Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1821
Peer-review started: November 29, 2023
First decision: January 15, 2024
Revised: January 29, 2024
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: May 15, 2024
Processing time: 161 Days and 22.8 Hours
Intraductal papillary neoplasm of the bile duct (IPNB) is a premalignant biliary-type epithelial neoplasm with intraductal papillary or villous growth. Currently reported local palliative therapeutic modalities, including endoscopic nasobiliary drainage, stenting and biliary curettage, endoscopic biliary polypectomy, percu
To assess the technical feasibility, efficacy, and safety of PTCS-BP for local palliative treatment of IPNB.
Patients with mucin-hypersecreting cast-like or polypoid type IPNB and receiving PTCS-BP between September 2010 and December 2019 were included. PTCS-BP was performed by using a half-moon type snare with a soft stainless-steel wire, and the tumor was snared and resected with electrocautery. The primary outcome was its feasibility, indicated by technical success. The secondary outcomes were efficacy, including therapeutic success, curative resection, and clinical success, and safety.
Five patients (four with mucin-hypersecreting cast-like type and one with polypoid type IPNB) were included. Low- and high-grade intraepithelial neoplasia (HGIN) and recurrent IPNB with invasive carcinoma were observed in one, two, and two patients, respectively. Repeated cholangitis and/or obstructive jaundice were presented in all four patients with mucin-hypersecreting cast-like type IPNB. All five patients achieved technical success of PTCS-BP. Four patients (three with mucin-hypersecreting cast-like type and one with polypoid type IPNB) obtained therapeutic success; one with mucin-hypersecreting cast-like type tumors in the intrahepatic small bile duct and HGIN had residual tumors. All four patients with mucin-hypersecreting IPNB achieved clinical success. The patient with polypoid type IPNB achieved curative resection. There were no PTCS-BP-related serious adverse events.
PTCS-BP appears to be feasible, efficacious, and safe for local palliative treatment of both mucin-hypersecreting cast-like and polypoid type IPNB.
Core Tip: Technical feasibility, efficacy, and safety of percutaneous transhepatic cholangioscopy-assisted biliary polypectomy (PTCS-BP) for local palliative treatment of intraductal papillary neoplasm of the bile duct (IPNB) were assessed. PTCS-BP was performed in four patients with mucin-hypersecreting cast-like type and one with polypoid type IPNB. All five patients achieved technical success, and four patients achieved therapeutic success. All the four patients with mucin-hypersecreting cast-like type IPNB achieved clinical success, and the patient with polypoid type IPNB achieved curative resection. Therefore, PTCS-BP appears to be feasible, efficacious, and safe for local palliative treatment of both cast-like and polypoid type IPNB.
- Citation: Ren X, Qu YP, Zhu CL, Xu XH, Jiang H, Lu YX, Xue HP. Percutaneous transhepatic cholangioscopy-assisted biliary polypectomy for local palliative treatment of intraductal papillary neoplasm of the bile duct. World J Gastrointest Oncol 2024; 16(5): 1821-1832
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1821.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1821
Intraductal papillary neoplasm of the bile duct (IPNB) is a premalignant biliary-type epithelial neoplasm with intraductal papillary or villous growth[1-6]. IPNBs with associated invasive cancer and low-grade (LGIN) or high-grade intraepithelial neoplasia (HGIN) account for 60.0% and 36.0% of IPNB cases, respectively. The 5-year survival rate is 22% and 100% for IPNB with and without invasive cancer, respectively[7]. Therefore, timely management is required, especially for IPNB with high-grade dysplasia.
At present, surgery with radical resection or liver transplantation are the two main treatment approaches for IPNB[7]. IPNB patients not suitable for radical surgery or liver transplantation may receive palliative managements, including endoscopic nasobiliary drainage, stenting, and biliary curettage after endoscopic sphincterotomy, and percutaneous biliary drainage to relieve mechanical obstruction[8-10]. In addition, local therapies including laser ablation[11], iridium-192 intraluminal therapy[10], argon plasma coagulation[12,13], photodynamic therapy[14,15], and endoscopic retrograde cholangiopancreatography (ERCP)-guided radiofrequency ablation have also been reported for the management of IPNB[16]. However, the above-mentioned managements are mostly described in case reports with certain limitations. In addition, there is no consensus on the optimal treatment for mucin-hypersecreting IPNB, for which the 5-year survival rate is only 19%[17]. New management strategies that provide more reliable efficacy are required.
Recently, peroral cholangioscopy with snare electric resection has been developed to remove biliary polyps, and this technique is called cholangioscopy-assisted biliary polypectomy[18]. In addition, cholangioscopic electrocoagulation was successfully applied to remove biliary papillomatosis in a patient with multiple papillary neoplasms with silt-like mucus in the hilar and intrahepatic bile ducts diagnosed on choledochoscopy via T-tube tract[19].
Since its introduction in the mid-1970s, percutaneous transhepatic cholangioscopy (PTCS) has been applied for removal of intrahepatic stones (IHS)[20-23] and treatment of bilioenteric anastomotic strictures[24,25]. Moreover, choledochoscopy-assisted techniques were developed as an assisting tool for local therapy of IPNB[23]. These techniques included choledochoscopy via T-tube tract with laser ablation therapy for biliary papillomatosis[11] and direct cholangioscopy with argon-plasma coagulation therapy for IPNB to reduce mucin production[12]. We applied PTCS in combination with snare electric resection in 2001 for the treatment of biliary polyps including gallbladder polyps and tentatively called this technique PTCS-assisted biliary polypectomy (PTCS-BP). Moreover, since September 2010, we have started to adopt this technique for the management of IPNB including mucin-hypersecreting cast-like growing and non-mucin-producing polypoid type tumors in order to find out a better local palliative modality to treat these types of IPNB. In the present study, we aimed to assess the technical feasibility, efficacy, and safety of PTCS-BP for the local palliative treatment of IPNB including cast-like and polypoid type tumors.
This was a retrospective study of patients with IPNB who received PTCS-BP at our hospital between September 2010 and December 2019. The study protocol was approved by the Ethics Committee of Heilongjiang Provincial Hospital. All information of patients at hospitalization and during follow-up visits was collected from the hospital medical records.
The inclusion criteria were the following: (1) Patients who were diagnosed with mucin-hypersecreting cast-like growing type IPNB with or without invasive cancer, repeated cholangitis and/or obstructive jaundice, and abnormality of liver function tests (LFTs) indicating cholestasis or patients who were diagnosed with polypoid type IPNB with dysplasia; and (2) patients who received PTCS-BP intervention. Patients with incomplete medical records were excluded.
IPNB was diagnosed based on clinical presentations, laboratory and imaging examinations, ERCP, percutaneous transhepatic cholangiography, and PTCS. The imaging diagnosis of IPNB was based on a previous literature[5]. The mucus was identified by ERCP, percutaneous transhepatic biliary drainage (PTBD), and PTCS. The IPNB location and extent were determined by PTCS. IPNB was pathologically diagnosed on the resected specimens during PTCS-BP. Histologically, IPNB was classified into LGIN and HGIN, and associated invasive carcinoma[4]. IPNB was classified into four types, including gastric type, intestinal type (IT), pancreatobiliary type (PT), and oncocytic types (OT)[4].
IPNB is macroscopically categorized into four types: Polypoid, cast-like growing, superficial-spreading, and cystic[3]. Of the four types, polypoid type with pedunculated, or sessile polypoid tumors, and cast-like growing type with a longitudinal extent of tumor involvement over twice the height of the tumor filling the ductal lumen[3] are suitable for PTCS-BP. Thus, only these two types of IPNB were included in the present study. In addition, IPNB was also classified into extrahepatic duct (EHD), intrahepatic duct (IHD), and diffuse type, with the diffuse type being located over a wide range of IHD and EHD[26].
PTBD was performed to enable percutaneous access for PTCS. The bile duct was punctured at the right anterior-superior branch or the left peripheral branch by a 19-gauge puncture needle (Hakko, Nagano, Japan) under ultrasound and fluoroscopic guidance. A 7Fr pigtail catheter (Hakko, Nagano, Japan) was placed in the bile duct. One week later, stepwise sinus tract dilatation was performed by a plastic bougie (Cook Medical LLC. Bloomington, IN, United States) until the caliber reached 18 Fr in two sessions within a 2-3 d interval. Afterwards, the 18 Fr indwelling catheter (Sumitomo, Tokyo, Japan) was left in place for PTCS. PTCS was performed 2 wk after PTBD by using a 4.9 mm external diameter cholangioscope with a 2 mm-diameter working channel (ED-270F, Fujifilm, Tokyo, Japan) to identify the location, extent, and morphologic appearance of the tumor and take targeted biopsies.
Then, PTCS-BP procedure was performed by using a half-moon type snare with a soft stainless-steel wire (SD-7P/8P or SD-221L, Olympus Medial Systems Corp, Tokyo, Japan), and the tumor was resected with electrocautery [power settings of 15 watts, blended current (blend 1 mode) for PSD 30 Electrosurgical Unit from Olympus Medial Systems Corp or a fractionated cutting current (effect 2, cut duration 1, cut interval 4) for ENDO CUT® Q from Erbe Elektromedizin GmbH Inc., Tübingen, Germany] under PTCS. For a mucin-hypersecreting cast-like growing tumor, a step-by-step attempt was made until the tumor was successfully removed. For a polypoid tumor, the snare was placed to the basis of the tumor and then slowly tightened, and the tumor was electrically resected. Liver and biliary ultrasound and/or computed tomography examinations were performed on the third day after PTCS-BP procedure to rule out biloma caused by biliary injury.
The primary outcome was technical feasibility, indicated by the technical success of the procedure (i.e., the success for the endoscopists to accomplish PTCS-BP procedure for IPNB). The secondary outcomes were the efficacy, including therapeutic success, curative resection, and clinical success, and safety. Therapeutic success was defined when there was barely any residual macroscopic tumor for cast-like type or no residual tumors for polypoid type after PTCS-BP. Curative resection was defined as the successful removal of polypoid type IPNB without any residue tumor and lymph node metastasis during at least a 3–6-month follow-up period. Clinical success was defined as the improvement of IPNB-related symptoms (i.e., cholangitis and/or jaundice) and cholestasis (LFTs or imaging data) lasting for more than 3 months for mucin-hypersecreting cast-like type IPNB after the removal of the catheter or replacement of the 18Fr catheter with a 7 Fr catheter. Clinical success was assessed based on the improvement of cholangitis and/or jaundice and cholestasis in patients with mucin-hypersecreting cast-like IPNB. The improvement of cholangitis was defined as no recurrent cholangitis for at least 3 months after PTCS-BP. The improvement of cholestasis was defined as a decrease in γ-glutamyl transferase, alkaline phosphatase, or total bilirubin level by > 70% or in the dilatated bile duct caliber by > 1/2 two wk after the PTCS-BP. In addition, the bile drainage volume, and the duration of catheter-free and indwelling drainage catheter in patients with mucin-hypersecreting cast-like IPNB were also measured.
Safety was evaluated by the occurrence and severity of adverse events, which were defined as any unexpected clinical manifestations or laboratory and imaging abnormalities that occurred during the preparation for PTCS-BP and during the PTCS-BP procedure. Adverse events were graded as mild, moderate, or severe as previously described[27,28].
The patient was discharged from the hospital 3-4 d after the end of PTCS-BP procedure, with the PTCS catheter being placed at least for 3 months. The clinical manifestations, the amount of bile drainage during the indwelling period of the catheter, LFTs, and imaging examinations were evaluated 2 wk after hospital discharge and patients were followed up every 3 months for at least 1 year. The catheter was clamped at least for 2 wk 3-6 months after PTCS-BP and removed or exchanged for a 7 Fr catheter if there was no cholestasis or cholangitis. In addition, PTCS was repeated within 3 to 6 months to observe the status of the resected tumor.
Overall, 45 patients were diagnosed with IPNB between September 1999 and December 2021. Of them, five patients, 2 male and 3 female, with an average age of 60.2 (range 48-76) years old, underwent PTCS-BP for IPNB and thus were included in the present study. Their baseline characteristics, past interventional procedures, and laboratory results are shown in Supplementary Table 1. Polypoid type and mucin-hypersecreting cast-like type IPNB were detected, respectively, in one and four patients. Four patients had IHS which was completely cleared by PTCS. Two patients were incidentally found to have IHD type IPNB during PTCS treatment for IHS.
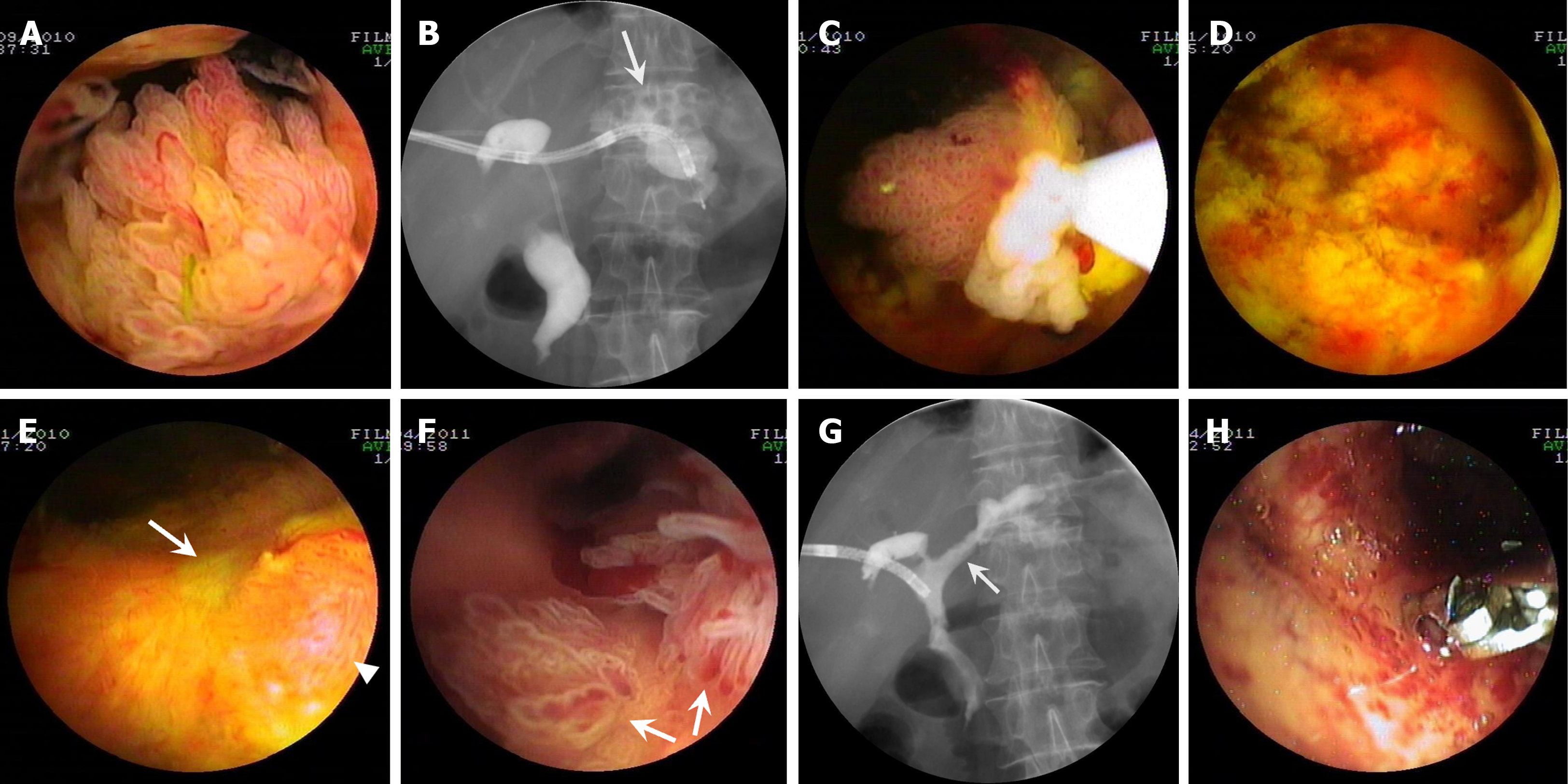
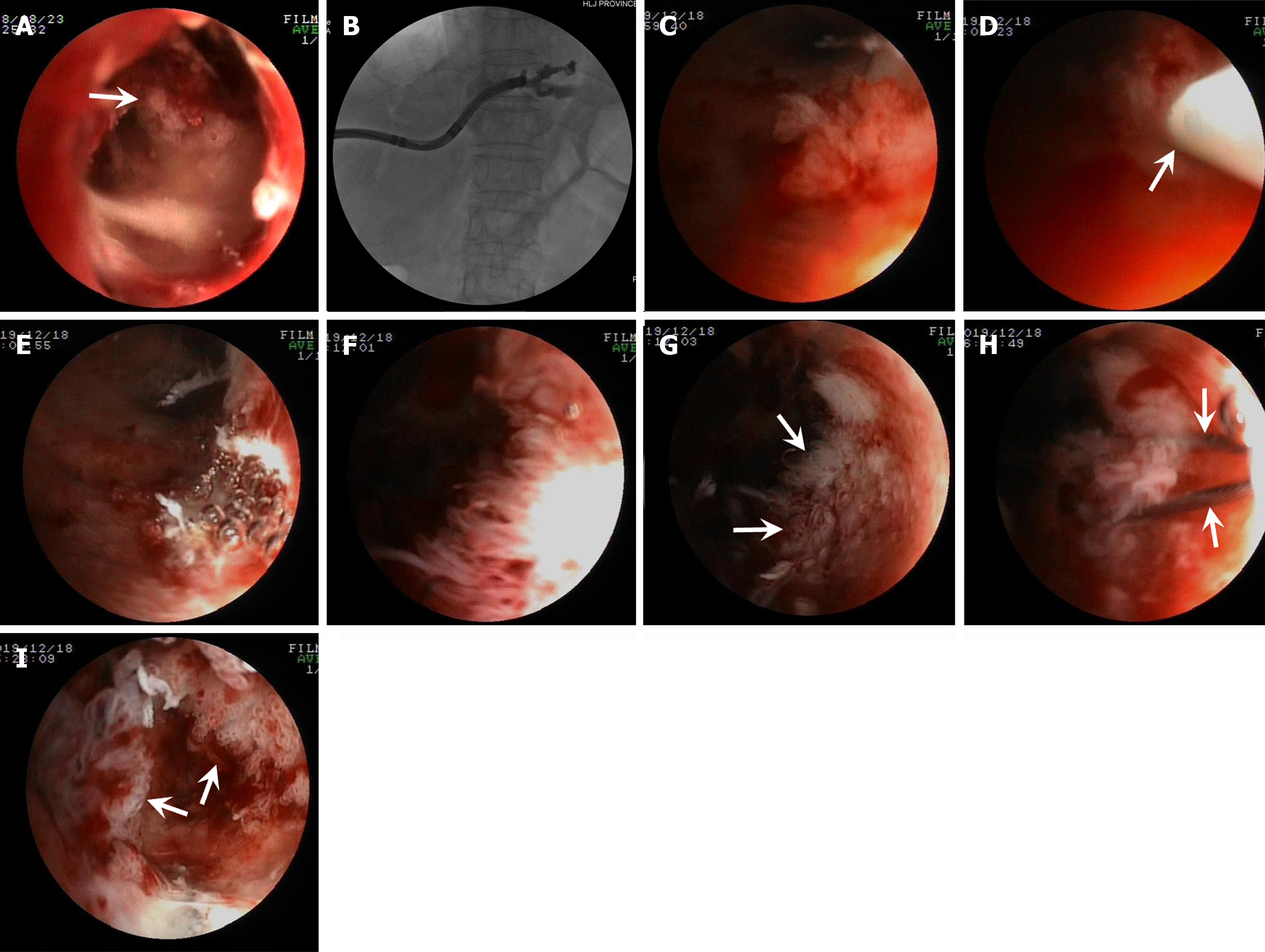
Patient one: Cast-like type IPNB. PTCS showed innumerable long and slender villous and frond-like tumors (Figure 1A) distributed in the center of the common hepatic duct and left hepatic duct and a large amount of viscous mucus (Video 1) with multiple biliary stones in the left IHD (Figure 1B). PTCS-BP was performed 40 d after PTCS diagnosis. The patient received five PTCS-BP sessions (Figure 1C) during a 2-month period until all tumors were successfully removed (Figure 1D; Video 1). Repeated PTCS 2 wk after the fifth PTCS-BP session showed that scars had formed with a minimal proliferated tumor (Figure 1E). Pathological examination revealed LGIN and HGIN with MUC2 and MUC5AC expressions. Five months later, PTCS showed a few newly growing leaf-like tumors with HGIN (Figure 1F) at the location of the removed tumor. Cholangiography showed a reduced biliary tract caliber with a stenotic stiff and rigid ductal wall suspected of an invasive lesion in the left hepatic duct (Figure 1G). Then, a biopsy was repeated. The pathological examination revealed IPNB with invasive carcinoma (Figure 1H; Table 1).
| Case No. | Histopathological findings | Subtype | Immunohistochemistry profiles | |||||
| Ki67 | P53 | MUC1 | MUC2 | MUC5AC | MUC6 | |||
| 11 | IPNB with HGIN was diagnosed. 5 PTCS-BP sessions were performed. The tubular adenocarcinoma was identified in biopsies taken from the bile duct wall where the tumor was resected under PTCS 5 months later | IT | 0.2 | - | - | + | + | - |
| 22 | IPNB with mucinous adenocarcinoma was observed in resected specimens | IT | 0.9 | - | + | + | + | + |
| 3 | IPNB with LGIN was diagnosed with resected specimens | OT | 0.1 | - | - | - | + | - |
| 42 | IPNB with mucinous adenocarcinoma was identified in resected specimens under PTCS | PT | 0.3 | - | + | + | + | + |
| 5 | IPNB with HGIN was diagnosed in resected specimens. The patient developed intrahepatic bile duct-stomach fistula 32 months (August 2022) post- PTCS-BP due to drain obstruction induced by thick mucus | IT | 0.7 | - | - | + | + | - |
Video 1: PTCS showing that patient one has cast-like growing tumors and multiple delicate villous and frond-like tumors with vascular images distributed in the center of the common hepatic duct and the left hepatic bile duct. There is excessively thick mucin in the dilated bile duct. Step-by-step PTCS-BP of the tumors is performed with technical success. Two months after the end of PTCS-BP, repeated PTCS shows that therapeutic success has been successfully achieved.
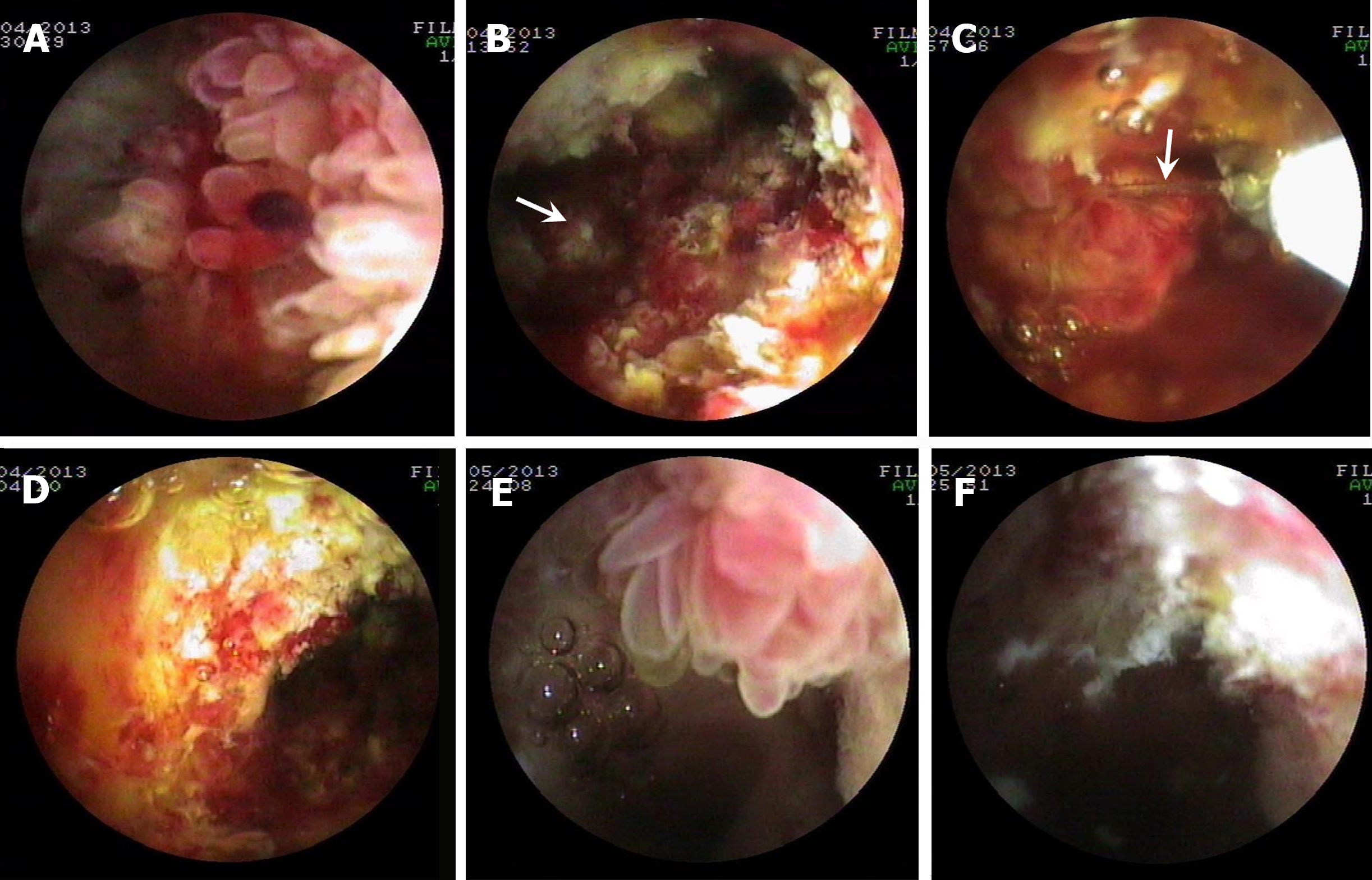
Patient two: Cast-like type IPNB. Multiple papillary (Figure 2A) and polypoid mass (Figure 2C) and frond-like tumors (Figure 2E) with a large amount of mucus located in the hilar bile duct and common hepatic duct were detected by PTCS. Two PTCS-BP sessions were performed with successful resection of the tumor (Figure 2B, D, and F; Supplementary Table 2). Histopathologic examination showed IPNB with mucinous adenocarcinoma in one resected specimen. Other specimens were associated with LGIN or HGIN, with positive expression of MUC1, MUC-2, MUC5AC, and MUC-6 (Table 1).
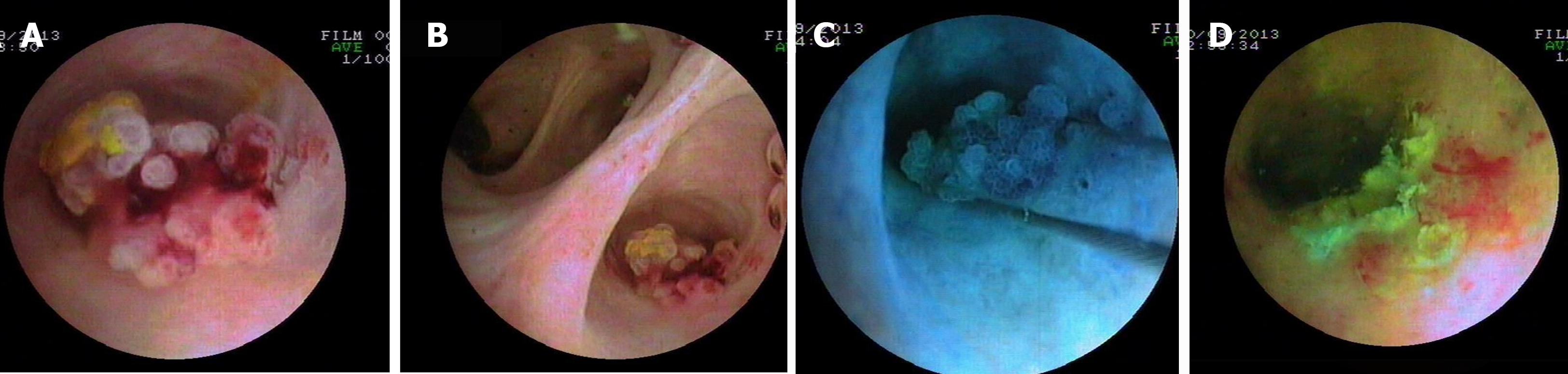
Patient three: Polypoid type IPNB. Two polypoid masses (1.0 cm2 × 1.2 cm2 and 0.5 cm2 × 0.6 cm2) (Figure 3A-C) in the right anterior superior branch and its adjacent branch bile duct were detected during PTCS treatment for IHS, which were successfully removed with PTCS-BP (Figure 3D; Supplementary Table 2). Histopathological and immunohistochemical examinations revealed IPNB with LGIN and OT subtype and positive expression of MUC5AC, respectively (Table 1). Repeated PTCS 6 months after PTCS-BP showed no evidence of tumor.
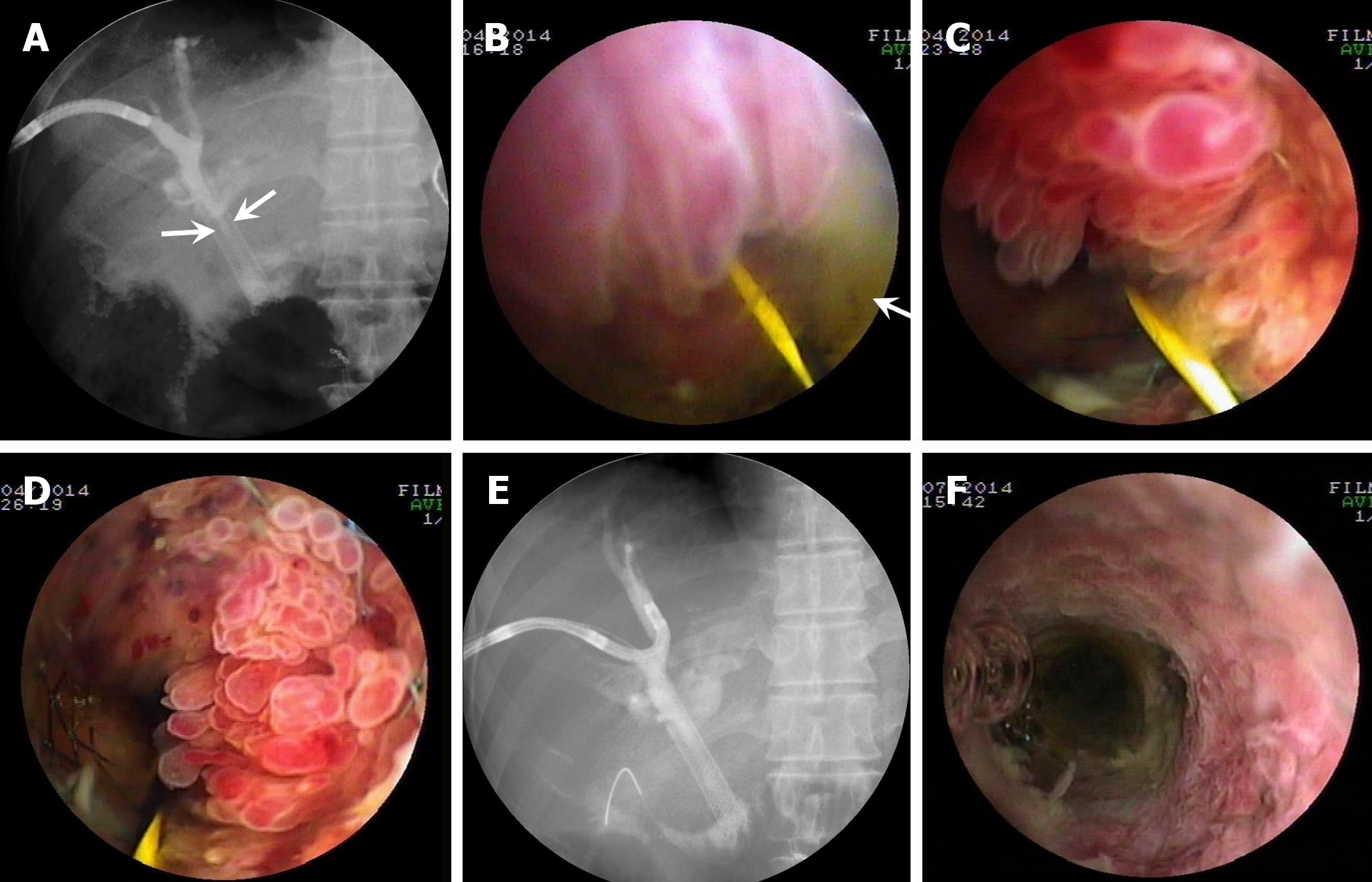
Patient four: Cast-like type IPNB. The patient received a transanastomotic self-expandable metal stent (SEMS) for postoperative recurrence IPNB (Supplementary Table 1). Cholangiography showed the SEMS luminal stricture (Figure 4A). PTCS showed a large amount of mucus and multiple villous (Figure 4B), frond-like (Figure 4C), and fish-egg like protrusions (Figure 4D) in the stent lumen. The lesions protruding into the stent lumen were successfully resected with PTCS-BP. Pathological examination revealed IPNB that was associated with LGIN or HGIN in several specimens, and mucinous adenocarcinoma in one specimen, which was positive for the expression MUC1, MUC-2, MUC5AC, and MUC-6 (Table 1). Cholangiography and repeated PTCS both showed a patent metal stent with no significant tumorous elevations in the stent lumen 3 months after PTCS-BP (Figure 4E and F).
Patient five: Cast-like type IPNB. Biliary tumors (Figure 5A) were detected when PTCS passed through a stenotic bile duct for stone removal. Cholangiography showed the stricture located in the left IHD (Figure 5B). PTCS-BP was carried out 16 months later, which successfully resected several frond-like or villous tumors (Figure 5C-H) but was unable to successfully resect tumors located in the small lumen (Figure 5I). Pathological examination showed HGIN and IT subtype with positive expression of MUC2 and MUC5AC. The patient developed rare intrahepatic biliary-stomach fistula between left peripheral bile duct and stomach 32 months later (Table 1).
The five IPNB patients received a total of 11 PTCS-BP sessions with an average of 2.2 (range 1-5) sessions, and all (100.0%) achieved technical success of PTCS-BP (Supplementary Table 2). Moreover, PTCS-BP successfully resected cast-like tumors filling the ductal lumen, including the tumors surrounding biliary-enteric anastomosis or located in the metal stent lumen, and polypoid tumors in IHD, except for tumors located in the small biliary lumen.
Four (80%) patients (3 cast-like tumors and 1 polypoid tumor) achieved therapeutic success (Supplementary Table 2). One (20.0%) patient had residual lesions due to its location in the relatively small intrahepatic ductal lumen (Figure 5I). One patient with polypoid type IPNB achieved curative resection. Four patients (100%) with mucin-hypersecreting IPNB through PTCS-BP achieved clinical success (Supplementary Table 2), although one of them had residual lesions. The dilatated bile duct caliber was reduced by more than 50% in two of the patients with improvement of cholestasis. In patient one, cholangiography showed obvious improvement of the bile duct dilation due to decreased mucus secretion (Figure 1G). However, invasive carcinoma developed 5 months after the last PTCS-BP. In addition, the mucus components decreased in the bile of two patients. The bile drainage volume increased from 200 to 300 mL/d to 500 to 600 mL/d in these two patients after PTCS-BP.
The five patients were followed up for an average of 24.4 months (8.0-37.0 months). The indwelling PTCS catheters were exchanged for an indwelling 7 Fr catheter 7 and 3 months after the end of PTCS-BP, respectively, in two patients, and the indwelling PTCS catheter was continuously placed in the one patient. The catheters were removed 6 and 3 months after the end of PTCS-BP in the remaining two patients, with the catheter-free duration being 31 and 25 months, respectively (Supplementary Table 2). All five patients experienced a total of ten onsets of cholangitis with an average of 2 (ranging 1-3) during the follow-up period (Supplementary Table 2). The cholangitis episodes were associated with IHS recurrence in one patient (patient three) and with hilar bile duct stricture in another (patient two). Cholangitis episodes appeared to be directly related to thick mucus in two patients including one (patient five) with residual tumor.
Bacteremia developed in two patients during the preparation for PTCS-BP. Three patients experienced cholangitis (Supplementary Table 3). No PTCS-BP-related serious adverse events were observed. Three patients died 8, 15, and 28 months after hospital discharge (Supplementary Table 3).
In the present report, we present the feasibility, efficacy, and safety of PTCS-BP in five IPNB patients with polypoid and cast-like type IPNB in the extrahepatic or intrahepatic bile duct. All five patients (100.0%) achieved technical success, indicating the feasibility of the PTCS-BP technique. Moreover, therapeutic success was achieved in four (80.0%) patients including three (75.0%) with mucin-hypersecreting cast-like tumors and one with polypoid tumors, which curative resection was achieved. We applied PTCS-BP for palliative therapy of cast-like type IPNB with mucin hypersecretion in four patients (100.0%) and achieved clinical success although one of them had residual lesions. There were no major safety issues in all patients.
Single or scattered growing polypoid type IPNB tumors, especially pedunculated and semipedunculated polypoid tumors with LGIN and HGIN, are easy to snare and thus likely to be successfully removed with PTCS-BP, achieving curative resection. In one of the five patients, all two non-mucin-producing polypoid tumors with LGIN located in the intrahepatic large bile duct were successfully removed with PTCS-BP, and curative resection (Figure 3) was achieved.
Mucobilia is a herald sign of IPNB[29] and can be directly identified by endoscopy or PTBD. In our case series, four IPNB patients with excessive thick mucus in the bile duct were detected by ERCP, PTBD, and PTCS. Prior to PTCS-BP, drainage was difficult for patients as the thick mucus frequently obstructed the catheter. Endoscopic biliary curettage with a balloon sweep has been used to remove mucus or tumor fragments in the bile duct[26,29]. However, for patients with significant bile duct dilatation, the balloon sweep is usually ineffective, and irrigation with 1% N-acetyl-cysteine in the bile duct is required[12].
Successful resection with PTCS-BP is not easy for cast-like type tumors filling the ductal lumen. In our experience, when long and slender multiple villous tumors are snared, an assistant is required to slowly tighten the snare steel wire while the operator slightly pushes out the snare sheath to avoid the tumors slipping off the snare. More importantly, the proximal end of the snare should be pressed down slightly while the distal end of the loop should not be uplifted. PTCS mainly relies on irrigation rather than air supply to maintain a satisfactory operative field of vision. PTCS-BP is sometimes attempted in a visual field-free state after the tumor has been snared. Therefore, it is very important for the assistant and operator to cooperate with each other. For PTCS-BP of cast-like tumors filling the ductal lumen, rotation of the cholangioscopic axis in a clockwise or anticlockwise direction and adjustment of the cholangioscope up to the best location are required to snare the tumor. In patient one, the multiple delicate villous and frond-like tumors were successfully removed with PTCS-BP (Video 1; Figure 1D). Only one patient (patient five) with cast-like type tumors associated had residual lesions (Figure 5I) after the tumor removal (Figure 5C-H) because some tumors were located in a relatively narrow space. We successfully performed PTCS-BP of multiple tumors inside the metal stent in patient four. The repeated PTCS showed the stent patency without tumor within the stent after 3 months (Figure 4F). Our experience indicates that PTCS-BP can be performed within the metal stent lumen.
Notably, in patient one, most of the resected specimens were pathologically associated with LGIN with only a few associated with HGIN, and IPNB with invasive carcinoma was diagnosed through further PTCS biopsies at bile duct wall 5 months later. A similar pathological phenomenon was observed in other two patients (patients two and four). These findings indicate that pathological examinations of biopsied or electrically resected specimens cannot always accurately diagnose IPNB as the same tumor may exhibit different pathological changes or stages[30]. In addition, among the four patients with mucin-hypersecreting IPNB, patient one with IT subtype had both MUC2 and MUC5AC. Two other patients (patients two and four) had IT and PT histological subtype, respectively, and were positive for mucin core proteins MUC1, MUC2, MUC5AC, and MUC6. In our case series, two MUC1-positive patients with postoperative recurrence and invasive adenocarcinoma had poor survival.
We believe that PTCS-BP is efficacious and superior to other resection methods for the treatment of polypoid and mucin-hypersecreting cast-like growing IPNB tumors in a large bile duct. Among the present five patients, two were catheter-free 6 and 3 months later, and two exchanged from an 18-Fr catheter to a 7-Fr indwelling catheter 7 and 3 months later, respectively. The last patient with residual lesions continued to have an indwelling PTCS catheter. In the four patients with mucin-hypersecreting cast-like tumors, two had increased bile drainage volume, while the other two patients with the bile duct caliber reduced by more than 50%. Nine onsets of cholangitis developed in the four patients with cast-like type IPNB (Supplementary Table 2) during follow up period, in which the frequency of cholangitis tended to be lower than that before PTCS-BP.
It was observed that one patient (patient five) with residual lesions also achieved clinical success. However, he developed cholangitis 31 months post PTCS-BP, most likely due to poor drainage caused by the accumulation of the mucus and recurrence of the stone. Then, stone removal and mucus clearance were performed by PTCS. One month later, he developed rare intrahepatic biliary tree-stomach fistula, which might have developed from the obstruction of PTCS catheter caused by the thick mucus and subsequent IHD perforation into the adjacent stomach under the mechanical pressure exerted by the excessive mucus accumulation within the stenotic bile duct.
Minor adverse events occurred in three patients during the preparation process for PTCS (Supplementary Table 3). The most common adverse events were bacteremia and cholangitis. Therefore, PTCS-BP is safe for the treatment of polypoid type and cast-like mucin-hypersecreting type IPNB. However, when performing PTCS-BP, one needs to pay particular attention to cholestasis due to bile duct perforation and major hemorrhage due to physical effects or electrical damage to parallel vessels such as arteries and portal veins. During PTCS-BP, a special half-moon type snare with a soft stainless-steel wire is used to snare the soft tumor. To avoid damage to the bile duct wall, we used relatively weak blended current (blend 1 mode) or weak electrocoagulation (ENDO CUT, effect 2) to resect the tumors, and no PTCS-BP-related serious adverse events such as perforation or massive bleeding occurred. The bile duct wall can be accidentally enrolled into the snares, causing damage to the bile duct wall and perforation during electrical resection although this never happened in our cases. If perforation occurs, bile leakage may occur in the extrahepatic bile duct, and cholestasis or biloma may develop in the intrahepatic bile duct, which can be easily diagnosed detected by ultrasound and computed tomography. For bile leakage, an indwelled PTCS drainage catheter can be placed to keep the bile duct decompressed. Biloma generally does not need particular treatment due to biliary decompression through the PTCS catheter; however, additional percutaneous drainage is required for a large biloma (e.g., > 3 cm) or infection due to a biloma. It should also be kept in mind that major hemorrhage due to electrical damage to the parallel blood vessels such as arteries and portal veins might occur during PTCS-BP although our experience suggests that the parallel blood vessels are relatively safe. If major bleeding occurs due to electric injuries of blood vessels, emergency vascular intervention or surgery is required. In addition, bleeding caused by a biliovenous fistula due to blood vessel injury during the establishment of the percutaneous access can be avoided by guiding the puncture needle path under ultrasound during PTBD.
The following limitations should be kept in mind. First, the retrospective nature of the present study could bring bias into the result analysis. Second, the PTCS-BP procedure was difficult for successful resection of cast-like growing type masses within the small ductal lumen or diffuse IPNB involving the confluence of bile duct branches or short tumors such as carpet-like tumors and superficial-spreading type small papillary tumors. Third, the number of the present case series was relevantly small although it represents the largest so far reported.
PTCS-BP appears to be feasible, efficacious, and safe for local palliative treatment of both mucin-hypersecreting cast-like and polypoid type IPNB.
Currently reported local palliative therapeutic modalities, including endoscopic or percutaneous therapy to relieve mechanical obstruction, for intraductal papillary neoplasm of the bile duct (IPNB), are not efficacious due to various limitations.
Since 2010, we have applied percutaneous transhepatic cholangioscopy (PTCS)-assisted biliary polypectomy (PTCS-BP) technique for local palliative treatment of IPNB, including mucin-hypersecreting cast-like type and polypoid type tumors. Here we wish to share our experience including the feasibility, clinical efficacy, and safety of the technique with peer-physicians and endoscopists.
To assess the technical feasibility, efficacy, and safety of PTCS-BP for local palliative treatment or radical resection of IPNB.
PTCS-BP was performed by using a half-moon type snare with a soft stainless-steel wire in four patients with mucin-hypersecreting cast-like type and one with polypoid type IPNB. The tumors were snared and resected with electrocautery.
All five patients achieved technical success of PTCS-BP. Three with mucin-hypersecreting cast-like type and one with polypoid type IPNB obtained therapeutic success; one with cast-like type tumors in the small bile duct had residual tumors. All four patients with mucin-hypersecreting IPNB achieved clinical success. The patient with polypoid type IPNB achieved curative resection.
PTCS-BP appears to be feasible, efficacious, and safe for local palliative treatment of both mucin-hypersecreting cast-like and polypoid type IPNB. The new theory is that PTCS-BP can be used feasibly, efficaciously, and safely for the treatment of mucin-hyper secreting cast-like type and one with polypoid type IPNB. The novel point is that we, for the first time, applied PTCS-BP technique to treat IPNB.
Our findings indicate that PTCS-BP is superior to other resection methods for the treatment of mucin-hypersecreting cast-like and polypoid type IPNB tumors. Future studies with a larger number of patients are required to confirm our findings.
We would like to express our appreciation to Professor Kazuei Ogoshi (Niigata Cancer Center Hospital, Niigata, Japan), who passed away on July 27, 2021, for his continuous instructions and support during the development of our PTCS technique over the years. He will be missed forever. We would also like to thank Professor Tatsuo Yamakawa (Teikyo University Hospital at Mizonokuchi, Kanagawa, Japan) for his dedicated guidance on the PTCS technique.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujita K, Japan S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O, Miyazaki M. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014;2014:459091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Nakanuma Y, Kakuda Y, Uesaka K. Characterization of Intraductal Papillary Neoplasm of the Bile Duct with Respect to the Histopathologic Similarities to Pancreatic Intraductal Papillary Mucinous Neoplasm. Gut Liver. 2019;13:617-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Kim H, Lim JH, Jang KT, Kim MJ, Lee J, Lee JY, Choi D, Lim HK, Choi DW, Lee JK, Baron R. Morphology of intraductal papillary neoplasm of the bile ducts: radiologic-pathologic correlation. Abdom Imaging. 2011;36:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Nakanuma Y, Uesaka K, Kakuda Y, Sugino T, Kubota K, Furukawa T, Fukumura Y, Isayama H, Terada T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Park HJ, Kim SY, Kim HJ, Lee SS, Hong GS, Byun JH, Hong SM, Lee MG. Intraductal Papillary Neoplasm of the Bile Duct: Clinical, Imaging, and Pathologic Features. AJR Am J Roentgenol. 2018;211:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Gordon-Weeks AN, Jones K, Harriss E, Smith A, Silva M. Systematic Review and Meta-analysis of Current Experience in Treating IPNB: Clinical and Pathological Correlates. Ann Surg. 2016;263:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Krawczyk M, Ziarkiewicz-Wróblewska B, Podgórska J, Grzybowski J, Gierej B, Krawczyk P, Grąt M, Kornasiewicz O, Skalski M, Wróblewski T. Intraductal papillary neoplasm of the bile duct - A comprehensive review. Adv Med Sci. 2021;66:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | D'Abrigeon G, Blanc P, Bauret P, Diaz D, Durand L, Michel J, Larrey D. Diagnostic and therapeutic aspects of endoscopic retrograde cholangiography in papillomatosis of the bile ducts: analysis of five cases. Gastrointest Endosc. 1997;46:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Tsuchida K, Yamagata M, Saifuku Y, Ichikawa D, Kanke K, Murohisa T, Tamano M, Iijima M, Nemoto Y, Shimoda W, Komori T, Fukui H, Ichikawa K, Sugaya H, Miyachi K, Fujimori T, Hiraishi H. Successful endoscopic procedures for intraductal papillary neoplasm of the bile duct: a case report. World J Gastroenterol. 2010;16:909-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Adioui T, Seddik H, Baba H, Slioui B, Ali AA, El Hamdi FZ, Benkirane A, Zentar A. Successful surgical treatment of extrahepatic biliary papillomatosis diagnosed with endoscopic retrograde cholangiopancreatography: a case report. J Med Case Rep. 2014;8:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Meng WC, Lau WY, Choi CL, Li AK. Laser therapy for multiple biliary papillomatosis via choledochoscopy. Aust N Z J Surg. 1997;67:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Brauer BC, Fukami N, Chen YK. Direct cholangioscopy with narrow-band imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct (with videos). Gastrointest Endosc. 2008;67:574-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Jazrawi SF, Nguyen D, Barnett C, Tang SJ. Novel application of intraductal argon plasma coagulation in biliary papillomatosis (with video). Gastrointest Endosc. 2009;69:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Bechmann LP, Hilgard P, Frilling A, Schumacher B, Baba HA, Gerken G, Zoepf T. Successful photodynamic therapy for biliary papillomatosis: a case report. World J Gastroenterol. 2008;14:4234-4237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Cheong CO, Lim JH, Park JS, Park SW, Kim HK, Kim KS. Volume-reserving Surgery after Photodynamic Therapy for Biliary Papillomatosis: A Case Report. Korean J Gastroenterol. 2015;66:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Natov NS, Horton LC, Hegde SR. Successful endoscopic treatment of an intraductal papillary neoplasm of the bile duct. World J Gastrointest Endosc. 2017;9:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E, Lee SG, Min YI. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | D'Souza LS, Korman A, Benias PC, Carr-Locke DL. A novel technique for biliary polypectomy. Endoscopy. 2017;49:E244-E245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Zhou X, Wu C, Yuan C, Hu B. Electrocoagulation of biliary papillomatosis during choledochoscopy. Endoscopy. 2019;51:390-391. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Yeh YH, Huang MH, Yang JC, Mo LR, Lin J, Yueh SK. Percutaneous trans-hepatic cholangioscopy and lithotripsy in the treatment of intrahepatic stones: a study with 5 year follow-up. Gastrointest Endosc. 1995;42:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Huang MH, Chen CH, Yang JC, Yang CC, Yeh YH, Chou DA, Mo LR, Yueh SK, Nien CK. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Ren X, Tang XF, SI LJ. The percutaneous cholangioscopy therapy in treating intra and extra hepatic calculi. Zhonghua Xiaohuaneijing Zazhi. 2004;21:13-16. [DOI] [Full Text] |
| 23. | Choi JH, Lee SK. Percutaneous transhepatic cholangioscopy: does its role still exist? Clin Endosc. 2013;46:529-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Kim JH, Lee SK, Kim MH, Song MH, Park DH, Kim SY, Lee SS, Seo DW, Bae JS, Kim HJ, Han J, Sung KB, Min YI. Percutaneous transhepatic cholangioscopic treatment of patients with benign bilio-enteric anastomotic strictures. Gastrointest Endosc. 2003;58:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Yamauchi H, Kida M, Miyata E, Okuwaki K, Iwai T, Minato N, Tadehara M, Watanabe M, Imaizumi H, Koizumi W. Endoscopic Balloon Dilation for Benign Bilioenteric Stricture: Outcomes and Factors Affecting Recurrence. Dig Dis Sci. 2019;64:3557-3567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Kim JR, Lee KB, Kwon W, Kim E, Kim SW, Jang JY. Comparison of the Clinicopathologic Characteristics of Intraductal Papillary Neoplasm of the Bile Duct according to Morphological and Anatomical Classifications. J Korean Med Sci. 2018;33:e266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199-S202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1340] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 28. | Oh HC, Lee SK, Lee TY, Kwon S, Lee SS, Seo DW, Kim MH. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy. 2007;39:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Tsou YK, Liu NJ, Wu RC, Lee CS, Tang JH, Hung CF, Jan YY. Endoscopic retrograde cholangiography in the diagnosis and treatment of mucobilia. Scand J Gastroenterol. 2008;43:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Ren X, Zhu CL, Qin XF, Jiang H, Xia T, Qu YP. Co-occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: A case report and review of the literature. World J Clin Cases. 2019;7:102-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |