Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1647
Peer-review started: November 18, 2023
First decision: December 22, 2023
Revised: January 8, 2024
Accepted: February 19, 2024
Article in press: February 19, 2024
Published online: April 15, 2024
Processing time: 144 Days and 16.6 Hours
Hepatocellular carcinoma (HCC) is one of the leading causes of death due to its complexity, heterogeneity, rapid metastasis and easy recurrence after surgical resection. We demonstrated that combination therapy with transcatheter arterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), Epclusa, Lenvatinib and Sintilimab is useful for patients with advanced HCC.
A 69-year-old man who was infected with hepatitis C virus (HCV) 30 years previously was admitted to the hospital with abdominal pain. Enhanced computed tomography (CT) revealed a low-density mass in the right lobe of the liver, with a volume of 12.9 cm × 9.4 cm × 15 cm, and the mass exhibited a “fast-in/fast-out” pattern, with extensive filling defect areas in the right branch of the portal vein and an alpha-fetoprotein level as high as 657 ng/mL. Therefore, he was judged to have advanced HCC. During treatment, the patient received three months of Epclusa, three TACE treatments, two HAIC treatments, three courses of sintilimab, and twenty-one months of lenvatinib. In the third month of treatment, the patient developed severe side effects and had to stop immunotherapy, and the Lenvatinib dose had to be halved. Postoperative pathological diagnosis indicated a complete response. The patient recovered well after the operation, and no tumor recurrence was found.
Multidisciplinary conversion therapy for advanced enormous HCC caused by HCV infection has a significant effect. Individualized drug adjustments should be made during any treatment according to the patient's tolerance to treatment.
Core Tip: The present study describes the excellent efficacy of conversion therapy including interventional therapy, anti-hepatitis C virus therapy, and immunotargeted therapy for advanced giant hepatocellular carcinoma (HCC). After 21 months of conversion therapy, the patient underwent surgical resection completely and the postoperative pathology suggested a complete response. In summary, conversion therapy is a promising treatment option for unresectable HCC patients.
- Citation: Chu JH, Huang LY, Wang YR, Li J, Han SL, Xi H, Gao WX, Cui YY, Qian MP. Pathologically successful conversion hepatectomy for advanced giant hepatocellular carcinoma after multidisciplinary therapy: A case report and review of literature. World J Gastrointest Oncol 2024; 16(4): 1647-1659
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1647.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1647
At present, primary liver cancer is the fourth most common malignant tumor in China and the second most common cause of death[1]. It has seriously threatened the life and health of Chinese people, and hepatocellular carcinoma (HCC) accounts for the majority of of this disease[2]. Several liver diseases, such as hepatitis virus infection, alcoholism, and nonalcoholic fatty liver disease, are often associated with the development of HCC[3-5]. When diagnosed, more than 80% of patients are in the advanced stage when they are diagnosed and have obvious resistance to conventional chemotherapy and radiotherapy[6-8].
Conversion therapy is an effective method for transforming unresectable HCC into resectable HCC[9,10]. However, large tumors and portal vein invasion contribute to a poor prognosis in all stages of HCC[11]. In recent years, immunotargeted therapy has become an attractive and promising approach because it can improve the efficacy of antitumor drugs[12]. Lenvatinib, a newly developed polytyrosine kinase inhibitor, has a stronger effect on tumor vasculature and a greater tumor regression effect than does sorafenib[13]. Moreover, the development of immunotherapy has also promoted the treatment of advanced HCC[14]. Sintilimab, a programmed cell death factor-1 (PD-1) inhibitor, was introduced into clinical practice in 2018 and has now become an important therapy for patients with advanced HCC[15].
For people with hepatitis C virus (HCV), antiviral treatment can help prevent further damage to the liver from the virus. The earlier the time of antiviral treatment, the better the therapeutic effect. Epclusa is a single tablet fixed-dose compound preparation composed of two potent active antiviral ingredients. The drug has few side effects and a 98% cure rate for HCV[16].
Transcatheter arterial chemoembolization (TACE) is the main treatment method for primary liver cancer patients who cannot be treated by radical surgery[17]. TACE is less traumatic than the other surgical methods, significantly improves the therapeutic efficacy of patients and prolongs patient survival. In hepatic arterial infusion chemotherapy (HAIC), which is another local interventional therapy used in treating HCC, chemical drugs are delivered directly to the tumor vessel through hepatic arterial infusion. In 2013, EACH released the FOLFOX regimen (oxaliplatin + calcium folinate + 5-fluorouracil), which has good efficacy in the treatment of advanced HCC, and confirmed for the first time that the FOLFOX protocol can improve the survival rate of HCC patients[18].
To our knowledge, few studies have reported that patients with giant advanced HCC caused by hepatitis C successfully underwent surgical resection and achieved a pathological complete response after individualized conversion therapy.
A 69-year-old male patient who presented with abdominal pain for several days was admitted to the Shanghai Tenth People's Hospital on November 9, 2021.
The patient presented with abdominal pain that started 1 wk prior to hospitalization, and ultrasound indicated a space-occupying lesion in the right liver, so he was hospitalized.
The man underwent subtotal gastrectomy 30 years prior to treating the bleeding from a gastric ulcer, and the patient was infected with HCV through an intraoperative blood transfusion and visited a primary doctor. However, he did not receive any therapy for HCV. Before the tumor was discovered, the patient was in poor health and had cirrhosis resulting in hypersplenism and ascites, atrial fibrillation, and malnutrition.
The patient had been smoking and drinking for more than 40 years and had stopped smoking 10 years prior. The patient had no significant family history.
Physical examination revealed light pressure pain in the right upper abdomen and a hard liver upon palpation.
Venous blood was collected, and the indicators in Table 1 were measured.
| Variables | Value | Unit |
| AFP | 657 | ng/mL |
| WBC | 3.18 | 109/L |
| Hemoglobin | 104 | g/L |
| Platelet | 95 | 109/L |
| ALT | 36.7 | U/L |
| AST | 102.8 | U/L |
| ALP | 212 | U/L |
| γ-GTP | 111.1 | U/L |
| Albumin | 25.7 | g/L |
| T-BIL | 15.3 | umol/L |
| D-BIL | 7.6 | umol/L |
| PT | 13.6 | s |
| APTT | 31.2 | s |
| TT | 19.1 | s |
| Fibrinogen | 2.16 | s |
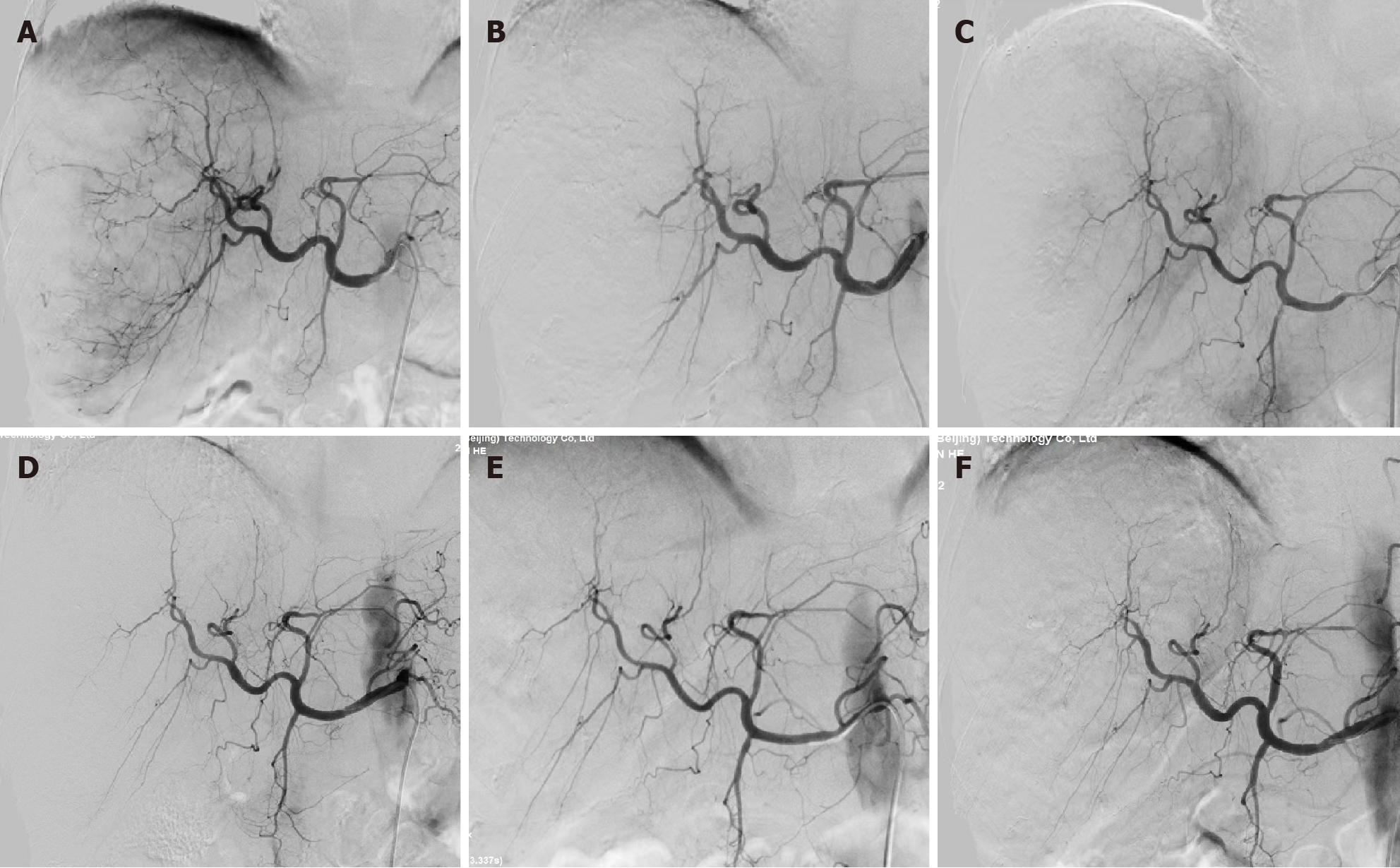
Enhanced computed tomography (CT) of the upper abdomen showed a low-density mass in the right lobe of the liver, with a volume of 12.9 cm × 9.4 cm × 15 cm; this mass exhibited a “fast-in/fast-out” pattern, with extensive filling defect areas in the right branch of the portal vein (Figure 1A and B). In addition, CT revealed that the total liver volume was 2764 cm³, the residual liver tissue was 786 cm³, and the residual liver tissue/total liver volume was 0.2843 (Figure 2).
The patient was diagnosed with advanced HCC (T4N0M0, stage IIIB) according to the 8th edition of the American Joint Committee on Cancer TNM Classification for HCC. The tumor was judged to be unresectable because the patient had an insufficient volume of his remaining healthy liver, and he had many basic diseases and malnutrition and was Child-Pugh stage B (Table 2)[19].
| Variables | 1 | 2 | 3 |
| Hepatic encephalopathy (stage) | None | 1-2 | 3-4 |
| Ascites | None | mild | Moderate-severe |
| T-BIL (umol/L) | < 34 | 34-51 | < 51 |
| Albumin (g/L) | < 35 | 28-35 | < 28 |
| Prothrombin time (s) | < 14 | 14-18 | < 18 |
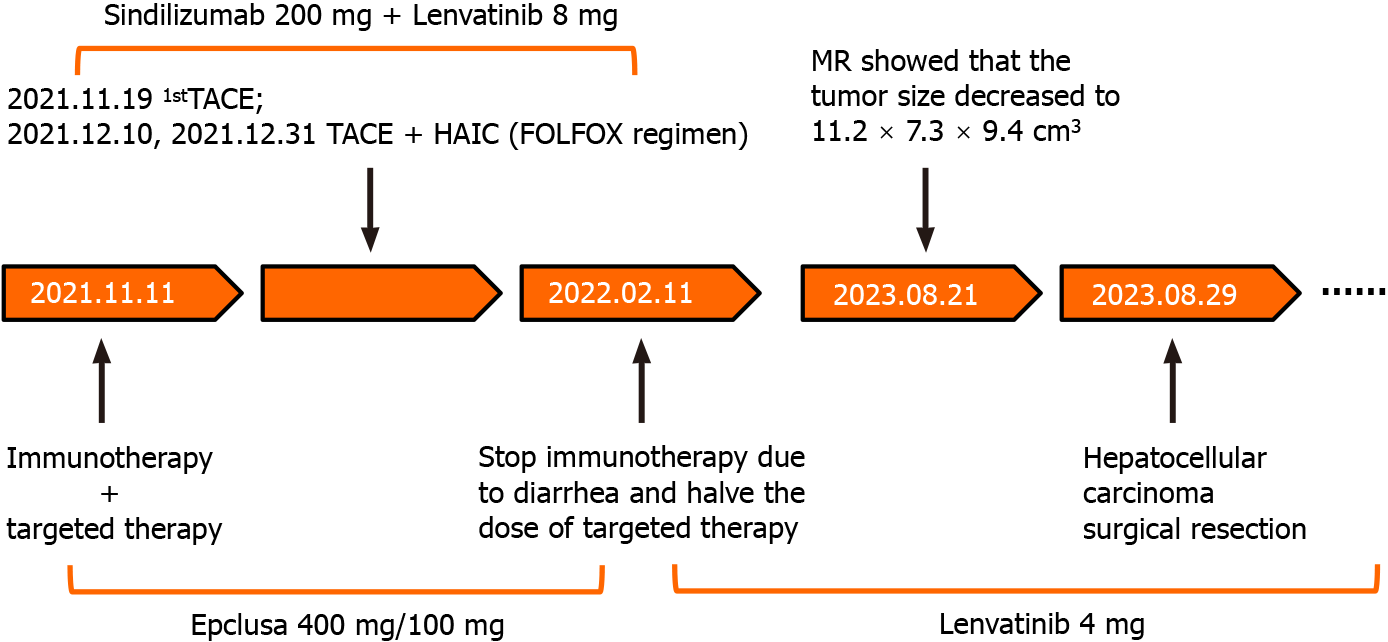
Symptomatic supportive treatment was given to improve the general condition of the patient. From the first day of hospitalization, 20 mg of albumin was administered intravenously daily for 5 consecutive days, and his albumin concentration increased from 25.7 g/L to 37 g/L. After a comprehensive analysis of the patient’s condition, TACE with epirubicin was performed first. To further increase the antitumor efficacy, the patient was given intravenous Sintilimab 200 mg of intravenous Sintilimab in combination with oral Lenvatinib 8 mg of oral lenvatinib after the first TACE treatment. During the whole treatment period, the patient received a total of 3 TACE and 2 HAIC treatments (FOLFOX regimen, oxaliplatin 150 mg + calcium folinate 200 mg + fluorouracil 3 g micropump) treatments (Figure 3). Epclusa was administered orally, 1 tablet once daily, for 3 months from the time that HCC was initially diagnosed, at which point the patient’s HCV RNA test became negative, and the patient did not develop any adverse reactions.
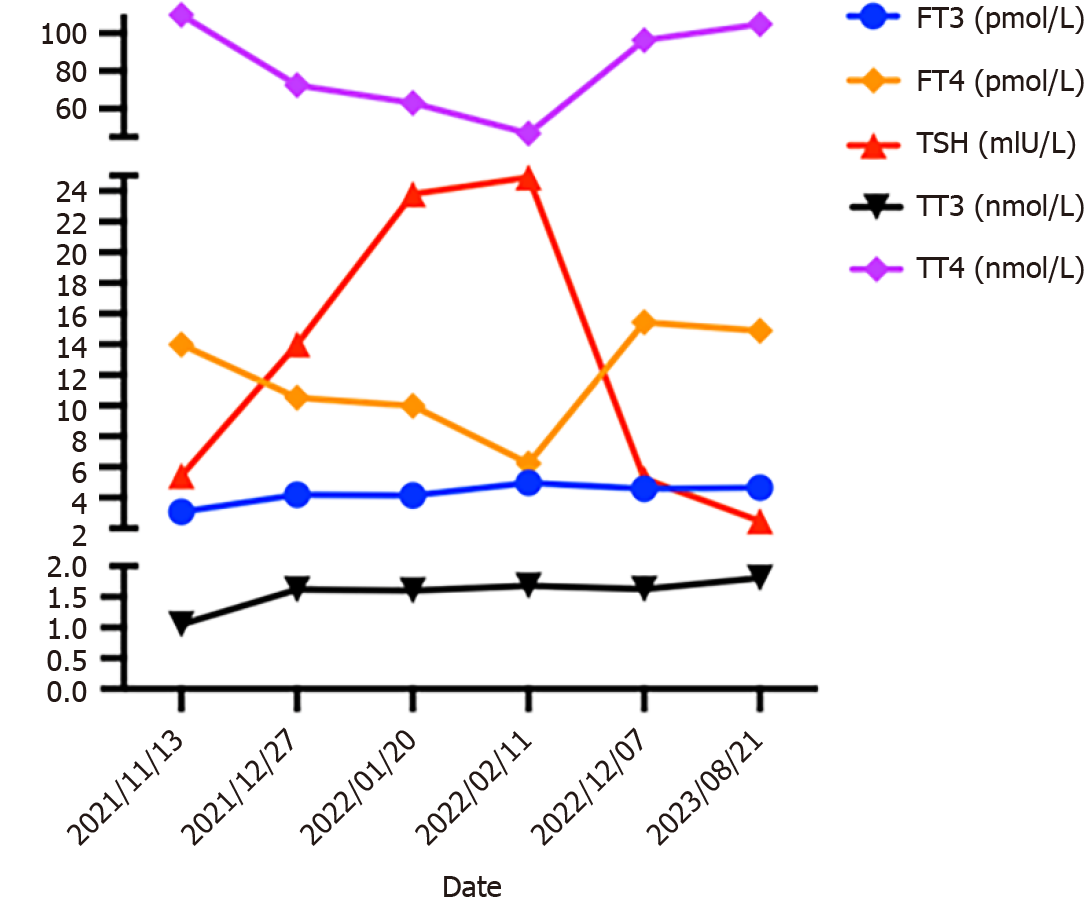
After 3 months, CT three-dimensional CT reconstruction of the liver and volume calculations showed that the tumor size was 14 cm × 9.6 cm × 12 cm (Figure 1C and D); the total liver volume was 1975 cm3; the residual liver tissue was 650 cm3; and the residual liver tissue/total liver volume was 0.3291 (Figure 2). This finding demonstrated the effectiveness of the combined treatment regimen. Unfortunately, the patient presented with mild neutropenia (neutrophil: 1.47 × 109), thrombocytopenia [platelet (PLT): 33 × 109], grade 3 diarrhea, and moderate hypothyroidism (Figure 4) at the point where the immunotherapy had to be discontinued and the oral lenvatinib dose was halved. Surprisingly, after three months of anti-HCV therapy with oral Epclusa, his HCV RNA level decreased to normal, and the drug was discontinued. For neutropenia, human granulocyte colony-stimulating factor was injected as a treatment, and the neutrophil count returned to the normal range after treatment. Recombinant human thrombopoietin as an injection was used to treat the thrombocytopenia, but the PLT was still at a low level after two treatments, which may be related to the patient’s hypersplenism. Furthermore, we used smectite powder and bifico to treat his Grade 3 diarrhea. Euthyrox is one of the most commonly used drugs for the treatment of hypothyroidism and is used for the treatment of patients with subclinical hypoth
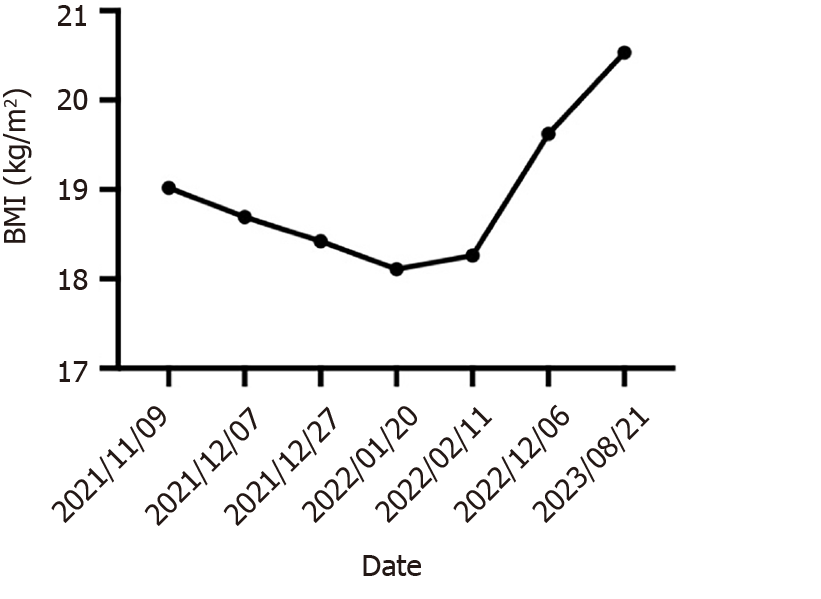
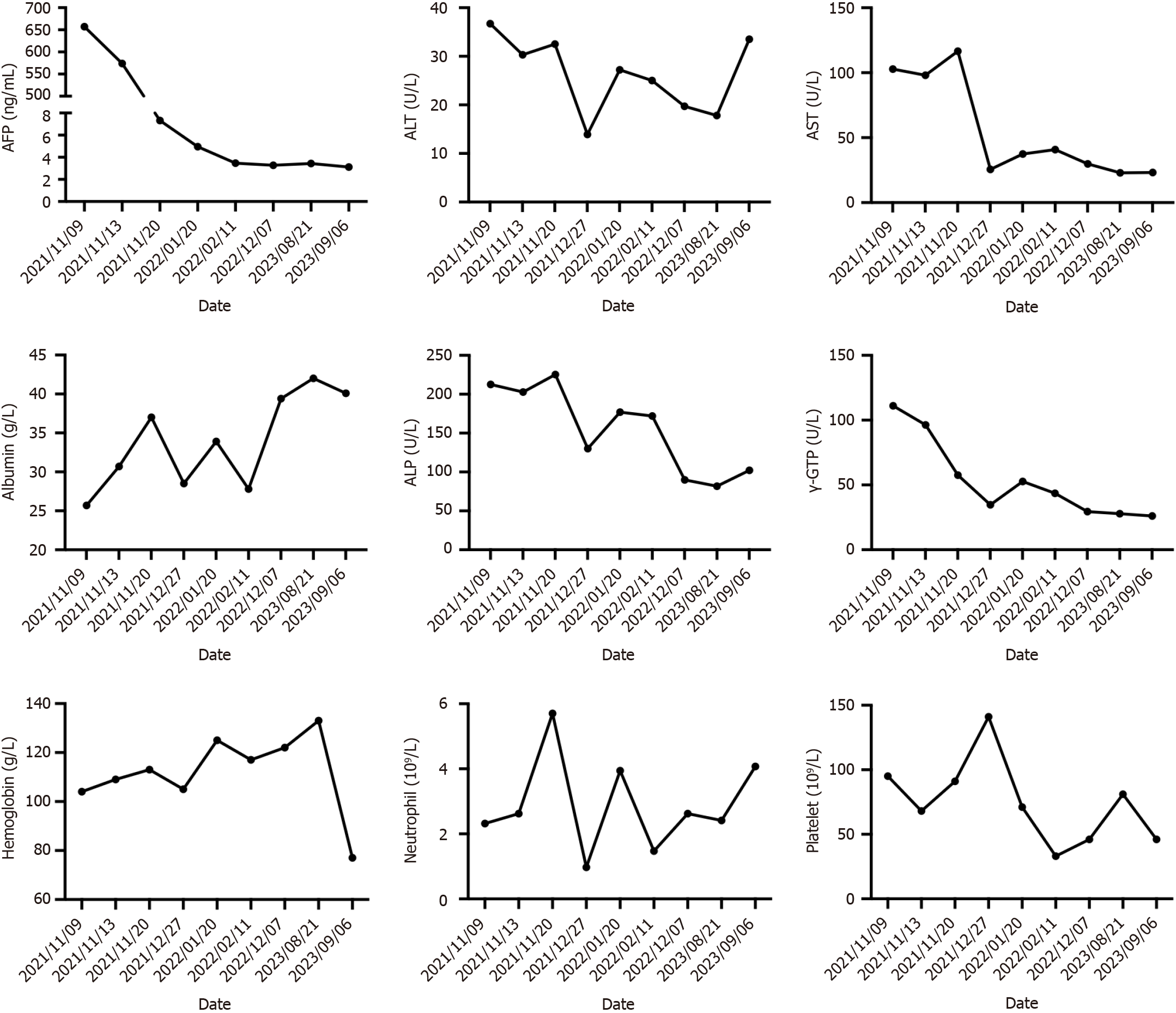
After up to 21 months of treatment, the patient's liver function (Child-Pugh A) and nutritional status were significantly improved, especially his albumin concentration and weight (Figure 5), which reached the highest values during treatment, and liver enhanced magnetic resonance imaging revealed that the tumor volume had decreased to 11.2 cm × 7.3 cm × 9.4 cm (Figure 1E and F). Some of the test indicators obtained during the whole treatment of the patient are shown in Figure 6. The patient underwent partial hepatectomy one month after the end of the discontinuation of oral lenvatinib. The operation time was 236 min, and the bleeding volume was 500 mL. Two units of red blood cells were given intraoperatively.
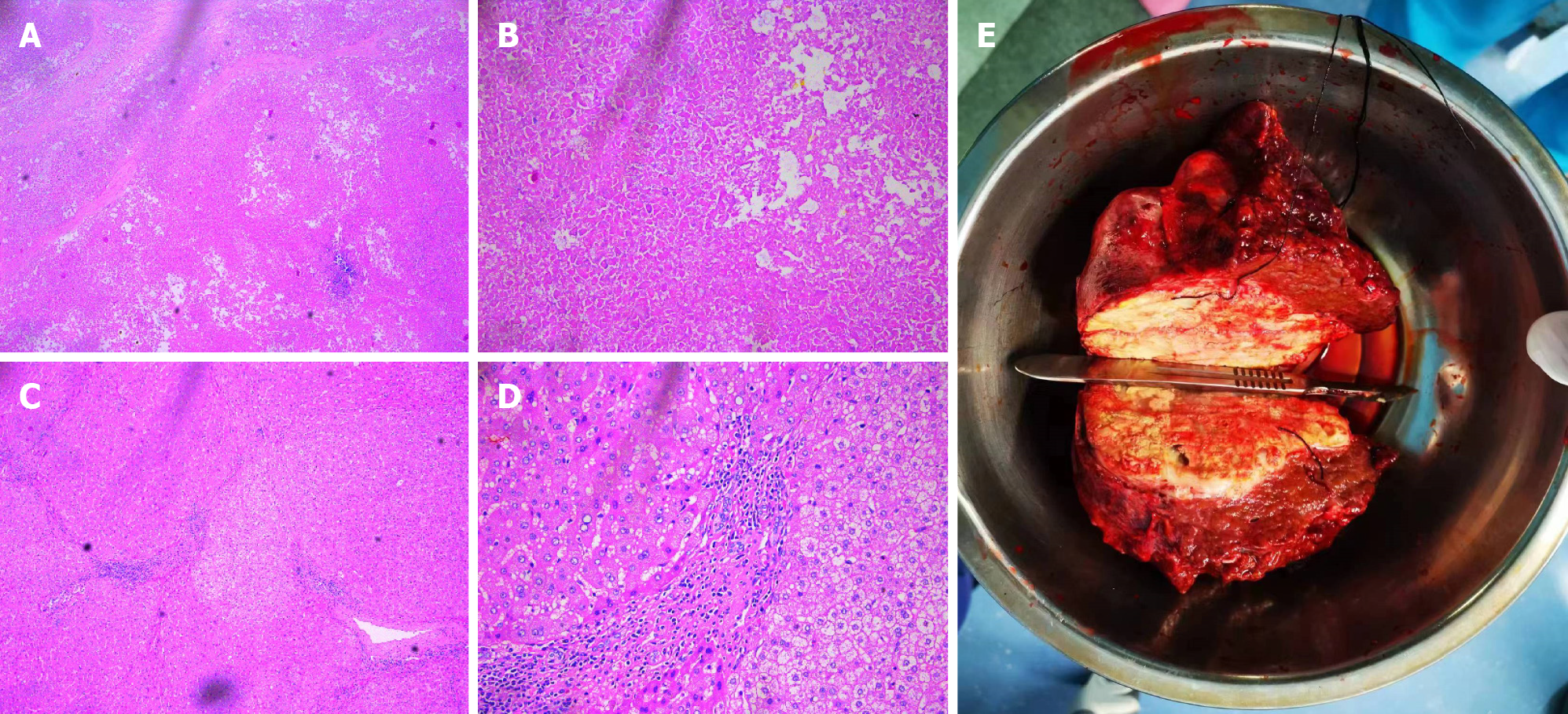
The postoperative pathological diagnosis revealed extensive coagulation necrosis of the tumor tissue because of the combination therapy. The arteries contained a lot of lipiodol deposition. The noncancerous liver tissue exhibited a micronodular pattern, indicating liver cirrhosis. However, no active cancer cells were found upon pathological examination (Figure 7). In other words, the patient achieved a complete pathological response.
The patient was discharged from the hospital on the 9th d after surgery without serious complications. All tumor marker levels were within the normal range. We used a timeline figure to summarize the patient’s clinical characteristics and treatment (Figure 8).
HCV are a group of single-stranded RNA viruses that belong to the Flaviviridae family, and HCV does not integrate with host chromosomes in vivo, as compared with those of hepatitis B virus group (HBV); therefore, the clinical characteristics of HCV-associated HCC and HBV-associated HCC may differ. It has been suggested that patients with HCV-associated HCC are older than patients without HCV-associated HCC[20], have a better prognosis, and have a lower risk of recurrence[21]. A sustained virological response can reduce the incidence of HCC in most patients with HCV. Therefore, the introduction of a direct-acting antiviral agent into HCV therapy makes HCV curable. As a result, for large HCV-associated unresectable HCC patients, the addition of antiviral therapy to conversion therapy may be beneficial.
HCC conversion therapy mainly includes immunotherapy, targeted therapy, interventional therapy, radiation therapy, traditional Chinese medicine therapy, etc. Conversion therapy for advanced liver cancer has also been described in detail by other clinicians (Table 3)[22-30], suggesting the importance of individualized therapy and multidisciplinary management in achieving surgical resection. Wei et al[22] successfully performed R0 hepatectomy after 5 months of conversion therapy in a 67-year-old man who had bulky bilobar HCC with right hepatic artery anatomic variation and achieved partial remission. Ning et al[23] performed two-stage associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for a male patient with large, unresectable HCC with HBV after TACE and immunotherapy, and the postoperative pathological diagnosis suggested a complete response. This result suggested that ALPPS may provide another salvage option for future HCC conversion therapy.
| Ref. | Age | Sex | Underlying liver disease | Tumor size | Symptom | Previous treatment | Pathological response rate |
| Wei et al[22] | 67 | Male | Hepatitis B | 7.2 cm × 7.1 cm × 6.4 cm | Abdominal discomfort | HAIC, TACE, Immunotargeted therapy | PR |
| Ning et al[23] | 48 | Male | Hepatitis B | 16.2 cm × 11.2 cm | Palpable mass | TACE, Immunotargeted therapy | PR |
| Ning et al[23] | 54 | Male | Hepatitis B | 14.9 cm × 11.8 cm | Abdominal pain | TACE, Immunotargeted therapy | CR |
| Zhou et al[24] | 69 | Male | Hepatitis B | 12.7 cm × 10.3 cm | None | HAIC, TAE, Immunotargeted therapy | PR |
| Wu et al[25] | 41 | Male | Hepatitis B | 7.1 cm × 5.5 cm × 5.1 cm | Abdominal pain | Immunotargeted therapy | CR |
| Chen et al[26] | 49 | Male | Hepatitis B | 7.1 cm × 5.5 cm | None | Immunotargeted therapy | PR |
| Chen et al[27] | 52 | Male | Hepatitis B | 11.3 cm × 12.0 cm × 11.9 cm | Abdominal pain | TACE, Targeted therapy | CR |
| Chen et al[27] | 42 | Male | Hepatitis B | 11.4 cm × 8.9 cm × 10.0 cm | None | HAIC, TACE, Targeted therapy | PR |
| Yano et al[28] | 68 | Male | None | 7.2 cm in S1 | None | Immunotargeted therapy | PR |
| Zhang et al[29] | 67 | Male | None | The largest was ≥ 10 cm | Epigastric distention | TACE, antiviral therapy | PR |
| Tomonari et al[30] | 69 | Male | Alcoholic cirrhosis | 7.3 cm | Abdominal pain | TAE, targeted therapy | PR |
| Tomonari et al[30] | 71 | Female | Nonalcoholic steatohepatitis | 5.8 cm in S3 | None | TAE, targeted therapy | PR |
| Tomonari et al[30] | 73 | Male | Alcoholic cirrhosis | 5.2 cm in S5 | None | TAE, targeted therapy | PR |
At present, there have been breakthroughs in the systemic treatment of liver cancer that range from molecular targeted drugs to immune checkpoint inhibitors; however, single-target or immunotherapy effects are limited[31]. Numerous studies have confirmed that combined immunotargeted therapy can improve the treatment and prolong the overall survival of HCC patients, but its efficacy still cannot be used to meet clinical needs[32,33]. To further meet the patient's expectations for a good prognosis of the disease, several scholars have proposed that the combination of immunotargeted therapy and local therapy may be a potential treatment option.
Interventional therapy, including TACE and HAIC, is an effective method for treating liver cancer. TACE involves the use of a mixture of chemical drugs and iodized oil or drug-loaded microspheres to block tumor blood supply vessels, and these microspheres continuously release high concentrations of chemotherapeutic drugs to cause tumor ischemia necrosis and shrinkage[34]. In addition, TACE may improve the immune microenvironment of tumors and stimulate the exposure of neoantigens to enhance the effectiveness of immunotherapy, and the antitumor angiogenesis effect of targeted therapy and immunotherapy may also help reduce the recurrence of tumors caused by tumor angiogenesis after TACE[35]. On the other hand, HAIC involves selective insertion of a catheter into the hepatic artery without embolization or continuous infusion of chemotherapy drugs alone. Notably, HAIC is associated with less severe grade 3-4 adverse reactions than TACE, especially liver function damage, and provides necessary conditions for liver reserve function for subsequent combined treatment of unresectable liver cancer[36]. Recently, clinical experts have actively explored local treatment based on interventional therapy combined with immunotargeted therapy for HCC, which has shown good efficacy[37].
Vascular endothelial growth factor receptor, fibroblast growth factor receptor, and PLT-derived growth factor receptor alpha are the loci of lenvatinib blockade. In 2018, the REFLECT study showed that the overall survival of the patients in the lenvatinib group was not inferior to that of patients in the sorafenib group; thus, lenvatinib became the second first-line drug for unresectable HCC, overcoming the treatment dilemma associated with HCC[38]. Common side effects of lenvatinib include hypertension, hemorrhage, hypothyroidism, and diarrhea[39]. In this patient, Grade 3 diarrhea and moderate hypothyroidism occurred during the use of this drug. Hence, we halved the dose of lenvatinib after 3 months of standard treatment. After the use of this personalized treatment, the patient's treatment tolerance was significantly improved.
PD-1 inhibitors are immune checkpoint inhibitors that can prevent tumor cells from hiding and enhance the body's immune response to tumor cells by blocking the binding of PD-1 and ligands on tumor cells to play an antitumor role. PD-1 inhibitors have shown good antitumor potential in many advanced tumors[40]. The microenvironment of HCC can induce the expression of vascular endothelial growth factor and promote tumor angiogenesis, during which the expression of a variety of tumor inhibitory receptors, including PD-1 and cytotoxic T lymphocyte associated antigen 4, is upregulated[14]. PD-1 expression was found during treatment of patients with middle-stage and advanced HCC with immune checkpoint inhibitors. Theoretically, actively blocking the binding of PD-1 to its ligand can effectively improve the prognosis of patients with middle-stage and advanced HCC. Sintilimab, as one of the most commonly used PD-1 inhibitors, has become a treatment option for patients with advanced HCC and has achieved good results. The side effects of sintilimab are an issue that clinicians cannot ignore. Common side effects include diarrhea, hypothyroidism, thrombocytopenia, and liver and kidney damage[41]. In this case, the patient developed severe diarrhea, hypothyroidism, and thrombocytopenia during standard-dose immunotargeted therapy. In addition to the side effects of immunotherapy, thrombocytopenia may also be related to the hypersplenism caused by liver cirrhosis in patients. After the injection of recombinant human thrombopoietin and oral leucogen, the number of PLT increased at the initial stage of immunotherapy. However, PLT count continued to decrease after 1 month of treatment, and the immunotherapy had to be stopped after comprehensively considering the patient's situation. After stopping the drug treatment, the patient's PLTs gradually recovered.
As the world's first oral anti-HCV drug for treating all 1-6 genotypes of hepatitis C, Epclusa has had excellent clinical performance[16]. It can be used alone in patients without cirrhosis or compensatory cirrhosis or in combination with ribavirin in patients with uncompensatory cirrhosis. Epclusa has achieved a good curative efficacy in all genotypes of patients, and it is expected to eliminate genotyping tests and improve the cure rate of hepatitis C patients[42]. After three months of oral administration of Epclusa, the patient’s HCV RNA test became negative. Therefore, the drug was no longer used in follow-up treatment, and the patient did not experience common adverse effects such as headache, fatigue or nausea during treatment.
In this patient, tumor shrinkage and marked improvement in liver function were observed following immunotherapy, targeted therapy, or anti-HCV therapy combined with interventional therapy. The improvement in liver function may be due to the death of tumor cells and the regeneration of healthy liver cells. When alanine aminotransferase and aspartate aminotransferase levels are elevated during antitumor therapy, we recommend the use of glutathione and polyene phosphatidylcholine to promote liver cell repair and regeneration. Moreover, based on the Response Evaluation Criteria in Solid Tumors, the patient was judged to exhibit complete remission[43].
In summary, multidisciplinary treatments, which include conversion hepatectomy, TACE, HAIC, Epicla and immunotargeted therapy, could be potential treatment options for advanced HCC. For serious adverse drug reactions, individual treatments, such as drug adjustment, should be carried out at the same time as active symptomatic treatment to find a long-term treatment plan suitable for patients. In addition, this paper has several limitations. First, patient indicators should be tested more frequently to determine the most suitable time for surgery. Second, long-term follow-up of patients should be conducted after surgery. Third, due to the poor physical condition of the patient, no needle biopsy of liver tissue was performed before conversion therapy.
This study describes the case of an unresectable advanced HCC patient who underwent complete conversion therapy and surgical resection completely, and the postoperative pathology suggested a complete response. Moreover, the drug dose should be reasonably adjusted according to the patient's body tolerance and adverse reactions to better complete the conversion therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liakina V, Lithuania; Oley MH, Indonesia S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Li C, Qu L, Farragher C, Vella A, Zhou B. MicroRNA Regulated Macrophage Activation in Obesity. J Transl Int Med. 2019;7:46-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Ogawa E, Nomura H, Nakamuta M, Furusyo N, Kajiwara E, Dohmen K, Kawano A, Ooho A, Azuma K, Takahashi K, Satoh T, Koyanagi T, Ichiki Y, Kuniyoshi M, Yanagita K, Amagase H, Morita C, Sugimoto R, Kato M, Shimoda S, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Development of Hepatocellular Carcinoma in Patients Aged 75-84 Years With Chronic Hepatitis C Treated With Direct-Acting Antivirals. J Infect Dis. 2022;226:431-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Zhang H, Xu K, Xiang Q, Zhao L, Tan B, Ju P, Lan X, Liu Y, Zhang J, Fu Z, Li C, Wang J, Song J, Xiao Y, Cheng Z, Wang Y, Zhang S, Xiang T. LPCAT1 functions as a novel prognostic molecular marker in hepatocellular carcinoma. Genes Dis. 2022;9:151-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Qiu L, Zhan K, Malale K, Wu X, Mei Z. Transcriptomic profiling of peroxisome-related genes reveals a novel prognostic signature in hepatocellular carcinoma. Genes Dis. 2022;9:116-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Liu R, Qian MP, Cui YY. Protein kinases: The key contributors in pathogenesis and treatment of nonalcoholic fatty liver disease-derived hepatocellular carcinoma. Metabolism. 2023;147:155665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Chen J, Gao G, Zhang Y, Dai P, Huang Y. Comprehensive analysis and validation of SNX7 as a novel biomarker for the diagnosis, prognosis, and prediction of chemotherapy and immunotherapy response in hepatocellular carcinoma. BMC Cancer. 2023;23:899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Xu Y, Yang Y, Ouyang J, Zhou Y, Li L, Ye F, Yang H, Huang Z, Zhou A, Zhang W, Zhou J, Zhao X, Zhao H. Reclassification of therapeutic response of unresectable hepatocellular carcinoma to anti-angiogenic therapy and immunotherapy using alpha RECIST. Eur Radiol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Takada H, Yamashita K, Osawa L, Komiyama Y, Muraoka M, Suzuki Y, Sato M, Kobayashi S, Yoshida T, Takano S, Maekawa S, Enomoto N. Significance of the autoantibody assay in predicting the development of immune-related adverse events in patients receiving atezolizumab plus bevacizumab combination therapy for unresectable hepatocellular carcinoma. Hepatol Res. 2024;54:162-173. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Peng Y, Tang H, Huang Y, Yuan X, Wang X, Ran Z, Deng W, Liu R, Lan X, Shen H, Zhang J. CT-derived extracellular volume and liver volumetry can predict posthepatectomy liver failure in hepatocellular carcinoma. Insights Imaging. 2023;14:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Wang W, Ye CH, Deng ZF, Wang JL, Zhang L, Bao L, Xu BH, Zhu H, Guo Y, Wen Z. CD4(+)CD25(+) regulatory T cells decreased future liver remnant after associating liver partition and portal vein ligation for staged hepatectomy. World J Gastrointest Surg. 2023;15:917-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 11. | Zhang D, Zhang Y, Luo Y, Qi E, Yu J, Liang P. Perfluoropentane/apatinib-encapsulated metal-organic framework nanoparticles enhanced the microwave ablation of hepatocellular carcinoma. Nanoscale Adv. 2023;5:4892-4900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Ouyang J, Yang Y, Zhou Y, Ye F, Wang Z, Li Q, Xu Y, Li L, Zhao X, Zhang W, Zhou A, Huang Z, Wang Y, Cai J, Zhao H, Zhou J. The MAPS-CRAFITY score: a novel efficacy predictive tool for unresectable hepatocellular carcinoma treated with targeted therapy plus immunotherapy. Hepatol Int. 2023;17:1519-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Limousin W, Laurent-Puig P, Ziol M, Ganne-Carrié N, Nahon P, Ait-Omar A, Seror O, Sidali S, Campani C, Blanc P, Lermine A, Marisa L, Zucman-Rossi J, Nault JC. Molecular-based targeted therapies in patients with hepatocellular carcinoma and hepato-cholangiocarcinoma refractory to atezolizumab/bevacizumab. J Hepatol. 2023;79:1450-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Kamal MA, Badary HA, Omran D, Shousha HI, Abdelaziz AO, El Tayebi HM, Mandour YM. Virtual Screening and Biological Evaluation of Potential PD-1/PD-L1 Immune Checkpoint Inhibitors as Anti-Hepatocellular Carcinoma Agents. ACS Omega. 2023;8:33242-33254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Ning S, Li X, Ma X, Liu J, Chang X. Efficacy of TACE Combined with Lenvatinib Plus Sintilimab for Hepatocellular Carcinoma with Tumor Thrombus in the Inferior Vena Cava and/or Right Atrium. J Hepatocell Carcinoma. 2023;10:1511-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Link JO, Taylor JG, Trejo-Martin A, Kato D, Katana AA, Krygowski ES, Yang ZY, Zipfel S, Cottell JJ, Bacon EM, Tran CV, Yang CY, Wang Y, Wang KW, Zhao G, Cheng G, Tian Y, Gong R, Lee YJ, Yu M, Gorman E, Mogalian E, Perry JK. Discovery of velpatasvir (GS-5816): A potent pan-genotypic HCV NS5A inhibitor in the single-tablet regimens Vosevi(®) and Epclusa(®). Bioorg Med Chem Lett. 2019;29:2415-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H, Huang M. TACE Combined with Lenvatinib and Camrelizumab for Unresectable Multiple Nodular and Large Hepatocellular Carcinoma (>5 cm). Technol Cancer Res Treat. 2023;22:15330338231200320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Pan Y, Yuan Z, Wang J, Ngai S, Hu Z, Sun L, Yang Z, Hu D, Chen M, Zhou Z, Zhang Y. Survival benefit and impact of adjuvant therapies following FOLFOX-HAIC-based conversion therapy with unresectable hepatocellular carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol. 2023;149:14761-14774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Kikugawa C, Uchikawa S, Kawaoka T, Kinami T, Yano S, Amioka K, Naruto K, Ando Y, Yamaoka K, Tsuge M, Kosaka Y, Ohya K, Mori N, Takaki S, Tsuji K, Kouno H, Kohno H, Morio K, Moriya T, Nonaka M, Aisaka Y, Masaki K, Honda Y, Naeshiro N, Hiramatsu A, Aikata H, Oka S. Outcomes of patients with Child-Pugh B and unresectable hepatocellular carcinoma undergoing first-line systemic treatment with sorafenib, lenvatinib, or atezolizumab plus bevacizumab. Oncology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Huo TI, Huang YH, Hsia CY, Su CW, Lin HC, Hsu CY, Lee PC, Lui WY, Loong CC, Chiang JH, Chiou YY, Lee SD. Characteristics and outcome of patients with dual hepatitis B and C-associated hepatocellular carcinoma: are they different from patients with single virus infection? Liver Int. 2009;29:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Katsuta E, Tanaka S, Mogushi K, Matsumura S, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, Tanaka H, Tanabe M, Arii S. Age-related clinicopathologic and molecular features of patients receiving curative hepatectomy for hepatocellular carcinoma. Am J Surg. 2014;208:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Wei YG, Su H, Lv ZL, Liao XW, Zeng ZM, Jia YX, Huang HS, Shen XQ, Zhu GZ, Han CY, Ye XP, Peng T. Case Report: A case of hepatocellular carcinoma with aberrant right hepatic artery treated with transarterial chemoembolization and infusion chemotherapy separately to bilobar lesion combining with systemic therapies and sequential hepatectomy. Front Oncol. 2023;13:1165538. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Ning C, Liu G, Zhang J, Yang X, Xu Y, Zhao H. Case Report: The application of associating liver partition and portal vein ligation for staged hepatectomy in patients with hepatitis b virus-related hepatocellular carcinoma after undergoing treatment with an immune checkpoint inhibitor: a report of two cases. Front Immunol. 2023;14:1159885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Zhou Z, Xu X, Sun M, Liu Y, Liu Q, Chen C, Yin Y. Conversion therapy for massive hepatocellular carcinoma: A case report and literature review. Clin Case Rep. 2023;11:e7533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 25. | Wu Y, Ou S, Liao X, Han C, Yang C, Qin W, Tan Y, Lao Q, Peng T, Ye X. Massive Hepatocellular Carcinoma with Situs Inversus Totalis Achieved a Complete Response Following Camrelizumab Plus Apatinib and Combined with Two-Stage Hepatectomy: A Case Report. Pharmgenomics Pers Med. 2023;16:111-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Chen Z, Chen Z, Fan W, Zou Y, Zhang Y, Shi N, Jin H. Conversion surgery for advanced hepatocellular carcinoma after combination treatment of lenvatinib and camrelizumab: a case report. World J Surg Oncol. 2023;21:29. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Chen K, Luo CP, Ge DX, Wang KL, Luo Q, Li YZ, You XM, Xiang BD, Li LQ, Ma L, Zhong JH. Case report: Conversion therapy to permit resection of initially unresectable hepatocellular carcinoma. Front Oncol. 2022;12:946693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 28. | Yano S, Kawaoka T, Johira Y, Miura R, Kosaka M, Shirane Y, Murakami S, Amioka K, Naruto K, Ando Y, Kosaka Y, Yamaoka K, Kodama K, Uchikawa S, Fujino H, Ohno A, Nakahara T, Murakami E, Okamoto W, Yamauchi M, Imamura M, Mori K, Arihiro K, Kuroda S, Kobayashi T, Ohdan H, Aikata H. Advanced hepatocellular carcinoma with response to lenvatinib after atezolizumab plus bevacizumab. Medicine (Baltimore). 2021;100:e27576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Zhang JJ, Wang ZX, Niu JX, Zhang M, An N, Li PF, Zheng WH. Successful totally laparoscopic right trihepatectomy following conversion therapy for hepatocellular carcinoma: A case report. World J Clin Cases. 2021;9:6469-6477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Tomonari T, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Sogabe M, Okamoto K, Miyamoto H, Muguruma N, Saito Y, Imura S, Bando Y, Shimada M, Takayama T. Conversion therapy for unresectable hepatocellular carcinoma after lenvatinib: Three case reports. Medicine (Baltimore). 2020;99:e22782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Xu Q, Hu H, Mo Z, Chen T, He Q, Xu Z. A multifunctional nanotheranostic agent based on Lenvatinib for multimodal synergistic hepatocellular carcinoma therapy with remarkably enhanced efficacy. J Colloid Interface Sci. 2023;638:375-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 32. | Dong H, Jian Y, Wang M, Liu F, Zhang Q, Peng Z, Cheng N, Zhang W. Hepatic artery intervention combined with immune-targeted therapy is superior to sequential therapy in BCLC-C hepatocellular carcinoma. J Cancer Res Clin Oncol. 2023;149:5405-5416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Nong X, Zhang YM, Liang JC, Xie JL, Zhang ZM. Complete response by patients with advanced hepatocellular carcinoma after combination immune/targeted therapy and transarterial chemoembolization: two case reports and literature review. Transl Cancer Res. 2022;11:2973-2984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Hu Y, Pan T, Cai X, He QS, Zheng YB, Huang MS, Jiang ZB, Chen JW, Wu C. Addition of transarterial chemoembolization improves outcome of tyrosine kinase and immune checkpoint inhibitors regime in patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2023;14:1837-1848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 35. | Guo Y, Wu J, Liang L, Zhu K, Zhou J, Lin L, Chen Y, Cao B, He M, Lian H, Huang W, Cai M. Tyrosine-kinase inhibitor combined with iodine-125 seed brachytherapy for hepatocellular carcinoma refractory to transarterial chemoembolization: a propensity-matched study. Cancer Imaging. 2023;23:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 36. | Yang Z, Fu Y, Wu W, Hu Z, Pan Y, Wang J, Chen J, Hu D, Zhou Z, Chen M, Zhang Y. Comparison of hepatic arterial infusion chemotherapy with mFOLFOX vs. first-line systemic chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma. Front Pharmacol. 2023;14:1234342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 37. | Huang P, Huang X, Zhou Y, Yang G, Sun Q, Shi G, Chen Y. The Efficacy and Safety of Hepatic Arterial Infusion Chemotherapy Based on FOLFIRI for Advanced Intrahepatic Cholangiocarcinoma as Second-Line and Successive Treatment: A Real-World Study. Can J Gastroenterol Hepatol. 2022;2022:9680933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Takeda H, Nishijima N, Nasu A, Komekado H, Kita R, Kimura T, Kudo M, Osaki Y. Long-term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol Res. 2019;49:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | He Y, Lin W, Cai Z, Huang Y, You M, Lei M, Chen R. Cost-effectiveness analysis of transarterial chemoembolization combined with lenvatinib as the first-line treatment for advanced hepatocellular carcinoma. Front Pharmacol. 2023;14:1219694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Qiu Q, Wu C, Tang W, Ji L, Dai G, Gao Y, Chen E, Jiang H, Xie X, Zhang J. Development and validation of a risk-prediction model for immune-related adverse events in patients with non-small-cell lung cancer receiving PD-1/PD-L1 inhibitors. J Zhejiang Univ Sci B. 2023;24:935-942. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Sun C, Wang Q, Hou L, Zhang R, Chen Y, Niu L. A contrast-enhanced ultrasound-based nomogram for the prediction of therapeutic efficiency of anti-PD-1 plus anti-VEGF agents in advanced hepatocellular carcinoma patients. Front Immunol. 2023;14:1229560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Glombicki SE, Cohen PR. Sofosbuvir-Velpatasvir (Epclusa)-Associated Photosensitivity in a Hepatitis C Patient: Case Report and Review of Photosensitivity to Hepatitis C Antiviral Agents. Cureus. 2021;13:e16496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 43. | Zhao SM, Qiu LW, Zhao H, Gu WW, Yang XH, Gu ZX, Shi RF, Ni CF. Prognostic nomogram for hepatocellular carcinoma patients after transarterial chemoembolization based on des-γ-carboxy prothrombin reactivity and modified Response Evaluation Criteria in Solid Tumors. J Cancer Res Ther. 2021;17:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |