Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1763
Peer-review started: December 28, 2023
First decision: January 17, 2024
Revised: February 2, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: May 15, 2024
Processing time: 133 Days and 11 Hours
The models for assessing liver function, mainly the Child–Pugh (CP), albumin
To investigate and compare the prognostic performance of the above three models in thrombocytopenic HCC patients.
A total of 135 patients with thrombocytopenic HCC who underwent radical surgery were retrospectively analyzed. Preoperative scores on the CP, ALBI and PALBI classifications were estimated accordingly. Kaplan–Meier curves with log-rank tests and Cox regression models were used to explore the significant factors associated with overall survival (OS) and recurrence-free survival (RFS).
The preoperative platelet counts were significantly different among the CP, ALBI and PALBI groups. After a median follow-up of 28 mo, 39.3% (53/135) of the patients experienced postoperative recurrence, and 36.3% (49/135) died. Univariate analysis suggested that α-fetoprotein levels, tumor size, vascular invasion, and ALBI grade were significant predictors of OS and RFS. According to the multivariate Cox regression model, ALBI was identified as an independent prognostic factor. However, CP and PALBI grades were not statistically signi
The ALBI grade, rather than CP or PALBI grade, is a significant prognostic indicator for thrombocytopenic HCC patients.
Core Tip: Thrombocytopenia is a common finding in hepatocellular carcinoma (HCC) and may influence the prognostic value of the Child–Pugh (CP), albumin–bilirubin (ALBI), and platelet–ALBI (PALBI) grade. We showed that the preoperative platelet count was significantly different among the different CP, ALBI and PALBI groups of thrombocytopenic HCC patients. The statistically significant prognostic model was verified to be ALBI, rather than CP or PALBI. Therefore, ALBI grade is a significant prognostic indicator for thrombocytopenic HCC patients.
- Citation: Man ZR, Gong XK, Qu KL, Pang Q, Wu BQ. Albumin–bilirubin grade as a predictor of survival in hepatocellular carcinoma patients with thrombocytopenia. World J Gastrointest Oncol 2024; 16(5): 1763-1772
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1763.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1763
Hepatocellular carcinoma (HCC) represents 85%–90% of all liver cancer cases and is an essential health problem with a growing incidence worldwide[1,2]. HCC could be due to various causes, mainly chronic hepatitis B or C, alcoholic or nonalcoholic fatty liver, and cirrhosis[3,4]. To date, hepatectomy is the preferred radical therapy for HCC patients in the early and middle stages, and the postoperative 5-year survival rate is approximately 70%[5,6].
Most cases of HCC occur in patients with a background of chronic hepatic diseases. Therefore, perioperative liver dysfunction is one of the common reasons for postoperative morbidity and mortality in patients with HCC. Abnormal liver function is also one of the crucial factors for treatment options and long-term outcomes in HCC patients[7]. Previously, several noninvasive tools have been proposed to estimate liver function, tumor stage, treatment choice, and outcome in HCC patients. The Child–Pugh (CP) grade is among the most commonly used models[8,9]. The CP grade is widely used in cirrhotic patients, but it is not suitable for assessing tumor patients. In the CP classification, ascites and encephalopathy are subjective variables, and the levels of albumin (ALB) and ascites are inter-related elements. Therefore, the clinical application of the CP grade in treating HCC is limited and unsatisfactory. In terms of this situation, recently, the ALB–bilirubin (ALBI) and platelet–ALBI (PALBI) combinations have been established in sequence to evaluate the preoperative liver function and prognosis of HCC patients[10,11]. It has been demonstrated that the two emerging models are superior to the classical CP grade in the assessment of preoperative liver function, posthepatectomy liver failure, and outcomes in HCC patients following various treatment modalities[12-15].
Thrombocytopenia is a known noninvasive factor associated with the severity of liver disease. However, platelets have dual effects on liver tumorigenesis and progression. On the one hand, platelets promote tumor growth and angiogenesis by releasing numerous growth factors, such as α-granules and dense granules[16,17]. Thrombocytosis is associated with adverse outcomes in several solid tumors, including HCC[18]. On the other hand, thrombocytopenia is a usual manifestation of HCC and has been reported to be a valuable predictive factor of poor survival and postoperative recurrence[19]. Recently, the platelet count (PLT) was shown to be significantly correlated with hepatic function indices total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), etc., as well as the CP, ALBI and PALBI grades in HCC[18,20]. Therefore, PLT influences the prognostic value of the three models in HCC. The present study first explored and compared the prognostic significance of the three models in thrombocytopenic HCC patients.
A retrospective cohort study of thrombocytopenic HCC patients who underwent radical surgery at the First Affiliated Hospital of Bengbu Medical College between January 2015 and December 2019 was conducted. The inclusion criteria for patients were as follows: (1) Diagnosed pathologically with HCC; (2) underwent radical surgery for the first time; (3) not receiving preoperative antitumor treatment modalities, including targeted treatment and interventional therapy; and (4) preoperative thrombocytopenia (PLT < 100 × 109/L). The exclusion criteria for patients were as follows: (1) Perioperative mortality; (2) repeat hepatectomy; (3) tumor lesions at other sites; (4) incomplete calculations of the CP, ALBI or PALBI grade; and (5) malnutrition or hematological disorders; prophylactic platelet transfusions; antiplatelet drugs; etc. The study conformed with transparent reporting of a multivariable prediction model for individual prognosis or diagnosis guidelines[21] and the Declaration of Helsinki[22]. The Institutional Review Boards of Bengbu Medical College approved this study, No. 2019-055. All the included patients signed informed consent for the operation.
The following baseline information was extracted: age; sex; etiology (hepatitis B virus (HBV) or other); size and number of tumors; presence of vascular invasion; presence of ascites; cirrhosis; and hematological indicators, including preoperative α-fetoprotein (AFP), PLT, TBIL, ALT, AST and ALB levels; and the prothrombin time–international normalized ratio. Preoperative scores and grades of ALBI[10] and PALBI[11] were calculated and evaluated according to the corresponding formulas.
All patients were regularly followed up until December 2021. The follow-up interval, treatment course and salvage treatment were consistent with previous studies[18].
All the statistical analyses were carried out with SPSS version 22.0. The dichotomous variables were compared using the
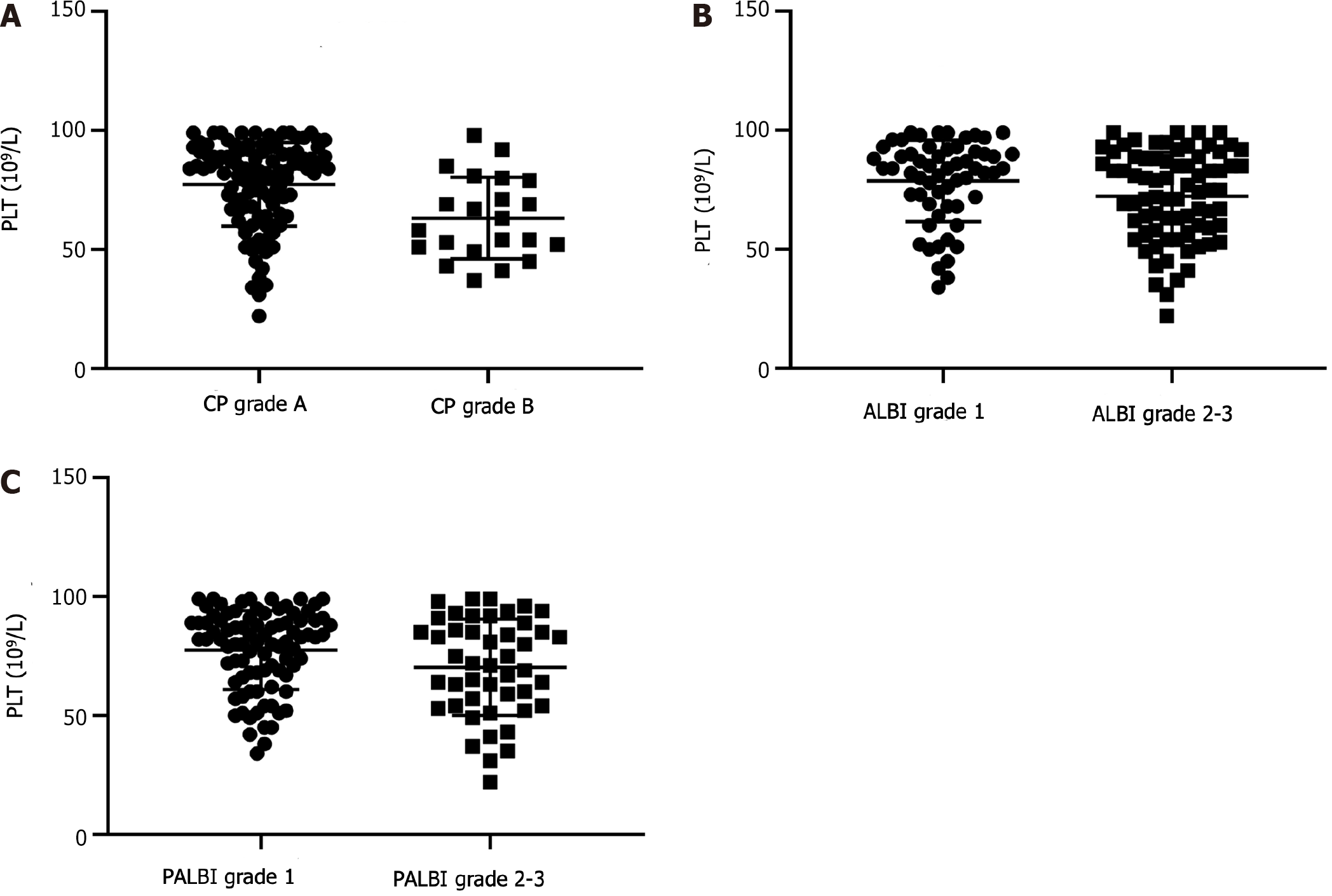
A total of 135 HCC patients with thrombocytopenia were recruited in the current cohort, and the basic information is shown in Table 1. The cohort was composed of 113 men and 22 women, and the mean age was 53.7 ± 9.8 years. There were 113 (83.7%) patients with grade A CP and 22 (16.3%) with grade B CP. According to the ALBI classification, patients were divided into grade 1 (n = 59, 43.7%) and grade 2/3 (n = 76, 56.3%) groups. In terms of the PALBI grade, 89 (65.9%) patients had a grade 1 lesion, and 46 (34.1%) had a grade 2/3 lesion. After a median follow-up of 28 mo, 53 (39.3%) patients experienced postoperative recurrence, and 50 (37.0%) patients died (37 from recurrent and progressive tumors, 6 from metastasis, 4 from liver failure, and 3 from upper gastrointestinal hemorrhage). There were significant differences in the PLT among the different groups (CP, ALBI and PALBI) (Figure 1).
| Variables | Overall |
| Etiology: HBV/others | 118/17 |
| Sex: Male/female | 113/22 |
| Age (yr) | 53.7 ± 9.8 |
| Age: ≥ 60/< 60 yr | 37/98 |
| ALT (U/L) | 38.0 (26.0-63.2) |
| AST (U/L) | 40.0 (29.4-63.0) |
| TBIL (µmol/L) | 14.8 (11.8-20.1) |
| ALB (g/L) | 38.9 (34.1-42.0) |
| PLT (109/L) | 80 (62-90) |
| AFP: ≥ 400/< 400 ng/mL | 43/92 |
| Ascites: Yes/no | 29/106 |
| Tumor size: > 5/≤ 5 cm | 64/71 |
| Tumor number: Multiple/single | 20/115 |
| Vascular invasion: Yes/no | 15/120 |
| Cirrhosis: Yes/no | 89/46 |
| CP grade (A/B) | 113/22 |
| ALBI score | -2.53 (-2.76 to -2.15) |
| ALBI grade (1/2-3) | 59/76 |
| PALBI score | -2.65 (-2.82 to -2.44) |
| PALBI grade (1/2-3) | 89/46 |
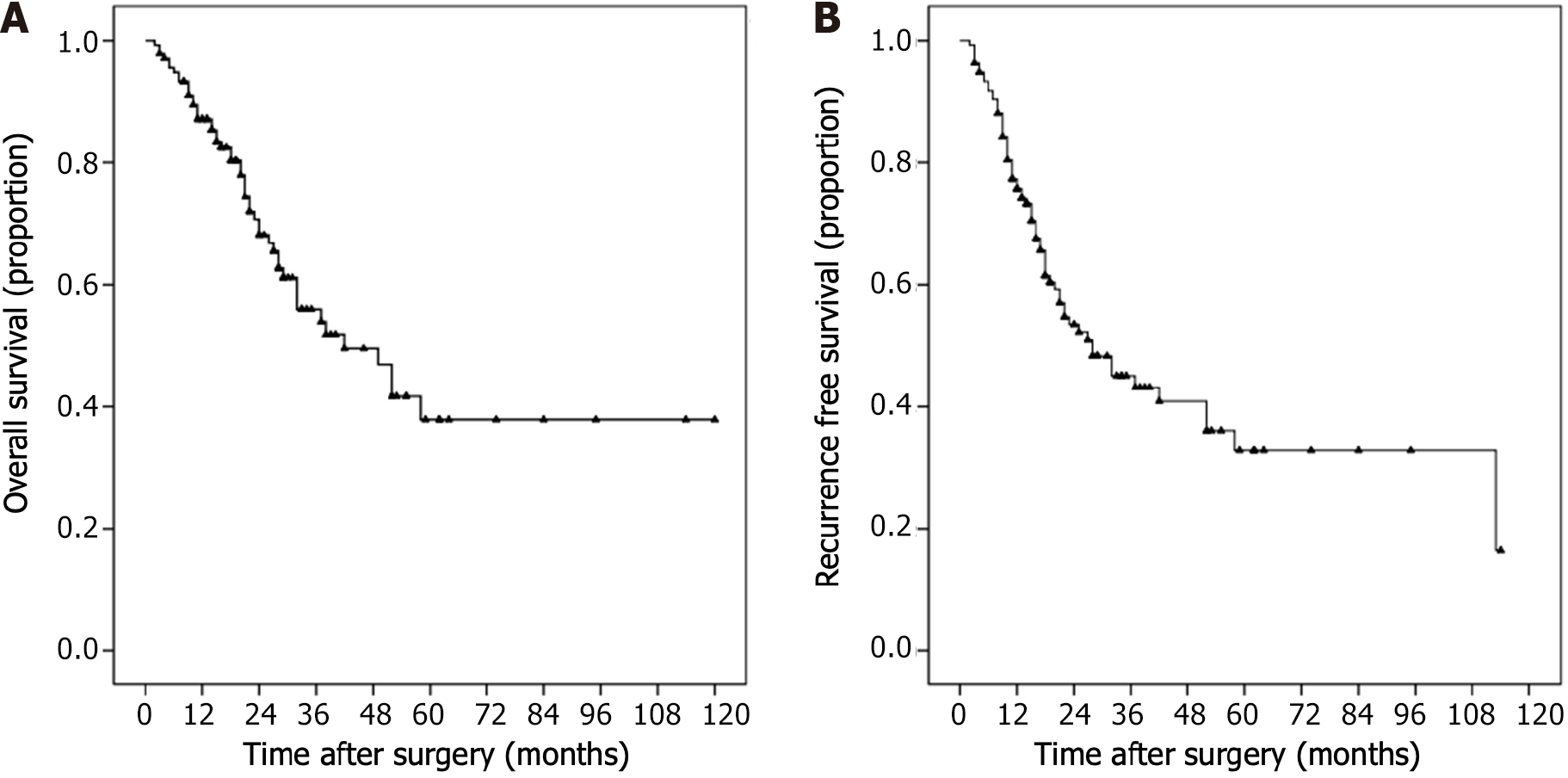
Figure 2 shows the Kaplan–Meier survival curves of the whole population. The OS rates at 1, 2 and 3 years were 87.1%, 68.1% and 55.9%, respectively, and the RFS rates at 1, 2 and 3 years were 75.7%, 53.4% and 45.0%, respectively.
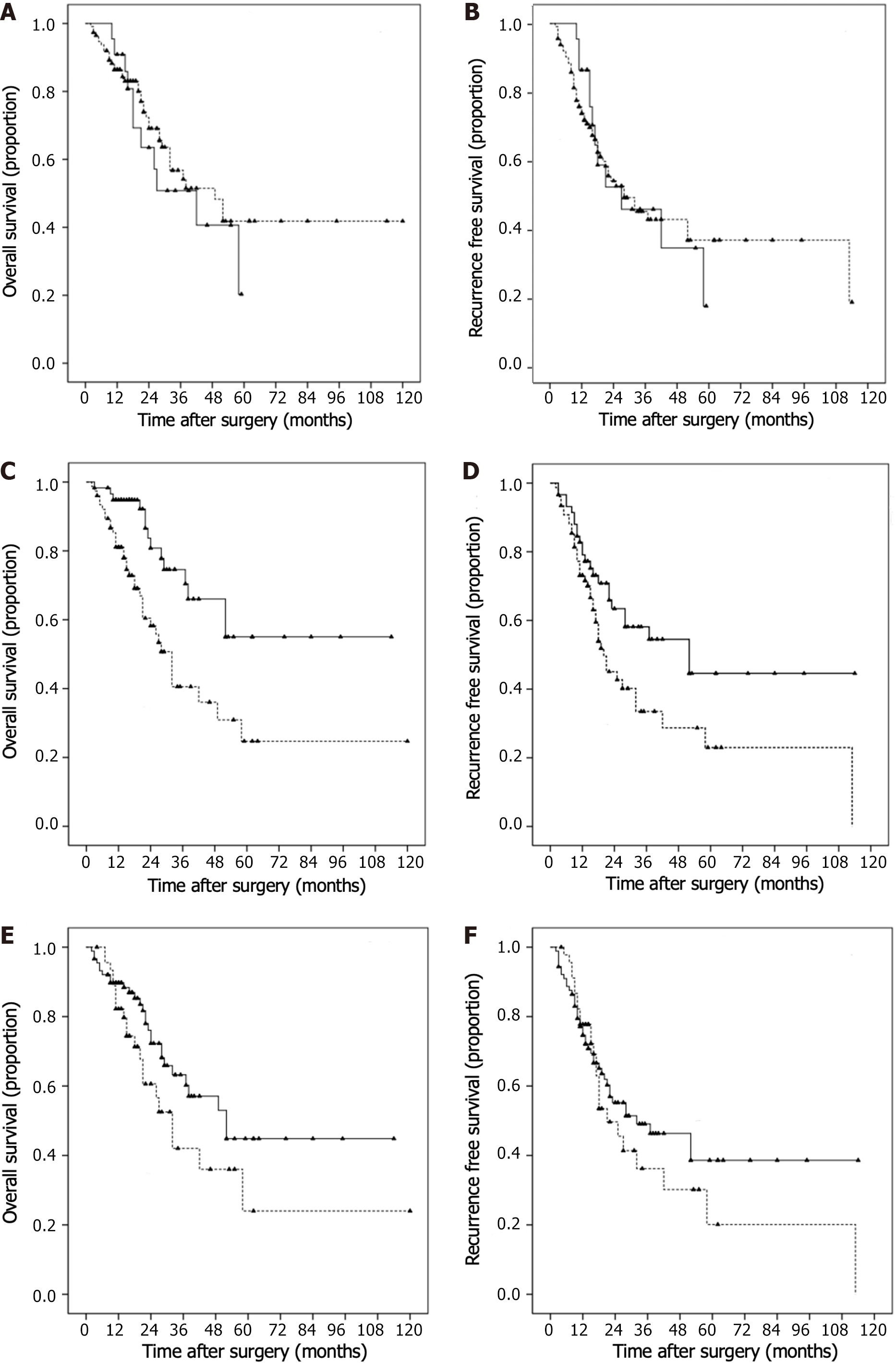
Kaplan–Meier survival curves and log-rank analyses demonstrated that the CP and PALBI grades (P > 0.05; Figure 3A–D) were not significant predictors of OS and RFS. However, ALBI grade (Figure 3E and F; P < 0.05) was found to be a significant predictor of OS and RFS. Univariate Cox regression identified AFP, tumor size, vascular invasion and ALBI grade as valuable predictors of OS and RFS. Ascites was also a significant predictor of worse RFS.
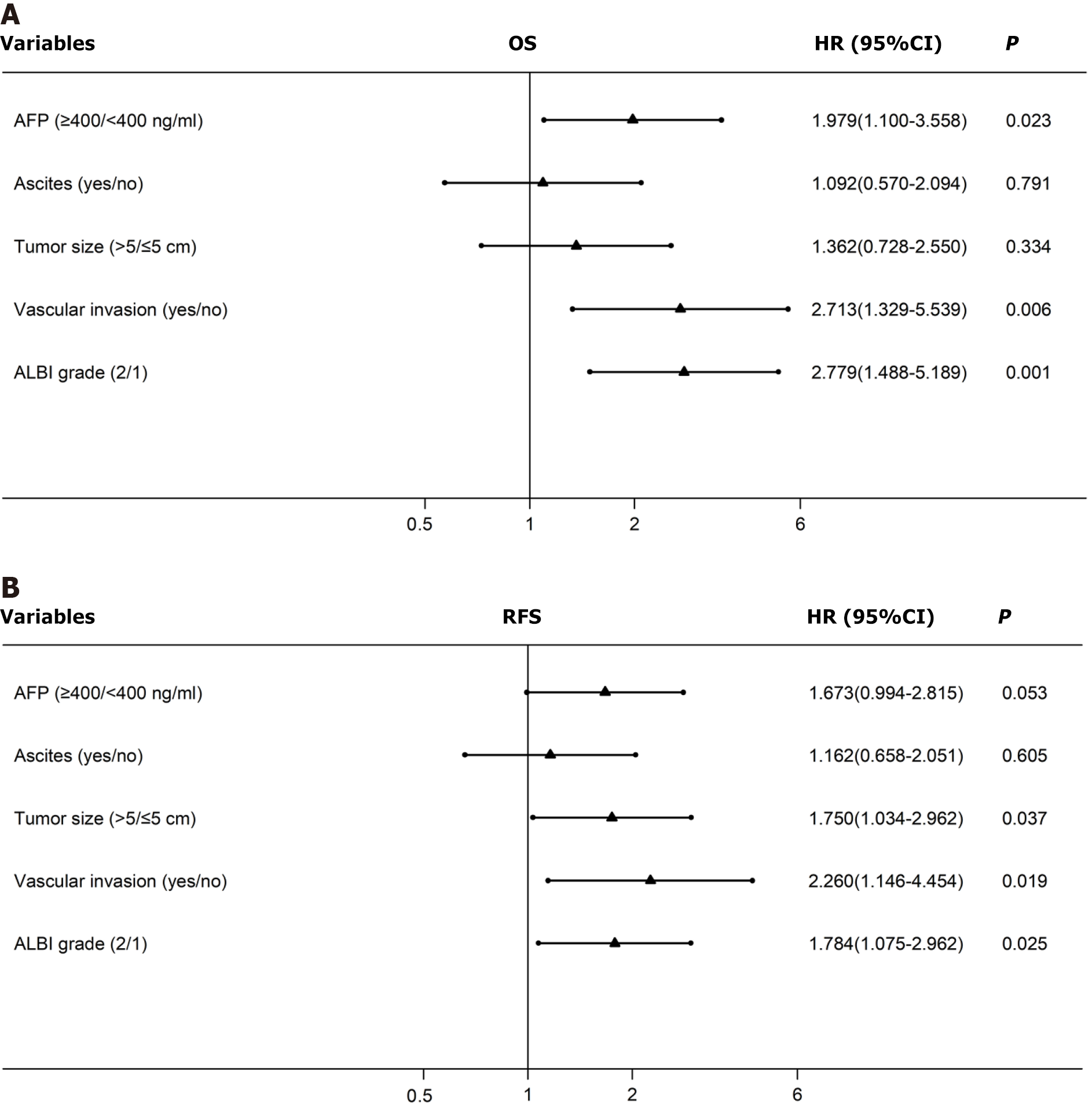
Cox multivariate analyses were conducted, and the results are shown in the forest plot (Figure 4). The AFP level [hazard ratio (HR) 1.98, 95%CI 1.10–3.56] (P = 0.023), vascular invasion (HR 2.71, 95%CI: 1.33–5.54) (P = 0.006), and ALBI grade (HR 2.78, 95%CI: 1.49–5.19) (P = 0.001) were found to be independent predictors of OS. In contrast, tumor size (HR 1.75, 95%CI: 1.03–2.96) (P = 0.037), vascular invasion (HR 2.26, 95%CI: 1.15–4.45) (P = 0.019), and ALBI grade (HR 1.78, 95%CI: 1.08–2.96) (P = 0.025) were found to be independent predictors of RFS (Table 2).
| Variables | OS | RFS | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Etiology (HBV/others) | 1.908 (0.755-4.824) | 0.172 | 1.449 (0.687-3.058) | 0.330 |
| Sex (male/female) | 1.510 (0.728-3.129) | 0.268 | 1.245 (0.649-2.389) | 0.509 |
| Age (> 60/≤ 60 years) | 0.598 (0.297-1.204) | 0.150 | 0.553 (0.292-1.050) | 0.070 |
| ALT (> 40/≤ 40 U/L) | 1.250 (0.710-2.198) | 0.441 | 1.272 (0.778-2.083) | 0.786 |
| AST (> 40/≤ 40 U/L) | 1.027 (0.585-1.802) | 0.927 | 1.092 (0.672-1.770) | 0.916 |
| TBIL (> 17/≤ 17 µmol/L) | 1.411 (0.800-2.487) | 0.234 | 1.156 (0.527-1.418) | 0.566 |
| ALB (< 35/≥ 35 g/L) | 1.029 (0.566-1.871) | 0.924 | 1.074 (0.635-1.818) | 0.789 |
| AFP (≥ 400/< 400 ng/mL) | 1.881 (1.054-3.357) | 0.032a | 1.863 (1.128-3.077) | 0.015a |
| Ascites (yes/no) | 1.692 (0.908-3.152) | 0.098 | 1.788 (1.054-3.033) | 0.031a |
| Tumor size (> 5/≤ 5 cm) | 1.919 (1.080-3.411) | 0.026a | 2.189 (1.330-3.602) | 0.002a |
| Tumor number (multiple/single) | 1.265 (0.645-2.481) | 0.494 | 1.161 (0.620-2.175) | 0.641 |
| Vascular invasion (yes/no) | 2.384 (1.181-4.812) | 0.015a | 2.509 (1.306-4.820) | 0.006a |
| Cirrhosis (yes/no) | 1.006 (0.563-1.796) | 0.984 | 1.127 (0.668-1.899) | 0.654 |
| CP grade (B/A) | 1.279 (0.654-2.504) | 0.472 | 0.998 (0.533-1.868) | 0.994 |
| ALBI grade (2/1) | 2.704 (1.448-5.047) | 0.002a | 1.768 (1.066-2.932) | 0.027a |
| PALBI grade (2-3/1) | 1.666 (0.945-2.940) | 0.078 | 1.250 (0.758-2.060) | 0.382 |
When accompanied by chronic injury or cirrhosis, HCC outcome is associated not only with tumor characteristics but also with hepatic function[7]. Previously, the CP classification has been widely applied to estimate hepatic reserve function, determine treatment, and evaluate the outcome of HCC[8,9,23]. In recent years, it has been shown that CP grade is not satisfactory[24]. In terms of a large HCC cohort, Johnson et al[10] recently established the ALBI classification for HCC[10]. Subsequent research has shown that ALBI grade is superior to CP for prediction of HCC outcomes[13,14,25]. In the HCC staging systems, which include the Barcelona Clinic Liver Cancer and the Cancer of Liver Italian Program stages, the ALBI classification was proposed as a replacement for the CP grade, and in the HCC tumor–node–metastasis staging system, the ALBI grade was added as an external supplement[26,27]. PALBI classification is also a more accurate prognostic tool than the CP in HCC patients[15,28].
Platelets are associated with the aggressive biological behavior of several types of cancer. However, in liver tumors, platelets have dual regulatory effects. On the one hand, platelets have been proven to accelerate hepatic fibrosis and tumor progression by releasing growth factors normally stored within platelet granules, including platelet-derived growth factor and serotonin[29,30]. Platelets also facilitate angiogenesis, aggressiveness and metastasis of liver tumors via these stimulants[31-33]. In contrast, platelets inhibit HCC growth through P2Y12-dependent CD40L release[34]. Consistent with these findings, we recently showed that, compared with those with normal PLTs, both high and low PLTs were significantly related to worse survival in patients with HCC[18].
Patients with hepatic cirrhosis and tumors, such as thrombocytopenia and functional changes in platelets, have profound alterations in primary hemostasis[35,36], which makes the assessment of platelets more challenging. PLTs are significantly correlated with liver function and CP, ALBI and PALBI grades[18,20]. Therefore, the PLT influences the prognostic performance of the three models. Recently, we showed that PALBI grade was closely related to OS and RFS in HCC patients, excluding those with thrombocytopenia, and that the order of the models used for identifying survival was as follows: PALBI, ALBI and CP grade[18]. However, in HCC patients with thrombocytopenia, the PALBI grade had a low predictive value[18]. The prognostic abilities of these three models for thrombocytopenic HCC have never been investigated previously. In this study, we found that a higher ALBI grade was significantly associated with worse OS and RFS in thrombocytopenic HCC patients. However, the CP and PALBI grades were not significant prognostic models. Multivariate analyses further identified ALBI grade as a prognostic factor independent of tumor size, AFP level, vascular invasion, and ascites. The underlying reasons that enable ALBI rather than CP or PALBI grade to determine outcome in patients with HCC and thrombocytopenia have not been well established. As the ALBI grade is an index of liver function irrespective of the presence and severity of underlying liver cirrhosis, thrombocytopenia may have less impact on ALBI grade than on CP and PALBI grades.
In previous research, the importance of the ALBI in treating HCC has been emphasized. We recently summarized 12 studies involving > 20 000 HCC patients and demonstrated that a higher preoperative ALBI grade was related to a greater incidence of posthepatectomy liver failure and mortality[37]. Wong et al[38] indicated that ALBI grade was a better predictor of severe hepatic failure and 30-d mortality than was the PALBI grade in patients with HCC[38]. The ALBI grade had superior discriminatory potential compared with the CP grade for differentiating outcomes among HCC patients receiving drug-eluting embolic transarterial chemoembolization (TACE)[39], TACE combined with cryoablation or sorafenib[40,41], and thermal ablation[42].
There were several limitations to our study. First, this was a retrospective study with a small sample size and single-center design. Second, only HCC patients who underwent radical hepatectomy were included. However, the superior performance of the ALBI grade in thrombocytopenic HCC patients receiving other treatments has not been validated. Third, the majority of the included patients had HBV infection. Therefore, comparisons among the three models in patients with other causes, such as hepatitis C virus, require further study. Fourth, other therapies for HCC after recurrence may influence patient outcomes. However, therapeutic information for most patients was missing after recurrence.
The ALBI grade, rather than CP or PALBI grade, is an effective prognostic indicator for thrombocytopenic HCC patients. Future research should focus on whether the outcome of thrombocytopenic HCC could be improved by decreasing the ALBI grade.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elshimi E, Egypt S-Editor: Li L L-Editor: Kerr C P-Editor: Zhang XD
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4092] [Article Influence: 584.6] [Reference Citation Analysis (6)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 3. | Papatheodoridis GV, Voulgaris T, Papatheodoridi M, Kim WR. Risk Scores for Hepatocellular Carcinoma in Chronic Hepatitis B: A Promise for Precision Medicine. Hepatology. 2020;72:2197-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Gilles H, Garbutt T, Landrum J. Hepatocellular Carcinoma. Crit Care Nurs Clin North Am. 2022;34:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 5. | Kloeckner R, Galle PR, Bruix J. Local and Regional Therapies for Hepatocellular Carcinoma. Hepatology. 2021;73:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. Biosci Trends. 2021;15:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1215] [Article Influence: 202.5] [Reference Citation Analysis (1)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6038] [Article Influence: 862.6] [Reference Citation Analysis (3)] |
| 9. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 10. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2006] [Article Influence: 200.6] [Reference Citation Analysis (0)] |
| 11. | Liu PH, Hsu CY, Hsia CY, Lee YH, Chiou YY, Huang YH, Lee FY, Lin HC, Hou MC, Huo TI. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol. 2017;32:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 12. | Kumada T, Toyoda H, Tada T, Yasuda S, Tanaka J. Changes in Background Liver Function in Patients with Hepatocellular Carcinoma over 30 Years: Comparison of Child-Pugh Classification and Albumin Bilirubin Grade. Liver Cancer. 2020;9:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, Lai PBS, Mazzaferro V, García-Fiñana M, Kudo M, Kumada T, Roayaie S, Johnson PJ. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 14. | Peng Y, Wei Q, He Y, Xie Q, Liang Y, Zhang L, Xia Y, Li Y, Chen W, Zhao J, Chai J. ALBI vs child-pugh in predicting outcome of patients with HCC: A systematic review. Expert Rev Gastroenterol Hepatol. 2020;14:383-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Liu R, Li R, Zhang M, Liu W, Li H, Li D. Prognostic Value of Platelet-Albumin-Bilirubin Grade in Child-Pugh A and B Patients With Hepatocellular Carcinoma: A Meta-Analysis. Front Oncol. 2022;12:914997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Wang L, Wang X, Guo E, Mao X, Miao S. Emerging roles of platelets in cancer biology and their potential as therapeutic targets. Front Oncol. 2022;12:939089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Morris K, Schnoor B, Papa AL. Platelet cancer cell interplay as a new therapeutic target. Biochim Biophys Acta Rev Cancer. 2022;1877:188770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 18. | Pang Q, Liu S, Wang L, Pan H, Wang C, Zhou L, Lu Y, Liu H. The Significance of Platelet-Albumin-Bilirubin (PALBI) Grade in Hepatocellular Carcinoma Patients Stratified According to Platelet Count. Cancer Manag Res. 2020;12:12811-12822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Pang Q, Qu K, Bi JB, Liu SS, Zhang JY, Song SD, Lin T, Xu XS, Wan Y, Tai MH, Liu HC, Dong YF, Liu C. Thrombocytopenia for prediction of hepatocellular carcinoma recurrence: Systematic review and meta-analysis. World J Gastroenterol. 2015;21:7895-7906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Crowley M, Rayman S, Ross S, Crespo K, Syblis C, Sucandy I, Rosemurgy A. Does Preoperative Thrombocytopenia in Patients Undergoing Robotic Hepatectomy for Liver Tumors Predict Poor Outcomes? A Propensity-Score Match Analysis. Am Surg. 2022;88:1879-1884. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1652] [Cited by in RCA: 1878] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
| 22. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 18258] [Article Influence: 1521.5] [Reference Citation Analysis (0)] |
| 23. | Rebonato A, Graziosi L, Maiettini D, Marino E, De Angelis V, Brunese L, Mosca S, Metro G, Rossi M, Orgera G, Scialpi M, Donini A. Inflammatory Markers as Prognostic Factors of Survival in Patients Affected by Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Gastroenterol Res Pract. 2017;2017:4164130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Xavier SA, Vilas-Boas R, Boal Carvalho P, Magalhães JT, Marinho CM, Cotter JB. Assessment of prognostic performance of Albumin-Bilirubin, Child-Pugh, and Model for End-stage Liver Disease scores in patients with liver cirrhosis complicated with acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2018;30:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Zou H, Yang X, Li QL, Zhou QX, Xiong L, Wen Y. A Comparative Study of Albumin-Bilirubin Score with Child-Pugh Score, Model for End-Stage Liver Disease Score and Indocyanine Green R15 in Predicting Posthepatectomy Liver Failure for Hepatocellular Carcinoma Patients. Dig Dis. 2018;36:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Chan AW, Kumada T, Toyoda H, Tada T, Chong CC, Mo FK, Yeo W, Johnson PJ, Lai PB, Chan AT, To KF, Chan SL. Integration of albumin-bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Chan AW, Chong CC, Mo FK, Wong J, Yeo W, Johnson PJ, Yu S, Lai PB, Chan AT, To KF, Chan SL. Incorporating albumin-bilirubin grade into the cancer of the liver Italian program system for hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Ni JY, Fang ZT, An C, Sun HL, Huang ZM, Zhang TQ, Jiang XY, Chen YT, Xu LF, Huang JH. Comparison of albumin-bilirubin grade, platelet-albumin-bilirubin grade and Child-Turcotte-Pugh class for prediction of survival in patients with large hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Int J Hyperthermia. 2019;36:841-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Pang Q, Jin H, Wang Y, Dai M, Liu S, Tan Y, Liu H, Lu Z. Depletion of serotonin relieves concanavalin A-induced liver fibrosis in mice by inhibiting inflammation, oxidative stress, and TGF-β1/Smads signaling pathway. Toxicol Lett. 2021;340:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Lai Q, Vitale A, Manzia TM, Foschi FG, Levi Sandri GB, Gambato M, Melandro F, Russo FP, Miele L, Viganò L, Burra P, Giannini EG; Associazione Italiana per lo Studio del Fegato (AISF) HCC Special Interest Group. Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Cao L, Zhang Y, Mi J, Shi Z, Fang Z, Jia D, Pan Z, Peng P. α-Hederin inhibits the platelet activating factor-induced metastasis of HCC cells through disruption of PAF/PTAFR axis cascaded STAT3/MMP-2 expression. Pharmacol Res. 2022;178:106180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Pavlovic N, Rani B, Gerwins P, Heindryckx F. Platelets as Key Factors in Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, Leone V, Peiseler M, Surewaard BGJ, Rath D, Ali A, Wolf MJ, Drescher H, Healy ME, Dauch D, Kroy D, Krenkel O, Kohlhepp M, Engleitner T, Olkus A, Sijmonsma T, Volz J, Deppermann C, Stegner D, Helbling P, Nombela-Arrieta C, Rafiei A, Hinterleitner M, Rall M, Baku F, Borst O, Wilson CL, Leslie J, O'Connor T, Weston CJ, Chauhan A, Adams DH, Sheriff L, Teijeiro A, Prinz M, Bogeska R, Anstee N, Bongers MN, Notohamiprodjo M, Geisler T, Withers DJ, Ware J, Mann DA, Augustin HG, Vegiopoulos A, Milsom MD, Rose AJ, Lalor PF, Llovet JM, Pinyol R, Tacke F, Rad R, Matter M, Djouder N, Kubes P, Knolle PA, Unger K, Zender L, Nieswandt B, Gawaz M, Weber A, Heikenwalder M. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 34. | Ma C, Fu Q, Diggs LP, McVey JC, McCallen J, Wabitsch S, Ruf B, Brown Z, Heinrich B, Zhang Q, Rosato U, Wang S, Cui L, Berzofsky JA, Kleiner DE, Bosco DB, Wu LJ, Lai CW, Rotman Y, Xie C, Korangy F, Greten TF. Platelets control liver tumor growth through P2Y12-dependent CD40L release in NAFLD. Cancer Cell. 2022;40:986-998.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 35. | Zanetto A, Rinder HM, Campello E, Saggiorato G, Deng Y, Ciarleglio M, Wilson FP, Senzolo M, Gavasso S, Bulato C, Simioni P, Garcia-Tsao G. Acute Kidney Injury in Decompensated Cirrhosis Is Associated With Both Hypo-coagulable and Hyper-coagulable Features. Hepatology. 2020;72:1327-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 36. | Raparelli V, Basili S, Carnevale R, Napoleone L, Del Ben M, Nocella C, Bartimoccia S, Lucidi C, Talerico G, Riggio O, Violi F. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology. 2017;65:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 37. | Pang Q, Zhou S, Liu S, Liu H, Lu Z. Prognostic role of preoperative albumin-bilirubin score in posthepatectomy liver failure and mortality: a systematic review and meta-analysis. Updates Surg. 2022;74:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Wong WG, Perez Holguin RA, Tarren AY, Shen C, Vining C, Peng JS, Dixon ME. Albumin-bilirubin score is superior to platelet-albumin-bilirubin score and model for end-state liver disease sodium for predicting posthepatectomy liver failure. J Surg Oncol. 2022;126:667-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Young LB, Tabrizian P, Sung J, Biederman D, Bishay VL, Ranade M, Patel RS, Nowakowski FS, Fischman AM, Lookstein RA, Kim E. Survival Analysis Using Albumin-Bilirubin (ALBI) Grade for Patients Treated with Drug-Eluting Embolic Transarterial Chemoembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2022;33:510-517.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Huang Z, Zuo M, Ni J, Gu Y, Zhang T, Jiang Y, Zhuo S, An C, Huang J. Assessment in the Survival Outcome After Transarterial Chemoembolization Combined with Cryoablation for Hepatocellular Carcinoma (Diameter > 4cm) Based on the Albumin-Bilirubin Grade and Platelet-Albumin-Bilirubin grade: A Preliminary Study. Cancer Manag Res. 2020;12:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Wang Z, Fan Q, Wang M, Wang E, Li H, Liu L. Comparison between Child-Pugh Score and albumin-bilirubin grade in patients treated with the combination therapy of transarterial chemoembolization and sorafenib for hepatocellular carcinoma. Ann Transl Med. 2020;8:537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Zhang J, Zhao L, Zhou Y, Ding J, Zhang Q, Jing X. The comparison between albumin-bilirubin grade and Child-Pugh grade for assessing the prognosis of hepatocellular carcinoma after thermal ablation: a propensity score-matched analysis. Transl Cancer Res. 2022;11:2523-2535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |