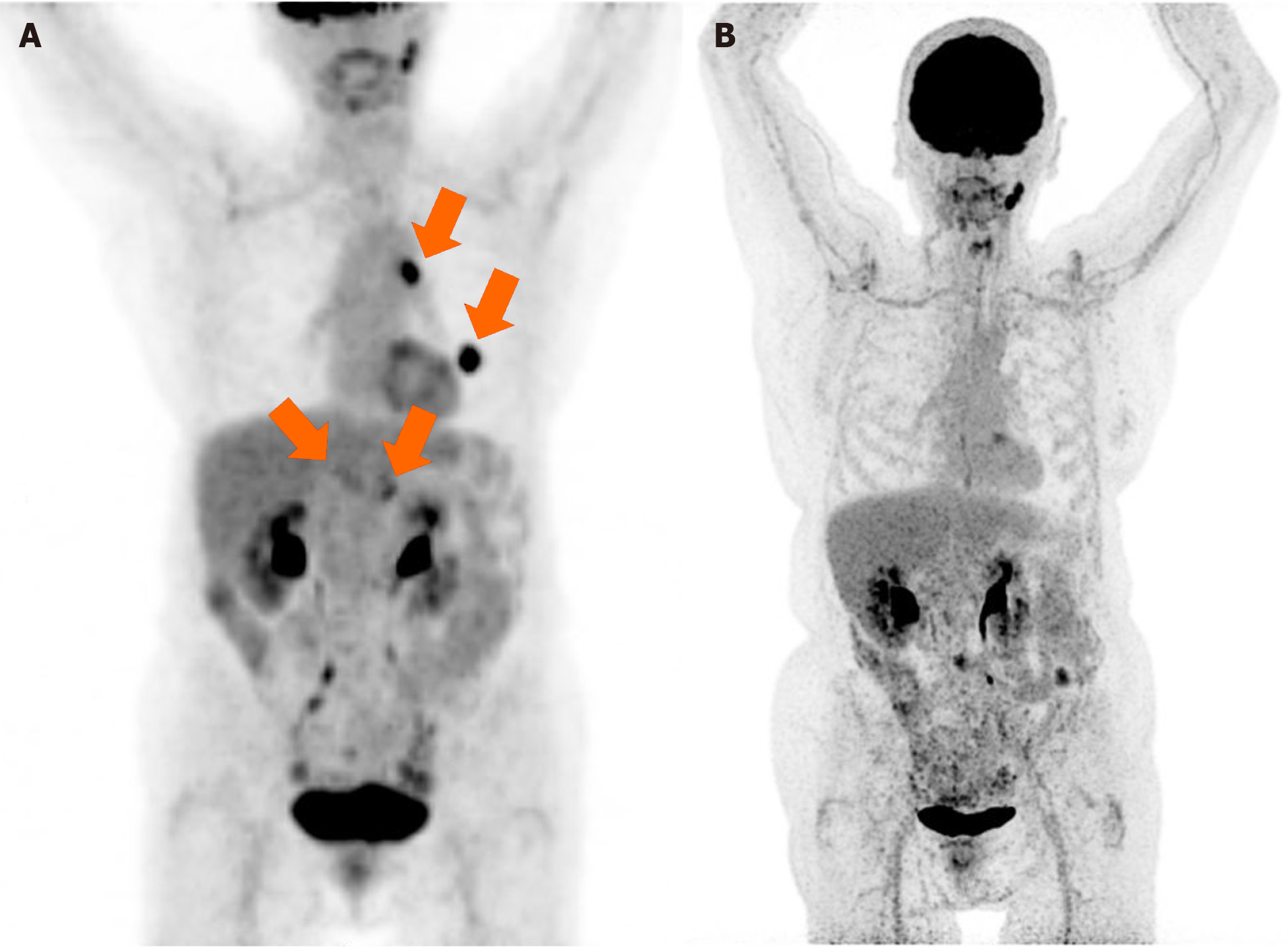
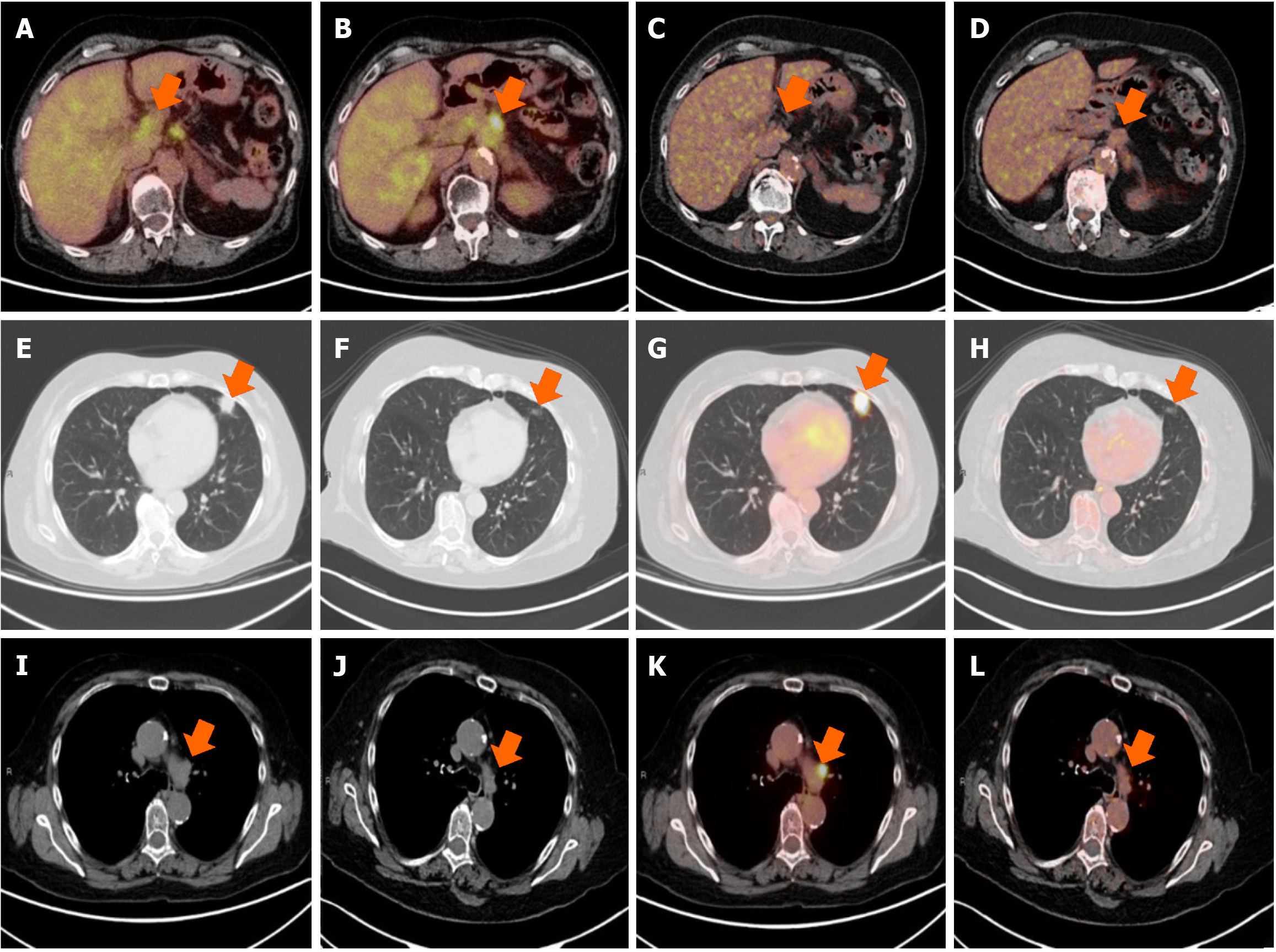
Enhanced computed tomography (CT) scan performed 18 months after diagnosis (8 months after the end of treatment) revealed a significant growth of a nodule located in the lingula of left lung (17 mm × 17.5 mm and spiculated contours). The scan also visualized a previously known pancreatic mass, approximately 40 mm × 30 mm that included the origin of the celiac trunk, exit of the hepatic artery, origin of the gastro-duodenal artery, and proximal region of the superior mesenteric artery, infiltrating and obliterating the mesenteric portal venous confluence. It was reported as bilateral adrenal hyperplasia of left predominance contacting the pancreatic mass, without ruling out infiltration, observing nonspecific mesenteric lymph nodes. One month later, positron emission tomography (PET)-CT evidenced metabolic progression of the disease, showing the presence of a pancreatic neoplastic lesion, together with an adenopathy in the hepatic hilum and two neoplastic lesions in the left lung and mediastinum. Thus, PET-CT revealed PDAC progression and the appearance of pulmonary lesions suggestive of a second primary tumor in the lung (Figures 1 and 2).
In April 2018, 20 months after diagnosis, CT-guided incisional biopsy in the left lung revealed infiltration by lung adenocarcinoma (TTF1+/P40-/CD56-). In May 2018, a second biopsy of adenopathy 4 L was performed. Anatomopathological study confirmed adenocarcinoma and metastasis compatible with bronchogenic origin (TTF1+; CK19+; CEA-). Therefore, both biopsies confirmed the presence of lung cancer with mediastinal metastases (clinically staged as T2N1). Additionally, immunohistochemical study showed 95% programmed death ligand 1 (PD-L1) positive neoplastic cells (antibody clone 22C3) in pulmonary lesions. Unfortunately, it was not possible to determine molecular parameters in the pancreatic tumor due to insufficient tumor tissue sample.
The final diagnosis was tumor progression of the initial PDAC, with metastasis involving the hepatic hilum and a second primary tumor, stage IIB lung cancer with mediastinal metastases (T2N1M0).
In June 2018, 12 months after chemoradiotherapy (i.e., 22 months since diagnosis), the patient started a two-year course of immunotherapy based on a 200 mg dose of pembrolizumab every three weeks, with the purpose of covering both the progression of PC and the appearance of lung cancer.
CA19-9 values decreased throughout the treatment with pembrolizumab, normal values were obtained after 7 cycles, and were then maintained throughout the treatment (Figure 3). Four months after starting immunotherapy (October 2018), PET-CT results evidenced a complete response, with no signs of tumor disease, which continued until the end of treatment in November 2020, after 29 months of treatment (Figures 1B and 2C-L). At 5.6 years since her diagnosis, the patient remains alive and free of disease. Figure 4 depicts the timeline of the most relevant events.
PDAC is one of the most lethal and chemoresistant cancers, and surgery is the only curative treatment. Neoadjuvant treatment is administered in some unresectable cases with local invasion in an attempt to meet the criteria for resection[11]. However, most patients with PDAC are not candidates for curative surgery, and the majority of those undergoing this surgery eventually relapse and/or develop metastases[12].
Immunotherapy with immune checkpoint inhibitors targeting the PD-1/PD-L1 axis, such as pembrolizumab, has been successful as monotherapy in some solid tumors, such as non-small cell lung cancer[13], melanoma[14], head and neck squamous cell cancer[15], and triple-negative breast cancer[16]. But, despite these promising results, a poor response has been observed in PDAC[17,18]. Various factors have been implicated, including the immunologically “cold” nature of PDAC, with scant infiltration of effector T-cells and a highly immunosuppressive microenvironment, favored by its intense desmoplastic reaction and high hypoxia[19]. PDAC is also characterized by immunosuppressive populations such as tumor-associated macrophages with M2 phenotype, myeloid-derived suppressor cells and regulatory T-cells[20,21].
The most actively investigated predictive biomarkers for treatment with anti-PD-1/anti-PD-L1 antibodies are defective DNA mismatch repair, high microsatellite instability and high tumor mutation burden, but these are observed in only around 1% of PDAC patients, in whom they have not always predicted a clinical benefit[18,19]. PD-L1 expression has also been related to the therapeutic response in other tumor types, but there is insufficient evidence on this biomarker in PDAC[22-24].
In recent years, it has been shown that chemotherapy and chemoradiotherapy are able to reprogram the tumor microenvironment in PDAC, making it immune-responsive[25] by releasing tumor antigens via apoptosis induction, decreasing infiltrating immunosuppressive populations, and increasing PD-L1 expression[26-28].
A phase Ib/II study of metastatic PDAC patients treated with GEM, nab-P, and pembrolizumab described a disease control rate of 100%, with three partial responses and an overall survival of 15 months[29]. Although the sample size was small (n = 11), these data suggested that the combination of PD-1/PD-L1 inhibitors with chemotherapy may yield clinical benefits in patients with PDAC. However, another study of GEM plus nab-P combined with nivolumab (anti-PD-1) in 50 patients with locally advanced or metastatic PDAC patients reported a disease control rate below 65% and an overall survival of 10 months[30]. These discrepant findings suggest that different PD-1 inhibitors might produce variable responses. Strikingly, Shang et al[22] described two patients with PDAC (stages III and IV) and positive PD-L1 expression but microsatellite stability whose tumor size was reduced after combined treatment with PD-1 inhibitor (tislelizumab) and chemotherapy, including GEM. Both patients underwent surgical resection, achieving R0 resection margins, and both remained alive at the time of publication. Likewise, another study reported a therapeutic response to the combination of anti-PD-1 (tislelizumab) and chemoradiotherapy in PDAC with microsatellite stability and non-high tumor mutation burden[31]. According to these data, biomarkers currently employed to predict the clinical benefit of anti-PD-1/anti-PD-L1 antibodies are inadequate for patients with PDAC, indicating the involvement of other mechanisms.
We report here a case of metastatic PDAC with a striking clinical response to PD-1 inhibition by pembrolizumab. The patient was diagnosed with unresectable locally advanced PDAC that was refractory to chemoradiotherapy, and progressed to metastasis, with the appearance of a second primary tumor in the lung. Given the severity of PDAC and the resistance shown to taxanes, GEM, and capecitabine, systemic treatment was administered with pembrolizumab to cover both tumor types. The patient showed a complete remission of both diseases after a few months of this treatment, and is currently alive and disease-free at 5.6 years.
The main study limitation was the lack of sufficient tumor tissue to evaluate the presence of some stablished predictive biomarkers. The high PD-L1 expression observed in the lung cancer may be responsible for the good response at pulmonary level, but we cannot specify the mechanism underlying the successful response at pancreatic levels. Authors have demonstrated the benefit of chemoradiotherapy in reshaping the tumor microenvironment in PDAC, and these conventional treatments might change the tumor from being immunologically cold to hot, favoring the effectiveness of this immunotherapy. It could also be hypothesized that our patient is one of the 1% of cases with a tumor phenotype that could potentially benefit from immunotherapy. Within these limitations, our study contributes data on the use of PD-1 inhibitors in metastatic PDAC. It should be noted that the United States Food and Drug Administration has approved pembrolizumab for unresectable or metastatic solid tumors, including PDAC, but only if the aforementioned biomarkers are present, while the European Medicines Agency has excluded PDAC as a candidate tumor for this immunotherapy. According to the present report, this approach can be of major benefit against PDAC, even in the absence of surgery and in the presence of metastasis. Further research is needed in a larger number of patients to identify predictive biomarkers of the response to anti-PD-1. Nevertheless, the striking response obtained in our patient supports the use of pembrolizumab plus chemoradiotherapy in PDAC, and suggests the need to review clinical protocols for this malignancy.
Immunotherapy based on PD-1/PD-L1 inhibition is not a widespread strategy against PDAC due to the poor response observed in monotherapy and the small patient population considered likely to obtain a therapeutic effect. To our best knowledge, this is the first reported case of a patient with metastatic PDAC previously treated with chemoradiotherapy and without therapeutic options showing exceptional sensitivity to pembrolizumab, and achieving a durable complete remission without curative surgery. This suggests the potential clinical benefit of this immunotherapy after chemoradiation in patients with PDAC.
The authors are grateful to Layla and Richard Davies for assistance with the English version. María Zambrano postdoctoral fellowship funded by the Spanish Ministry of Universities and Next Generation European Union funds supported the salary of Cristina Jiménez-Luna.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Batta A, India S-Editor: Li L L-Editor: A P-Editor: Zhang XD
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9963] [Article Influence: 4981.5] [Reference Citation Analysis (2)] |
| 2. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1262] [Article Influence: 180.3] [Reference Citation Analysis (39)] |
| 3. | Muñoz Martín AJ, Adeva J, Martínez-Galán J, Reina JJ, Hidalgo M. Pancreatic ductal adenocarcinoma: metastatic disease. Clin Transl Oncol. 2017;19:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 854] [Article Influence: 213.5] [Reference Citation Analysis (0)] |
| 5. | Leowattana W, Leowattana P, Leowattana T. Systemic treatment for advanced pancreatic cancer. World J Gastrointest Oncol. 2023;15:1691-1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Reference Citation Analysis (1)] |
| 6. | Beatty GL, Eghbali S, Kim R. Deploying Immunotherapy in Pancreatic Cancer: Defining Mechanisms of Response and Resistance. Am Soc Clin Oncol Educ Book. 2017;37:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Jimenez-Luna C, Prados J, Ortiz R, Melguizo C, Torres C, Caba O. Current Status of Immunotherapy Treatments for Pancreatic Cancer. J Clin Gastroenterol. 2016;50:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12:2777-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 9. | Zhao L, Singh V, Ricca A, Lee P. Survival Benefit of Pembrolizumab for Patients With Pancreatic Adenocarcinoma: A Case Series. J Med Cases. 2022;13:240-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Storandt MH, Tran N, Martin N, Jatoi A. Pembrolizumab near the end of life in patients with metastatic pancreatic cancer: a multi-site consecutive series to examine survival and patient treatment burden. Cancer Immunol Immunother. 2023;72:2515-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Atiq S, Atiq OO, Atiq ZO, Samad S, Atiq O. A Case of Metastatic Pancreatic Adenocarcinoma in Complete Remission Using Chemotherapy and Immunotherapy. Cureus. 2021;13:e13133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Papadoniou N, Kosmas C, Gennatas K, Polyzos A, Mouratidou D, Skopelitis E, Tzivras M, Sougioultzis S, Papastratis G, Karatzas G, Papalambros E, Tsavaris N. Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res. 2008;28:543-549. [PubMed] |
| 13. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leal TA, Riess JW, Jensen E, Zhao B, Pietanza MC, Brahmer JR. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol. 2021;39:2339-2349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 681] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 14. | Si L, Zhang X, Shu Y, Pan H, Wu D, Liu J, Mao L, Wang X, Wen X, Gu Y, Zhu L, Lan S, Cai X, Diede SJ, Dai H, Niu C, Li J, Guo J. Pembrolizumab in Chinese patients with advanced melanoma: 3-year follow-up of the KEYNOTE-151 study. Front Immunol. 2022;13:882471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 15. | Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy vs cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2081] [Article Influence: 346.8] [Reference Citation Analysis (0)] |
| 16. | Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, Winer EP, Mukai H, Tamura K, Armstrong A, Liu MC, Iwata H, Ryvo L, Wimberger P, Rugo HS, Tan AR, Jia L, Ding Y, Karantza V, Schmid P. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 17. | Wong W, Alouani E, Wei A, Ryu YK, Chabot JA, Manji GA. Future of immunotherapy in pancreas cancer and the trials, tribulations and successes thus far. Semin Oncol. 2021;48:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Chouari T, La Costa FS, Merali N, Jessel MD, Sivakumar S, Annels N, Frampton AE. Advances in Immunotherapeutics in Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 792] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 20. | Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, Allison JP, LeBleu VS, Kalluri R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017;8:15095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 480] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 21. | Ogawa Y, Masugi Y, Abe T, Yamazaki K, Ueno A, Fujii-Nishimura Y, Hori S, Yagi H, Abe Y, Kitago M, Sakamoto M. Three Distinct Stroma Types in Human Pancreatic Cancer Identified by Image Analysis of Fibroblast Subpopulations and Collagen. Clin Cancer Res. 2021;27:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 22. | Shang L, Li P, Fan J, Zhao C, Niu X, Bian Q, Yuan Z, Kong Y, Zhu T, Xu B, Dong J, Xiang H. Case report: Two PD-L1 positive unresectable advanced pancreatic carcinoma patients with microsatellite stability achieved R0 resection after PD-1 antibody plus chemotherapy as a successful downstaging therapy: A report of two cases. Front Immunol. 2022;13:946266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Quintanilha JCF, Storandt MH, Graf RP, Li G, Keller R, Lin DI, Ross JS, Huang RSP, Schrock AB, Oxnard GR, Chakrabarti S, Mahipal A. Tumor Mutational Burden in Real-World Patients With Pancreatic Cancer: Genomic Alterations and Predictive Value for Immune Checkpoint Inhibitor Effectiveness. JCO Precis Oncol. 2023;7:e2300092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, You L, Zheng L, Zhang T, Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 25. | Li K, Tandurella JA, Gai J, Zhu Q, Lim SJ, Thomas DL 2nd, Xia T, Mo G, Mitchell JT, Montagne J, Lyman M, Danilova LV, Zimmerman JW, Kinny-Köster B, Zhang T, Chen L, Blair AB, Heumann T, Parkinson R, Durham JN, Narang AK, Anders RA, Wolfgang CL, Laheru DA, He J, Osipov A, Thompson ED, Wang H, Fertig EJ, Jaffee EM, Zheng L. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy. Cancer Cell. 2022;40:1374-1391.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 26. | Mukherji R, Debnath D, Hartley ML, Noel MS. The Role of Immunotherapy in Pancreatic Cancer. Curr Oncol. 2022;29:6864-6892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 27. | Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905-4913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 345] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 29. | Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schütz E, Khemka V. Correction to: Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2019;37:797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wainberg ZA, Hochster HS, Kim EJ, George B, Kaylan A, Chiorean EG, Waterhouse DM, Guiterrez M, Parikh A, Jain R, Carrizosa DR, Soliman HH, Lila T, Reiss DJ, Pierce DW, Bhore R, Banerjee S, Lyons L, Louis CU, Ong TJ, O'Dwyer PJ. Open-label, Phase I Study of Nivolumab Combined with nab-Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin Cancer Res. 2020;26:4814-4822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 31. | Lu C, Zhu Y, Cheng H, Kong W, Zhu L, Wang L, Tang M, Chen J, Li Q, He J, Li A, Qiu X, Chen D, Meng F, Qian X, Liu B, Qiu Y, Du J. Case Report: Pathologic Complete Response to Induction Therapy in a Patient With Potentially Resectable Pancreatic Cancer. Front Oncol. 2022;12:898119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |