Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1613
Peer-review started: September 21, 2023
First decision: December 19, 2023
Revised: December 27, 2023
Accepted: February 22, 2024
Article in press: February 22, 2024
Published online: April 15, 2024
Processing time: 202 Days and 16.9 Hours
The combination of programmed cell death protein-1 (PD-1) inhibitor and che
To assess the comparative effectiveness and tolerability of combining PD-1 inhibitors with chemotherapy vs chemotherapy alone in patients with advanced gastric cancer, gastroesophageal junction (GEJ) cancer, or oesophageal carcinoma.
We searched the PubMed and Embase databases for studies that compared the efficacy and tolerance of PD-1 inhibitors in combination with chemotherapy vs chemotherapy alone in patients with advanced oesophageal or gastric cancer. We employed either random or fixed models to analyze the outcomes of each clinical trial, en
Nine phase 3 clinical trials (7016 advanced oesophageal and gastric cancer patients) met the inclusion criteria. Our meta-analysis demonstrated that the pooled PD-1 inhibitor + chemotherapy group had a significantly longer OS than the chemotherapy-alone group [hazard ratio (HR) = 0.76, 95% confidence interval (CI): 0.71-0.81]; the pooled PFS result was consistent with that of OS (HR = 0.67, 95%CI: 0.61-0.74). The count of patients achieving an objective response in the PD-1 inhibitor + chemotherapy group surpassed that of the chemotherapy-alone group [odds ratio (OR) = 1.86, 95%CI: 1.59-2.18]. AE incidence was also higher in the combination-therapy group than in the chemotherapy-alone group, regardless of whether ≥ grade 3 only (OR = 1.30, 95%CI: 1.07-1.57) or all AE grades (OR = 1.88, 95%CI: 1.39-2.54) were examined. We performed a subgroup analysis based on the programmed death-ligand 1 (PD-L1) combined positive score (CPS) and noted extended OS and PFS durations within the CPS ≥ 1, CPS ≥ 5, and CPS ≥ 10 subgroups of the PD-1 inhibitor + chemotherapy group.
In contrast to chemotherapy alone, the combination of PD-1 inhibitor and chemotherapy appears to present a more favorable option for initial or subsequent treatment in patients with gastric cancer, GEJ tumor, or oesophageal cancer. This holds true particularly for individuals with PD-L1 CPS scores of ≥ 5 and ≥ 10.
Core Tip: The combination of programmed cell death protein-1 (PD-1) inhibitor and chemotherapy is approved as a standard first- or second-line treatment in patients with advanced oesophageal or gastric cancer. However, it is unclear whether this combination is superior to chemotherapy alone. We assessed the comparative effectiveness and tolerability of combining PD-1 inhibitors with chemotherapy vs chemotherapy alone in patients with advanced gastric cancer, gastroesophageal junction cancer, or oesophageal carcinoma. Our analysis showed that immunotherapy combined with chemotherapy significantly prolonged patients’ overall survival and progression-free survival relative to the chemotherapy group, both in the overall population and in the combined positive score (CPS) ≥ 1, CPS ≥ 5, and CPS ≥ 10 subgroup.
- Citation: Zhang XM, Yang T, Xu YY, Li BZ, Shen W, Hu WQ, Yan CW, Zong L. Effectiveness and tolerability of programmed cell death protein-1 inhibitor + chemotherapy compared to chemotherapy for upper gastrointestinal tract cancers. World J Gastrointest Oncol 2024; 16(4): 1613-1625
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1613.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1613
Upper gastrointestinal tract (UGT) cancers stand as the prevailing form of malignancy, encompassing oesophageal squamous cell carcinoma (ESCC) and gastric/gastroesophageal junction (G/GEJ) adenocarcinomas[1]. According to Global Cancer Statistics 2020, an estimated 1089103 and 604100 new cases of gastric and oesophageal cancers, respectively, occur each year, and the annual numbers of new deaths are 768793 and 544076[2]. The presently employed methods for treating UGT cancers are surgery, chemotherapy, and radiotherapy. Even with continuous enhancements in chemoradiotherapy, the clinical results for ESCC and G/GEJ adenocarcinoma have not exhibited substantial advan
Immunotherapy is an evolving approach to cancer treatment and is designed to destroy cancer cells by strengthening patients’ immune capabilities. Various immunotherapies encompass the utilization of immune checkpoint inhibitor (ICIs), chimeric antigen receptor T-cell therapy, cancer vaccines, and adoptive cell therapy[4]. Programmed cell death protein-1 (PD-1) functions as a checkpoint receptor expressed on the surface of diverse immune cells. Blocking the interaction between PD-1 and programmed death-ligand 1 (PD-L1) serves as an effective means to bolster the immune response[5]. Anti-PD-1 antibodies such as nivolumab, pembrolizumab, sintilimab, camrelizumab, and tislelizumab demonstrated improved antitumor activity alongside manageable safety profiles in patients with oesophageal or gastric cancer[6].
Combinations of a PD-1 inhibitor and chemotherapy have gained approval as standard first or second-line treatment choices for patients with advanced oesophageal and gastric cancer in clinical settings[7]. While various studies have outlined the advantages of pairing PD-1 inhibitor therapy with chemotherapy, it remains undetermined whether the combination of a PD-1 inhibitor + chemotherapy surpasses the efficacy of chemotherapy alone. Tabernero et al[8] revealed that the combination of pembrolizumab with chemotherapy did not exhibit superiority over chemotherapy alone in terms of the tested overall survival (OS) and progression-free survival (PFS) endpoints. In a separate study involving patients with gastric or GEJ cancer, the addition of nivolumab to chemotherapy did not lead to a significant improvement in OS compared to the group treated with chemotherapy alone[9]. Therefore, it remains essential to ascertain whether chemo
To identify relevant studies, we performed a comprehensive literature search of the electronic databases PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Embase (embase.com/). Filter articles published before July 2023. We used search terms pertaining to diseases (e.g., GEJ adenocarcinoma, gastric, gastric cancer, gastro-oesophageal junction cancer, oesophageal squamous cell carcinoma, oesophageal cancer, oesophageal adenocarcinoma, oesophageal squamous cell carcinoma) or the therapies established for those diseases (e.g., chemotherapy, nivolumab, pembrolizumab, camrelizumab, sintilimab, tislelizumab). We also set up filters for clinical trials. The search results from each database were combined in Endnote X9, and duplicates were removed. For the rest of the literature, we evaluate whether it is qualified by reading the full text. In addition, we also searched the relevant literature manually to find other potentially relevant articles. The literature selection process was completed independently by two authors (Zhang XM and Yang T).
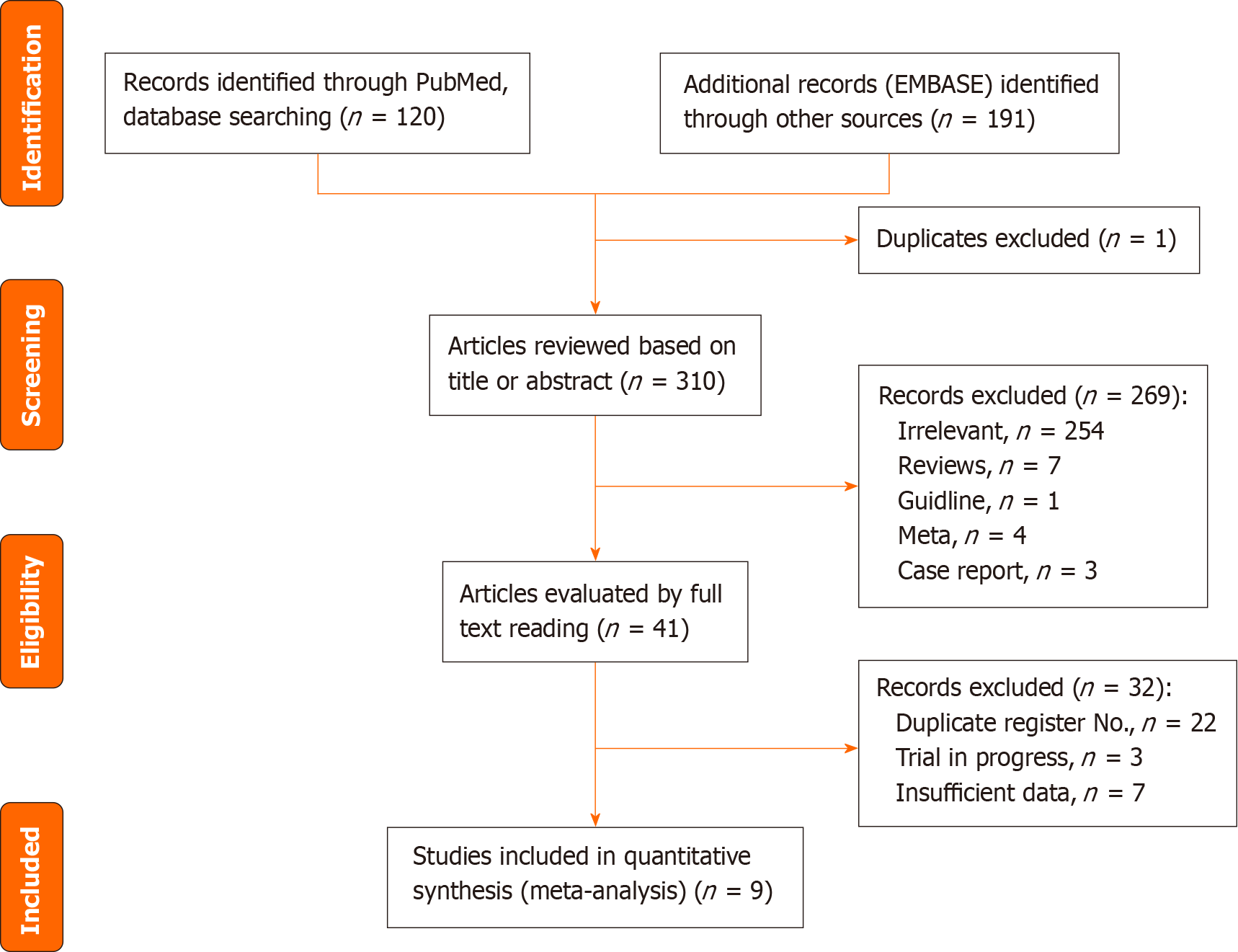
Eligible studies were selected following inclusion and exclusion criteria (Figure 1). A study was considered eligible for inclusion in the present meta-analysis if it met the following inclusion criteria: (1) The study was limited to oesophageal squamous cell carcinoma and/or G/GEJ adenocarcinoma; (2) The study was a phase III clinical trial; (3) The study included a sample of more than 500 patients; (4) The study encompassed at least one group receiving a combination of PD-1 inhibitor and chemotherapy, alongside one group receiving chemotherapy alone; (5) The study’s basic data (e.g., trial year, sample size, patient sex distribution, tumor types, trial abbreviation, registry number) were complete. Additionally, it was essential for the study to acquire the hazard ratio (HR) along with its corresponding 95% confidence interval (CI) for OS and PFS, as well as comparable data for objective response rate (ORR) and adverse events (AEs); and (6) The study was reported in English.
The exclusion criteria were as follows: (1) The literature type was meta-analysis, review or letter; (2) Lack of HR values and 95%CIs for OS and PFS comparisons between the two groups, or the number of patients who achieved ORRs and the number of AEs between the two groups were not reported; (3) The study was a non-randomized clinical trial; (4) The study concerned the results of radiotherapy; (5) The study included only results from special patient populations, such as elderly patients; and (6) Research lacking sufficient published data suitable for analysis.
Data were extracted independently by two of the authors (Zhang XM and Yang T). The extraction process encompassed the following items in accordance with the inclusion criteria: (1) General characteristics of the studies: Name of the first author, publication year, trial abbreviation, and registry number; (2) The patients’ baseline characteristics: Age, sex, sample size, tumor type, intervention arm, chemotherapy regimens, and PD-L1 combined positive scores (CPSs); and (3) Outcomes: We extracted the HRs and their 95%CIs for each study’s OS, PFS, the number of patients who achieved an objective response, all AEs, grade > 3 AEs, and the counts of patients with a PD-L1 CPS of ≥ 5 or ≥ 10.
We evaluated the potential bias in each study using Cochrane Collaboration’s tool (Review Manager 5.3). The primary criteria for this assessment included random sequence generation, allocation concealment, participant blinding, outcome assessment blinding, incomplete outcome data, and selective reporting, as well as other biases. This assessment assigned grades according to levels of risk, i.e., high, low, or unclear risk.
Stata ver. 12.0 software was used to perform all statistical tests. For the quantitative aggregation of the survival outcomes, the HRs and their 95%CIs were combined as the effective value. The significance of the pooled OR or HR was assessed using the Z-test, where P-values < 0.05 were deemed statistically significant. To evaluate the global heterogeneity between studies, we used the χ2-based Q-test (P > 0.10 was considered a lack of heterogeneity) and the I2 test (I2 ≤ 50% indicated low heterogeneity, while I2 > 50% indicated high heterogeneity). We employed the fixed-effects model if the heterogeneity was low; otherwise, we used the random-effects model. Funnel plots were generated to assess the presence of publication bias in the outcomes. An asymmetrical plot indicated possible publication bias. Review of the funnel plots could not rule out the potential for publication bias for the efficacy of PD-1 inhibitors in the treatment of UGT cancers. Further, asymmetry in the funnel plot was evaluated through Egger’s test and Begg’s test, with P-values < 0.05 indicating significance. If publication bias existed, we assessed the effect of publication bias by using the trim-fill method. Additionally, sensitivity analyses were conducted to assess the stability of the results. The statistical methods of this study were reviewed by Guo-Lin Han from Medical records statistics room of Changzhi People’s Hospital.
A total of 310 articles were retrieved from the PubMed and Embase databases; one duplicate paper was removed, leaving 309 articles remaining. We initially screened the articles according to their titles and abstracts. As a result, we excluded 254 articles as irrelevant and 15 as either meta-analyses, case reports, guidelines, or reviews. We then screened the remaining 41 articles by reading them in full, resulting in the exclusion of 22 articles with the same trial registration number, 3 incomplete clinical trials, and 7 articles lacking the OS, PFS, ORR, and/or AE data required by this study. The final total was 9 articles[10-18]. Figure 1 is a flow diagram illustrating the inclusion and exclusion of articles.
All 9 of the articles included in this meta-analysis were reports of phase 3 clinical trials. Among them, 8 were multicenter studies and the remainder was a single-center study. The 9 articles were published between 2020 and 2022 and included a total of 7016 patients, with sample sizes ranging from 596 to 1581. Three articles compared nivolumab in combination with chemotherapy vs chemotherapy alone, while two articles compared pembrolizumab + chemotherapy vs chemo
| Ref. | Treatment stage | Sample size | Male/female | Median age (IQR) | Trial Abbr. | Register No. | Tumor type | Intervention arm | Control arm | CPS subgroup | Reported outcomes |
| Janjigian et al[12] | Phase 3 | 1581 (789/792) | 1100/481 | 62 (54-69) vs 61 (53-68) | CheckMate 649 | NCT02872116 | G/GEJ/oesophageal adenocarcinoma | Nivolumab + XELOX (capecitabine and oxaliplatin) or FOLFOX (leucovorin, fluorouracil, and oxaliplatin) | XELOX (capecitabine and oxaliplatin) or FOLFOX (leucovorin, fluorouracil, and oxaliplatin) | ≥ 5, ≥ 1 | OS, PFS, ORR |
| Kang et al[11] | Phase 3 | 724 (362/362) | 523/201 | 63.5 vs 65 | ATTRACTION-4 | NCT02746796 | G/GEJ | Nivolumab plus oxaliplatin and capecitabine/s-1 | Placebo plus oxaliplatin and capecitabine/s-1 | NA | OS, PFS |
| Doki et al[18] | Phase 3 | 645 (321/324) | 528/117 | 64 (40-90) vs 64 (26-81) | CheckMate 648 | NCT03143153 | Advanced esophageal squamous-cell carcinoma | Nivolumab + chemotherapy (fluorouracil + cisplatin) | Chemotherapy | NA | OS, PFS, ORR |
| Shitara et al[10] | Phase 3 | 763 (257/250) | 257/250 | 62 (22-83) vs 62.5 (23-87) | KEYNOTE-062 | NCT02494583 | G/GEJ | Pembrolizumab + cisplatin + fluorouracil or capecitabine | Cisplatin + fluorouracil or capecitabine | ≥ 1, ≥ 10 | OS, PFS, ORR |
| Sun et al[16] | Phase 3 | 749 (373/376) | 625/124 | 64 (28-94) vs 62 (27-89) | KEYNOTE-590 | NCT03189719 | Advanced oesophageal cancer | Pembrolizuma + chemotherapy (5-fluorouracil plus cisplatin) | Placebo + chemotherapy (5-fluorouracil plus cisplatin) | CPS < 10, CPS ≥ 10 | OS, PFS, ORR |
| Xu et al[15] | Phase 3 | 650 (327/323) | NA | NA | ORIENT-16 | NCT03745170 | Advanced G/GEJ adenocarcinoma | Sintilimab combined with chemotherapy (CapeOX: oxaliplatin + capecitabine) | Placebo and chemotherapy (CapeOX: oxaliplatin + capecitabine) | CPS ≥ 5 | OS, PFS, ORR |
| Lu et al[14] | Phase 3 | 659 (327/332) | 567/92 | 63 (57-67) vs 63 (56-67) | ORIENT-15 | NCT03748134 | Oesophageal squamous cell carcinoma | Sintilimab combined with chemotherapy (cisplatin + paclitaxel or cisplatin + 5-fluorouracil) | Placebo and chemotherapy (cisplatin + paclitaxel or cisplatin + 5-fluorouracil) | CPS < 10, CPS ≥ 10, CPS < 5, CPS ≥ 5, CPS < 1, CPS ≥ 1 | OS, PFS, ORR |
| Luo et al[13] | Phase 3 | 596 (298/298) | 523/73 | 62 (56-66) vs 62 (56-67) | ESCORT | NCT03691090 | Esophageal squamous cell carcinoma | Camrelizumab combined with paclitaxel and cisplatin | Placebo and paclitaxel and cisplatin | TPS | OS, PFS, ORR |
| Xu et al[17] | Phase 3 | 649 (326/323) | 563/86 | 64 (59-68) vs 65 (58-70) | RATIONALE-306 | NCT03783442 | Esophageal squamous-cell carcinoma | Tislelizumab + chemotherapy [a platinum agent (cisplatin or oxaliplatin) + a fluoropyrimidine (fluorouracil or capecitabine) or paclitaxel] | Chemotherapy [a platinum agent (cisplatin or oxaliplatin) + a fluoropyrimidine (fluorouracil or capecitabine) or paclitaxel] | TAP | OS, PFS, ORR |
Next, we evaluated the risk of bias for each study, and the outcomes are illustrated in Figure 2. One of the sources of bias originated from the absence of blinding, which notably impacted the study quality. The precision of results in another study could have been influenced due to participant withdrawals or losses. Additional biases could have arisen from factors such as an uneven geographic distribution of participants, poor or incomplete patient compliance, or other underlying reasons.
All 9 of the clinical trials provided HR and 95%CI values for the OS and PFS outcomes of patients treated with a PD-1 inhibitor + chemotherapy compared to chemotherapy alone. We pooled the HR values from the 9 clinical trials using randomized- or fixed-effects models, and the results showed that the pooled PD-1 inhibitor + chemotherapy group had longer OS than the pooled chemotherapy-alone group (HR = 0.76, 95%CI: 0.71-0.81). Figure 3A shows the combined forest plot of the OS data. The combined outcome for patients’ PFS corresponded with that of their OS (HR = 0.67, 95%CI: 0.61-0.74), as depicted in Figure 3B.
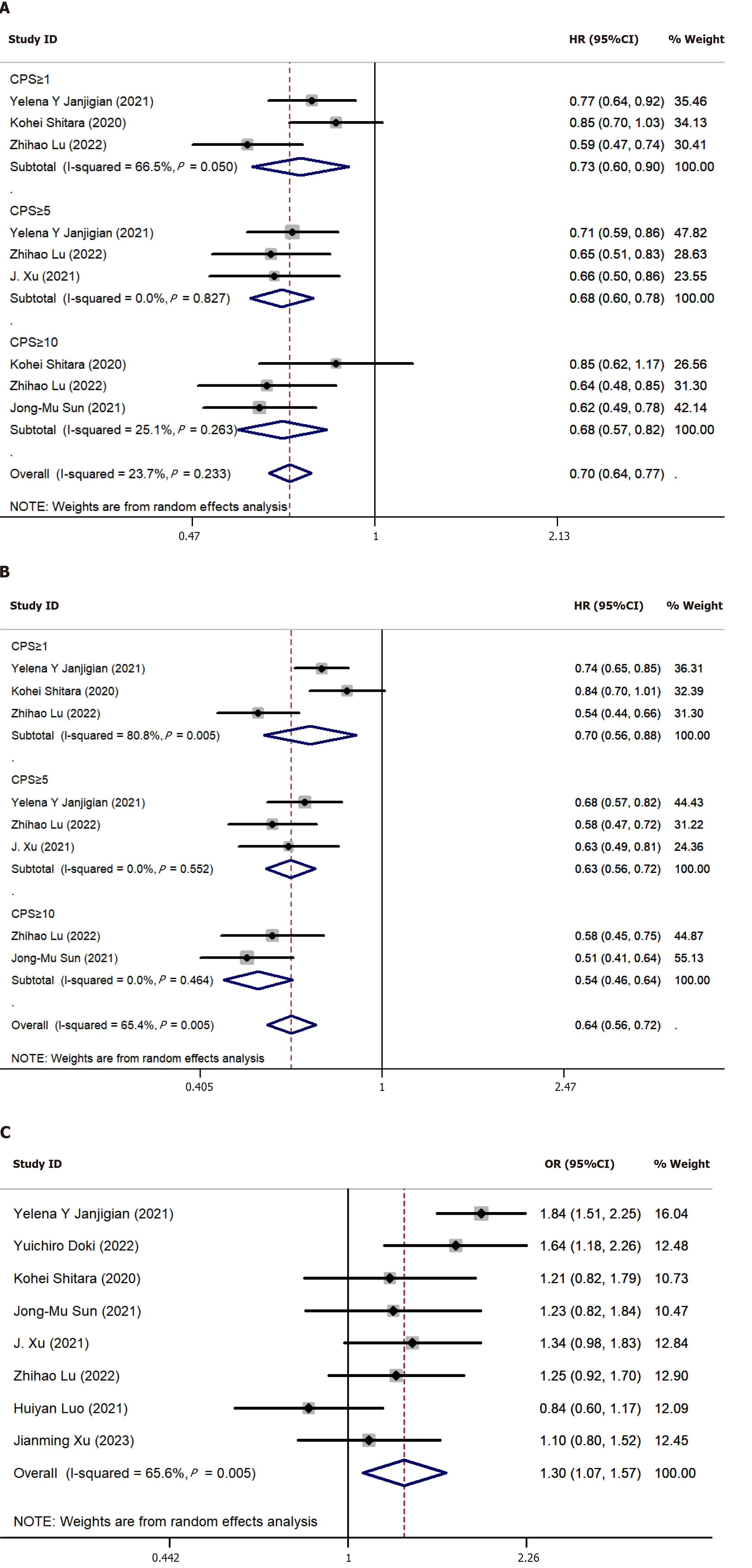
Of the 9 studies, 8 provided ORR data, and we gathered the count of patients who obtained an objective response in each study. The findings suggested that the count of individuals achieving an objective response in the PD-1 inhibitor + chemotherapy group exceeded that in the chemotherapy-alone group (OR = 1.86, 95%CI: 1.59-2.18) (Figure 3C). Regarding the AEs observed in the studies, we aggregated both the AEs of all grades and those rated ≥ grade 3. As shown in Figure 3D, the meta-analysis of AEs indicated that the frequency of AEs in the PD-1 inhibitor + chemotherapy group exceeded that in the chemotherapy-alone group. This held true for AEs of all grades (OR = 1.88, 95%CI: 1.39-2.54) as well as for AEs rated ≥ grade 3 (OR = 1.30, 95%CI: 1.07-1.57) in Figure 4C.
We employed the patients’ PD-L1 CPS scores as a measure to assess the efficacy of immunotherapy against tumors. Of the 9 clinical trials, 5 reported treatment outcomes in different PD-L1 CPS subgroups. Consequently, we conducted a subgroup analysis of OS and PFS based on the patients’ PD-L1 CPS scores. The subgroup analysis of OS (Figure 4A) indicated that patients treated with a PD-1 inhibitor + chemotherapy exhibited prolonged OS compared to those treated with chemotherapy alone, irrespective of the subgroup CPS ≥ 1 (HR = 0.73, 95%CI: 0.60-0.90, P = 0.002), CPS ≥ 5 (HR = 0.68, 95%CI: 0.60-0.78, P < 0.001), or CPS ≥ 10 (HR = 0.68, 95%CI: 0.57-0.82, P < 0.001). The outcomes of our subgroup analysis for PFS (Figure 4B) aligned with those for OS across all three groups: CPS ≥ 1 (HR = 0.70, 95%CI: 0.56-0.88, P = 0.002), CPS ≥ 5 (HR = 0.63, 95%CI: 0.56-0.72, P < 0.001), and CPS ≥ 10 (HR = 0.54, 95%CI: 0.46-0.64, P < 0.001).
To minimize the effects of publication bias, we expanded database searches and performed a thorough search for unpublished studies, and used funnel plot to quantify the potential presence of publication bias. We utilized funnel plots of the OS and PFS data to evaluate potential publication bias (Supplementary Figure 1). The outcomes of the Begg’s test (OS: P = 0.25; PFS: P = 0.532) and Egger’s test (OS: P = 0.336; PFS: P = 0.512) for both the OS and PFS funnel plots indicated the absence of substantial publication bias in this meta-analysis.
The Stata sensitivity analysis (Supplementary Figure 2) revealed that the exclusion of any of the 9 articles had a minimal impact on the overall effect size. This suggested that the sensitivity of this meta-analysis was low and that the removal of a single article did not significantly alter the results. In order to further explore the source of heterogeneity of OS and PFS, we used meta-regression to analyze whether the year of publication, sample size, and tumor type were the source of heterogeneity of OS and PFS. The results of meta-regression showed that the year of publication, sample size, and tumor type were not sources of heterogeneity in OS (year of publication: P = 0.167; sample size: P = 0.233; tumor type: P = 0.247) and PFS (year of publication: P = 0.574; sample size: P = 0.609; tumor type: P = 0.914).
The combination of ICIs with chemotherapy has emerged as a potent targeted therapy for advanced or metastatic gas
Several clinical trials of PD-1 inhibitors in combination with chemotherapy vs chemotherapy alone have been complet
The ORR is the proportion of patients who respond to treatment, which is a key measure of a drug therapy’s effectiveness. The results of our meta-analysis demonstrated that the PD-1 inhibitor + chemotherapy group had superior ORR to that of the chemotherapy-alone group. We also conducted an analysis of the incidence of AEs between the two treatment approaches. This revealed a heightened occurrence of AEs in patients receiving immunotherapy combined with chemotherapy compared to those undergoing chemotherapy alone. This trend was consistent whether we examined AEs of all grades (OR = 1.88, 95%CI: 1.39-2.54) or evaluated AEs rated ≥ grade 3 (HR = 1.30, 95%CI: 1.07-1.57). The AEs of immunotherapy are referred to as immune-related (ir)AEs. These irAEs have been documented to be generally mild and manageable, often not necessitating specific interventions. More importantly, they are noted to have minimal impact on treatment efficacy[20,21].
PD-L1 expression levels are frequently employed as predictive biomarkers to ascertain whether patients are likely to derive benefits from immunotherapy. The CPS score has been indicated to be a more effective method of assessing PD-L1 expression than the tumor proportion score (TPS)[22]. In the present meta-analysis, we therefore conducted subgroup analyses of OS and PFS based on the CPS scores for PD-L1. The outcomes demonstrated that within the subgroup with a CPS ≥ 5 or CPS ≥ 10, patients in the immunotherapy + chemotherapy group exhibited longer OS periods. The PFS subgroup analysis revealed that the patients in the subgroup with a CPS ≥ 10 were more likely to benefit from immunotherapy + chemotherapy. Based on the results of our analysis, PD-1 inhibitor + chemotherapy can be recommended as a suitable first-line treatment for patients with PD-L1 CPS scores of ≥ 5 or ≥ 10. This study has two limitations. First, not all of the included articles provided survival data for CPS subgroups, opening the possibility of bias in our CPS subgroup analysis. Second, the limited number of articles focused on the same tumor type precluded the possibility of conducting a subgroup analysis based on tumor type.
The outcomes of this meta-analysis illustrated that patients receiving a PD-1 inhibitor + chemotherapy experienced notably prolonged OS and PFS compared to patients treated with chemotherapy alone. The ORR and the rate of AEs were both higher in the group treated with a PD-1 inhibitor + chemotherapy than in the chemotherapy-alone group. For the subgroups with a CPS ≥ 5 or CPS ≥ 10, the patients in the immunotherapy + chemotherapy group had longer OS and PFS. Considering both efficacy and safety, PD-1 inhibitor + chemotherapy could potentially emerge as a preferable first-line treatment option for patients diagnosed with gastric cancer, GEJ tumor, or esophageal cancer, particularly for those with a PD-L1 CPS score of ≥ 5 or ≥ 10.
Programmed cell death protein-1 (PD-1) inhibitor has shown effective anti-tumor immune activity by blocking the interaction of PD-1 with programmed death ligand 1 (PD-L1), and it combined with chemotherapy has been approved as a standard first- or second-line treatment option for patients with gastric cancer, gastroesophageal junction (GEJ) cancer and advanced esophageal cancer.
Several clinical trials of PD-1 inhibitors combined with chemotherapy vs chemotherapy alone have been completed or are ongoing. However, in terms of efficacy, it is controversial whether PD-1 inhibitor plus chemotherapy can significantly prolong patients’ overall survival (OS) and progression-free survival (PFS) compared to chemotherapy alone.
To compare the efficacy and safety of PD-1 inhibitor combined with chemotherapy and chemotherapy alone, and provide treatment options for patients with advanced gastric cancer, GEJ cancer and advanced esophageal cancer.
We searched PubMed and Embase databases for clinical trials comparing the efficacy and safety of PD-1 inhibitors combined with chemotherapy and chemotherapy alone in gastric cancer, GEJ tumors, and esophageal cancer. The effect value of OS, PFS, objective response rate and adverse events were combined using random or fixed effects models. The significance of the pooled odds ratio or hazard ratio was assessed using the Z-test. We used the χ2-based Q-test and the I2 test to evaluate the heterogeneity between studies. Funnel plots were generated to assess the presence of publication bias in the outcomes. Additionally, sensitivity analyses were conducted to assess the stability of the results.
A total of 9 clinical trials were included in this study. Compared with the chemotherapy group, the PD-1 inhibitor + chemotherapy group had longer OS and PFS, and more patients achieved objective response rates. In addition, the number of adverse reactions in the combined treatment group was higher than that in the chemotherapy group alone. The results of subgroup analysis showed that compared with the subgroup of combined positive score (CPS) ≥ 1, patients in the CPS ≥ 5 and CPS ≥ 10 subgroups were able to achieve better therapeutic outcomes with PD-1 inhibitor combined with chemotherapy.
The efficacy of PD-1 inhibitor combined with chemotherapy was superior to the chemotherapy group alone in patients with gastric cancer, GEJ tumors, and esophageal cancer. Subgroups with PD-L1 CPS ≥ 5 and ≥ 10 were more likely to benefit from PD-1 inhibitor combined with chemotherapy.
Our findings provide reference for the treatment of patients with advanced gastric cancer, GEJ tumors, and advanced esophageal cancer. Therefore, our next step is to conduct a randomized controlled trial of PD-1 inhibitors combined with chemotherapy for gastric cancer and GEJ tumors to further improve the management of these patients.
We would like to thank Guo-Lin Han for statistical contributions to this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ebrahimifar M, Iran; Keikha M, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Zhao YQ
| 1. | Qiu HB. Safety and efficacy of tislelizumab plus chemotherapy for first-line treatment of advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Thorac Cancer. 2020;11:3419-3421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64565] [Article Influence: 16141.3] [Reference Citation Analysis (176)] |
| 3. | Chen M, Li C, Sun M, Li Y, Sun X. Recent developments in PD-1/PD-L1 blockade research for gastroesophageal malignancies. Front Immunol. 2022;13:1043517. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Jin X, Liu Z, Yang D, Yin K, Chang X. Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front Immunol. 2022;13:948647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (17)] |
| 5. | Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L, Wang S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 291] [Reference Citation Analysis (0)] |
| 6. | Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 7. | Yuan H, Lu S, Shi M, Yang Z, Liu W, Ni Z, Yao X, Hua Z, Feng R, Zheng Y, Wang Z, Sah BK, Chen M, Zhu Z, He C, Li C, Zhang J, Yan C, Yan M. Sintilimab combined neoadjuvant intraperitoneal and systemic chemotherapy in gastric cancer with peritoneal metastasis. Future Oncol. 2023;19:2517-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Tabernero J, Shen L, Elimova E, Ku G, Liu T, Shitara K, Lin X, Boyken L, Li H, Grim J, Ajani J. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022;18:3255-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Shah MA, Kennedy EB, Alarcon-Rozas AE, Alcindor T, Bartley AN, Malowany AB, Bhadkamkar NA, Deighton DC, Janjigian Y, Karippot A, Khan U, King DA, Klute K, Lacy J, Lee JJ, Mehta R, Mukherjee S, Nagarajan A, Park H, Saeed A, Semrad TJ, Shitara K, Smyth E, Uboha NV, Vincelli M, Wainberg Z, Rajdev L. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:1470-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 107] [Article Influence: 53.5] [Reference Citation Analysis (35)] |
| 10. | Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 864] [Article Influence: 172.8] [Reference Citation Analysis (1)] |
| 11. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 482] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 12. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1888] [Article Influence: 472.0] [Reference Citation Analysis (1)] |
| 13. | Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH; ESCORT-1st Investigators. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021;326:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 461] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 14. | Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, Wang B, Sun G, Ji Y, Cao G, Liu H, Cui T, Li N, Qiu W, Li G, Hou X, Luo H, Xue L, Zhang Y, Yue W, Liu Z, Wang X, Gao S, Pan Y, Galais MP, Zaanan A, Ma Z, Li H, Wang Y, Shen L; ORIENT-15 study group. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 216] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 15. | Xu JM, Jiang H, Pan Y, Gu K, Cang S, Han L, Shu Y, Li J, Zhao J, Pan H, Luo S, Qin Y, Guo Q, Bai Y, Ling Y, Guo Y, Li Z, Liu Y, Wang Y, Zhou H. LBA53 Sintilimab plus chemotherapy (chemo) vs chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study. Ann Oncol. 2021;32:S1331. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 16. | Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K; KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 894] [Article Influence: 223.5] [Reference Citation Analysis (0)] |
| 17. | Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, Park SR, Ping L, Jiang Y, Zhang J, Wu X, Yao Y, Shen L, Kojima T, Gotovkin E, Ishihara R, Wyrwicz L, Van Cutsem E, Jimenez-Fonseca P, Lin CY, Wang L, Shi J, Li L, Yoon HH. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023;24:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 127] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 18. | Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 640] [Article Influence: 213.3] [Reference Citation Analysis (2)] |
| 19. | Guo X, Yang B, He L, Sun Y, Song Y, Qu X. PD-1 inhibitors plus oxaliplatin or cisplatin-based chemotherapy in first-line treatments for advanced gastric cancer: A network meta-analysis. Front Immunol. 2022;13:905651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Aggarwal S. Adverse effects of immuno-oncology drugs-Awareness, diagnosis, and management: A literature review of immune-mediated adverse events. Indian J Cancer. 2019;56:S10-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Urwyler P, Earnshaw I, Bermudez M, Perucha E, Wu W, Ryan S, Mcdonald L, Karagiannis SN, Taams LS, Powell N, Cope A, Papa S. Mechanisms of checkpoint inhibition-induced adverse events. Clin Exp Immunol. 2020;200:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Yamashita K, Iwatsuki M, Harada K, Eto K, Hiyoshi Y, Ishimoto T, Nagai Y, Iwagami S, Miyamoto Y, Yoshida N, Komohara Y, Ajani JA, Baba H. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |