Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1683
Peer-review started: December 30, 2023
First decision: January 16, 2024
Revised: January 29, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: May 15, 2024
Processing time: 130 Days and 17.5 Hours
Despite colorectal cancer’s (CRC) high global incidence, residents of low- and middle- income countries, as well as low-income minorities in advanced economies have low screening rates. Observational studies demonstrate that in these groups higher incidence of CRC is observed, yet screening rates remain low for consistent reasons. Low income, low educational background, and lack of awareness in combination with inadequate social security of certain population groups impede access and compliance rates to CRC screening. On the other hand, despite the global availability of multiple screening approaches (colonoscopy, sigmoidoscopy, faecal occult blood test, faecal immunochemical test, computed tomography-colonography, etc.) with proven diagnostic validity, many low-income countries still lack established screening programs. The absence of screening guidelines in these countries along with the heterogeneity of guidelines in the rest of the world, demonstrate the need for global measures to tackle this issue comprehensively. An essential step forward is to develop a global approach that will link specific elements of screening with the incidence and available resources in each country, to ensure the achievement of at least a minimum screening program in low-income countries. Utilizing cheaper, cost-effective techniques, which can be carried out by less specialized healthcare providers, might not be equivalent to endoscopy for CRC screening but seems more realistic for areas with fewer resources. Awareness has been highlighted as the most pivotal element for the effective implementation of any screening program concerning CRC. Moreover, multiple studies have demonstrated that outreach strategies and community-based educational programs are associated with encouraging outcomes, yet a centrally coordinated expansion of these programs could provide more consistent results. Additionally, patient navigator programs, wherever implemented, have increased CRC screening and improved follow-up. Therefore, global coordination and patient education seem to be the main areas on which policy making needs to focus.
Core Tip: The high colorectal cancer (CRC) mortality rate worldwide, despite the adequate availability of modern screening, diagnostic and treatment options, raises important research and clinical questions. In both low- and middle- income countries (LMICs), and minority populations of high-income countries, the paradox of underutilization of existing screening methods was observed. The lack of global, homogeneous guidelines and the absence of guidelines addressing CRC screening at the national level for LMICs, contribute to the confusing status in the management of screening of aforementioned populations. Global efforts should focus on medical education and vigilance of medical healthcare providers and patients, on exploring the socio-economic aspects, and on the use of technological innovations in telemedicine and artificial intelligence in clinical practice.
- Citation: Rozani S, Lykoudis PM. Overcoming geographical and socioeconomic limitations in colorectal cancer screening. World J Gastrointest Oncol 2024; 16(5): 1683-1689
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1683.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1683
Colorectal cancer (CRC) is the fourth most common cancer and the second most common cause of cancer-related death worldwide. Obesity, lack of exercise and smoking increase the risk of CRC[1]. The epidemiology of CRC varies considerably between different regions of the world. It appears to have an increased incidence in developed countries, while age of onset and gender inequalities are also prominent[2,3]. Predominantly in developed countries, 10% of CRC cases occur at an early age (around 50 years of age) with the incidence of the disease increasing progressively, often in the form of advanced disease arising from the left colon[4]. The incidence of CRC is increasing progressively in low- and middle-income countries (LMICs). Dissimilar to high income countries (HICs), the actual data from epidemiology in LMICs remain unknown or unclear due to difficulty in tracking patients and loss of patients to follow-up[5]. Despite the high global CRC incidence, residents of LMICs, as well as low-income minorities in advanced economies have low screening rates[6]. Observational studies demonstrate that in these groups higher incidence of CRC is observed, yet screening rates remain low for consistent reasons[6,7]. Concerning screening, early detection of the disease contributes significantly to reducing the incidence and mortality of this cancer[6-8]. Multiple factors are involved in this variation, including exposure to risk factors, demographic parameters, genetic mutations and susceptibility, as well as consequent impact on prognosis and response to treatment[2,9]. In particular, screening by means of endoscopy and fecal occult blood tests (FOBT) can effectively reduce CRC incidence and mortality rates[6]. Over its course, the disease becomes symptomatic mostly at an advanced stage. Therefore, the global implementation of screening programs, aimed at increasing early detection and reducing the morbidity and mortality from CRC, are gaining ground in the prevention of this cancer in both HICs and LMICs[4].
Modernization of management of certain types of cancer, including CRC, has led to significantly better oncological outcomes. Techniques for surgical treatment, which remains the cornerstone in the management of CRC, as well as medical treatments have impressively improved. However, screening will always be the most cost-effective approach, firstly because it can detect premalignant conditions and reduce the incidence of the disease, and secondly because it can lead to early detection, thus maximizing the efficacy of aforementioned treatment options. Therefore, this editorial places particular emphasis on screening strategies.
Low income, low educational background, and lack of awareness in combination with inadequate social security of certain population groups impede access and compliance rates to CRC screening[10,11]. Insufficient population education and lackluster effort to raise awareness of the population to improve health, create additional obstacles[12]. The differences in the management and screening methods of CRC testify to the systemic differences in terms of equity and equality in access to quality health services[13].The majority of studies support the view that the low educational level of patients contributes to the neglect of the disease and their recourse to screening[14]. Inequalities in access to diagnostic and treatment facilities in most LMICs are primarily due to a lack of resources streamed to respective health care system and cancer care. The absence of quality care in these countries discourages residents from resorting to health facilities and health professionals[13,14].
The particular challenges that national systems of these countries have to face are mainly social, cultural and structural in nature, such as poverty and the ignorance of patients about new screening techniques that have been integrated into the national health system. In addition, thorough discussion of symptoms or screening tests may involve cultural biases and fear, contributing to lower colon cancer screening[12]. It also seems that the lower social strata with a low level of education have distorted views and superstitions regarding diseases in general, and cancer treatment in particular Gender is an additional factor as fear of social stigmatization due to the disease is mainly observed in female patients[14].
Geographical factors hinder proper access to healthcare facilities due to long distances, with travel costs that are mainly borne by patients. Other financial difficulties are added to geographical causes, where for instance medical expenses for cancer expenses are covered by patients in total, in lack of provision through respective healthcare and social insurance systems[13,15]. Ensuring equity in optimal access and treatment is also compromised by substandard health services and infrastructures aimed at screening, diagnosis, surgical and conservative management, preventing more and more patients from resorting to health infrastructures, since they consider they will receive suboptimal treatment[13,15]. Both inequitable patient access and inadequate training of health care providers are driven by these financial barriers and significantly affect the health care system, leading to disparities in the delivery of effective CRC prevention efforts[16]. The recourse to said health services is affected by the socioeconomic classification of populations[17,18]. Therefore, both exposure to health risks and the adoption of health behaviors and disease self-management are affected[19]. Undoubtedly, they include gender criteria and the concept of ethnicity due to its social construction[17-20].
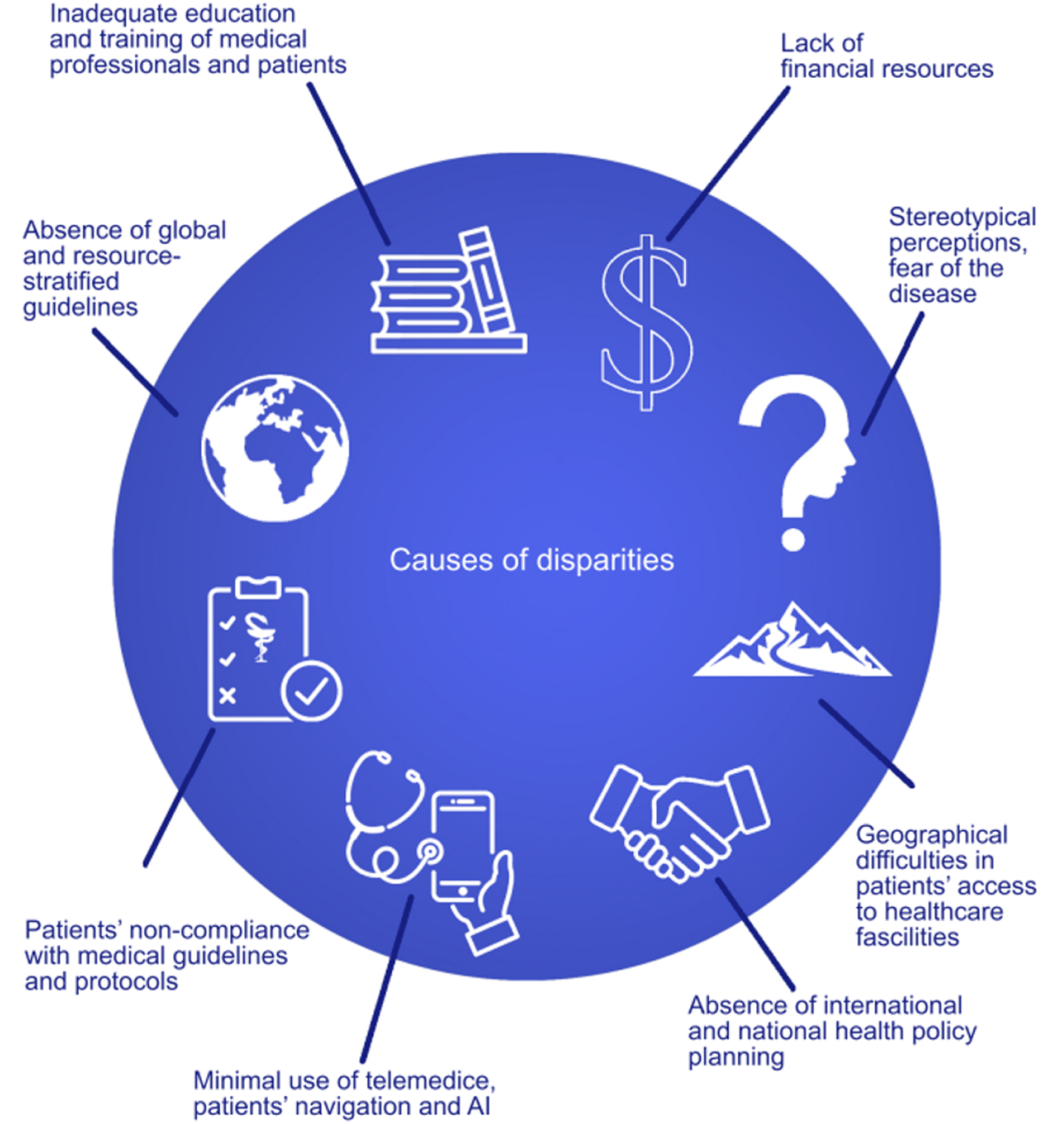
Among minority populations within HICs, inequalities appear to have identical causes to those of LMICs (race/ethnicity, socioeconomic status, geographic location), especially among populations of African American descent[21,22]. In addition to African populations, Hispanic minority groups in the Americas with low education rates and high levels of poverty also showed significant variation in access to CRC screening[23-25]. In addition to common causes, the lack of stable and robust employment and health insurance, and the fear of burdening health providers and services with more debt, prevent these populations, especially those of Latin American origin, from accessing health services[26]. Moreover, the ambiguity and lack of understanding of medical instructions and uncertainty about the results of screening further exacerbate the problem[26,27]. Improved education and opportunities would lead to a better understanding of modifiable risk factors and inform additional primary prevention or risk stratification strategies, that could promote the benefits of screening in these populations[27-29]. Figure 1 summarizes the pivotal causes of inequalities.
Particular emphasis should be placed on both medical and patient education. Studies supported by collaboration between a funding agency, an academic institution and community organizations, could provide access to colonoscopy for uninsured patients of minority populations. At the same time, this condition could be a lucrative ground for better medical education in screening and endoscopy for residents and qualified physicians[30].
Better training of health professionals will make health providers more efficient in screening and improve the quality of services provided. At the same time, better trained physicians and nurses will be communicators that raise awareness and educate the target population about the clinical benefits of prevention[31]. The above effort should be closely supervised by other health services and stakeholders so that it is adequately controlled based on quality indicators. The implementation of an organized CRC screening program in LMICs, with clear instructions to patients and clear criteria for inclusion (presence of symptoms, gender, age) and exclusion of patients (previous diagnosis of inflammatory bowel disease or hereditary CRC syndromes, individual history of CRC, previous colorectal resection surgery) for screening physicians would greatly assist in this direction[31].
The education of vulnerable population groups could also be supported by the development of a unified model of patient compliance with screening guidelines based on the Sociocultural Health Behavior Model. This model will be stratified according to population dynamics [educational level, family status, health needs, health beliefs, socio-economic factors (quality of health services, affordability of health insurance, availability of health care resources)][32]. Based on these, an international plan will be organized that will oversee the recourse of patients to healthcare services and the decision-making algorithm that doctors follow.
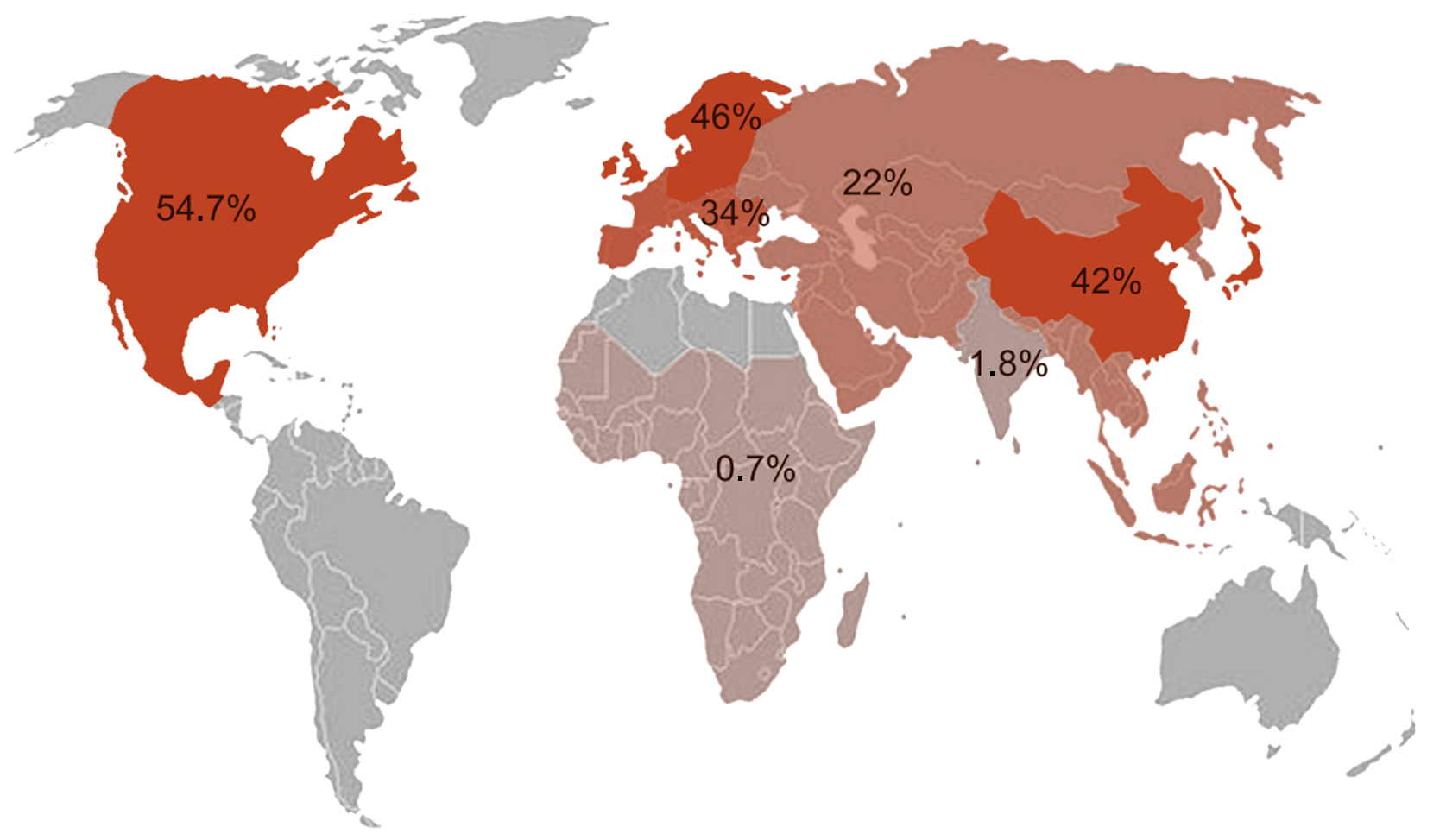
A summary of current data on geographic variations regarding access to colorectal cancer screening is depicted in Figure 2. In an effort to adopt a more global approach that would link screening data with epidemiological indicators, such as incidence, and take into account available state resources, the study of Selmouni et al[33] is considered an important contribution. Following improvement in the medical education of healthcare professionals, strategic outreach to selected population groups in a routine primary care setting resulted in higher patient compliance to diagnostic protocols [faecal immunochemical test (FIT), colonoscopy compliance].
Furthermore, in terms of healthcare economics, this study could also help in the assessment of financial impact on each national health system, dealing with large numbers of referrals, as well as cost-effectiveness analysis of existing screening methods, with the aim of utilizing the most economically accessible and most effective ones. More cost-effective screening tests that can also be performed by less trained health professionals, could be offered by smaller providers or primary care services. In turn, these could be available in geographically remote areas, therefore facilitating access[34].
From a socio-economic perspective, the adoption of a design-budget impact model in the formulation of global CRC screening guidelines, presupposes the implementation of national programs based on per capita income and government budget[35]. Leveraging strategies that have been tested in other areas, such as Pennsylvania's implementation of the Affordable Care Act, which provides funding to states to expand Medicaid eligibility to citizens over 65 at the poverty line, could contribute to the implementation of a minimum screening program per country as well as a more global effort to use CRC testing services, and a more comprehensive understanding of important factors associated with CRC testing among vulnerable population groups in both HICs and LMICs[34]. Therefore, the most effective and least expensive strategies will be adopted, such as FIT and colonoscopies over FOBT[36]. In this way, guidelines could be formulated for the implementation of a basic control program per country that takes into account available funds.
Undoubtedly, the coronavirus disease 2019 pandemic has once again brought to the fore the importance of integrating telemedicine and asynchronous healthcare services into everyday practice. Services such as text reminders and web-based communication portals, navigating patients to cost-effective and accessible CRC screening interventions targeted to specific populations, would provide excellent solutions to better manage patients with regards to both screening and compliance to follow-up[37].
Another practice of proven efficacy is the one suggested by Fernandez et al[38], involving telephone navigation. The 2-1-1 service increased the access of patients-particularly those of low-income - to cancer screening services, including colon cancer. The study demonstrated that the navigation intervention resulted in a significantly greater completion of any required diagnostic and counseling service, including that of CRC, and therefore we believe that it could be an important aid towards the timely access of oncology patients of lower social strata to health services.
In addition to telemedicine, one-time computer-tailored intervention may offer better prospects for CRC screening[39]. Patient navigation to CRC screening, facilitates access and eventually preserves resources that can be streamed to strengthen other key actions[37,40]. In particular, regarding poor patient compliance due to geographical mobility difficulties, the introduction of FIT mail could be pivotal[41]. Consequently, better healthcare delivery would be observed for both socio-economically vulnerable groups and for groups that are underserved due to geographical distance.
Finally, artificial intelligence and especially Computer Aided Detection systems can significantly assist in the evolution of colonoscopy screening[42], and in reducing devastating healthcare costs. Therefore, research should look into this evolving field, aiming to define ways of incorporating these innovative and promising technologies.
In conclusion, disparities in access to health care and screening services for the above populations can be alleviated by leveraging optimal educational models that target both physicians and patients. Optimizing screening approaches as well as mitigating disparities in order to improve patient access to screening services are pivotal. These are considered of paramount importance, in order to implement treatment approaches, such as surgical or medical treatment, in the most beneficial way. Close cooperation of governmental and non-governmental agencies, together with healthcare providers under their close supervision, will promote sufficient medical education, increase quality of services, and remove any fear or mistrust that patients might feel. The shaping of screening models according to the socioeconomic status of target populations can lead to a robust international screening plan, ultimately reducing mortality from CRC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Engida YE, Ethiopia; Safarzadeh Kozani P, Iran S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 2976] [Article Influence: 496.0] [Reference Citation Analysis (3)] |
| 2. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (2)] |
| 3. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 376] [Article Influence: 125.3] [Reference Citation Analysis (6)] |
| 4. | Sinicrope FA. Increasing Incidence of Early-Onset Colorectal Cancer. N Engl J Med. 2022;386:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 269] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 5. | Kwakye G, Dally CK. Colorectal cancer screening in sub-Saharan Africa. Lancet Glob Health. 2022;10:e938-e939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, Chen H, Dai M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021;522:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 7. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 960] [Article Influence: 137.1] [Reference Citation Analysis (2)] |
| 8. | Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An Update on the Epidemiology, Molecular Characterization, Diagnosis, and Screening Strategies for Early-Onset Colorectal Cancer. Gastroenterology. 2021;160:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 9. | Song M. Global epidemiology and prevention of colorectal cancer. Lancet Gastroenterol Hepatol. 2022;7:588-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 10. | The Lancet Gastroenterology Hepatology. Controversy over colonoscopy for colorectal cancer screening. Lancet Gastroenterol Hepatol. 2022;7:1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1522] [Reference Citation Analysis (3)] |
| 12. | Akanbi M, Santiago Rivera OJ, Dutta A, Pratiti R. A Review of Community Awareness for Colorectal Cancer Screening and Prevention in North and Central Asian Countries. Cureus. 2023;15:e40540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 13. | Knaul F, Frenk J, Shulman L. Closing the Cancer Divide: A Blueprint to Expand Access in Low- and Middle-Income Countries. Rochester. 2011. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2055430. |
| 14. | Khan SZ, Lengyel CG. Challenges in the management of colorectal cancer in low- and middle-income countries. Cancer Treat Res Commun. 2023;35:100705. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Romero Y, Trapani D, Johnson S, Tittenbrun Z, Given L, Hohman K, Stevens L, Torode JS, Boniol M, Ilbawi AM. National cancer control plans: a global analysis. Lancet Oncol. 2018;19:e546-e555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 16. | White PM, Itzkowitz SH. Barriers Driving Racial Disparities in Colorectal Cancer Screening in African Americans. Curr Gastroenterol Rep. 2020;22:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 872] [Article Influence: 436.0] [Reference Citation Analysis (1)] |
| 18. | Carethers JM, Doubeni CA. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology. 2020;158:354-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 19. | Kizub DA, Naik S, Abogan AA, Pain D, Sammut S, Shulman LN, Martei YM. Access to and Affordability of World Health Organization Essential Medicines for Cancer in Sub-Saharan Africa: Examples from Kenya, Rwanda, and Uganda. Oncologist. 2022;27:958-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Deo SVS, Kumar S, Bhoriwal S, Shukla NK, Sharma A, Thulkar S, Das P, Bhagat P, Dhall K, Pathy S, Mohanti BK. Colorectal Cancers in Low- and Middle-Income Countries-Demographic Pattern and Clinical Profile of 970 Patients Treated at a Tertiary Care Cancer Center in India. JCO Glob Oncol. 2021;7:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Doubeni CA, Selby K, Gupta S. Framework and Strategies to Eliminate Disparities in Colorectal Cancer Screening Outcomes. Annu Rev Med. 2021;72:383-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:627-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 361] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 23. | Nicot-Cartsonis MS, Digbeu BDE, Raji MA, Kuo YF. Disparities in Late-Stage Breast and Colorectal Cancer Diagnosis Among Hispanic, Non-Hispanic White, and Non-Hispanic Black Patients: a Retrospective Cohort Study of Texas Medicare Beneficiaries. J Racial Ethn Health Disparities. 2023;10:3168-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Cénat JM, Dromer É, Darius WP, Dalexis RD, Furyk SE, Poisson H, Mansoub Bekarkhanechi F, Shah M, Diao DG, Gedeon AP, Lebel S, Labelle PR. Incidence, factors, and disparities related to cancer among Black individuals in Canada: A scoping review. Cancer. 2023;129:335-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Napit K, Ratnapradipa KL, King KM, Ramos AK, Luma LBL, Dinkel D, Robinson T, Schabloske L, Tchouankam T, Watanabe-Galloway S. Qualitative Analysis of Colorectal Cancer Screening for African American and Hispanic Populations in Nebraska: an Application of the PRECEDE Framework. J Cancer Educ. 2023;38:1767-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | De La Torre CL, Dumbauld JN, Haughton J, Gupta S, Nodora J, Giacinto RE, Ramers C, Bharti B, Wells K, Lopez J, Díaz M, Moody J, Arredondo EM. Development of a Group-Based Community Health Worker Intervention to Increase Colorectal Cancer Screening Among Latinos. Hisp Health Care Int. 2021;19:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Petrick JL, Barber LE, Rosenberg L. What Are the Factors Underlying Colorectal Cancer Health Disparities? Cancer Prev Res (Phila). 2022;15:561-563. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Heintzman JD, Ezekiel-Herrera DN, Quiñones AR, Lucas JA, Carroll JE, Gielbultowicz SH, Cottrell EC, Marino M. Disparities in Colorectal Cancer Screening in Latinos and Non-Hispanic Whites. Am J Prev Med. 2022;62:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Wyatt LC, Patel S, Kranick JA, Raveis VH, Ravenell JE, Yi SS, Kwon SC, Islam NS. Disparities in colorectal cancer screening among South Asians in New York City: a cross-sectional study. J Cancer Educ. 2022;37:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | McClellan DA, Ojinnaka CO, Pope R, Simmons J, Fuller K, Richardson A, Helduser JW, Nash P, Ory MG, Bolin JN. Expanding Access to Colorectal Cancer Screening: Benchmarking Quality Indicators in a Primary Care Colonoscopy Program. J Am Board Fam Med. 2015;28:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Ribeiro U Jr, Safatle-Ribeiro AV, Sorbello M, Kishi PHR, Cohend DD, Mattar R, Castilho VLP, Goncalves EMDN, Kawaguti F, Marques CFS, Alves VAF, Nahas SC, Eluf-Neto J. Implementation of an organized colorectal cancer screening program through quantitative fecal immunochemical test followed by colonoscopy in an urban low-income community: Guidance and strategies. Clinics (Sao Paulo). 2023;78:100278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Ma GX, Zhu L, Lin TR, Tan Y, Do P. Multilevel Pathways of Colorectal Cancer Screening Among Low-Income Vietnamese Americans: A Structural Equation Modeling Analysis. Cancer Control. 2021;28:10732748211011077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Selmouni F, Amrani L, Sauvaget C, Bakkar M, El Khannoussi B, Souadka A, Benkabbou A, Majbar MA, Belekhel L, Lucas E, Muwonge R, Chami Khazraji Y, Mohsine R, Bennani M, Sankaranarayanan R, Bekkali R, Basu P. Delivering colorectal cancer screening integrated with primary health care services in Morocco: Lessons learned from a demonstration project. Cancer. 2022;128:1219-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Eom KY, Rothenberger SD, Jarlenski MP, Schoen RE, Cole ES, Sabik LM. Enrollee characteristics and receipt of colorectal cancer testing in Pennsylvania after adoption of the Affordable Care Act Medicaid expansion. Cancer Med. 2023;12:15455-15467. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Ngan TT, Ramanathan K, Saleh MRBM, Schliemann D, Ibrahim Tamin NSB, Su TT, Donnelly M, O'Neill C. Budget impact analysis of a home-based colorectal cancer screening programme in Malaysia. BMJ Open. 2023;13:e066925. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Jahn B, Sroczynski G, Bundo M, Mühlberger N, Puntscher S, Todorovic J, Rochau U, Oberaigner W, Koffijberg H, Fischer T, Schiller-Fruehwirth I, Öfner D, Renner F, Jonas M, Hackl M, Ferlitsch M, Siebert U; Austrian Colorectal Cancer Screening Model Group. Effectiveness, benefit harm and cost effectiveness of colorectal cancer screening in Austria. BMC Gastroenterol. 2019;19:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Qian F, Gates M, Bisner S, Collins E, Vora S, Dacus H. Benefits, Cost, and Activities of Patient Navigation (PN) Program for Colorectal Cancer Screening at the Charles B. Wang Community Health Center (CBWCHC). J Immigr Minor Health. 2020;22:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Fernandez ME, Savas LS, Atkinson JS, Ricks KB, Ibekwe LN, Jackson I, Castle PE, Jobe D, Vernon SW. Evaluation of a 2-1-1 Telephone Navigation Program to Increase Cancer Control Behaviors: Results From a Randomized Controlled Trial. Am J Health Promot. 2022;36:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Rawl SM, Christy SM, Perkins SM, Tong Y, Krier C, Wang HL, Huang AM, Laury E, Rhyant B, Lloyd F, Willis DR, Imperiale TF, Myers LJ, Springston J, Skinner CS, Champion VL. Computer-tailored intervention increases colorectal cancer screening among low-income African Americans in primary care: Results of a randomized trial. Prev Med. 2021;145:106449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Rice K, Sharma K, Li C, Butterly L, Gersten J, DeGroff A. Cost-effectiveness of a patient navigation intervention to increase colonoscopy screening among low-income adults in New Hampshire. Cancer. 2019;125:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Saurat JH, Gluckman E, Bussel A, Didierjean L, Puissant A. The lichen planus-like eruption after bone marrow transplantation. Br J Dermatol. 1975;93:675-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Rao HB, Sastry NB, Venu RP, Pattanayak P. The role of artificial intelligence based systems for cost optimization in colorectal cancer prevention programs. Front Artif Intell. 2022;5:955399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |