Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2225
Peer-review started: December 17, 2023
First decision: January 10, 2024
Revised: January 30, 2024
Accepted: March 5, 2024
Article in press: March 5, 2024
Published online: May 15, 2024
Processing time: 143 Days and 23.1 Hours
Hepatocellular carcinoma (HCC), a major contributor to cancer-related deaths, is particularly prevalent in Asia, largely due to hepatitis B virus infection. Its pro
The patient presented with large HCC complicated by intratumoral bleeding. Treatment involved a multidisciplinary approach, providing individualized care. The strategy included drug-eluting bead transarterial chemoembolization, sorafenib-targeted therapy, laparoscopic partial hepatectomy, and standardized sintilimab monoclonal antibody therapy. Six months after treatment, the patient achieved complete radiological remission, with significant symptom relief. Imag
This study demonstrated effective multidisciplinary treatment for massive HCC with intratumoral bleeding, providing insights for future similar cases.
Core Tip: In this study, a unique multidisciplinary approach was demonstrated for treating a patient with massive hepatocellular carcinoma (HCC) complicated by hemorrhage. Treatment included combined drug-eluting bead transarterial chemoembolization, targeted therapy, laparoscopic surgery, and monoclonal antibody therapy. This method achieved complete radiological remission and significant symptom relief, offering a novel and effective strategy for managing complex HCC patients. This report provides valuable insights for the treatment of similar conditions, paving the way for future clinical practice.
- Citation: Kou XS, Li FF, Meng Y, Zhao JM, Liu SF, Zhang L. Multidisciplinary comprehensive treatment of massive hepatocellular carcinoma with hemorrhage: A case report and review of literature. World J Gastrointest Oncol 2024; 16(5): 2225-2232
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2225.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2225
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide[1]. Representing 75%-85% of all primary liver cancers, it predominantly occurs in Asia, with more than 50% of cases occurring in China. The primary cause in Asia is hepatitis B virus (HBV) infection[2], while in the United States and Europe, hepatitis C virus and alcohol are common risk factors, with nonalcoholic steatohepatitis also emerging as a recognized risk[3,4]. In addition to these causes, the importance of prevention of HCC cannot be overstated[5]. The prognosis of HCC remains poor across all regions of the world, with incidence and mortality rates being roughly equivalent. From 1990 to 2019, there was a decline in mortality, incidence, and prevalence. The global health care burden caused by viral hepatitis is decreasing[6,7]. HBV, a DNA virus, can induce chronic necrotic inflammation, promote hepatocellular mutations, and lead to HCC[8]. Advances in diagnostic techniques and treatment options, including hepatic resection, liver transplantation, radiofrequency ablation (RFA), transarterial chemoembolization (TACE), external radiotherapy, and molecular targeted therapies, have improved patient prognoses[9]. Combined immunotherapy is now a standard treatment for advanced HCC[10]. However, larger HCCs remain challenging to treat, often require advanced surgical skills and are associated with increased risks of bleeding, prolonged surgery and hospital stays, and increased mortality, thus leading to poorer outcomes[9,11,12].
We report the case of a patient diagnosed with massive HCC with rupture and hemorrhage. After evaluating and discussing treatment options, the patient underwent multidisciplinary treatment, including drug-eluting bead TACE (DEB-TACE), laparoscopic partial hepatectomy, and standardized sintilimab monoclonal antibody combined with sorafenib therapy. The patient is currently in good health, without abdominal pain, discomfort, or signs of tumor recurrence, and remains under close follow-up.
A 40-year-old male patient was admitted to the hospital with a hepatic lesion. He experienced sudden abdominal pain, dizziness, and fatigue and was initially diagnosed with liver cancer at a local hospital, where he underwent transarterial embolization.
The patient improved postoperatively but was later diagnosed with massive HCC with rupture and hemorrhage at our institution.
He had a history of untreated hepatitis B, with tests showing positive Hepatitis B surface antigen, e antibody, and core antibody, but negative HBV DNA. Liver function was normal.
The patient denied any family history of malignant tumors.
Physical examination revealed tenderness under the right costal margin but no palpable mass, splenomegaly, or shifting dullness.
After admission, the patient underwent basic laboratory tests, which did not reveal any abnormal findings.
The patient's imaging findings were negative except for the liver.
Based on various clinical data, including patient complaints, medical history, and auxiliary examinations, the patient was ultimately diagnosed with ruptured massive HCC accompanied by bleeding.
After a comprehensive assessment of the patient's condition and considering the large size of the tumor, a right hepatectomy was deemed unsuitable due to insufficient remaining liver tissue volume (< 40%), which could lead to a high probability of liver failure. Consequently, it was recommended to proceed with TACE combined with Sindilizumab monoclonal antibody and Lenvatinib treatment. The patient was also prescribed daily Entecavir capsules (0.5 mg orally) for long-term use, with no associated adverse reactions observed.
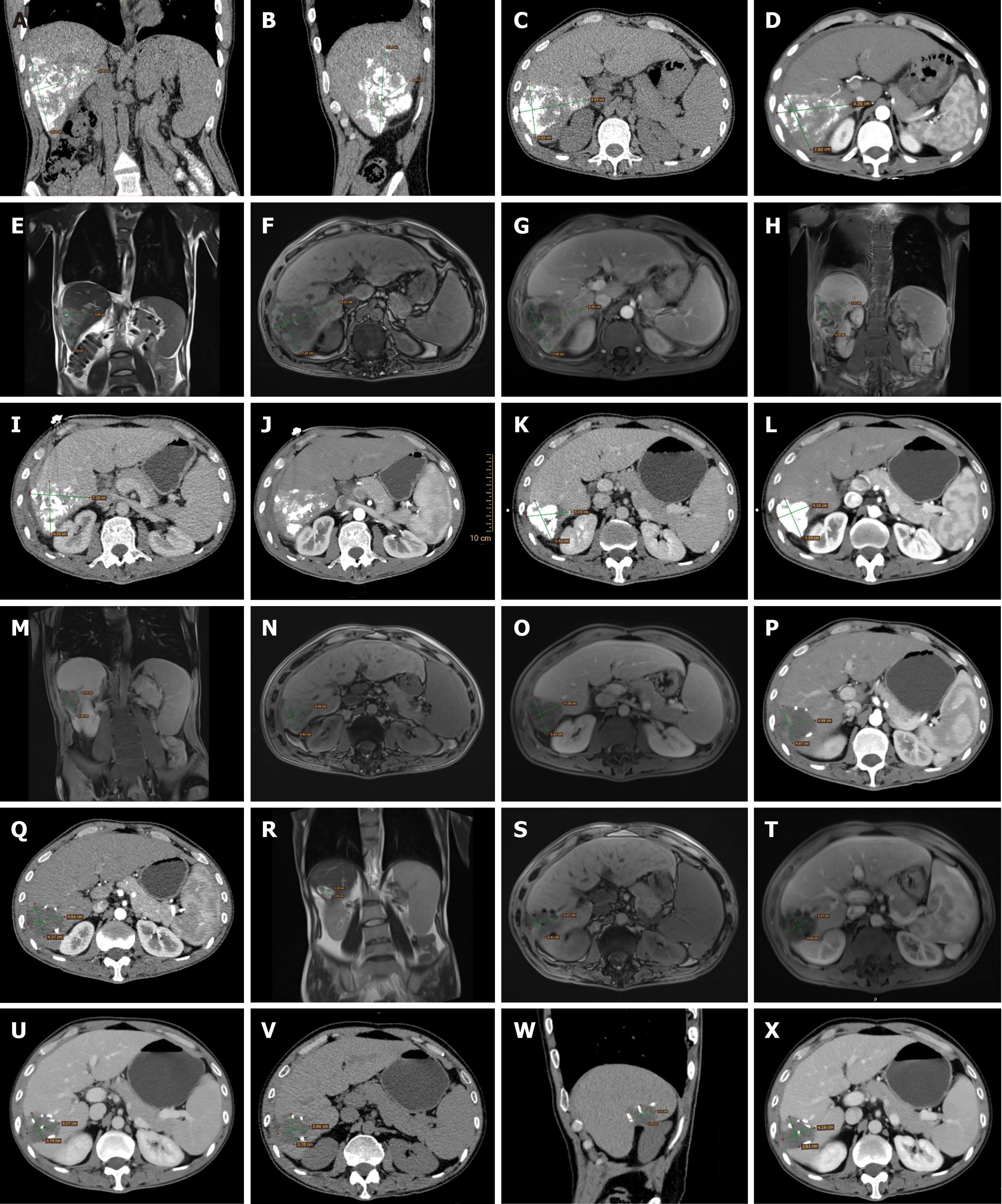
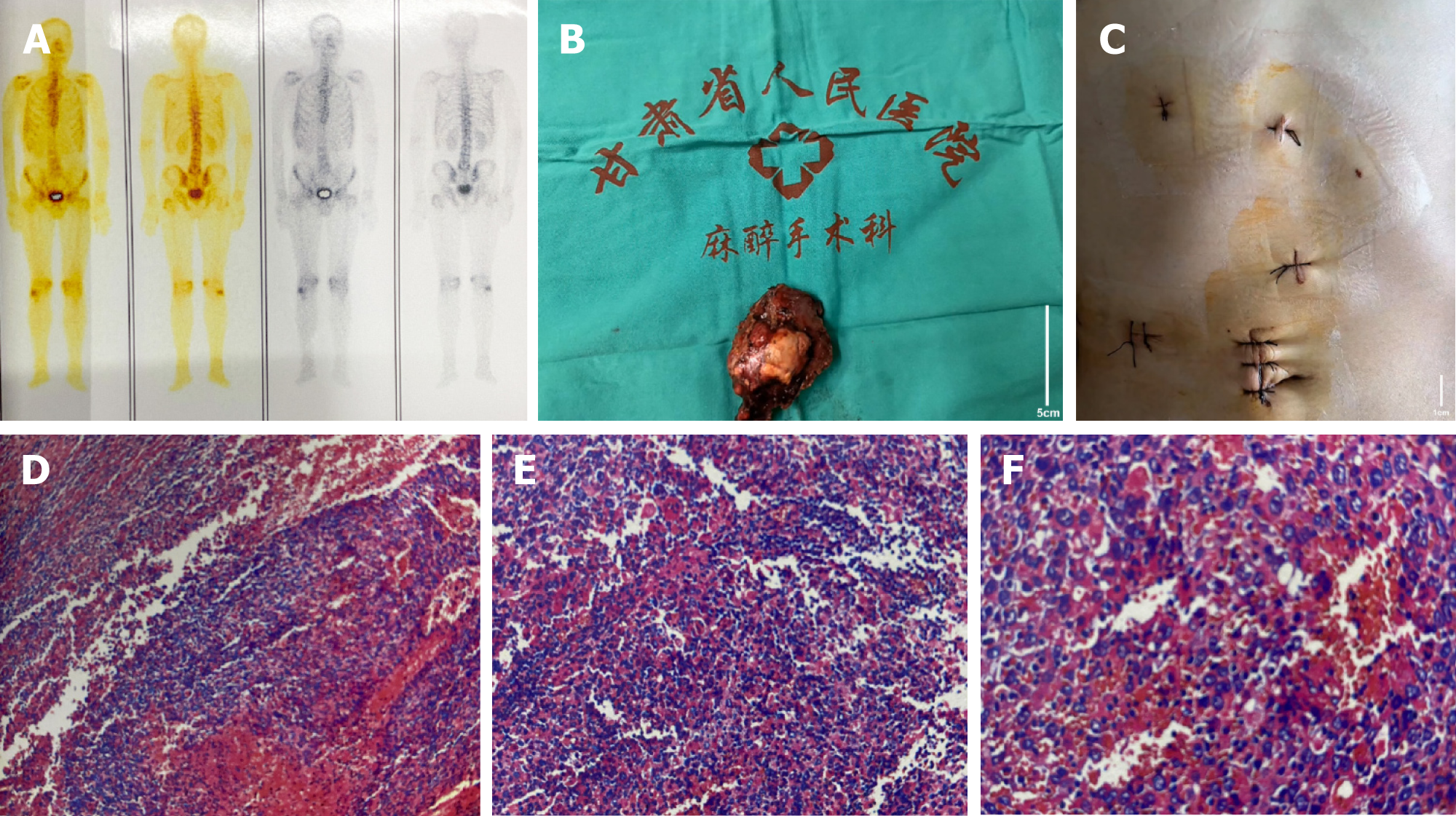
One month post-intervention, in collaboration with the interventional radiology department, drug-eluting microspheres loaded with doxorubicin were used to embolize the hepatic lesions and the main tumor artery, yielding good results. Once the condition stabilized, abdominal computed tomography (CT) and magnetic resonance imaging (MRI) were performed, locating the liver tumor in segments S6-S7 (Figure 1A-H). Three months later, after completing the second round of Sindilizumab monoclonal antibody and Lenvatinib treatment, an abdominal CT follow-up showed a reduction in tumor size (Figure 1I and J). The third round of Sindilizumab monoclonal antibody treatment was completed by the fourth month. During the fifth month post-surgery, upon hospital admission, another abdominal MRI was conducted, revealing further reduction in tumor size and compensatory hypertrophy of the left liver, indicating a postoperative residual liver volume of > 40% (Figure 1K-O). Three months postsurgery, the whole-body bone scan indicated no obvious metastatic lesions (Figure 2A).
After thorough preoperative preparation, our surgical team, in coordination with the anesthesiology and surgery team, performed a laparoscopic resection of malignant liver tumor. During the laparoscopy, a nodular lesion approximately 4 cm × 3 cm in size was observed in segment S6 of the right liver lobe, characterized by a hard texture and limited mobility. The initial step involved freeing the hepatic portal structures, intermittently obstructing hepatic portal blood flow for 15 min, and 5 min intervals. Using an ultrasonic scalpel, an incision was made 1 cm from the tumor's edge through the liver capsule and parenchyma. Local blood vessels and bile ducts were ligated and severed, followed by the complete excision of the tumor. Hemostasis and irrigation of the surgical site were performed, and after ensuring no active bleeding, the specimen was removed. An abdominal drainage tube was placed, and the abdomen was closed. The surgery lasted for 190 min with an estimated blood loss of about 800 mL. During the procedure, 1.5 units of red blood cell suspension and 275 mL of plasma were transfused.
Intraoperative images of the tumor and postoperative incision are shown in Figure 2B and C. Postoperative pathology results indicated a major pathologic response of the tumor, with no evidence of cancerous tissue involvement at the liver resection margins (Figure 2D-F).
Six months after admission, a follow-up abdominal CT showed fluid accumulation in the surgical area (Figure 1P), prompting treatment with Sindilizumab monoclonal antibody. In the seventh month of the treatment timeline, additional Sindilizumab treatment was administered, followed by another session in the eighth month (Figure 1Q). A follow-up abdominal MRI in the ninth month revealed a decrease in lesion size (Figure 1R-T), and Sindilizumab treatment continued. After eleven months from the start of treatment, another examination was conducted (Figure 1U). Continuous Sindilizumab treatment was maintained up to the twelfth month, with no adverse reactions noted. Fifteen months after the initial treatment, an abdominal CT scan indicated stability in the postoperative hepatic region, with no signs of recurrence (Figure 1V-X). The patient's imaging presentation was similar to that of previous studies[13].
Des-gamma-carboxy prothrombin (PIVKA-II) remains valuable in diagnosing alpha-fetoprotein (AFP)-negative HCC, as its levels correlate with certain pathological features indicative of tumor aggressiveness and poor prognosis. PIVKA-II is also useful in evaluating the effectiveness of liver cancer surgery. Serum ferritin (FER) plays a significant role in predicting the prognosis and survival of patients with advanced liver cancer and can be used in conjunction with AFP and PIVKA-II for a comprehensive assessment of treatment efficacy and prognosis. In this patient, levels of AFP, FER, and PIVKA-II showed a marked downward trend following treatment. Liver function fluctuated during the treatment period, with instances of elevation and reduction, but normalized after administration of hepatoprotective drugs such as ammonium glycyrrhizinate and acetylcysteine. On the last check-up, the aforementioned indicators were all within the normal range, suggesting effective disease control (Table 1). The patient continues to be closely monitored.
| AFP (< 8.78 g/m) | FER (21.81-274.66 g/m) | PIVKA-II (13.62-40.38 AU/mL) | ALT (9-50 U/L) | AST (15-40 U/L) | γ-GT (10-60 U/L) | ALP (45-125 U/L) | |
| POM 1 | > 2000 | 417.69 | 80.53 | 40 | 21 | 52.33 | 95 |
| POM 2 | > 2000 | 450.84 | 73.78 | 58 | 52 | 76.2 | 139 |
| POM 3 | > 2000 | 237.77 | 453.92 | 30 | 25 | 53.1 | 116 |
| POM 4 | 140.84 | 272.95 | 35.43 | 56 | 40 | 47.06 | 126 |
| POM 4.5 | 8.11 | 285.44 | 10.81 | 26 | 17 | 41.4 | 87 |
| POM 5 | 2.05 | 181.46 | 17.04 | 47 | 33 | 31.6 | 104 |
| POM 6 | 1.22 | 225.3 | 33.28 | 63 | 47 | 65.88 | 110 |
| POM 7 | 0.75 | 197.6 | 26.96 | 23 | 15 | 46 | 105 |
| POM 12 | 52.12 | 0.84 | 20.73 | 64.29 | 32.34 | 70.97 | 125.61 |
| POM 13 | 80.03 | 0.95 | 26.54 | 36.61 | 21.01 | 59.61 | 117.39 |
Current data indicate that although the incidence and mortality rates of liver cancer are declining annually, it is one of the cancers with high diagnostic and mortality rates in our country[14]. The primary treatment modalities for liver cancer include radical therapies such as hepatectomy, liver transplantation, ablative therapy, TACE, radiotherapy, and systemic therapy[2,15,16]. In the early stages of HCC, RFA treatment can be considered[16]. At present, there is no uniform treatment standard for large-volume liver cancer. The larger the liver cancer lesion is, the more challenging the treatment becomes[17]. HCC is commonly associated with chronic liver disease, necessitating multidisciplinary collaboration and individualized assessment to achieve maximal tumor eradication without significantly compromising liver function. Typically, the treatment approach is determined by the treating clinician’s personal experience and expertise, leading to significant variation and heterogeneity in treatment protocols and long-term outcomes[18]. Conversion therapy refers to transforming unresectable liver cancer into resectable liver cancer, and one of the pathways for patients with inter
The patient was treated by the HCC treatment team at the hospital, where an in-depth analysis and discussion were conducted on the patient with massive right liver cancer. The team also summarized the current state of liver cancer conversion therapy, aiming to provide a reference for the use of the MDT approach in the clinical management of large liver cancers.
The patient initially underwent two rounds of transformational therapy with TACE in combination with sintilimab and lenvatinib, achieving the goal of reducing the tumor size to safely perform surgical resection. If the tumor remains stable three months after downstaging treatment, treatment is considered effective[20,21]. Studies have shown that the majority of patients with BCLC-B subclassification HCC can also benefit from TACE[22]. Moreover, compared with sorafenib, lenvatinib generally has a superior overall efficacy[23]. Studies have indicated that combining TACE with lenvatinib and sintilimab for the treatment of HCC can effectively control tumor progression and prolong the survival time of patients[24]. DEB-TACE is considered to be less harmful to liver function and has a lower incidence of doxorubicin-related side effects. There was no significant difference in the indications for these two treatments compared to traditional TACE. However, DEB-TACE is more effective at blocking tumor blood vessels. The slow release of anticancer drugs during DEB-TACE provides a continuous antitumor effect, increasing the safety of the process. DEB-TACE can significantly improve the overall survival period of HCC patients who have undergone a similar number of traditional TACE procedures[25]. Wang et al[26] have reported that the use of lenvatinib and sintilimab for conversion therapy in patients with initially inoperable mid- to late-stage local HCC is safe and feasible. However, the optimal duration of conversion therapy remains controversial. Some surgeons believe that surgery should be performed as soon as the criteria for operability are met to minimize the risk of disease progression and drug-induced liver damage[27]. Therefore, the most rational and individualized conversion therapy plan should be formulated based on the patient's own disease and various other conditions. The implementation of all conversion therapy plans often requires joint discussion and planning by multiple disciplines, including hepatobiliary surgery, oncology, interventional radiology, and radiology. To reduce the risk of tumor progression, patients should undergo surgery as soon as possible or when the tumor shrinks to a size that is close to a resectable range[26]. Although a future liver remnant (FLR) ≥ 20% is feasible in a healthy liver, for a liver preda
In summary, our experience with a 40-year-old male patient presenting with massive HCC with rupture and hemorrhage illustrates the importance of personalized treatment plans. Despite the complexity of his case, which included no treatment for hepatitis B and the risk of liver failure, our MDT approach enabled us to carefully balance tumor factors, basic liver function, and the patient's physical condition. By combining TACE, sintilimab monoclonal antibody therapy, and lenvatinib therapy, along with antiviral treatment, we successfully managed his condition without significant adverse effects. This case study underscores the potential of personalized, MDT-based strategies for improving outcomes in patients with advanced, initially high-risk liver cancer. Such approaches can increase downstaging conversion rates and resectability, providing valuable insights for the treatment of mid- to late-stage massive liver cancer.
We express our heartfelt thanks to Professor Lan Zhang and Sheng-Fen Liu for her exceptional guidance and insight. Our appreciation extends to all the authors for their significant contributions to this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maida M, Italy S-Editor: Qu XL L-Editor: A P-Editor: Zhang YL
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11419] [Article Influence: 3806.3] [Reference Citation Analysis (4)] |
| 2. | Torimura T, Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2042-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 3. | Coffin P, He A. Hepatocellular Carcinoma: Past and Present Challenges and Progress in Molecular Classification and Precision Oncology. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, Quek J, Xiao J, Dan YY, Siddiqui MS, Sanyal AJ, Muthiah MD, Loomba R, Huang DQ. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 5. | Cabibbo G, Maida M, Genco C, Antonucci M, Cammà C. Causes of and prevention strategies for hepatocellular carcinoma. Semin Oncol. 2012;39:374-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (1)] |
| 7. | Konyn P, Ahmed A, Kim D. The current trends in the health burden of primary liver cancer across the globe. Clin Mol Hepatol. 2023;29:358-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Reference Citation Analysis (0)] |
| 8. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1-441. [PubMed] |
| 9. | Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Girardi DM, Sousa LP, Miranda TA, Haum FNC, Pereira GCB, Pereira AAL. Systemic Therapy for Advanced Hepatocellular Carcinoma: Current Stand and Perspectives. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Nagasue N, Kohno H, Chang YC, Taniura H, Yamanoi A, Uchida M, Kimoto T, Takemoto Y, Nakamura T, Yukaya H. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann Surg. 1993;217:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 214] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Xu G, Mao Y. Giant hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2021;10:583-584. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Agnello F, Salvaggio G, Cabibbo G, Maida M, Lagalla R, Midiri M, Brancatelli G. Imaging appearance of treated hepatocellular carcinoma. World J Hepatol. 2013;5:417-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2198] [Article Influence: 732.7] [Reference Citation Analysis (1)] |
| 15. | Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 898] [Article Influence: 179.6] [Reference Citation Analysis (2)] |
| 16. | Cabibbo G, Maida M, Genco C, Alessi N, Peralta M, Butera G, Galia M, Brancatelli G, Genova C, Raineri M, Orlando E, Attardo S, Giarratano A, Midiri M, Di Marco V, Craxì A, Cammà C. Survival of patients with hepatocellular carcinoma (HCC) treated by percutaneous radio-frequency ablation (RFA) is affected by complete radiological response. PLoS One. 2013;8:e70016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Pandrowala S, Patkar S, Goel M, Mirza D, Mathur SK. Surgical resection for large hepatocellular carcinoma and those beyond BCLC: systematic review with proposed management algorithm. Langenbecks Arch Surg. 2023;408:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Davila JA, Kramer JR, Duan Z, Richardson PA, Tyson GL, Sada YH, Kanwal F, El-Serag HB. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57:1858-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Bureau of Medical Administration, National Health Commission of the People's Republic of China. [Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:367-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 20. | Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, Roberts J, Reich DJ, Schwartz ME, Mieles L, Lee FT, Florman S, Yao F, Harper A, Edwards E, Freeman R, Lake J. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 301] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Yao FY, Hirose R, LaBerge JM, Davern TJ 3rd, Bass NM, Kerlan RK Jr, Merriman R, Feng S, Freise CE, Ascher NL, Roberts JP. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Biolato M, Gallusi G, Iavarone M, Cabibbo G, Racco S, De Santis A, Corte CD, Maida M, Attili AF, Sangiovanni A, Cammà C, La Torre G, Gasbarrini A, Grieco A. Prognostic ability of BCLC-B Subclassification in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Ann Hepatol. 2018;17:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Cappuyns S, Corbett V, Yarchoan M, Finn RS, Llovet JM. Critical Appraisal of Guideline Recommendations on Systemic Therapies for Advanced Hepatocellular Carcinoma: A Review. JAMA Oncol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 24. | Zhang M, Lai W, Zhang J, Hu B, Huang L, Chu C. Efficacy Investigation of TACE Combined with Lenvatinib and Sintilimab in Intermediate-Stage Hepatocellular Carcinoma. Dis Markers. 2022;2022:6957580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | Chen J, Lai L, Zhou C, Luo J, Wang H, Li M, Huang M. Safety, efficacy, and survival of drug-eluting beads-transarterial chemoembolization vs. conventional-transarterial chemoembolization in advanced HCC patients with main portal vein tumor thrombus. Cancer Imaging. 2023;23:70. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Wang L, Wang H, Cui Y, Liu M, Jin K, Xu D, Wang K, Xing B. Sintilimab plus Lenvatinib conversion therapy for intermediate/locally advanced hepatocellular carcinoma: A phase 2 study. Front Oncol. 2023;13:1115109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 27. | Takamoto T, Hashimoto T, Sano K, Maruyama Y, Inoue K, Ogata S, Takemura T, Kokudo N, Makuuchi M. Recovery of liver function after the cessation of preoperative chemotherapy for colorectal liver metastasis. Ann Surg Oncol. 2010;17:2747-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB; American Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Kobayashi T. Long-term Survival Analysis of Pure Laparoscopic Versus Open Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: A Single-center Experience. Ann Surg. 2015;262:e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Di Sandro S, Danieli M, Ferla F, Lauterio A, De Carlis R, Benuzzi L, Buscemi V, Pezzoli I, De Carlis L. The current role of laparoscopic resection for HCC: a systematic review of past ten years. Transl Gastroenterol Hepatol. 2018;3:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Schwarz L, Bubenheim M, Zemour J, Herrero A, Muscari F, Ayav A, Riboud R, Ducerf C, Regimbeau JM, Tranchart H, Lermite E, Petrovai G, Suhol A, Doussot A, Capussotti L, Tuech JJ, Le Treut YP; FRENCH association. Bleeding Recurrence and Mortality Following Interventional Management of Spontaneous HCC Rupture: Results of a Multicenter European Study. World J Surg. 2018;42:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Wang P, Moses AS, Li C, Chen S, Qi X, Xu K, Shao HB, Han XJ. Prognosis factors of predicting survival in spontaneously ruptured hepatocellular carcinoma. Hepatol Int. 2022;16:1330-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |