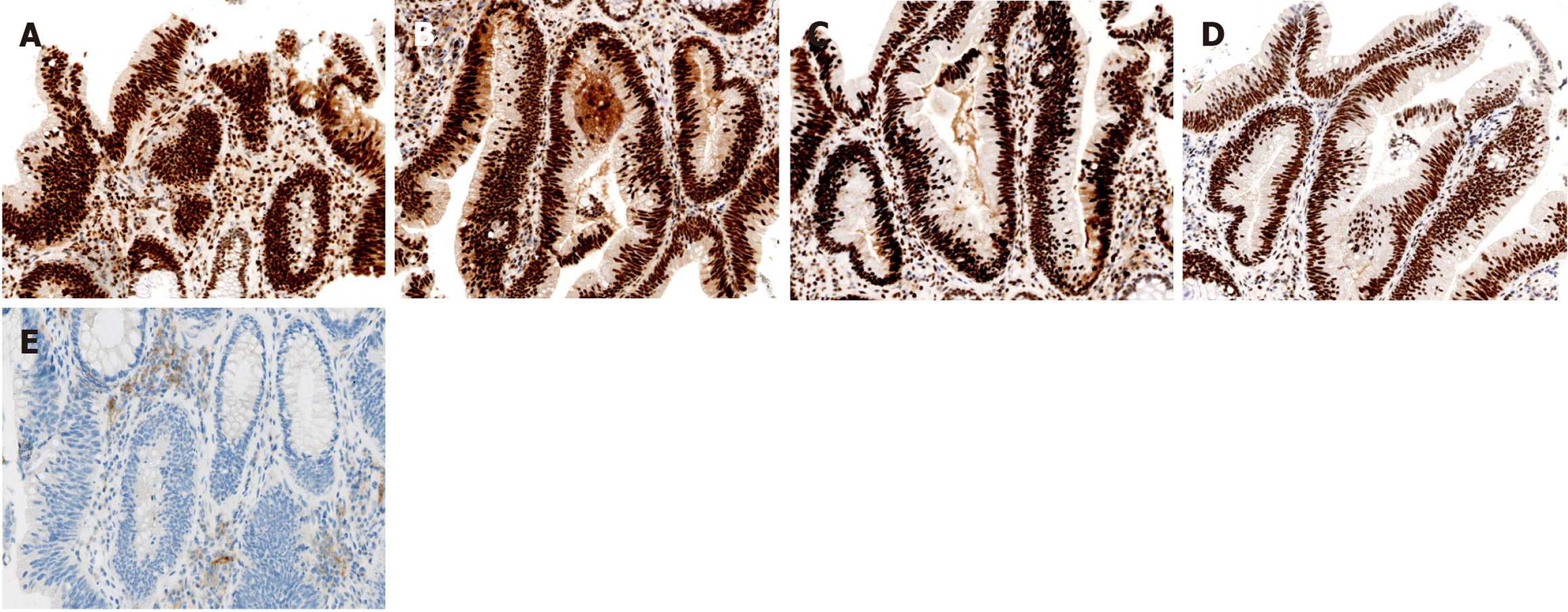Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2219
Peer-review started: November 14, 2023
First decision: January 31, 2024
Revised: February 8, 2024
Accepted: March 14, 2024
Article in press: March 14, 2024
Published online: May 15, 2024
Processing time: 177 Days and 8.4 Hours
According to the latest report, colorectal cancer is still one of the most prevalent cancers, with the third highest incidence and mortality worldwide. Treatment of advanced rectal cancer with distant metastases is usually unsatisfactory, espec
We report a case of a pMMR rectal adenocarcinoma with metastases of multiple lymph nodes, including the left supraclavicular lymph node, before treatment in a 70-year-old man. He received full courses of chemoradiotherapy (CRT) followed by 4 cycles of programmed death 1 inhibitor Tislelizumab, and a pathologic complete response (pCR) was achieved, and the lesion of the left supraclavicular lymph node also disappeared.
pMMR advanced rectal cancer with preserved intact distant metastatic lymph nodes may benefit from full-course CRT combined with immunotherapy.
Core Tip: Rectal cancer is a clinically common malignancy and the mainstream treatment methods are chemoradiotherapy (CRT) and surgery. Advanced rectal cancer is often associated with distant organs and lymph node metastasis. We report a case of mismatch repair proficient rectal cancer with left supraclavicular lymph node metastasis. The patient received 4 cycles of CRT combined with immunotherapy and achieved pCR. This case provides a rare clinical experience for such patients.
- Citation: Zhong WT, Lv Y, Wang QY, An R, Chen G, Du JF. Chemoradiotherapy plus tislelizumab for mismatch repair proficient rectal cancer with supraclavicular lymph node metastasis: A case report. World J Gastrointest Oncol 2024; 16(5): 2219-2224
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2219.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2219
Clinical trials in the treatment of advanced solid tumors using programmed death 1 (PD-1) inhibitors are being conducted extensively. Recent results have shown that PD-1 blockade antibody has significant therapeutic efficacy in patients with mismatch repair deficient (dMMR) and microsatellite instability-high (MSI-H) colorectal cancer (CRC)[1,2], but these patients account for only 15% of all CRC patients[3]. In addition, clinical trials of immunotherapy for patients with mismatch repair proficient (pMMR) CRC are deficient. Here, we report a case of a pMMR rectal adenocarcinoma with metastases of multiple lymph nodes before treatment, including the left supraclavicular lymph node, in a 70-year-old man. He received full courses of chemoradiotherapy (CRT) followed by 4 cycles of PD-1 inhibitor Tislelizumab, a complete pathological response was achieved, and the lesion of the left supraclavicular lymph node also disappeared.
A 70-year-old Chinese man was admitted to our hospital complaining of difficulty in defecation for 1 month.
Upon admission, the patient underwent endoscopy, which detected a cyclo-intestinal luminal growth mass, which was diagnosed as a poorly differentiated adenocarcinoma by pathologic examination. 18F-FDG PET/CT was performed and metastasis to the left supraclavicular lymph node was found. The patient underwent enterostomy due to difficulty in defecation.
The patient had hypertension for more than 10 years, and kept taking medicine to control his blood pressure.
The patient denied any family history of rectal cancer or psychological or genetic disorders.
All relevant physical examinations showed no significant abnormality and vital signs were stable.
Tumor markers in serum including carcinoembryonic antigen, cancer antigen 199, CA125 and CA153 were all within the normal ranges, whereas the level of CA724 increased to 8.9 ng/mL (reference range 0-5.3 ng/mL).
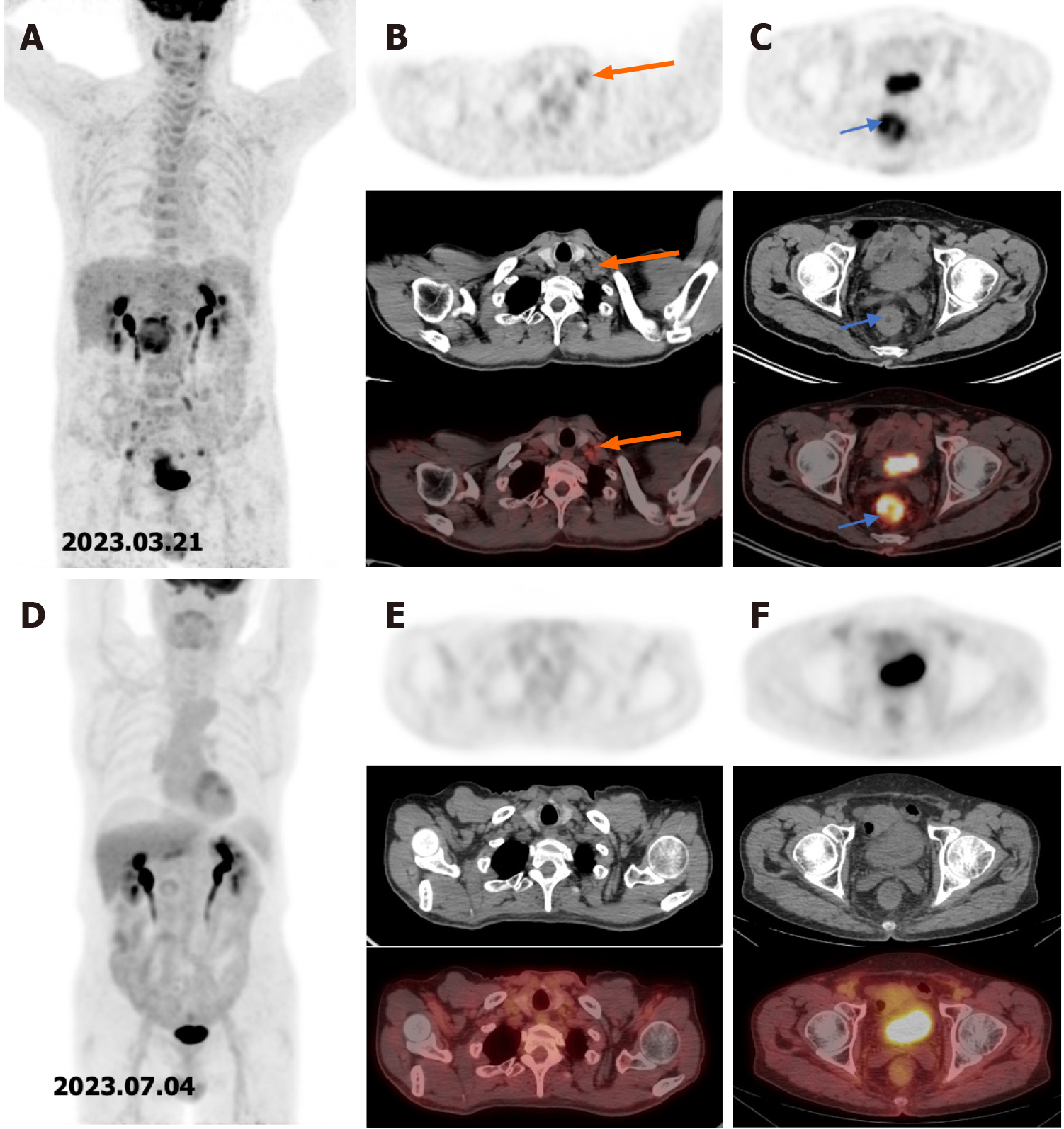
18F-FDG PET/CT was performed to detect metastases (Figure 1A). It revealed metastases of multiple lymph nodes, including the left supraclavicular lymph node (Figure 1B) and a high uptake in the rectum (Figure 1C).
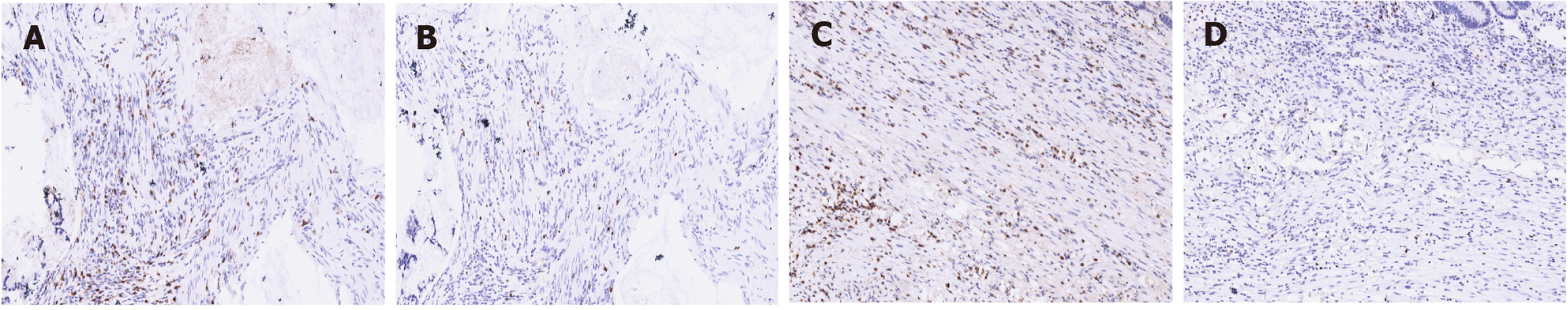
The immunohistochemistry demonstrated that the patient was positive for all four proteins associated with mismatch repair (MMR) status (Figure 2A-D), suggesting a pMMR rectal cancer, and the tumor proportion score of programmed cell death-ligand 1 was 8% (Figure 2E). We then compared the changes in CD8+ T cell/effector regulatory T cell (CD8/eTreg) ratio before and after treatment, and found that the ratio increased from 2.79 to 3.26 (Figure 3).
Combined with the medical history, the patient was diagnosed with rectal cancer with the left supraclavicular lymph node metastasis, which was staged as cT3N2bM1a (c-stage IVA), according to the AJCC 8th TNM staging system.
The patient received 4 cycles of CRT with capecitabine and radiotherapy at a dose of 50.4 Gy, followed by PD-1 inhibitor Tislelizumab 200 mg once every three weeks.
The patient did not have any adverse effects related to the PD-1 inhibitor during the follow-up. After finishing the last treatment, the patient was admitted to the hospital for re-examination, unexpectedly, there was no significant uptake of the previous metastases of the multiple lymph nodes, including the left supraclavicular lymph node (Figure 1D and E) and no uptake of the rectal lesion could be seen on the 18F-FDG PET/CT (Figure 1F). He then underwent radical resection of the rectal lesion, and no tumor cell was found under microscope, which suggested that the patient achieved a pCR.
The 5-year survival rate of CRC patients is still low in spite of various treatments used[4,5]. Based on the mutation patterns, CRC can be categorized into two distinct groups: the patients with dMMR, which tends to have high overall tumor mutation burden, and the patients with pMMR, with much lower tumor mutation burden[6]. dMMR tumors can be certified by the lack of immunohistochemical staining of the MMR proteins MLH1, MSH2, MSH6, or PMS2, which tend to have a high density of immune cell infiltration, known as "hot tumor", thus these patients have a better prognosis generally, but the number of the patients was low[7].
Currently, many phase 2 and 3 clinical trials have been carried out in the treatment of various solid tumors, including CRC, using PD-1 inhibitors. In a phase 2 clinical trial by Cercek, 100% dMMR/MSI-H rectal cancer patients, who received PD-1 inhibitor treatment and standard CRT followed by surgery, achieved clinical complete response, and no patient had disease progression or recurrence[8]. The KEYNOTE 016 study[9] evaluated the efficacy of pembrolizumab in metastatic CRC patients. All patients with dMMR/MSI-H metastatic CRC displayed favorable antitumor activity with an objective remission rate (ORR) of 40% (4/10), while none of the patients with pMMR or microsatellite stable tumor achieved ORR. The latest PANDORA trial[10] also indicated the efficacy of a neoadjuvant therapy with PD-1 inhibitor following CRT in patients with locally advanced rectal cancer (LARC), and 34.5% (19/55) of the patients achieved pCR. The above trials have shown positive therapeutic effects in dMMR/MSI-H CRC patients treated with PD-1 inhibitor alone or combined with other treatments, but pMMR CRC patients showed little benefit from immunotherapy. The main reason for this phenomenon is the “cold” characteristic of pMMR tumor. Therefore, how to induce pMMR tumor to be “hot” has been a major concern.
As observed in the VOLTAGE-A study[11], 37 patients received 5 cycles of CRT and Nivolumab and about 30% of the patients achieved pCR. Similarly, the study by Gao et al[12] confirmed the effectiveness of immunotherapy for pMMR LARC. One possible explanation is that the induction of tumor cell death after radiotherapy increases the release of tumor antigens and pro-inflammatory mediators which promote infiltration of tumor-infiltrating lymphocytes at the primary site and triggers anti-tumor responses[13,14]. Simultaneously, according to recent studies, maintaining the integrity of the lymph nodes is significant to immunotherapy efficiency[15,16], which suggested that immunotherapy induces activation of migratory dendritic cell in tumor-draining lymph node and increases anti-tumor capacity by decreasing Treg and activating effector T cells in the sentinel lymph node and peripheral blood, increasing anti-tumor capacity.
We presented a rare case of pMMR advanced rectal cancer with distant lymph node metastasis treated with CRT and Tislelizumab with good efficacy. Immunotherapy combined with standard CRT as a conversion therapy might be a promising treatment in the advanced rectal cancer.
We appreciate the cooperation of patient and his family during the treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hidaka E, Japan S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1797] [Article Influence: 359.4] [Reference Citation Analysis (0)] |
| 2. | Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, Liu M, Lin JZ, Wang FL, Li C, Quan TT, Xi SY, Zhang HZ, Pan ZZ, Wang F, Xu RH. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. 2023;8:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 3. | Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 724] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 4. | Leowattana W, Leowattana P, Leowattana T. Systemic treatment for metastatic colorectal cancer. World J Gastroenterol. 2023;29:1569-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 5. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9900] [Article Influence: 4950.0] [Reference Citation Analysis (2)] |
| 6. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6659] [Article Influence: 512.2] [Reference Citation Analysis (0)] |
| 7. | Lin A, Zhang J, Luo P. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front Immunol. 2020;11:2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 8. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 888] [Article Influence: 296.0] [Reference Citation Analysis (0)] |
| 9. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7227] [Article Influence: 722.7] [Reference Citation Analysis (0)] |
| 10. | Grassi E, Zingaretti C, Petracci E, Corbelli J, Papiani G, Banchelli I, Valli I, Frassineti GL, Passardi A, Di Bartolomeo M, Pietrantonio F, Gelsomino F, Carandina I, Banzi M, Martella L, Bonetti AV, Boccaccino A, Molinari C, Marisi G, Ugolini G, Nanni O, Tamberi S. Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the PANDORA trial. ESMO Open. 2023;8:101824. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, Fukuoka S, Yuki S, Komatsu Y, Homma S, Taketomi A, Uemura M, Kato T, Fukui M, Wakabayashi M, Nakamura N, Kojima M, Kawachi H, Kirsch R, Yoshida T, Suzuki Y, Sato A, Nishikawa H, Ito M, Yoshino T. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin Cancer Res. 2022;28:1136-1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 12. | Gao J, Zhang X, Yang Z, Zhang J, Bai Z, Deng W, Chen G, Xu R, Wei Q, Liu Y, Han J, Li A, Liu G, Sun Y, Kong D, Yao H, Zhang Z. Interim result of phase II, prospective, single-arm trial of long-course chemoradiotherapy combined with concurrent tislelizumab in locally advanced rectal cancer. Front Oncol. 2023;13:1057947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | MOLE RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 809] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, Harrington KJ. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 563] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 15. | Rahim MK, Okholm TLH, Jones KB, McCarthy EE, Liu CC, Yee JL, Tamaki SJ, Marquez DM, Tenvooren I, Wai K, Cheung A, Davidson BR, Johri V, Samad B, O'Gorman WE, Krummel MF, van Zante A, Combes AJ, Angelo M, Fong L, Algazi AP, Ha P, Spitzer MH. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell. 2023;186:1127-1143.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 226] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 16. | Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, Fong L, Nolan GP, Engleman EG. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell. 2017;168:487-502.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 745] [Article Influence: 93.1] [Reference Citation Analysis (0)] |