Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1849
Peer-review started: December 19, 2023
First decision: January 15, 2024
Revised: January 23, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: May 15, 2024
Processing time: 142 Days and 23.5 Hours
Lymph node (LN) staging in rectal cancer (RC) affects treatment decisions and patient prognosis. For radiologists, the traditional preoperative assessment of LN metastasis (LNM) using magnetic resonance imaging (MRI) poses a challenge.
To explore the value of a nomogram model that combines Conventional MRI and radiomics features from the LNs of RC in assessing the preoperative metastasis of evaluable LNs.
In this retrospective study, 270 LNs (158 nonmetastatic, 112 metastatic) were randomly split into training (n = 189) and validation sets (n = 81). LNs were classified based on pathology-MRI matching. Conventional MRI features [size, shape, margin, T2-weighted imaging (T2WI) appearance, and CE-T1-weighted imaging (T1WI) enhancement] were evaluated. Three radiomics models used 3D features from T1WI and T2WI images. Additionally, a nomogram model combining conventional MRI and radiomics features was developed. The model used univariate analysis and multivariable logistic regression. Evaluation employed the receiver operating characteristic curve, with DeLong test for comparing diagnostic performance. Nomogram performance was assessed using calibration and decision curve analysis.
The nomogram model outperformed conventional MRI and single radiomics models in evaluating LNM. In the training set, the nomogram model achieved an area under the curve (AUC) of 0.92, which was significantly higher than the AUCs of 0.82 (P < 0.001) and 0.89 (P < 0.001) of the conventional MRI and radiomics models, respectively. In the validation set, the nomogram model achieved an AUC of 0.91, significantly surpassing 0.80 (P < 0.001) and 0.86 (P < 0.001), respectively.
The nomogram model showed the best performance in predicting metastasis of evaluable LNs.
Core Tip: We have developed and validated a predictive model that combines radiomic features with conventional magnetic resonance imaging features. This model has shown promising results in preoperative assessment of lymph node metastasis (LNM), improving the accuracy of LNM evaluation by radiologists. Additionally, the study has focused on individual LNs and has the potential to provide information on both the quantity and location of metastatic LNs.
- Citation: Ye YX, Yang L, Kang Z, Wang MQ, Xie XD, Lou KX, Bao J, Du M, Li ZX. Magnetic resonance imaging-based lymph node radiomics for predicting the metastasis of evaluable lymph nodes in rectal cancer. World J Gastrointest Oncol 2024; 16(5): 1849-1860
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1849.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1849
Rectal cancer (RC) ranks 8th among the 36 different types of cancer in terms of both new cases and deaths[1]. Lymph node metastasis (LNM) is correlated with advanced tumor staging and unfavorable prognosis (seer.cancer.gov). Accurate preoperative assessment of LNs is crucial for selecting the treatment plan.
Magnetic resonance imaging (MRI) is the preferred method for the preoperative evaluation of RC, enabling the visualization of LNs with a diameter ≥ 5 mm. Guidelines highlight the assessment of LNM based on size, borders, and morphological characteristics[2]. However, importantly, > 50% of these LNs are nonmetastatic[3-5]. RC specimen studies show that only approximately 6% of total LNs can be evaluated[6], leading radiologists to primarily focus on these limited nodes for staging. The accuracy of assessing LNM based on MRI remains limited, as indicated by a meta-analysis reporting a sensitivity of approximately 73% and specificity of approximately 74% for MRI in diagnosing LNM[7-9]. Therefore, there is a risk of diagnostic insufficiency and overdiagnosis[10]. Evaluating LNs with a diameter < 5 mm is challenging, as they may not be visible on MRI, and the metastasis rate for all small LNs is less than 15%[11-14]. Importantly, there is no evidence suggesting that small LNs independently contribute to node staging beyond the evaluable LNs. Improving diagnostic accuracy is the crucial first step for radiologists in making accurate clinical decisions, considering the unique nature of evaluable LNs.
Radiomics extracts nonvisual information from medical images and transforms it into quantitative features that reveal pathophysiological potential[15]. Recent studies indicate that radiomics models can predict LNM using tumor information[16-19]. However, they only confirm the presence of LNM and do not provide precise details on quantity or location. To redress this insufficiency, it is paramount to prioritize the analysis of radiomics information specific to LNs. Presently, there is a lack of research targeting LN radiomics. The purpose of this study was to utilize MRI-based LN radiomics to predict the metastasis in evaluable LNs.
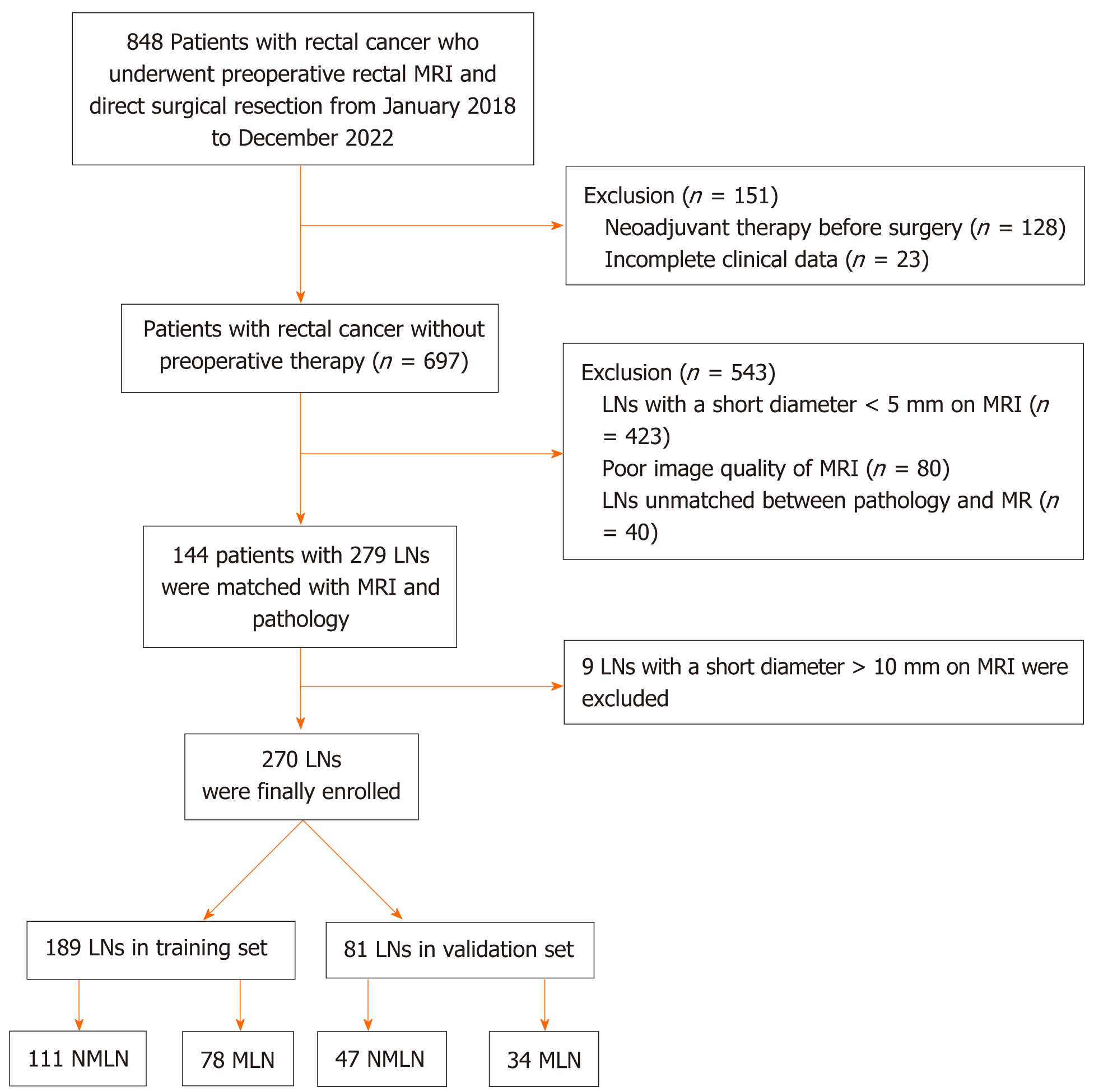
This retrospective study received approval from the institutional review board, and a waiver of written informed consent was obtained. Between January 2018 and December 2022, we continuously enrolled 848 patients with resectable RC from Nanjing Medical University Affiliated Cancer Hospital. The inclusion criteria were as follows: (1) Confirmed diagnosis of rectal adenocarcinoma through histopathology; (2) undergoing curative surgery for RC; (3) an MRI examination 2 wk before surgery with the MRI report indicating N stage positivity; and (4) complete clinical data, including carcinoembryonic antigen and surgical approaches. The exclusion criteria were as follows: (1) Incomplete clinical data; (2) neoadjuvant therapy before surgery; (3) poor image quality of MRI; (4) pelvic LNs with a short diameter on MRI less than 5 mm; and (5) LNs unmatched between pathology and MRI. Finally, we enrolled 144 patients with RC. Patients were allocated to the training and validation sets in a 7:3 ratio (Table 1).
| Characteristic | All patients | Training set | Validation set | P value |
| n = 144 | n = 100 | n = 44 | ||
| Age in yr | 59 ± 10 | 59 ± 9 | 60 ± 10 | 0.514 |
| Sex | 0.805 | |||
| Male | 97 (67.4) | 68 (68.0) | 29 (65.9) | |
| Female | 47 (32.6) | 32 (32.0) | 15 (34.1) | |
| CEA in ng/mL1 | 4.0 (2.4, 7.8) | 3.8 (2.3, 9.3) | 0.787 | |
| Location | 0.165 | |||
| Upper | 31 (21.5) | 22 (22.0) | 9 (20.5) | |
| Middle | 66 (45.8) | 41 (41.0) | 25 (56.8) | |
| Lower | 47 (32.6) | 37 (37.0) | 10 (22.7) | |
| Surgical approach | 0.489 | |||
| Dixon | 120 (83.3) | 82 (82.0) | 38 (86.4) | |
| Miles | 21 (14.6) | 15 (15.0) | 6 (13.6) | |
| Hartman | 3 (2.1) | 3 (3.0) | 0 (0.0) | |
| Surgical specimen histological type | 0.877 | |||
| Ulcerative | 96 (66.7) | 68 (68.0) | 28 (63.6) | |
| Infiltrative | 6 (4.2) | 4 (4.0) | 2 (4.5) | |
| Nodular | 42 (29.2) | 28 (28.0) | 14 (31.8) | |
| Differentiation | 0.689 | |||
| Well | 8 (5.6) | 5 (5.0) | 3 (6.8) | |
| Moderate | 86 (59.7) | 62 (62.0) | 24 (54.5) | |
| Poor | 50 (34.7) | 33 (33.0) | 17 (38.6) | |
| Pathological T stage | 0.579 | |||
| T1 | 7 (4.9) | 4 (4.0) | 3 (6.8) | |
| T2 | 39 (27.1) | 29 (29.0) | 10 (22.7) | |
| T3 | 84 (58.3) | 59 (59.0) | 25 (56.8) | |
| T4 | 14 (9.7) | 8 (8.0) | 6 (13.6) | |
| Pathological N status | 0.293 | |||
| Positive | 53 (36.8) | 34 (34.0) | 19 (43.2) | |
| Negative | 91 (63.2) | 66 (66.0) | 25 (56.8) |
Patients underwent total mesenteric excision and were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Patients in the non-LN metastasis (NLNM) group had no regional LNM, while patients in the positive LN group had at least one regional LNM. Patients in the positive LN group underwent assessment by two gastrointestinal radiologists, namely, reader ZK, boasting 25 years of experience, and reader MQW, with 27 years of experience. These radiologists adhered to the guidelines[2], aiming to identify nonfused suspicious LNM on MRI with a short diameter of ≥ 5 mm. Subsequently, the identified suspicious LNM were compared with the number and location of regionally LNM mentioned in the pathology report, including the peritumoral area, superior rectal artery region, and lateral pelvic wall. Only the nodes that were manually matched and agreed upon by both radiologists were considered metastatic. Given the reliable correlation observed between LNs with a short axis exceeding 1 cm and metastasis on MRI[13], the analysis excluded 9 LNs with a diameter greater than 1 cm. A total of 270 LNs (158 nonmetastatic and 112 metastatic) were included and randomly allocated to training set (111 nonmetastatic and 78 metastatic) and validation set (47 nonmetastatic and 34 metastatic) at a 7:3 ratio (Table 1). The flowchart of LNs collection is illustrated in Figure 1.
Patients were instructed to observe a fasting period prior to the examination. To suppress intestinal peristalsis, a 10 mg dosage of anisodamine hydrochloride was administered via intramuscular injection 10 min before examination with the 3.0 T MR scanner (Ingenia; Philips Medical Systems, Best, Netherlands). The imaging protocols consisted of axial T1-weighted imaging (T1WI) with the following parameters: Repetition time (TR) of 594 ms, echo time (TE) of 10 ms, field of view (FOV) of 300, matrix size of 376 × 296, slice thickness of 4 mm, and slice gap of 0.4 mm. For oblique axial T2-weighted imaging (T2WI), the parameters were a TR of 4203 ms, a TE of 102 ms, an FOV of 220, a matrix size of 256 × 256, a slice thickness of 4 mm, and no slice gap. Axial contrast-enhanced imaging utilized a TR of 4.5 ms, a TE of 1.4 ms, an FOV of 230, a matrix size of 268 × 250, a slice thickness of 5 mm, and a slice gap of 1 mm. Contrast enhancement involved the intravenous administration of gadopentetate dimeglumine (Magnevist; Bayer Healthcare, Leverkusen, Germany) at a flow rate of 1.5 mL/s, with a dose of 0.2 mL/kg body weight. A high-pressure injector was used to administer a 20 mL saline flush; then the contrast-enhanced scan was performed 1 min after administration of the contrast agent.
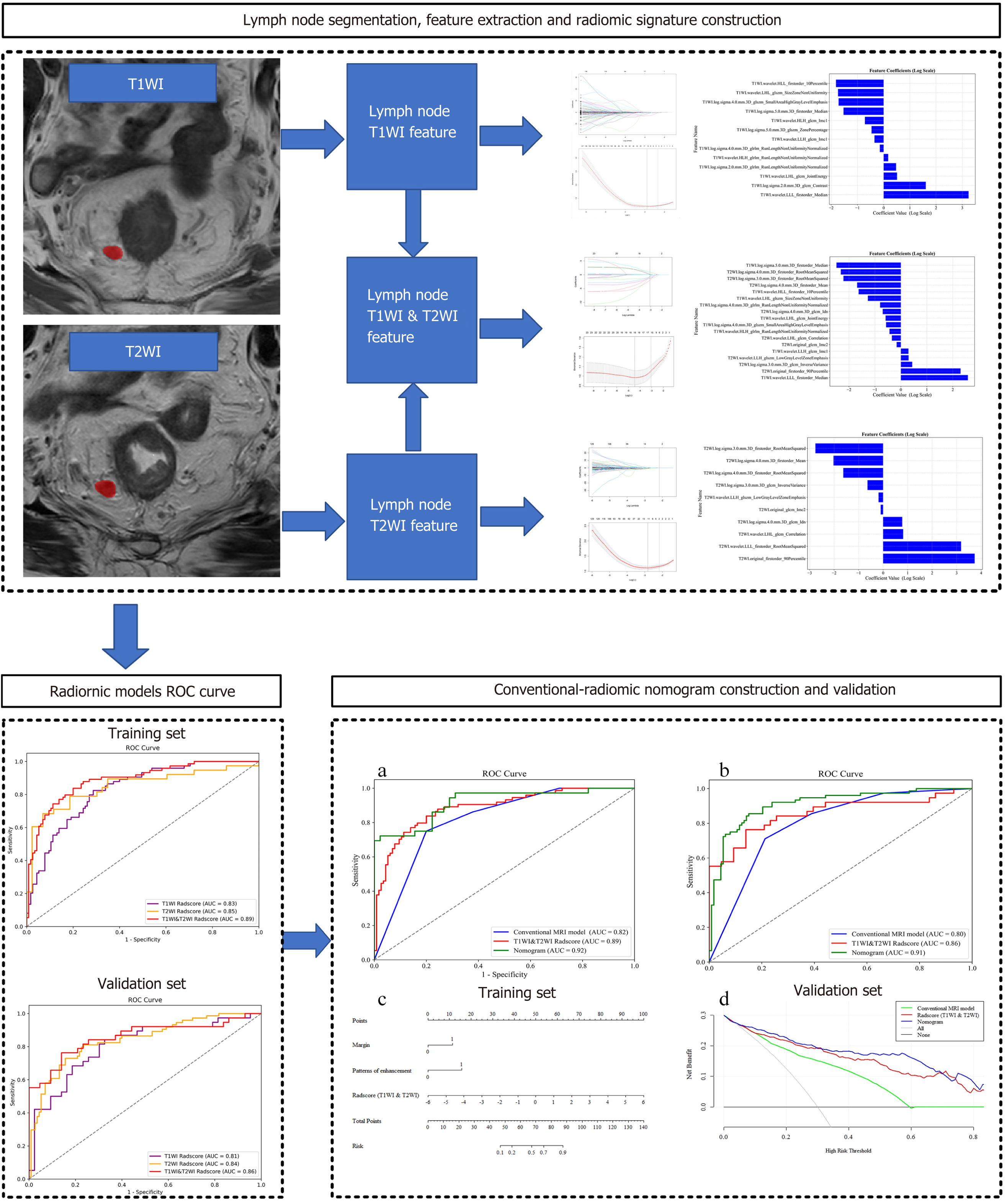
Two radiologists (reader XDX, with 8 years of experience in diagnosing RC with MRI; reader YXY, with 5 years of similar experience) conducted a qualitative analysis of image features. Although the presence of rectal adenocarcinoma tumors was confirmed, the radiologists were not informed of the tissue pathology of postoperative LNs or the study design of the images. They independently evaluated the conventional MRI features of LNs on T2WI and CE-T1WI, including: (1) Size (short axis/Long axis), (2) shape (round, nonround); (3) margin smooth or nonsmooth (lobulated, spiculated and indistinct)[20]; (4) T2WI heterogeneous appearance (absent/present); and (5) enhancement appearance (homogeneous/heterogeneous). A flowchart of radiomics process is shown in Figure 2. Radiomics feature analyses were as follows: LNs in both datasets were manually segmented by Reader ZXL using ITK-SNAP (version 3.6.0; http://www.itksnap.org) software for radiomics feature extraction. The segmentation process was performed on T1WI and T2WI. To assess the variability of intrareader, interreader, and radiomics features, all LNs from the test set were subjected to repeated segmentation by Reader ZXL and segmentation by MD. The second segmentation was conducted 1 mo after the initial segmentation. The intrareader and interreader agreements were evaluated using the intraclass correlation coefficient. Both readers were unaware of the histopathologic classification of all LNs. The radiomics features were extracted from segmented MRI voxels using the PyRadiomics open-source Python package (version 3.1.0; Harvard Medical School). Before extraction of the features, z score normalization was applied to normalize the MRI images. The “N4ITK” bias field correction method was applied to correct the intensity inhomogeneity caused by variations in the magnetic field[21]. To ensure consistent scale and orientation during the extraction of three-dimensional features, the images were resampled with a voxel size of 1 mm × 1 mm × 1 mm. A total of 2000 features were extracted from the T1WI and T2WI sequences, including 14 shape features, 18 first-order statistics, 14 GLDM features, 22 GLCM features, 16 GLRLM features, 16 GLSZM features, and 688 wavelet features.
Categorical variables were compared using the Chi-square test or Fisher’s exact test, while the normality of data distribution was assessed using the Kolmogorov-Smirnov test. For quantitative data, an independent sample t test or Mann-Whitney U test was utilized for comparisons. Univariate analysis was applied to examine the differences in conventional MRI features between nonmetastatic and metastatic LNs in the training set. The homogeneity of variance in radiomics features was evaluated using Levene’s test. Dimensionality reduction and optimal feature selection were conducted using the LASSO regression algorithm. The features with nonzero coefficients were linearly combined to calculate the radiomics score (Radscore) for each LN. A multivariable logistic regression was employed in the training set to construct a nomogram model for preoperative LNM, incorporating both morphological features and the Radscore. The odds ratios (ORs) and corresponding 95% confidence intervals (95%CIs) were calculated for each independent factor. The performance values of the morphological model, Radscore, and nomogram model in predicting LNM were obtained using receiver operating characteristic curves. The area under the curve (AUC) differences between different models were compared using the DeLong test. The clinical utility of the models was assessed using decision curve analysis (DCA) by calculating the net benefits. Statistical analyses were conducted using SPSS (version 23.0; IBM Corp., Armonk, NY, United States) and R software (version 3.6.1; R Project for Statistical Computing, www.r-project.org). A significant difference was indicated by P < 0.05.
The conventional MRI features of and LNM groups are presented in Table 2. A significant difference was observed between the groups in terms of size, margin, T2WI heterogeneous appearance, and enhancement appearance (all P < 0.05) in the training set. Exceptionally, no significant difference was found in shape (P >0.05). Multivariable analysis showed that margin (OR = 4.184; 95%CI: 1.831, 10.105; P < 0.001) and enhancement appearance (OR = 7.709; 95%CI: 3.200, 20.561; P < 0.001) remained independent conventional MRI features predictors associated with LNM (Table 3).
| Features | Training set | Validation set | ||||
| NLNM, n = 111 | LNM, n = 78 | P value | NLNM, n = 47 | LNM, n = 34 | P value | |
| Size | ||||||
| Short axis1 in mm | 6.10 (5.40, 7.20) | 7.80 (6.60, 9.00) | < 0.001 | 5.70 (5.10, 7.40) | 7.25 (6.57, 8.67) | < 0.001 |
| Long-axis in mm | 7.63 ± 1.72 | 9.13 ± 1.94 | < 0.001 | 7.55 ± 1.79 | 8.67 ± 1.43 | 0.004 |
| Shape | 0.105 | 0.185 | ||||
| Non-round | 82 (73.88) | 29 (37.17) | 31 (65.95) | 27 (79.41) | ||
| Round | 29 (26.12) | 49 (62.83) | 16 (34.04) | 7 (20.59) | ||
| Margin | < 0.001 | 0.001 | ||||
| Clear | 55 (49.54) | 13 (16.67) | 24 (51.00) | 4 (14.70) | ||
| Unclear | 56 (50.46) | 65 (83.33) | 23 (49.00) | 30 (85.30) | ||
| T2WI heterogeneous signal | < 0.001 | 0.029 | ||||
| Absent | 63 (56.76) | 17 (21.79) | 25 (53.19) | 5 (14.71) | ||
| Present | 48 (43.24) | 61 (78.21) | ||||
| Patterns of enhancement | < 0.001 | < 0.001 | ||||
| Homogeneous | 65 (58.55) | 8 (10.26) | 32 (68.08) | 8 (23.52) | ||
| Heterogeneous | 46 (41.45) | 70 (89.74) | 15 (31.92) | 26 (76.48) | ||
| Radiomics score | -1.28 ± 1.28 | 0.62 ± 1.41 | < 0.001 | -1.29 ± 1.27 | 0.60 ± 1.60 | < 0.001 |
| Intercept and variable | Conventional MRI model | Nomogram model | ||||
| Coefficient | Odds ratio (95%CI) | P value | Coefficient | Odds ratio (95%CI) | P value | |
| Intercept | -6.429 | 0.002 (0.001, 0.012) | < 0.001 | -4.270 | 0.014 (0.001, 0.113) | < 0.001 |
| Short axis | 0.478 | 1.612 (0.962, 2.745) | 0.072 | 0.088 | 1.092 (0.650, 1.845) | 0.739 |
| Long-axis | 0.009 | 1.010 (0.679, 1.520) | 0.963 | 0.178 | 1.194 (0.794, 1.806) | 0.394 |
| Margin | 1.431 | 4.184 (1.831, 10.105) | 0.001 | 1.278 | 3.588 (1.592, 8.443) | 0.003 |
| T2WI heterogeneous signal | 0.309 | 1.362 (0.586, 3.120) | 0.467 | 0.556 | 1.744 (0.793, 3.856) | 0.166 |
| Patterns of enhancement | 2.042 | 7.709 (3.200, 20.561) | < 0.001 | 1.477 | 4.380 (2.004, 9.983) | < 0.001 |
| Radiomics score | NA | NA | NA | 0.839 | 2.314 (1.795, 3.086) | < 0.001 |
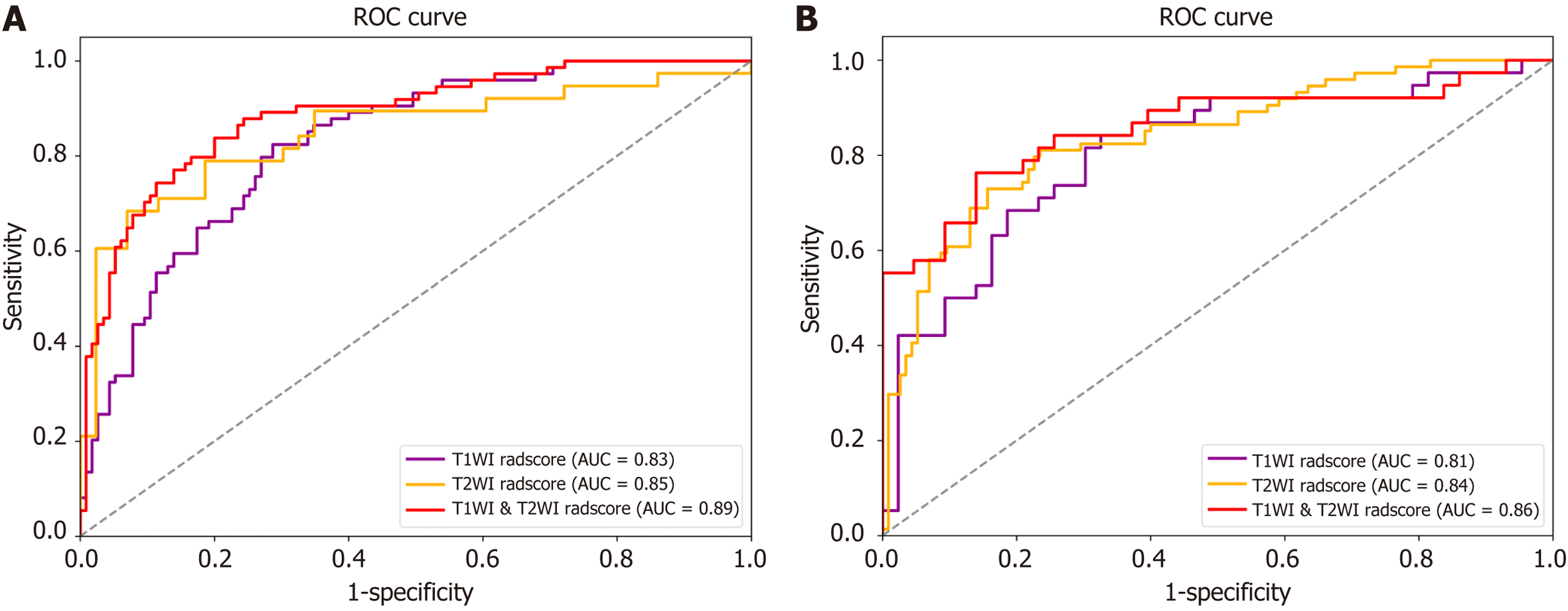
The T1WI-Radscore included 13 features, the T2WI-Radscore included 10 features, and the T1WI & T2WI-Radscore included 18 features (Supplementary Figure 1). The AUC for the T1WI & T2WI-Radscore was higher than that of the T1WI-Radscore and T2WI-Radscore in the training set (0.89 vs 0.83 and 0.89 vs 0.85, respectively) and validation set (0.86 vs 0.81 and 0.86 vs 0.84, respectively) (Figure 3 and Table 4).
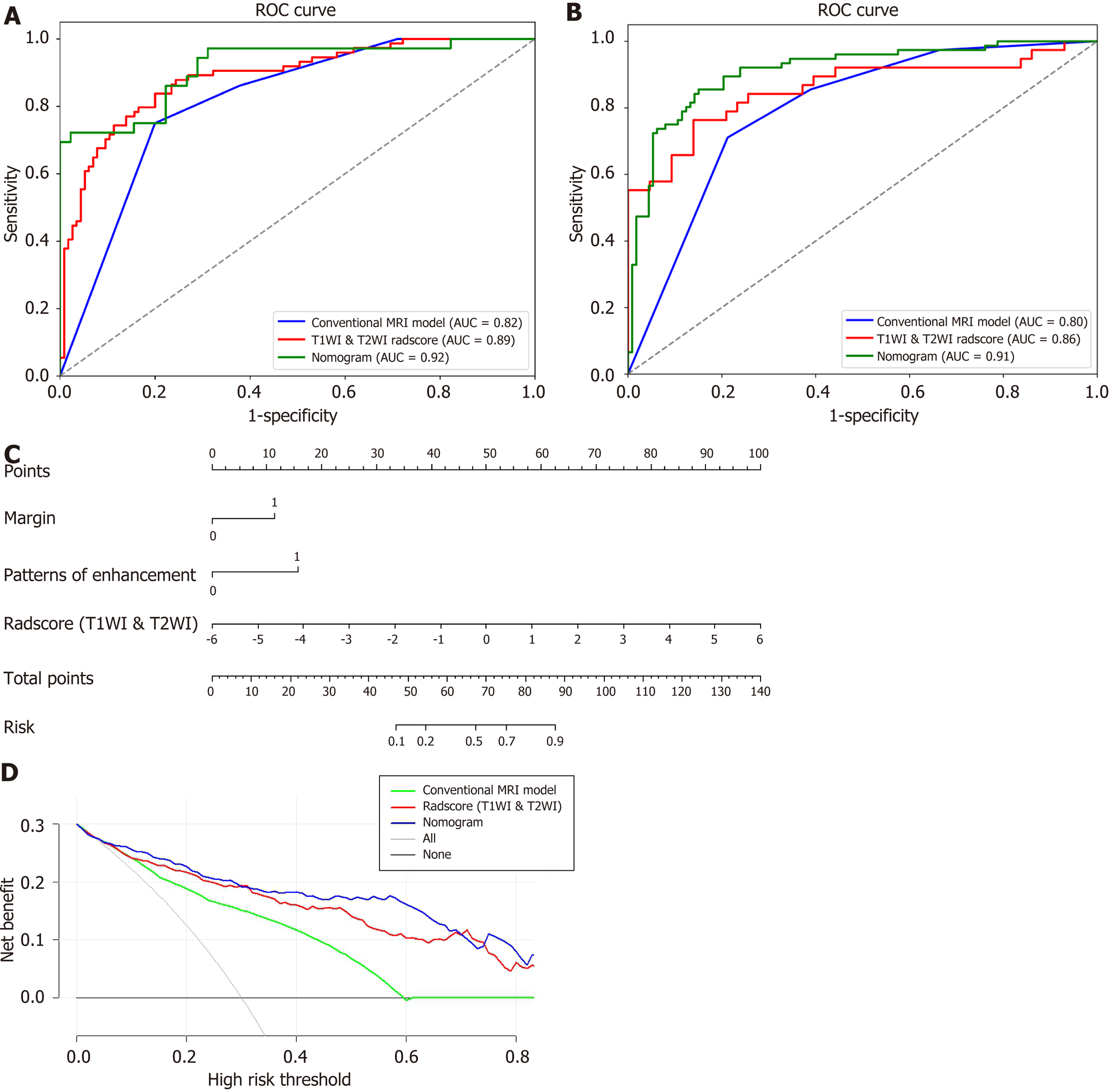
| Data set | Model | AUC (95%CI) | Sensitivity, % | Specificity, % | Accuracy, % |
| Training cohort | Conventional MRI model | 0.82 (0.76, 0.87) | 75.0 | 80.0 | 77.8 |
| T1WI radiomics model | 0.83 (0.79, 0.87) | 59.5 | 82.6 | 73.5 | |
| T2WI radiomics model | 0.85 (0.79, 0.91) | 63.1 | 93.0 | 79.0 | |
| T1WI & T2WI Radiomics model | 0.89 (0.84, 0.93) | 74.3 | 86.1 | 81.5 | |
| Nomogram model | 0.92 (0.84, 0.99) | 72.2 | 91.1 | 82.8 | |
| Validation cohort | Conventional MRI model | 0.80 (0.76, 0.83) | 71.0 | 78.8 | 75.7 |
| T1WI radiomics model | 0.81 (0.75, 0.86) | 60.5 | 83.7 | 72.8 | |
| T2WI radiomics model | 0.84 (0.80, 0.88) | 62.2 | 87.0 | 77.2 | |
| T1WI & T2WI Radiomics model | 0.86 (0.79, 0.92) | 65.8 | 90.7 | 79.0 | |
| Nomogram model | 0.91 (0.81, 0.96) | 81.6 | 86.7 | 84.7 |
The margin, enhancement appearance, and T1WI & T2WI-Radscore were integrated to build a nomogram model (Figure 4A). The nomogram model was obtained using the following equation: Nomogram model = -4.270 + (T1WI & T2WI-Radscore × 0.839) + (Margin × 1.278) + (enhancement appearance × 1.477). The DCA based on the three models is presented in Figure 4D. The diagnostic performances of the nomogram models are shown in Table 4 and Figure 4A and B. The AUC of the T1WI & T2WI-Radscore was higher than that of the conventional MRI model in the training set (0.89 vs 0.82, P < 0.001) and validation set (0.86 vs 0.80, P < 0.001). The AUC of the nomogram was higher than that of the conventional MRI model in the training set (0.92 vs 0.82, P < 0.001) and validation set (0.91 vs 0.80, P < 0.001). The AUC of the nomogram was higher than that of the T1WI & T2WI-Radscore in the training set (0.92 vs 0.89, P < 0.001) and validation set (0.91 vs 0.86, P < 0.001) (Figure 4).
In this study, we found that the optimal performance in preoperatively diagnosing LNM in patients with RC was provided by the nomogram model, followed by radiomics features alone. Meanwhile, both approaches outperformed conventional MRI model alone in both the training and validation set. These findings indicated that radiomics features derived from MRI could serve as an effective complement to MRI imaging features for the preoperative diagnosis of LNM in patients with RC.
Adequate morphological information is essential for the accurate diagnosis of LNM. Previous studies revealed specific characteristics observed in LNM, such as calcification and cystic changes, which contribute to heterogeneous enhancement[22,23]. Metastatic LNs typically exhibit irregular and indistinct borders with lobulated contours, resulting in a nonsmooth appearance[3,24,25]. LN heterogeneous T2WI signals have been recognized as indicators of LNM[23,26]. Our study also identified significant differences in both factors between the NLNM and LNM groups. However, it is intriguing to note that the heterogeneous T2WI signal did not exhibit independent predictive capability on its own. One possible explanation is that the influence of heterogeneous T2WI signals on LNM is encompassed by heterogeneous enhancement features. The heterogeneous enhancement feature exhibits a more stable impact in predicting LNM. Furthermore, our research results emphasize the significant clinical diagnostic value of using intravenous contrast agents as recommended in guidelines[27]. Multifactor logistic regression analysis revealed enhancement and boundary features as independent predictors for LNM, with a validation set AUC of 0.80. These findings align with previous research, which obtained a similar AUC of 0.82 for morphological evaluation in assessing LNM[2]. Despite the relatively low AUC values of the morphological model in both datasets, it still provides reasonably good classification performance.
Our research revealed that the AUC based on the LN T1WI & T2WI Radscore was consistently higher than the AUC based on the T1WI radscore and T2WI Radscore. This implies that combining radiological features of T1WI and T2WI imaging may provide more useful information for predicting LNM. Previous research has also demonstrated the superior predictive value of multiparametric MRI radiomics compared to single-parameter radiomics. In our study, we developed a model using T1WI and T2WI imaging, and it exhibited similar AUC values in both the training and validation datasets, indicating a high level of reproducibility for the model. This could be attributed to the inherent stability of LNs in T1WI and T2WI imaging, which are less susceptible to variations in scanning parameters, image preprocessing techniques, and ROI segmentation methods. The DWI sequence was not included in the study. In fact, DWI only aids in LN detection without providing predictive value for LNM[28]. Although some studies have affirmed the diagnostic value of tumor DWI radiomics in LNM[18,29], our preliminary exploration showed the poor ability of DWI LN radiomics for LNM. The matching rate between preoperative imaging and postoperative specimens of LNs in RC is notably low as demonstrated by a recent study that reported only 47% agreement[30]. Few studies have reported LN radiomics in predicting LNM in RC. Wang et al[31] found that LN CT radiomics outperformed morphological features in predicting lateral LNM. Zhu et al[32] showed that all visible LN MRI radiomics performed better than tumor radiomics in predicting LNM. We used MRI guidelines and pathology reports to correlate LNs and assess the predictive efficacy of MRI radiomics for LNM on a per-node basis. In our study, the LN T2WI-radscore was found to outperform morphological features in predicting LNM. This indicates that radiomics may capture some microscopic tumor characteristics of LNs that are not visible on MRI. Previous research has supported the superiority of integrating multiple predictive indicators in a nomogram for LNM compared to using a single Radscore[17,33]. Our study yielded similar results, with the nomogram demonstrating superior predictive efficacy in diagnosing LNM compared to the T1WI & T2WI Radscore. The integration of Conventional MRI features into the nomogram offers personalized risk evaluation for per-node metastasis. This holds immense importance for patients who have enlarged lateral LNs, as it has the potential to avoid genitourinary dysfunction resulting from unnecessary LN dissection in those with negative lateral LNs[34].
In our research, we found the nomogram combining Conventional MRI features and MRI radiomics, making up for the limitation of assessing Metastasis of Evaluable LNs only according to Conventional MRI features of the LN itself, could improve the accuracy of preoperative assessing metastasis of evaluable LNs; this improvement may be because MRI radiomics can reflect tumor microscopic characteristics in the evaluable LNs.
The nomogram model of conventional MRI features combined with radiomics features on T1WI and T2WI of RC-related evaluable LNs showed promising performance in assessing LNM preoperatively.
Lymph node (LN) staging in rectal cancer (RC) affects treatment decisions and patient prognosis. For radiologists, the traditional preoperative assessment of LN metastasis (LNM) using magnetic resonance imaging (MRI) poses a challenge.
The accuracy of assessing LNM based on MRI remains limited. A meta-analysis demonstrated a sensitivity of approximately 77% and specificity of approximately 71% when using MRI to diagnose metastasis in evaluable LNs. Therefore, there is a risk of diagnostic insufficiency and overdiagnosis.
To explore the value of a nomogram model that combines Conventional MRI and radiomics features from the LNs of RC in assessing the preoperative metastasis of evaluable LNs.
A total of 270 LNs (158 LNM and 112 metastatic) were included and randomly allocated to training set (111 nonmetastatic and 78 metastatic) and validation set (47 nonmetastatic and 34 metastatic) at a 7:3 ratio. Radiomic features were extracted from T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) images of individual LN. The least absolute shrinkage and selection operator regression analysis was used for feature selection. Multivariate logistic regression analysis was used to develop the Rad-score and nomogram model. Receiver operating characteristic curves were constructed to evaluate the diagnostic performance of the models for predicting LNM. The performance of the nomogram was assessed using decision curve analysis (DCA).
The nomogram model outperformed conventional MRI and single radiomics models in evaluating LNM. In the training set, the nomogram model achieved an area under the curve (AUC) of 0.92, which was significantly higher than the AUCs of 0.82 (P < 0.001) and 0.89 (P < 0.001) of the conventional MRI and radiomics models, respectively. In the validation set, the nomogram model achieved an AUC of 0.91, significantly surpassing 0.80 (P < 0.001) and 0.86 (P < 0.001), respectively.
The nomogram model showed the best performance in predicting metastasis of evaluable LNs.
Radiomics holds great promise for transforming medical practice, especially for patients with RC. However, before its widespread adoption, challenges regarding sample size, model design, and robust multicenter validation sets must be addressed. To validate the proposed model externally, future prospective multicenter studies with larger sample sizes are crucial.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Computer science, artificial intelligence
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chien CR, Taiwan S-Editor: Chen YL L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64715] [Article Influence: 16178.8] [Reference Citation Analysis (177)] |
| 2. | Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 606] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 3. | Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 607] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 4. | Dahlbäck C, Korsbakke K, Alshibiby Bergman T, Zaki J, Zackrisson S, Buchwald P. Accuracy of magnetic resonance imaging staging of tumour and nodal stage in rectal cancer treated by primary surgery: a population-based study. Colorectal Dis. 2022;24:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Park JS, Jang YJ, Choi GS, Park SY, Kim HJ, Kang H, Cho SH. Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features. Dis Colon Rectum. 2014;57:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Langman G, Patel A, Bowley DM. Size and distribution of lymph nodes in rectal cancer resection specimens. Dis Colon Rectum. 2015;58:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Gröne J, Loch FN, Taupitz M, Schmidt C, Kreis ME. Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer with Magnetic Resonance Imaging. J Gastrointest Surg. 2018;22:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Bedrikovetski S, Dudi-Venkata NN, Kroon HM, Seow W, Vather R, Carneiro G, Moore JW, Sammour T. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | Zhuang Z, Zhang Y, Wei M, Yang X, Wang Z. Magnetic Resonance Imaging Evaluation of the Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:709070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Zhao L, Liang M, Yang Y, Zhao X, Zhang H. Histogram models based on intravoxel incoherent motion diffusion-weighted imaging to predict nodal staging of rectal cancer. Eur J Radiol. 2021;142:109869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Lahaye MJ, Engelen SM, Kessels AG, de Bruïne AP, von Meyenfeldt MF, van Engelshoven JM, van de Velde CJ, Beets GL, Beets-Tan RG. USPIO-enhanced MR imaging for nodal staging in patients with primary rectal cancer: predictive criteria. Radiology. 2008;246:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Zhang C, Zheng Z, Ye F, Liu Y, Zou S, Zhou C. Chemical shift effect predicting lymph node status in rectal cancer using high-resolution MR imaging with node-for-node matched histopathological validation. Eur Radiol. 2017;27:3845-3855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Liu Y, Wen Z, Yang X, Lu B, Xiao X, Chen Y, Yu S. Lymph node metastasis in rectal cancer: comparison of MDCT and MR imaging for diagnostic accuracy. Abdom Radiol (NY). 2019;44:3625-3631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Zhuang Z, Ma X, Zhang Y, Yang X, Wei M, Deng X, Wang Z. Technique to match mesorectal lymph nodes imaging findings to histopathology: node-by-node comparison. J Cancer Res Clin Oncol. 2023;149:3905-3914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 3570] [Article Influence: 446.3] [Reference Citation Analysis (0)] |
| 16. | Zhang YC, Li M, Jin YM, Xu JX, Huang CC, Song B. Radiomics for differentiating tumor deposits from lymph node metastasis in rectal cancer. World J Gastroenterol. 2022;28:3960-3970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Zhao W, Xu H, Zhao R, Zhou S, Mei S, Wang Z, Zhao F, Xiao T, Huang F, Qiu W, Tang J, Liu Q. MRI-based Radiomics Model for Preoperative Prediction of Lateral Pelvic Lymph Node Metastasis in Locally Advanced Rectal Cancer. Acad Radiol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Jia H, Jiang X, Zhang K, Shang J, Zhang Y, Fang X, Gao F, Li N, Dong J. A Nomogram of Combining IVIM-DWI and MRI Radiomics From the Primary Lesion of Rectal Adenocarcinoma to Assess Nonenlarged Lymph Node Metastasis Preoperatively. J Magn Reson Imaging. 2022;56:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 19. | Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, Ma ZL, Liu ZY. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol. 2016;34:2157-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1321] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 20. | Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 361] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 21. | Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4946] [Cited by in RCA: 3775] [Article Influence: 251.7] [Reference Citation Analysis (0)] |
| 22. | Chen Y, Wen Z, Liu Y, Yang X, Ma Y, Lu B, Xiao X, Yu S. Value of High-resolution MRI in Detecting Lymph Node Calcifications in Patients with Rectal Cancer. Acad Radiol. 2020;27:1709-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Zhang Z, Chen Y, Wen Z, Wu X, Que Y, Ma Y, Wu Y, Liu Q, Fan W, Yu S. MRI for nodal restaging after neoadjuvant therapy in rectal cancer with histopathologic comparison. Cancer Imaging. 2023;23:67. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Xu Q, Xu Y, Wang J, Sun H, Lin J, Xie S. Distinguishing mesorectal tumor deposits from metastatic lymph nodes by using diffusion-weighted and dynamic contrast-enhanced magnetic resonance imaging in rectal cancer. Eur Radiol. 2023;33:4127-4137. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Almlöv K, Woisetschläger M, Loftås P, Hallböök O, Elander NO, Sandström P. MRI Lymph Node Evaluation for Prediction of Metastases in Rectal Cancer. Anticancer Res. 2020;40:2757-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Xian MF, Zheng X, Xu JB, Li X, Chen LD, Wang W. Prediction of lymph node metastasis in rectal cancer: comparison between shear-wave elastography based ultrasomics and MRI. Diagn Interv Radiol. 2021;27:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Gollub MJ, Arya S, Beets-Tan RG, dePrisco G, Gonen M, Jhaveri K, Kassam Z, Kaur H, Kim D, Knezevic A, Korngold E, Lall C, Lalwani N, Blair Macdonald D, Moreno C, Nougaret S, Pickhardt P, Sheedy S, Harisinghani M. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY). 2018;43:2893-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Heijnen LA, Lambregts DM, Mondal D, Martens MH, Riedl RG, Beets GL, Beets-Tan RG. Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol. 2013;23:3354-3360. [RCA] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Fang Z, Pu H, Chen XL, Yuan Y, Zhang F, Li H. MRI radiomics signature to predict lymph node metastasis after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. Abdom Radiol (NY). 2023;48:2270-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Rutegård MK, Båtsman M, Blomqvist L, Rutegård M, Axelsson J, Ljuslinder I, Rutegård J, Palmqvist R, Brännström F, Brynolfsson P, Riklund K. Rectal cancer: a methodological approach to matching PET/MRI to histopathology. Cancer Imaging. 2020;20:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Wang D, Zhuang Z, Wu S, Chen J, Fan X, Liu M, Zhu H, Wang M, Zou J, Zhou Q, Zhou P, Xue J, Meng X, Ju S, Zhang L. A Dual-Energy CT Radiomics of the Regional Largest Short-Axis Lymph Node Can Improve the Prediction of Lymph Node Metastasis in Patients With Rectal Cancer. Front Oncol. 2022;12:846840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Zhu H, Zhang X, Li X, Shi Y, Zhu H, Sun Y. Prediction of pathological nodal stage of locally advanced rectal cancer by collective features of multiple lymph nodes in magnetic resonance images before and after neoadjuvant chemoradiotherapy. Chin J Cancer Res. 2019;31:984-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Zhang S, Tang B, Yu M, He L, Zheng P, Yan C, Li J, Peng Q. Development and Validation of a Radiomics Model Based on Lymph-Node Regression Grading After Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Int J Radiat Oncol Biol Phys. 2023;117:821-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 34. | Ma P, Yuan Y, Yan P, Chen G, Ma S, Niu X, Xu M, Yang K, Cai H. The efficacy and safety of lateral lymph node dissection for patients with rectal cancer: A systematic review and meta-analysis. Asian J Surg. 2020;43:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |