Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1690
Peer-review started: December 27, 2023
First decision: January 16, 2024
Revised: January 30, 2024
Accepted: March 11, 2024
Article in press: March 11, 2024
Published online: May 15, 2024
Processing time: 134 Days and 10.5 Hours
Severe immunosuppression is a hallmark of colorectal cancer (CRC). Myeloid-derived suppressor cells (MDSCs), one of the most abundant components of the tumor stroma, play an important role in the invasion, metastasis, and immune escape of CRC. MDSCs create an immunosuppressive microenvironment by inhibiting the proliferation and activation of immunoreactive cells, including T and natural killer cells, as well as by inducing the proliferation of immunosuppressive cells, such as regulatory T cells and tumor-associated macrophages, which, in turn, promote the growth of cancer cells. Thus, MDSCs are key con
Core Tip: Severe immunosuppression is a hallmark of colorectal cancer (CRC). Myeloid-derived suppressor cells (MDSCs), one of the most abundant components of the tumor stroma, play an important role in the invasion, metastasis, and immune escape of CRC. In this study, we focused on the mechanisms through which MDSCs contribute to the immunosuppressive microenvironment, current therapeutic approaches and technologies targeting MDSCs, and the therapeutic potential of modulating MDSCs in CRC treatment. This study provides ideas and methods to enhance survival rates in patients with CRC.
- Citation: Nie SC, Jing YH, Lu L, Ren SS, Ji G, Xu HC. Mechanisms of myeloid-derived suppressor cell-mediated immunosuppression in colorectal cancer and related therapies. World J Gastrointest Oncol 2024; 16(5): 1690-1704
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1690.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1690
Colorectal cancer (CRC) ranks as the third most common malignancy and the second leading cause of cancer-related deaths worldwide[1,2]. Standard treatments for CRC include surgery, chemotherapy, radiotherapy, and combinations thereof[3]. In recent years, significant advancements have been made in the diagnosis and treatment of CRC, particularly with the introduction of immunotherapy[4-6]. Pembrolizumab, for instance, has improved the median survival time for patients with metastatic CRC from 8.2 to 16.5 months, becoming established as the standard first-line treatment option for metastatic microsatellite instability-high (MSI-H) and mismatch repair-deficient (dMMR) CRC[7,8]. However, immune checkpoint inhibitors (ICIs) are only effective in CRC patients with MSI-H/dMMR, who account for approximately 15% of cases[9-12]. Furthermore, even among these patients, the response rate to ICI is only about 40%[13,14], contributing to a high mortality rate, especially in patients with stage IV CRC, who have a 5-year survival rate of only 14%[15]. Therefore, improving the efficacy of immunotherapy remains a critical challenge in improving the prognosis for CRC patients[16].
CRC is a highly malignant disease with a complex tumor microenvironment (TME), marked by interactions between the tumor, stromal, and immune cells. The major cellular components of the TME in CRC include tumor cells, myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs), cancer-associated fibroblasts, tumor-associated macrophages (TAMs), natural killer (NK) cells, and regulatory T cells (Tregs), among other immune cells[17,18]. Crosstalk between cancer cells and the TME is an important factor that contributes to tumor immune escape, metastasis, recurrence, and poor immunotherapy efficacy[19,20]. MDSCs, which originate from hematopoietic stem cells, are one of the most abundant and dominant components of the TME. Several studies have demonstrated that MDSCs can cause immunosuppression, which in turn is involved in CRC progression, recurrence, and metastasis[15,17,21,22]. Increased levels of circulating and tumor-infiltrating MDSCs have been observed in CRC patients[23,24]. Thus, targeted inhibition of MDSCs attenuates immunosuppression and activates antitumor immune responses, such as T and NK cells, which in turn enhances antitumor immunotherapy[25,26]. Inhibiting MDSC trafficking to the TME has been proposed as a novel strategy in microsatellite-stable CRC, with the potential to reprogram the immune system[27]. A previous study reported that a high-salt diet inhibited tumor growth in mice by reducing MDSC activity and enhancing antitumor immune surveillance[28]. Furthermore, targeting TAMs and granulocytic MDSCs (G-MDSCs) augments the effects of ICIs and programmed cell death protein 1 (PD-1) blockade in cholangiocarcinoma[29]. Therefore, this review focuses on MDSCs, exploring and discussing their roles and mechanisms in tumor progression, and examining the current application of pharmacological and non-pharmacological therapies aimed at inhibiting MDSCs, including combination therapies with ICIs. This discussion aims to provide therapeutic ideas and targets for improving the immunosuppressive microenvironment in CRC, thereby enhancing the efficacy of immunotherapy and improving patient prognosis.
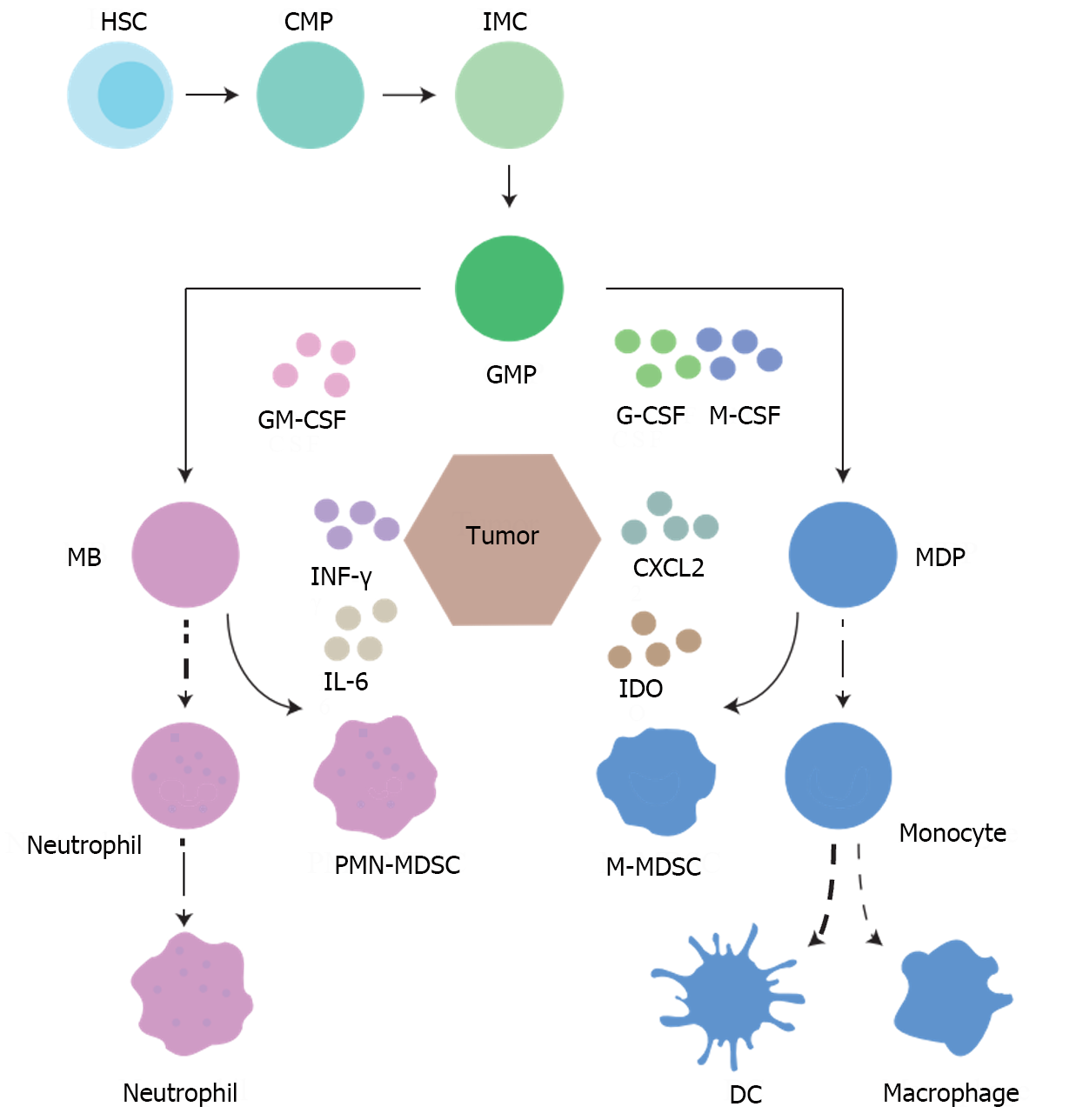
Under physiological conditions, hematopoietic progenitor cells in the bone marrow differentiate into common myeloid progenitors (Figure 1), and then undergo granulocyte-macrophage progenitor, myeloblast, and monocyte-DC progenitor processes that culminate in their differentiation into monocytes or neutrophils[30]. When a healthy human is subjected to acute infection or trauma, the bone marrow quickly releases large numbers of immature myeloid cells that differentiate into mature myeloid cells, such as polymorphonuclear neutrophils and monocytes, to help eliminate the acute pathological conditions[31,32]. However, in patients with tumors, continuous stimulation often leads to defective differentiation of immature myeloid cells, which eventually differentiate into MDSCs with immunosuppressive properties[33,34]. MDSCs are classified into two subsets: Monocytic MDSCs (M-MDSCs) and granulocytic polymorphonuclear MDSCs (PMN-MDSCs), with the latter comprising approximately 80% of the total MDSC population[35,36]. In mice, PMN-MDSCs are identified by the markers CD11b+Ly6G+Ly6Clow, whereas M-MDSCs are characterized as CD11b+Ly6G-Ly6C high[37-40]. In humans, PMN-MDSCs and M-MDSCs can be distinguished by their respective markers: CD14-CD11b+CD15+CD66b+CD33+ HLA-DR- for PMN-MDSCs and CD14+CD11b+CD15-CD66b-CD33+HLA-DR- for M-MDSCs[41-43].
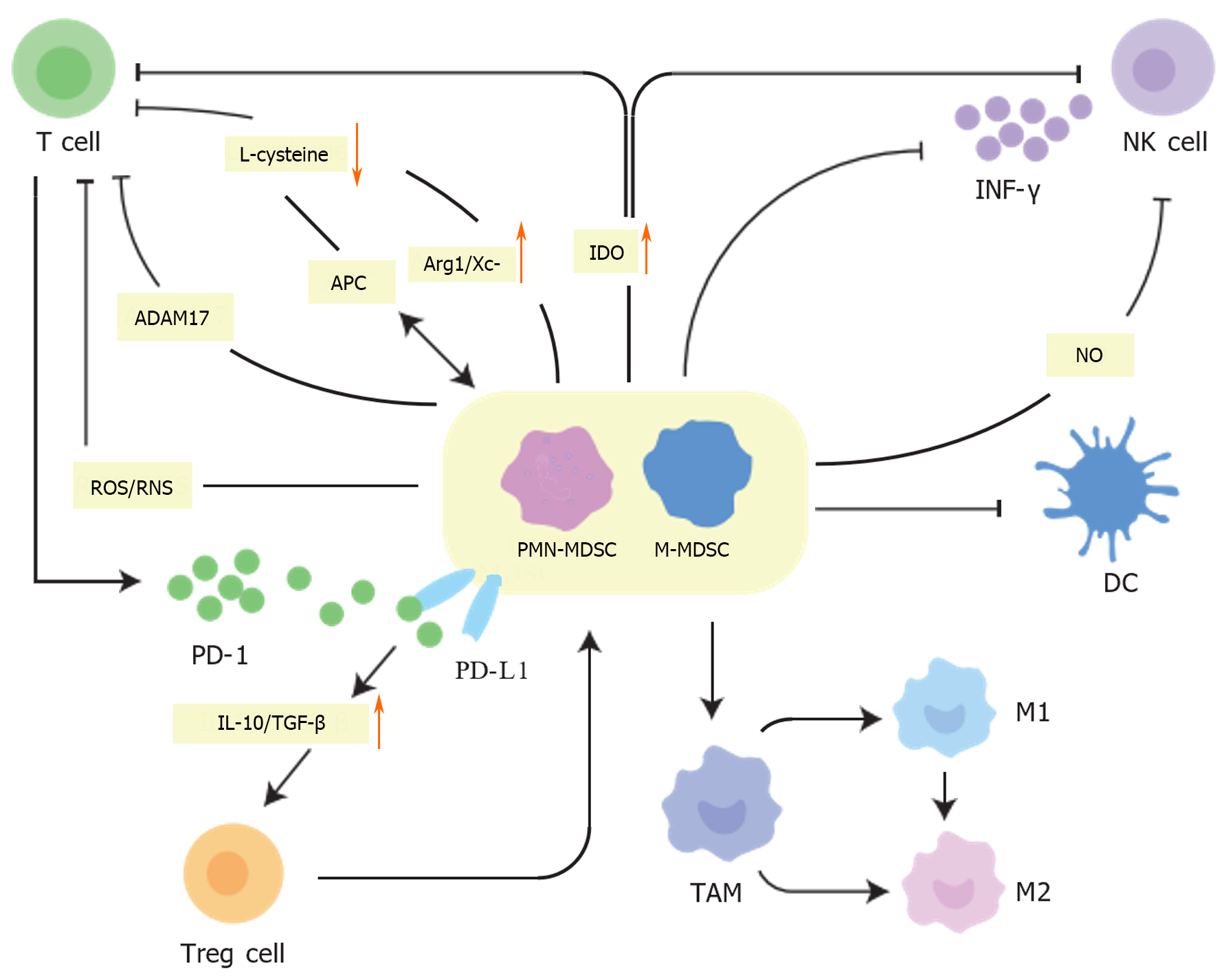
The hallmark of MDSCs is immunosuppression, primarily targeting immunoreactive cells such as T and NK cells, with a particular focus on T cells. They utilize multiple pathways that promote tumor immune evasion, leading to antitumor immune resistance[44,45]. However, PMN-MDSCs and M-MDSCs exert their immunosuppressive effects via different mechanisms. PMN-MDSCs mediate immunosuppression via the production of reactive oxygen species (ROS), peroxynitrite, and arginase 1 (ARG1), while M-MDSCs produce nitric oxide (NO) and immunoregulatory cytokines, including interleukin (IL)-10 and transforming growth factor beta (TGF-β). M-MDSCs also contribute to immunosuppression by upregulating the expression of immunoregulatory molecules such as programmed cell death 1 ligand 1 (PD-L1)[46]. Due to the induction of these tumor-derived growth factors and pro-inflammatory cytokines, the MDSC population is greatly expanded in the TME[47].
In addition to their immunosuppressive functions, MDSCs can also promote tumor progression by influencing remodeling and tumor angiogenesis through the production of vascular endothelial growth factor (VEGF), bFGF, Bv8, and MMP9[48-51]. MDSCs promote epithelial-mesenchymal transition (EMT) by activating the PI3K-AKT-mTOR pathway in cancer cells, thereby increasing the invasiveness and metastatic potential of breast cancer cells[52,53]. MDSCs also directly promote tumor growth and metastasis. A previous study confirmed that human MDSCs promote CRC development by enhancing CRC cell stemness and growth via exosomal S100A9[54]. Furthermore, MDSCs drive tumor progression by producing IL-6 (which activates STAT3 in cancer cells) and NO (which activates the Notch pathway and maintains STAT3 activation), thereby inducing stem cell-like features in breast cancer cells[55].
MDSC can recruit and induce other suppressive or regulatory cells (Figure 2), such as Tregs, and inhibit the immune function of various T cell types, including NK cells and CD8+ T cells, through multiple pathways, thereby affecting the immune function of patients with tumors[56,57]. Activated MDSCs within the TME express ARG1 and cystine–glutamate transporters (Xc-), thus depriving T cells of L-arginine and L-cysteine, which are essential for proliferation and activation[47,58]. T cells, which require cysteine for activation and function, can only acquire cysteine from antigen-presenting cells, such as macrophages and DCs, due to their inability to transport cysteine. However, MDSCs compete with these antigen-presenting cells for extracellular cysteine and are unable to export it, thus inhibiting T cell proliferation and activation[59]. MDSCs also express indoleamine 2,3-dioxygenase 1, a tryptophan-catabolizing enzyme with immunological functions capable of inhibiting the activity of T and NK cells under inflammatory conditions[60]. MDSCs also express ADAM17, which cleaves CD62L, thereby preventing naïve T cells from migrating to tumors or lymph nodes and hindering their development into effector T cells[61]. In addition, MDSCs release ROS and reactive nitrogen species, which downregulate the ζ chain expression on T cell receptors, dysregulating T cell function[36,62]. Conversely, activated MDSCs express PD-L1, which binds PD-1 on T cells, and secrete IL-10 and TGF-β to stimulate Treg activation and expansion[63]. Tregs, known for their immunosuppressive capabilities, release cytokines that suppress other immune cells, thereby inhibiting antitumor immune responses[64,65]. In a mouse model of colon carcinoma, interferon-γ (IFN-γ)-activated MDSCs were shown to promote the expansion and recruitment of Treg cells, possibly through the upregulation of major histocompatibility class 2 (MHC-II), IL-10, and TGF-β[66]. Further, a previous clinical trial showed that patients with advanced CRC have elevated levels of circulating MDSCs in their blood and that M-MDSCs are positively correlated with Tregs. These results suggest that MDSCs, and particularly M-MDSCs, are potential targets for CRC immunotherapy[67].
NK cells are cytolytic and cytokine-producing effector innate lymphoid cells with a critical role in immune activation against abnormal cells[68]. MDSCs produce TGF-β, which is a master regulator of NK cell functions in tumors[69]. Co-culture of MDSC with NK cells results in impaired tumor cytotoxic activity of NK cells and induces immune tolerance[70]. Further, MDSC can have either direct or indirect effects on angiogenesis through their interactions with NK and immunosuppressive activities[69]. MDSC-mediated NK cell incompetence is associated with the ability of MDSCs to downregulate CD247 expression on the surface of NK cells[71]. MDSCs also inhibit NK cells in hepatocellular carcinoma patients via the NKp30 receptor[72]. In addition, inhibition of MDSC trafficking has been shown to potentially enhance NK cell immunotherapy in head and neck cancer models[73]. MDSCs can also impair Fc receptor-mediated functions of NK cells by producing NO[74]. In addition, MDSCs can impair NK cell function and cytotoxicity by suppressing the production of IFN-γ from NK cells and decreasing the expression of NK group 2 member D[17,75].
DCs are specialized antigen-presenting cells that play a crucial role in activating T cells to drive antitumor responses[76]. However, multiple conditions and factors within the TME, including hypoxia, lactic acid build-up, and accumulation of adenosine, can cause DC abnormalities. Since MDSCs and DCs originate from a common progenitor cell, the observed reduction in mature DCs in cancer patients may result from this progenitor being skewed towards MDSC differentiation at the expense of DC maturation[77].
MDSCs can continue to differentiate into TAMs within the TME. TAMs can be categorized into two subsets: an M1 subset that inhibits tumor growth and an M2 subset that promotes tumor growth. The TME’s vascular distortion and rapid tumor cell growth cause hypoxia. This hypoxia then contributes to TME immunosuppression through the secretion of immunosuppressive factors, such as VEGF and TGF-β. VEGF, in particular, could enhance the infiltration of TAMs into tumor sites[78]. In the presence of MDSCs, macrophages tend to adopt an M2 or alternatively activated phenotype, which furthers tumor progression by decreasing the macrophage’s production of IL-12[79].
Elevated numbers of MDSCs are linked to poor prognosis and diminished response to treatment in several solid tumors[80,81]. MDSCs function as immunosuppressors by promoting immune evasion and resistance to cancer progression[82]. Presently, most treatments for tumors, especially CRC, rely on immunotherapy. The failure of immunotherapy is mainly related to the development of resistance to ICIs[83], which are designed to modulate and alter the response of T lymphocytes to tumors[84]. Anti-PD-1 and anti-PD-L1 antibodies represent the main types of ICIs. As discussed above, MDSCs can promote tumor immunosuppression, leading to ICI resistance by inhibiting T cell function[85]. Additionally, MDSCs increased PD-L1 expression on their surface, contributing to immunosuppression[84,86]. The immunosuppressive TME fosters resistance to anti-PD-1/PD-L1 therapies, and inhibiting MDSCs can synergize with PD-1/PD-L1 inhibitors to exert antitumor effects. HDAC expression in MDSCs promotes their differentiation into less inhibitory cells. HDAC inhibitors upregulate PD-1 or PD-L1 expression in tumors or immune cells and sensitize hormonal mice to anti-PD-1/PD-L1 therapy[87]. Kim et al[88] demonstrated that the removal of MDSCs results in the disappearance of tumor cells resistant to PD-1 antibody treatment. SLC25A22 knockout inhibits MDSC infiltration and function. The reduction of MDSCs through SLC25A22 knockout, particularly when combined with anti-PD1 therapy, synergistically induces CD8+ T-cell infiltration and IFN-γ expression, identifying SLC25A22 as a promising target for sensitizing KRAS-mutant CRC to immune checkpoint blockade therapy[89]. A growing body of research suggests that MDSCs are a potential therapeutic target for reducing tumor-promoting and immunosuppressive activities, as well as for boosting the efficacy of checkpoint inhibitors[90].
By inhibiting the recruitment and transport of MDSCs to tumor tissues and the spleen, immunosuppression can be reversed, and the antitumor activity of T and NK cells can be activated to inhibit tumor proliferation and enhance the efficacy of antitumor therapy. Previous studies have shown that VEGF, hypoxia, S100A8/A9, chemokine receptors, and CSF1-R inhibitors can inhibit the recruitment and transit of MDSCs. For instance, bevacizumab has been shown to significantly reduce G-MDSC levels in the peripheral blood of patients with non-small cell lung cancer[91]. In addition, chemotherapeutic agents also exhibit MDSC-inhibitory effects. For example, the combination of sulforaphane and doxorubicin has been shown to effectively inhibit breast cancer proliferation and MDSC aggregation while increasing CD8+ T cell levels[92]. Chemotherapy (cisplatin + pemetrexed) combined with a PD-1 checkpoint inhibitor inhibits the proliferation of malignant mesothelioma cells by reducing MDSC accumulation and angiogenesis[93]. Further, polypeptide nanoformulations containing doxorubicin and the immune regulator 1-methyl-DL-tryptophan have been shown to inhibit the recruitment of Tregs and MDSCs while increasing the frequency of tumor-infiltrating CD8+ T cells, thus exerting synergistic antitumor effects[94]. Blocking STAT3 signaling, for example, with the use of Embelin and Flubendazole, can reduce the levels of MDSCs and inhibit their activity. Embelin can directly reduce MDSC production, as well as their immunosuppressive activity, by inhibiting the C/EBPβ and STAT3 signaling pathways[95]. Meanwhile, Flubendazole reduces MDSC levels in tumor tissues via the inhibition of the transducer and activator signaling activity of STAT3[96]. IL-6 silences the TNFα-RIP1 necrotic pathway to maintain MDSC survival and accumulation by mediating activation of the STAT3-DNMT axis[97]. Thus, the inhibition of STAT3 activation suppresses MDSC levels. IL-17, on the other hand, can induce MDSC differentiation, inhibit MDSC proliferation, and promote apoptosis by activating STAT3[98]. A deficiency in CXCR2 hampers MDSC migration to tumor sites, significantly boosting the antitumor effects of PD-1[99]. Small molecules of traditional Chinese medicine also exhibit MDSC inhibitory effects. Nanoparticles containing curcumin can exert antitumor effects by inhibiting the recruitment and accumulation of MDSCs[100]. Carnosic acid reduces the proportion of MDSCs, enhances the function of CD8+ T cells by decreasing the levels of iNOS2, Arg-1, and MMP9, and enhances the cytotoxic effects of cisplatin on lung cancer cells[101]. Some anti-inflammatory and antifungal drugs also have the ability to inhibit MDSC aggregation. Terbinafine inhibits CRC proliferation by reversing intestinal fungal dysbiosis, inhibiting MDSC infiltration, and restoring antitumor immune responses[102]. The anti-inflammatory drug dimethyl itaconate protects against colitis-associated CRC by decreasing the number of macrophages and MDSCs[103]. OSU-53 (a PPAR-inactive derivative that stimulates AMPK kinase) has been shown to significantly reduce MDSCs in the spleens and tumors of EMT-6 mice[104]. LDK378 (an anaplastic lymphoma kinase inhibitor) partially blocks lipopolysaccharide-induced p38 phosphorylation, reduces cell surface CCR2 expression, and inhibits the migration of MDSCs to the spleen[105]. In addition, the targeted inhibition of SLC25A22, YTHDF2, and G-CSF inhibits MDSC migration and aggregation[89,106,107].
Previous studies have shown that chemotherapeutic agents, such as 5-Fluorouracil, docetaxel, and gemcitabine, can effectively deplete MDSCs. 5-Fluorouracil promotes MDSC apoptosis by upregulating the expression of Fas and p53 on these cells and increasing the infiltration of toxic T lymphocytes into tumor tissues[108]. Docetaxel promotes the polarization of MDSCs from an M1 (CCR7) to an M2 (CD206) type and increases the differentiation of macrophages towards an M1 phenotype[109]. Metformin, a drug commonly used to treat diabetes, inhibits MDSCs and M2-type macrophages in the CRC microenvironment by activating AMPK and inhibiting mTOR signaling[110]. Lenalidomide reduces the number of MDSCs and Tregs in lymphoma-loaded mice[111]. Sunitinib also reduces MDSC levels and restores the normal function of splenic T cells in mice[112]. Furthermore, the combination of OX40 (agonist anti-OX40 antibody) with belapectin (galectin-3 inhibitor) significantly reduces M-MDSCs levels and MHC-II hi macrophages thereby attenuating M-MDSC-induced immunosuppression[113]. Histamine dihydrochloride (a NOX2 inhibitor) in combination with low-dose IL-2, reduces M-MDSC levels in peripheral blood and enhances the antitumor efficacy of PD-1/PD-L1[114]. Apt/PDGss@pMOF (a tumor-targeting and light-responsive penetrable nanoplatform) can deplete MDSCs and reverse immunosuppression[115]. The application of DS-8273a (TRAIL-R2 agonistic antibody) results in the depletion of MDSCs in approximately 50% of patients, without affecting mature bone marrow cells or lymphocytes[116]. Amino-biophosphonates (MMP-9 inhibitors) inhibit tumor and bone marrow cell proliferation and attenuate immunosuppression[117]. The herbal molecule Baicalein decreases MDSC levels by regulating the Nrf2/HO-1 signaling pathway and NLRP3 expression in MDSCs[118]. The Shugan Jianpi Formula enhances immune surveillance by reducing CD8+ T cell apoptosis and tumor cell activity, inhibiting MDSC proliferation, and improving the survival of mice with breast cancer tumors[119]. In addition, the depletion of MDSCs and attenuation of immunosuppression can be achieved by targeting and inhibiting molecules closely related to MDSC function. NLRP3, for instance, promotes melanoma progression by inducing MDSC expansion and immune escape, yet its targeted inhibition can enhance the efficacy of PD-1[120]. IL4Rα is a key signaling molecule for MDSC survival; hence, blocking IL4Rα can directly deplete MDSCs and TAMs[121].
The effectiveness of immunotherapy can be enhanced by reducing the number of MDSCs and promoting the differentiation of immature myeloid cells. Angiotensin-converting enzymes or angiotensin receptor blockers can induce the maturation of myeloid cells towards non-suppressive neutrophils/monocytes, thus preventing them from becoming immature MDSCs[122]. The DHODH inhibitor, brequinar, prevents early myeloid progenitor cells from generating MDSCs and promotes their maturation, which, in turn, enhances the antitumor and anti-metastatic activity of PD-1 in ICI-resistant breast cancer models[123]. Vitamins A, D3, and E have been shown to reduce immature MDSCs and enhance the antitumor activity of T cells in both a mouse model and in patients with head and neck cancer[124,125]. Casein kinase 2 substantially reduces the number of PMN-MDSCs and TAMs, thereby enhancing the effectiveness of CTLA4 checkpoint inhibitors[126].
Another category involves the direct inhibition of the immunosuppressive activity of MDSCs. Celecoxib, (COX-2 inhibitor) inhibits MDSC amplification[127]. Entinostat inhibits the immunosuppressive activity of HER2+ breast cancer G-MDSCs and promotes a macrophage shift to the M1 type[128]. Sildenafil, (PDE-5 inhibitor) can downregulate the expression of ARG1, IL4Ra, and ROS, restore the antitumor activity of NK cells, and reduce the postoperative recurrence of abdominal malignancies[70]. Ibrutinib (a BTK inhibitor) can reverse MDSC-induced immunosuppression, increase CD8+ T cell infiltration, and enhance PD-L1 efficacy[129]. Compared with sorafenib, tivozanib (a c-Kit/SCF antagonist) significantly reduces the levels of Foxp3+ Tregs, MDSCs, and exhausted T cells, thereby reversing immunosuppression[130]. Cimetidine promotes MDSC apoptosis and inhibits lung cancer cell proliferation by inducing Fas/FasL expression on the surface of MDSCs[131]. IFN-α/β upregulates TRAIL expression on T cells and enhances the inhibitory effect of TNF-α on MDSC through the TRAIL-DR5 pathway[132]. The herbal molecule Curcuma kwangsiensis also induces MDSC apoptosis in the G0/G1 phase by upregulating caspase 3/9, PARP, and Bax and downregulating Bcl-xl[133,134]. Asparagus polysaccharide could induce MDSC apoptosis and attenuate immunosuppression through the toll-like receptor4 pathway[135]. In addition, application of Cimetidine[131], TJ-M2010-5 (MyD88 inhibitor)[136], MF-766 (EP4 antagonist)[137], low-dose IPI-145 (PI3Kδ/γ inhibitor)[138], mitochondria-targeted complex I inhibitors[139], and compound39 (potent GCN2 inhibitor)[140], or targeted inhibition of jagged[141], SCARB1 (scavenger receptor type B-1)[142], and ROS levels, inhibits MDSC activity[143] (Table 1).
| Function | Drug | Target pathway | Synergistic | Diseases | Ref. |
| Inhibition of MDSC recruitment | Bevacizumab | Non-small cell lung cancer | Koinis et al[91] | ||
| IL-17 | Breast cancer | Ma et al[98] | |||
| flubendazole | STAT3 | Melanoma | Li et al[96] | ||
| CXCR2 | Rhabdomyosarcoma | Highfill et al[99] | |||
| IL-6 | STAT3-DNMT | Colorectal cancer | Smith et al[97] | ||
| G-CSF | Colorectal cancer | Li et al[107] | |||
| Carnosic acid | Cisplatin | Lung cancer | Liu et al[101] | ||
| CDDP and PEM | Mesothelioma | Otsuka et al[93] | |||
| Dimethyl itaconate | Colorectal cancer | Wang et al[103] | |||
| Embelin | C/EBPβ and STAT3 | Colitis-associated cancer | Wu et al[95] | ||
| LDK378 | p38-GRK2-CCR2 | Sepsis | Hu et al[105] | ||
| Inhibition of YTHDF2 | Autoimmune hepatitis | Lyu et al[106] | |||
| Terbinafine | Colorectal cancer | Hu et al[102] | |||
| Targeting of SLC25A22 | Immunotherapeutic | KRAS-mutant colorectal cancer | Zhou et al[89] | ||
| Sulforaphane and doxorubicin | Breast cancer | Rong et al[92] | |||
| Polypeptide nanoformulation | Immunotherapeutic | Breast cancer and colon cancer | Feng et al[94] | ||
| OSU-53 | AMPK | Melanoma | Trikha et al[104] | ||
| Curcumin | Lung cancer | Wang et al[100] | |||
| MDSC depletion | 5-Fluorouracil | p53-Fas | Colorectal cancer | Yang et al[108] | |
| Docetaxel | Breast cancer | Kodumudi et al[109] | |||
| Metformin | Colorectal cancer | Kang et al[110] | |||
| Amino-biphosphonate | MMP-9 | Breast cancer | Melani et al[117] | ||
| Apt/PDGss@pMOF | Triple-negative breast cancer | Chen et al[115] | |||
| Baicalein | Nrf2/HO-1 | Lupus nephritis | Li et al[118] | ||
| DS-8273a | TRAIL-R2 | Pan-cancer | Dominguez et al[116] | ||
| Histamine | Anti-PD-1/PD-L1 | Pan-cancer | Grauers et al[114] | ||
| Shugan Jianpi Formula | Breast cancer | Lu et al[119] | |||
| OLT1177 | NLRP3 | Anti–PD-1 | Melanoma | Tengesdal et al[120] | |
| Aptamer | IL4Rα | Breast cancer | Roth et al[121] | ||
| Sunitinib | STAT5 | Renal cell carcinoma | Ko et al[112] | ||
| Lenalidomide | Cancer vaccine | Lymphomas | Sakamaki et al[111] | ||
| Inducting MDSC differentiation | ACEI | Colorectal Cancer | Bueno et al[112] | ||
| Brequinar | DHODH | Anti–PD-1 | Breast cancer | Colligan et al[123] | |
| Vitamins A, D3, and E | Head and neck cancer | Lathers et al[124]; Lee et al[125] | |||
| Casein kinase 2 | Anti-CTLA4 | Pan-cancer | Hashimoto et al[126] | ||
| Inhibiting MDSC activity | Celecoxib | COX-2 | Mesothelioma | Veltman et al[127] | |
| Entinostat | HDAC | HER2+ Breast Tumor | Sidiropoulos et al[128] | ||
| Cimetidine | Lung tumor | Zheng et al[131] | |||
| Compound39 | potent GCN2 inhibitor | Renal carcinoma | Jackson et al[140] | ||
| CTX014 | Jagged1/2 | Pan-cancer | Sierra et al[141] | ||
| Asparagus polysaccharide | Colorectal Cancer | Zhang et al[135] | |||
| Curcuma | TLR4-NF-κB | Pan-cancer | Jiang et al[134] | ||
| Ibrutinib | Anti-PD-L1 | Neuroblastoma | Ishfaq et al[129] | ||
| IFN-α/β | Anti-PD-1 | Colorectal cancer | Chen et al[132] | ||
| TJ-M2010-5 | Myd88 | Colorectal cancer | Wang et al[136] | ||
| Tivozanib | c-Kit/SCF | HCC | Kalathil et al[130] | ||
| Sildenafil | Phosphodiesterase-5 | Abdominal malignancies | Tai et al[70] | ||
| IPI-145 | PI3Kδ/γ | Anti-PD-L1 | Head and neck cancers | Davis et al[138] | |
| Mitochondrial complex I inhibitors | Melanoma | AbuEid et al[139] | |||
| MF-766 | Anti-PD-1 | Pan-cancer | Wang et al[137] | ||
| Lipoprotein Nanoparticle | Lung carcinoma | Plebanek et al[142] |
Treatments for CRC, such as chemotherapy, immunotherapy, and targeted therapy, are continuously evolving, yet surgery remains the preferred treatment option for patients with CRC[144]. In mouse models with CRC, MDSCs are known to be enriched in the peritoneal cavity, and are associated with poor prognosis after tumor resection[145]. On the other hand, chemotherapy and radiotherapy typically lead to tumor cell death by mechanisms such as inducing signi
CAR-T cells are genetically modified to express engineered receptors and chimeric constructs that recognize and react specifically to cancerous antigens in an MHC-independent manner and react specifically against them[149]. In recent years, many basic and clinical studies on the treatment of CRC with CAR-T cells have been published, with encouraging progress[150,151]. One study investigated CAR-T cell therapy in ten patients with metastatic carcinoembryonic antigen-positive CRC, seven of whom had stable disease after treatment. Two patients had stable disease for more than 30 wk, and positron emission tomography/computed tomography and magnetic resonance imaging analyses showed tumor shrinkage in two patients and a significant decrease in carcinoembryonic antigen levels in the majority of patients, which confirms the efficacy of CAR-T in the treatment of CRC[152]. Moreover, PD-1-TREM2-targeting scFv inhibited the activation of the PD-1/PD-L1 pathway. In addition, the secreted scFvs blocked the binding of ligands to TREM2 receptors present on MDSCs and TAMs, reduced the proportion of MDSCs and TAMs, and enhanced T-cell effector function, thereby mitigating immune resistance in the TME[153]. This demonstrates that CAR-T therapy can affect the TME via MDSC.
Many studies have suggested that disorders of intestinal microbiota play key roles in the pathogenesis of CRC[18,154,155]. The regulation of the intestinal flora also plays a role in improving the immunosuppressive microenvironment of tumors[156]. Recently, FMT has become a popular topic. In a mouse model of CRC, the application of terbinafine decreased fungus-induced MDSC infiltration and tumor load, whereas FMT in untreated with-terbinafine donor mice increased MDSC infiltration and promoted tumor proliferation[102]. Transplantation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated donor mouse feces into antibiotic-treated mice induces MDSCs and increases Tregs[157]. This suggests that intestinal microbiota can influence tumor growth through MDSC. Another study showed that a fecal suspension from the astragalus polysaccharide group inhibited tumor growth in melanoma mice, decreased MDSC, and increased CD8+ T cells in tumor tissues, confirming that FMT could reverse the tumor immunosuppressive microenvironment[158]. Therefore, we believe that FMT is a promising therapeutic approach for improving the tumor immunosuppressive microenvironment by inhibiting MDSC, thus exerting antitumor effects.
Tumors exploit various immunosuppressive pathways to actively evade immune recognition. MDSCs can create an immunosuppressive microenvironment in CRC by suppressing the immune function of T cells, NK cells, DCs, and macrophages, resulting in immune escape and resistance to immunotherapy. Therefore, therapeutic targeting of MDSCs presents a promising strategy to halt CRC progression and enhance the efficacy of immunotherapy. This involves preventing the expansion and accumulation of MDSCs, regulating their differentiation, and inhibiting their immunosuppressive activities. In this review, we focused on the role of MDSCs in CRC and the mechanisms through which they contribute to immunosuppression. We have also extensively discussed the currently available pharmacological and non-pharmacological treatments and strategies for targeting MDSC. This comprehensive analysis offers an objective understanding of the role of MDSCs in CRC and the methods to target MDSC-mediated suppression, ultimately aiming to improve the effectiveness of immunotherapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moldovan CA, Romania S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1339] [Article Influence: 334.8] [Reference Citation Analysis (5)] |
| 2. | Phipps O, Brookes MJ, Al-Hassi HO. Iron deficiency, immunology, and colorectal cancer. Nutr Rev. 2021;79:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Johdi NA, Sukor NF. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 2020;11:1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 4. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3008] [Article Influence: 501.3] [Reference Citation Analysis (3)] |
| 5. | Sun D, Zou Y, Song L, Han S, Yang H, Chu D, Dai Y, Ma J, O'Driscoll CM, Yu Z, Guo J. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm Sin B. 2022;12:378-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 6. | Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1220] [Article Influence: 203.3] [Reference Citation Analysis (0)] |
| 7. | Trullas A, Delgado J, Genazzani A, Mueller-Berghaus J, Migali C, Müller-Egert S, Zander H, Enzmann H, Pignatti F. The EMA assessment of pembrolizumab as monotherapy for the first-line treatment of adult patients with metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer. ESMO Open. 2021;6:100145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Ganesh K. Optimizing immunotherapy for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2022;19:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Lu W, Yu W, He J, Liu W, Yang J, Lin X, Zhang Y, Wang X, Jiang W, Luo J, Zhang Q, Yang H, Peng S, Yi Z, Ren S, Chen J, Siwko S, Nussinov R, Cheng F, Zhang H, Liu M. Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer. EMBO Mol Med. 2021;13:e12798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-5257. [PubMed] |
| 11. | Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 412] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 12. | Fan A, Wang B, Wang X, Nie Y, Fan D, Zhao X, Lu Y. Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci. 2021;17:3837-3849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 13. | Tian J, Chen JH, Chao SX, Pelka K, Giannakis M, Hess J, Burke K, Jorgji V, Sindurakar P, Braverman J, Mehta A, Oka T, Huang M, Lieb D, Spurrell M, Allen JN, Abrams TA, Clark JW, Enzinger AC, Enzinger PC, Klempner SJ, McCleary NJ, Meyerhardt JA, Ryan DP, Yurgelun MB, Kanter K, Van Seventer EE, Baiev I, Chi G, Jarnagin J, Bradford WB, Wong E, Michel AG, Fetter IJ, Siravegna G, Gemma AJ, Sharpe A, Demehri S, Leary R, Campbell CD, Yilmaz O, Getz GA, Parikh AR, Hacohen N, Corcoran RB. Combined PD-1, BRAF and MEK inhibition in BRAF(V600E) colorectal cancer: a phase 2 trial. Nat Med. 2023;29:458-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 107] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 14. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4937] [Article Influence: 617.1] [Reference Citation Analysis (0)] |
| 15. | Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 279] [Reference Citation Analysis (0)] |
| 16. | Rastin F, Javid H, Oryani MA, Rezagholinejad N, Afshari AR, Karimi-Shahri M. Immunotherapy for colorectal cancer: Rational strategies and novel therapeutic progress. Int Immunopharmacol. 2024;126:111055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 17. | Yin K, Xia X, Rui K, Wang T, Wang S. Myeloid-Derived Suppressor Cells: A New and Pivotal Player in Colorectal Cancer Progression. Front Oncol. 2020;10:610104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Zhou Z, Chen J, Yao H, Hu H. Fusobacterium and Colorectal Cancer. Front Oncol. 2018;8:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Czajka-Francuz P, Cisoń-Jurek S, Czajka A, Kozaczka M, Wojnar J, Chudek J, Francuz T. Systemic Interleukins' Profile in Early and Advanced Colorectal Cancer. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Fathi M, Pustokhina I, Kuznetsov SV, Khayrullin M, Hojjat-Farsangi M, Karpisheh V, Jalili A, Jadidi-Niaragh F. T-cell immunoglobulin and ITIM domain, as a potential immune checkpoint target for immunotherapy of colorectal cancer. IUBMB Life. 2021;73:726-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu K, Hu X, Bu Z, Peng J, Ren X, Zhang Z. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell. 2022;40:424-437.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 237] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 22. | Ai L, Mu S, Wang Y, Wang H, Cai L, Li W, Hu Y. Prognostic role of myeloid-derived suppressor cells in cancers: a systematic review and meta-analysis. BMC Cancer. 2018;18:1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Toor SM, Syed Khaja AS, El Salhat H, Bekdache O, Kanbar J, Jaloudi M, Elkord E. Increased Levels of Circulating and Tumor-Infiltrating Granulocytic Myeloid Cells in Colorectal Cancer Patients. Front Immunol. 2016;7:560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Toor SM, Khalaf S, Murshed K, Abu Nada M, Elkord E. Myeloid Cells in Circulation and Tumor Microenvironment of Colorectal Cancer Patients with Early and Advanced Disease Stages. J Immunol Res. 2020;2020:9678168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Kalathil SG, Thanavala Y. Importance of myeloid derived suppressor cells in cancer from a biomarker perspective. Cell Immunol. 2021;361:104280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Zhao S, Li S, Yang J, Gao W, Chen Z. GM-CSF-mediated inducement of bone marrow MDSCs by TSA and effect on survival of graft in mice. Eur J Med Res. 2022;27:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | Johnson B. Targeting Myeloid-Derived Suppressor Cell Trafficking as a Novel Immunotherapeutic Approach in Microsatellite Stable Colorectal Cancer. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | He W, Xu J, Mu R, Li Q, Lv DL, Huang Z, Zhang J, Wang C, Dong L. High-salt diet inhibits tumour growth in mice via regulating myeloid-derived suppressor cell differentiation. Nat Commun. 2020;11:1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, Pavelko KD, Li Y, O'Brien D, Wang C, Graham RP, Smoot RL, Dong H, Ilyas S. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130:5380-5396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 30. | Cao P, Sun Z, Zhang F, Zhang J, Zheng X, Yu B, Zhao Y, Wang W. TGF-β Enhances Immunosuppression of Myeloid-Derived Suppressor Cells to Induce Transplant Immune Tolerance Through Affecting Arg-1 Expression. Front Immunol. 2022;13:919674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 31. | Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 430] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 32. | Tesi RJ. MDSC; the Most Important Cell You Have Never Heard Of. Trends Pharmacol Sci. 2019;40:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 33. | Musolino C, Allegra A, Pioggia G, Gangemi S. Immature myeloid-derived suppressor cells: A bridge between inflammation and cancer (Review). Oncol Rep. 2017;37:671-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Neo SY, Jing X, Tong L, Tong D, Gao J, Chen Z, De Los Santos MC, Burduli N, De Souza Ferreira S, Wagner AK, Alici E, Rolny C, Cao Y, Lundqvist A. Tumor MHC class I expression alters cancer-associated myelopoiesis driven by host NK cells. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | Atretkhany KS, Nosenko MA, Gogoleva VS, Zvartsev RV, Qin Z, Nedospasov SA, Drutskaya MS. TNF Neutralization Results in the Delay of Transplantable Tumor Growth and Reduced MDSC Accumulation. Front Immunol. 2016;7:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2369] [Cited by in RCA: 2867] [Article Influence: 220.5] [Reference Citation Analysis (0)] |
| 37. | Barnett JD, Jin J, Penet MF, Kobayashi H, Bhujwalla ZM. Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol. 2020;13:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 39. | Su Z, Ni P, Zhou C, Wang J. Myeloid-Derived Suppressor Cells in Cancers and Inflammatory Diseases: Angel or Demon? Scand J Immunol. 2016;84:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Wang H, Ji J, Zhuang Y, Zhou X, Zhao Y, Zhang X. PMA induces the differentiation of monocytes into immunosuppressive MDSCs. Clin Exp Immunol. 2021;206:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 42. | Singh L, Muise ES, Bhattacharya A, Grein J, Javaid S, Stivers P, Zhang J, Qu Y, Joyce-Shaikh B, Loboda A, Zhang C, Meehl M, Chiang DY, Ranganath SH, Rosenzweig M, Brandish PE. ILT3 (LILRB4) Promotes the Immunosuppressive Function of Tumor-Educated Human Monocytic Myeloid-Derived Suppressor Cells. Mol Cancer Res. 2021;19:702-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol. 2018;200:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 44. | Zhao W, Jin L, Chen P, Li D, Gao W, Dong G. Colorectal cancer immunotherapy-Recent progress and future directions. Cancer Lett. 2022;545:215816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 45. | Consonni FM, Porta C, Marino A, Pandolfo C, Mola S, Bleve A, Sica A. Myeloid-Derived Suppressor Cells: Ductile Targets in Disease. Front Immunol. 2019;10:949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 46. | Stevenson MM, Valanparambil RM, Tam M. Myeloid-Derived Suppressor Cells: The Expanding World of Helminth Modulation of the Immune System. Front Immunol. 2022;13:874308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Tang H, Li H, Sun Z. Targeting myeloid-derived suppressor cells for cancer therapy. Cancer Biol Med. 2021;18:992-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, Iannello A, Iwamoto Y, Cortez-Retamozo V, Kamm RD, Pittet MJ, Raulet DH, Weinberg RA. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016;6:630-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 49. | Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 455] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 50. | Shojaei F, Ferrara N. Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res. 2008;68:5501-5504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Takanari K, Hashizume R, Hong Y, Amoroso NJ, Yoshizumi T, Gharaibeh B, Yoshida O, Nonaka K, Sato H, Huard J, Wagner WR. Skeletal muscle derived stem cells microintegrated into a biodegradable elastomer for reconstruction of the abdominal wall. Biomaterials. 2017;113:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Luo A, Meng M, Wang G, Han R, Zhang Y, Jing X, Zhao L, Gu S, Zhao X. Myeloid-Derived Suppressor Cells Recruited by Chemokine (C-C Motif) Ligand 3 Promote the Progression of Breast Cancer via Phosphoinositide 3-Kinase-Protein Kinase B-Mammalian Target of Rapamycin Signaling. J Breast Cancer. 2020;23:141-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Liu H, Wang Z, Zhou Y, Yang Y. MDSCs in breast cancer: an important enabler of tumor progression and an emerging therapeutic target. Front Immunol. 2023;14:1199273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 54. | Wang Y, Yin K, Tian J, Xia X, Ma J, Tang X, Xu H, Wang S. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv Sci (Weinh). 2019;6:1901278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 55. | Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, Wan S, Wei S, Wang Y, Liu Y, Staroslawska E, Szubstarski F, Rolinski J, Grywalska E, Stanisławek A, Polkowski W, Kurylcio A, Kleer C, Chang AE, Wicha M, Sabel M, Zou W, Kryczek I. Myeloid-Derived Suppressor Cells Endow Stem-like Qualities to Breast Cancer Cells through IL6/STAT3 and NO/NOTCH Cross-talk Signaling. Cancer Res. 2016;76:3156-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 56. | He ZN, Zhang CY, Zhao YW, He SL, Li Y, Shi BL, Hu JQ, Qi RZ, Hua BJ. Regulation of T cells by myeloid-derived suppressor cells: emerging immunosuppressor in lung cancer. Discov Oncol. 2023;14:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 57. | Siret C, Collignon A, Silvy F, Robert S, Cheyrol T, André P, Rigot V, Iovanna J, van de Pavert S, Lombardo D, Mas E, Martirosyan A. Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Front Immunol. 2019;10:3070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 58. | Xu M, Zhao Z, Song J, Lan X, Lu S, Chen M, Wang Z, Chen W, Fan X, Wu F, Chen L, Tu J, Ji J. Interactions between interleukin-6 and myeloid-derived suppressor cells drive the chemoresistant phenotype of hepatocellular cancer. Exp Cell Res. 2017;351:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 717] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 60. | Ju JM, Nam G, Lee YK, Jung M, Chang H, Kim W, Shon WJ, Lim JY, Kim JY, Chang J, Min CK, Lee DS, Choi K, Shin DM, Choi EY. IDO1 scavenges reactive oxygen species in myeloid-derived suppressor cells to prevent graft-versus-host disease. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells: Targets for therapy. Oncoimmunology. 2013;2:e24117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 62. | Jiang H, Zhu M, Guo P, Bi K, Lu Z, Li C, Zhai M, Wang K, Cao Y. Impaired myeloid-derived suppressor cells are associated with recurrent implantation failure: A case-control study. J Reprod Immunol. 2021;145:103316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Lim YJ, Koh J, Choi M, Kim S, Chie EK. Prognostic stratification based on the levels of tumor-infiltrating myeloid-derived suppressor cells and PD-1/PD-L1 axis in locally advanced rectal cancer. Front Oncol. 2022;12:1018700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Yang J, Bae H. Drug conjugates for targeting regulatory T cells in the tumor microenvironment: guided missiles for cancer treatment. Exp Mol Med. 2023;55:1996-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 65. | Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 561] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 66. | Mota Reyes C, Demir E, Çifcibaşı K, Istvanffy R, Friess H, Demir IE. Regulatory T Cells in Pancreatic Cancer: Of Mice and Men. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 67. | Siemińska I, Węglarczyk K, Walczak M, Czerwińska A, Pach R, Rubinkiewicz M, Szczepanik A, Siedlar M, Baran J. Mo-MDSCs are pivotal players in colorectal cancer and may be associated with tumor recurrence after surgery. Transl Oncol. 2022;17:101346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 428] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 69. | Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression. Front Immunol. 2019;10:771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 70. | Tai LH, Alkayyal AA, Leslie AL, Sahi S, Bennett S, Tanese de Souza C, Baxter K, Angka L, Xu R, Kennedy MA, Auer RC. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology. 2018;7:e1431082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 71. | Vaknin I, Blinder L, Wang L, Gazit R, Shapira E, Genina O, Pines M, Pikarsky E, Baniyash M. A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood. 2008;111:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 533] [Cited by in RCA: 526] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 73. | Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, Horn LA, Palena C, Schlom J, Maeda DY, Zebala JA, Clavijo PE, Allen C. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin Cancer Res. 2020;26:1420-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 74. | Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S, Abood D, Landi I, Hsu V, Duggan M, Wesolowski R, Old M, Howard JH, Yu L, Stasik N, Olencki T, Muthusamy N, Tridandapani S, Byrd JC, Caligiuri M, Carson WE. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin Cancer Res. 2018;24:1891-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 75. | Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336-4342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 76. | Li E, Yang X, Du Y, Wang G, Chan DW, Wu D, Xu P, Ni P, Xu D, Hu Y. CXCL8 Associated Dendritic Cell Activation Marker Expression and Recruitment as Indicators of Favorable Outcomes in Colorectal Cancer. Front Immunol. 2021;12:667177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 77. | Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 447] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 78. | Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res. 2019;25:5449-5457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 341] [Article Influence: 56.8] [Reference Citation Analysis (1)] |
| 79. | Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 80. | Yi M, Li T, Niu M, Mei Q, Zhao B, Chu Q, Dai Z, Wu K. Exploiting innate immunity for cancer immunotherapy. Mol Cancer. 2023;22:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 81. | Hao Z, Li R, Wang Y, Li S, Hong Z, Han Z. Landscape of Myeloid-derived Suppressor Cell in Tumor Immunotherapy. Biomark Res. 2021;9:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 82. | Starska-Kowarska K. The Role of Different Immunocompetent Cell Populations in the Pathogenesis of Head and Neck Cancer-Regulatory Mechanisms of Pro- and Anti-Cancer Activity and Their Impact on Immunotherapy. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Liu K, Yuan S, Wang C, Zhu H. Resistance to immune checkpoint inhibitors in gastric cancer. Front Pharmacol. 2023;14:1285343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Tellez RSL, Reynolds L, Piris MA. Myeloid-derived suppressor cells (MDSCs): what do we currently know about the effect they have against anti-PD-1/PD-L1 therapies? Ecancermedicalscience. 2023;17:1556. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | Ozbay Kurt FG, Lasser S, Arkhypov I, Utikal J, Umansky V. Enhancing immunotherapy response in melanoma: myeloid-derived suppressor cells as a therapeutic target. J Clin Invest. 2023;133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 86. | Iwata T, Kondo Y, Kimura O, Morosawa T, Fujisaka Y, Umetsu T, Kogure T, Inoue J, Nakagome Y, Shimosegawa T. PD-L1(+)MDSCs are increased in HCC patients and induced by soluble factor in the tumor microenvironment. Sci Rep. 2016;6:39296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 87. | Adeshakin AO, Adeshakin FO, Yan D, Wan X. Regulating Histone Deacetylase Signaling Pathways of Myeloid-Derived Suppressor Cells Enhanced T Cell-Based Immunotherapy. Front Immunol. 2022;13:781660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774-11779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 552] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 89. | Zhou Q, Peng Y, Ji F, Chen H, Kang W, Chan LS, Gou H, Lin Y, Huang P, Chen D, Wei Q, Su H, Liang C, Zhang X, Yu J, Wong CC. Targeting of SLC25A22 boosts the immunotherapeutic response in KRAS-mutant colorectal cancer. Nat Commun. 2023;14:4677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 90. | Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 91. | Koinis F, Vetsika EK, Aggouraki D, Skalidaki E, Koutoulaki A, Gkioulmpasani M, Georgoulias V, Kotsakis A. Effect of First-Line Treatment on Myeloid-Derived Suppressor Cells' Subpopulations in the Peripheral Blood of Patients with Non-Small Cell Lung Cancer. J Thorac Oncol. 2016;11:1263-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 92. | Rong Y, Huang L, Yi K, Chen H, Liu S, Zhang W, Yuan C, Song X, Wang F. Co-administration of sulforaphane and doxorubicin attenuates breast cancer growth by preventing the accumulation of myeloid-derived suppressor cells. Cancer Lett. 2020;493:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Otsuka K, Mitsuhashi A, Goto H, Hanibuchi M, Koyama K, Ogawa H, Ogino H, Saijo A, Kozai H, Yoneda H, Tobiume M, Kishuku M, Ishizawa K, Nishioka Y. Anti-PD-1 antibody combined with chemotherapy suppresses the growth of mesothelioma by reducing myeloid-derived suppressor cells. Lung Cancer. 2020;146:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Feng X, Xu W, Liu J, Li D, Li G, Ding J, Chen X. Polypeptide nanoformulation-induced immunogenic cell death and remission of immunosuppression for enhanced chemoimmunotherapy. Sci Bull (Beijing). 2021;66:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 95. | Wu T, Wang C, Wang W, Hui Y, Zhang R, Qiao L, Dai Y. Embelin impairs the accumulation and activation of MDSCs in colitis-associated tumorigenesis. Oncoimmunology. 2018;7:e1498437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Li Y, Acharya G, Elahy M, Xin H, Khachigian LM. The anthelmintic flubendazole blocks human melanoma growth and metastasis and suppresses programmed cell death protein-1 and myeloid-derived suppressor cell accumulation. Cancer Lett. 2019;459:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Smith AD, Lu C, Payne D, Paschall AV, Klement JD, Redd PS, Ibrahim ML, Yang D, Han Q, Liu Z, Shi H, Hartney TJ, Nayak-Kapoor A, Liu K. Autocrine IL6-Mediated Activation of the STAT3-DNMT Axis Silences the TNFα-RIP1 Necroptosis Pathway to Sustain Survival and Accumulation of Myeloid-Derived Suppressor Cells. Cancer Res. 2020;80:3145-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 98. | Ma M, Huang W, Kong D. IL-17 inhibits the accumulation of myeloid-derived suppressor cells in breast cancer via activating STAT3. Int Immunopharmacol. 2018;59:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 99. | Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 601] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 100. | Wang T, Wang J, Jiang H, Ni M, Zou Y, Chen Y, Wu T, Ding D, Xu H, Li X. Targeted regulation of tumor microenvironment through the inhibition of MDSCs by curcumin loaded self-assembled nano-filaments. Mater Today Bio. 2022;15:100304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Liu W, Wu TC, Hong DM, Hu Y, Fan T, Guo WJ, Xu Q. Carnosic acid enhances the anti-lung cancer effect of cisplatin by inhibiting myeloid-derived suppressor cells. Chin J Nat Med. 2018;16:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Hu LP, Huang W, Wang X, Xu C, Qin WT, Li D, Tian G, Li Q, Zhou Y, Chen S, Nie HZ, Hao Y, Song J, Zhang XL, Sundquist J, Sundquist K, Li J, Jiang SH, Zhang ZG, Ji J. Terbinafine prevents colorectal cancer growth by inducing dNTP starvation and reducing immune suppression. Mol Ther. 2022;30:3284-3299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 103. | Wang Q, Li XL, Mei Y, Ye JC, Fan W, Cheng GH, Zeng MS, Feng GK. The anti-inflammatory drug dimethyl itaconate protects against colitis-associated colorectal cancer. J Mol Med (Berl). 2020;98:1457-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 104. | Trikha P, Plews RL, Stiff A, Gautam S, Hsu V, Abood D, Wesolowski R, Landi I, Mo X, Phay J, Chen CS, Byrd J, Caligiuri M, Tridandapani S, Carson W. Targeting myeloid-derived suppressor cells using a novel adenosine monophosphate-activated protein kinase (AMPK) activator. Oncoimmunology. 2016;5:e1214787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Hu J, Zhang W, Liu Y, Yang Y, Tan C, Wei X, Wang Y, Tan S, Liu M, Liu K, Zhang H, Xiao X. LDK378 inhibits the recruitment of myeloid-derived suppressor cells to spleen via the p38-GRK2-CCR2 pathway in mice with sepsis. Immunol Cell Biol. 2019;97:902-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Lyu Z, Huang B, Zhang J, Qian Q, Pu X, Cui N, Ou Y, Li B, You Z, Lian M, Tang R, Chen W, Zhao Z, Hou J, Gershwin ME, Zhang H, Xia Q, Ma X. Suppression of YTHDF2 attenuates autoimmune hepatitis by expansion of myeloid-derived suppressor cells. J Autoimmun. 2023;135:102993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 107. | Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng Q, Wang Y, Yuan W, Ma J. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell. 2016;7:130-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 108. | Yang Y, Zhang M, Zhang Y, Liu K, Lu C. 5-Fluorouracil Suppresses Colon Tumor through Activating the p53-Fas Pathway to Sensitize Myeloid-Derived Suppressor Cells to FasL(+) Cytotoxic T Lymphocyte Cytotoxicity. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 109. | Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583-4594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 110. | Kang J, Lee D, Lee KJ, Yoon JE, Kwon JH, Seo Y, Kim J, Chang SY, Park J, Kang EA, Park SJ, Park JJ, Cheon JH, Kim TI. Tumor-Suppressive Effect of Metformin via the Regulation of M2 Macrophages and Myeloid-Derived Suppressor Cells in the Tumor Microenvironment of Colorectal Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 111. | Sakamaki I, Kwak LW, Cha SC, Yi Q, Lerman B, Chen J, Surapaneni S, Bateman S, Qin H. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia. 2014;28:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526-3536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 113. | Sturgill ER, Rolig AS, Linch SN, Mick C, Kasiewicz MJ, Sun Z, Traber PG, Shlevin H, Redmond WL. Galectin-3 inhibition with belapectin combined with anti-OX40 therapy reprograms the tumor microenvironment to favor anti-tumor immunity. Oncoimmunology. 2021;10:1892265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Grauers Wiktorin H, Nilsson MS, Kiffin R, Sander FE, Lenox B, Rydström A, Hellstrand K, Martner A. Histamine targets myeloid-derived suppressor cells and improves the anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade. Cancer Immunol Immunother. 2019;68:163-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 115. | Chen Q, He Y, Wang Y, Li C, Zhang Y, Guo Q, Chu Y, Liu P, Chen H, Zhou Z, Zhou W, Zhao Z, Li X, Sun T, Jiang C. Penetrable Nanoplatform for "Cold" Tumor Immune Microenvironment Reeducation. Adv Sci (Weinh). 2020;7:2000411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 116. | Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein N, Kumar P, Gabrilovich DI. Selective Targeting of Myeloid-Derived Suppressor Cells in Cancer Patients Using DS-8273a, an Agonistic TRAIL-R2 Antibody. Clin Cancer Res. 2017;23:2942-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 117. | Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438-11446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 118. | Li D, Shi G, Wang J, Zhang D, Pan Y, Dou H, Hou Y. Baicalein ameliorates pristane-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res Ther. 2019;21:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 119. | Lu YT, Li J, Qi X, Pei YX, Shi WG, Lin HS. Effects of Shugan Jianpi Formula () on myeloid-derived suppression cells-mediated depression breast cancer mice. Chin J Integr Med. 2017;23:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Tengesdal IW, Menon DR, Osborne DG, Neff CP, Powers NE, Gamboni F, Mauro AG, D'Alessandro A, Stefanoni D, Henen MA, Mills TS, De Graaf DM, Azam T, Vogeli B, Palmer BE, Pietras EM, DeGregori J, Tan AC, Joosten LAB, Fujita M, Dinarello CA, Marchetti C. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 121. | Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 122. | Bueno V, Forones NM, Pawelec G. Alternative Chemotherapies: Angiotensin-Converting Enzyme Inhibitors Reduce Myeloid-Derived Suppressor Cells to Benefit Older Patients with Colorectal Cancer. Front Biosci (Landmark Ed). 2023;28:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 123. | Colligan SH, Amitrano AM, Zollo RA, Peresie J, Kramer ED, Morreale B, Barbi J, Singh PK, Yu H, Wang J, Opyrchal M, Sykes DB, Nemeth MJ, Abrams SI. Inhibiting the biogenesis of myeloid-derived suppressor cells enhances immunotherapy efficacy against mammary tumor progression. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 124. | Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 125. | Lee GY, Han SN. The Role of Vitamin E in Immunity. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 293] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 126. | Hashimoto A, Gao C, Mastio J, Kossenkov A, Abrams SI, Purandare AV, Desilva H, Wee S, Hunt J, Jure-Kunkel M, Gabrilovich DI. Inhibition of Casein Kinase 2 Disrupts Differentiation of Myeloid Cells in Cancer and Enhances the Efficacy of Immunotherapy in Mice. Cancer Res. 2018;78:5644-5655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 127. | Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 128. | Sidiropoulos DN, Rafie CI, Jang JK, Castanon S, Baugh AG, Gonzalez E, Christmas BJ, Narumi VH, Davis-Marcisak EF, Sharma G, Bigelow E, Vaghasia A, Gupta A, Skaist A, Considine M, Wheelan SJ, Ganesan SK, Yu M, Yegnasubramanian S, Stearns V, Connolly RM, Gaykalova DA, Kagohara LT, Jaffee EM, Fertig EJ, Roussos Torres ET. Entinostat Decreases Immune Suppression to Promote Antitumor Responses in a HER2+ Breast Tumor Microenvironment. Cancer Immunol Res. 2022;10:656-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 129. | Ishfaq M, Pham T, Beaman C, Tamayo P, Yu AL, Joshi S. BTK Inhibition Reverses MDSC-Mediated Immunosuppression and Enhances Response to Anti-PDL1 Therapy in Neuroblastoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 130. | Kalathil SG, Wang K, Hutson A, Iyer R, Thanavala Y. Tivozanib mediated inhibition of c-Kit/SCF signaling on Tregs and MDSCs and reversal of tumor induced immune suppression correlates with survival of HCC patients. Oncoimmunology. 2020;9:1824863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 131. | Zheng Y, Xu M, Li X, Jia J, Fan K, Lai G. Cimetidine suppresses lung tumor growth in mice through proapoptosis of myeloid-derived suppressor cells. Mol Immunol. 2013;54:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 132. | Chen J, Sun HW, Yang YY, Chen HT, Yu XJ, Wu WC, Xu YT, Jin LL, Wu XJ, Xu J, Zheng L. Reprogramming immunosuppressive myeloid cells by activated T cells promotes the response to anti-PD-1 therapy in colorectal cancer. Signal Transduct Target Ther. 2021;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 133. | Dong CX, Zhang WS, Sun QL, Jiang YJ, Fu Z, Zhang Y, Du J, Sun YX, Tao N, Yao Z. Structural characterization of a pectin-type polysaccharide from Curcuma kwangsiensis and its effects on reversing MDSC-mediated T cell suppression. Int J Biol Macromol. 2018;115:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 134. | Jiang S, Ma J, Li Y, Lu B, Du J, Xu J, Qin Z, Ning T, Dong C. A polysaccharide from native Curcuma kwangsiensis and its mechanism of reversing MDSC-induced suppressive function. Carbohydr Polym. 2022;297:120020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 135. | Zhang W, He W, Shi X, Li X, Wang Y, Hu M, Ma F, Tao N, Wang G, Qin Z. An Asparagus polysaccharide fraction inhibits MDSCs by inducing apoptosis through toll-like receptor 4. Phytother Res. 2018;32:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Wang L, Hu D, Xie B, Xie L. Blockade of Myd88 signaling by a novel MyD88 inhibitor prevents colitis-associated colorectal cancer development by impairing myeloid-derived suppressor cells. Invest New Drugs. 2022;40:506-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 137. | Wang Y, Cui L, Georgiev P, Singh L, Zheng Y, Yu Y, Grein J, Zhang C, Muise ES, Sloman DL, Ferguson H, Yu H, Pierre CS, Dakle PJ, Pucci V, Baker J, Loboda A, Linn D, Brynczka C, Wilson D, Haines BB, Long B, Wnek R, Sadekova S, Rosenzweig M, Haidle A, Han Y, Ranganath SH. Combination of EP(4) antagonist MF-766 and anti-PD-1 promotes anti-tumor efficacy by modulating both lymphocytes and myeloid cells. Oncoimmunology. 2021;10:1896643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 138. | Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, Silvin C, Van Waes C, Allen C. Anti-PD-L1 Efficacy Can Be Enhanced by Inhibition of Myeloid-Derived Suppressor Cells with a Selective Inhibitor of PI3Kδ/γ. Cancer Res. 2017;77:2607-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 139. | AbuEid M, McAllister DM, McOlash L, Harwig MC, Cheng G, Drouillard D, Boyle KA, Hardy M, Zielonka J, Johnson BD, Hill RB, Kalyanaraman B, Dwinell MB. Synchronous effects of targeted mitochondrial complex I inhibitors on tumor and immune cells abrogate melanoma progression. iScience. 2021;24:102653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Jackson JJ, Shibuya GM, Ravishankar B, Adusumilli L, Bradford D, Brockstedt DG, Bucher C, Bui M, Cho C, Colas C, Cutler G, Dukes A, Han X, Hu DX, Jacobson S, Kassner PD, Katibah GE, Ko MYM, Kolhatkar U, Leger PR, Ma A, Marshall L, Maung J, Ng AA, Okano A, Pookot D, Poon D, Ramana C, Reilly MK, Robles O, Schwarz JB, Shakhmin AA, Shunatona HP, Sreenivasan R, Tivitmahaisoon P, Xu M, Zaw T, Wustrow DJ, Zibinsky M. Potent GCN2 Inhibitor Capable of Reversing MDSC-Driven T Cell Suppression Demonstrates In Vivo Efficacy as a Single Agent and in Combination with Anti-Angiogenesis Therapy. J Med Chem. 2022;65:12895-12924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 141. | Sierra RA, Trillo-Tinoco J, Mohamed E, Yu L, Achyut BR, Arbab A, Bradford JW, Osborne BA, Miele L, Rodriguez PC. Anti-Jagged Immunotherapy Inhibits MDSCs and Overcomes Tumor-Induced Tolerance. Cancer Res. 2017;77:5628-5638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 142. | Plebanek MP, Bhaumik D, Bryce PJ, Thaxton CS. Scavenger Receptor Type B1 and Lipoprotein Nanoparticle Inhibit Myeloid-Derived Suppressor Cells. Mol Cancer Ther. 2018;17:686-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 143. | Ohl K, Tenbrock K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front Immunol. 2018;9:2499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 144. | Wagner S, Mullins CS, Linnebacher M. Colorectal cancer vaccines: Tumor-associated antigens vs neoantigens. World J Gastroenterol. 2018;24:5418-5432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (4)] |
| 145. | Xu P, He H, Gu Y, Wang Y, Sun Z, Yang L, Miao C. Surgical trauma contributes to progression of colon cancer by downregulating CXCL4 and recruiting MDSCs. Exp Cell Res. 2018;370:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 146. | Kho VM, Mekers VE, Span PN, Bussink J, Adema GJ. Radiotherapy and cGAS/STING signaling: Impact on MDSCs in the tumor microenvironment. Cell Immunol. 2021;362:104298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 147. | Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 148. | Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1646] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 149. | Aparicio C, Belver M, Enríquez L, Espeso F, Núñez L, Sánchez A, de la Fuente MÁ, González-Vallinas M. Cell Therapy for Colorectal Cancer: The Promise of Chimeric Antigen Receptor (CAR)-T Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 150. | Xu J, Meng Q, Sun H, Zhang X, Yun J, Li B, Wu S, Li X, Yang H, Zhu H, Aschner M, Relucenti M, Familiari G, Chen R. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis. 2021;12:1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 151. | Qin X, Wu F, Chen C, Li Q. Recent advances in CAR-T cells therapy for colorectal cancer. Front Immunol. 2022;13:904137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 152. | Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J, Luo Y, Zhang Q, Liu L, Qin H, Liu W, Wu F, Chen W, Pan F, Zhang X, Bie P, Liang H, Pecher G, Qian C. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers. Mol Ther. 2017;25:1248-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 153. | Chen J, Zhu T, Jiang G, Zeng Q, Li Z, Huang X. Target delivery of a PD-1-TREM2 scFv by CAR-T cells enhances anti-tumor efficacy in colorectal cancer. Mol Cancer. 2023;22:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 154. | Long X, Wong CC, Tong L, Chu ESH, Ho Szeto C, Go MYY, Coker OO, Chan AWH, Chan FKL, Sung JJY, Yu J. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. 2019;4:2319-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 155. | Bergsten E, Mestivier D, Donnadieu F, Pedron T, Barau C, Meda LT, Mettouchi A, Lemichez E, Gorgette O, Chamaillard M, Vaysse A, Volant S, Doukani A, Sansonetti PJ, Sobhani I, Nigro G. Parvimonas micra, an oral pathobiont associated with colorectal cancer, epigenetically reprograms human colonocytes. Gut Microbes. 2023;15:2265138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 156. | Singh A, Ashar H, Butcher JT, Ranjan A. Age-associated changes in the gut microbiome impact efficacy of tumor immunomodulatory treatments. Exp Gerontol. 2023;181:112268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 157. | Neamah WH, Busbee PB, Alghetaa H, Abdulla OA, Nagarkatti M, Nagarkatti P. AhR Activation Leads to Alterations in the Gut Microbiome with Consequent Effect on Induction of Myeloid Derived Suppressor Cells in a CXCR2-Dependent Manner. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 158. | Ding G, Gong Q, Ma J, Liu X, Wang Y, Cheng X. Immunosuppressive activity is attenuated by Astragalus polysaccharides through remodeling the gut microenvironment in melanoma mice. Cancer Sci. 2021;112:4050-4063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |