Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1479
Peer-review started: November 20, 2023
First decision: December 26, 2023
Revised: January 8, 2024
Accepted: March 1, 2024
Article in press: March 1, 2024
Published online: April 15, 2024
Processing time: 142 Days and 20.4 Hours
Our study investigated the role of FAM53B in regulating macrophage M2 polarization and its potential mechanisms in promoting pancreatic ductal adenocarcinoma (PDAC) metastasis.
To further investigate the role of FAM53B in regulating macrophage M2 polarization and its potential mechanism in promoting PDAC metastasis. Our goal is to determine how FAM53B affects macrophage M2 polarization and to define its underlying mechanism in PDAC metastasis.
Cell culture and various experiments, including protein analysis, immunohistochemistry, and animal model experiments, were conducted. We compared FAM53B expression between PDAC tissues and healthy tissues and assessed the correlation of FAM53B expression with clinical features. Our study analyzed the role of FAM53B in macrophage M2 polarization in vitro by examining the expression of relevant markers. Finally, we used a murine model to study the role of FAM53B in PDAC metastasis and analyzed the potential underlying mechanisms.
Our research showed that there was a significant increase in FAM53B levels in PDAC tissues, which was linked to adverse tumor features. Experimental findings indicated that FAM53B can enhance macrophage M2 polarization, leading to increased anti-inflammatory factor release. The results from the mouse model further supported the role of FAM53B in PDAC metastasis, as blocking FAM53B prevented tumor cell invasion and metastasis.
FAM53B promotes PDAC metastasis by regulating macrophage M2 polarization. This discovery could lead to the development of new strategies for treating PDAC. For example, interfering with the FAM53B signaling pathway may prevent cancer spread. Our research findings also provide important information for expanding our understanding of PDAC pathogenesis.
Core Tip: Our study investigates FAM53B in regulating macrophage M2 polarization and its potential mechanisms in promoting pancreatic ductal adenocarcinoma (PDAC) metastasis. Our research revealed a significant upregulation of FAM53B in PDAC tissues, which was associated with the malignant features of the tumors. Experimental findings indicated that FAM53B can enhance macrophage M2 polarization, leading to increased release of anti-inflammatory factors. Murine model results further confirmed the role of FAM53B in PDAC metastasis, as inhibiting FAM53B suppressed tumor invasion and metastasis. Therefore, FAM53B promotes PDAC metastasis by regulating macrophage M2 polarization.
- Citation: Pei XZ, Cai M, Jiang DW, Chen SH, Wang QQ, Lu HM, Lu YF. FAM53B promotes pancreatic ductal adenocarcinoma metastasis by regulating macrophage M2 polarization. World J Gastrointest Oncol 2024; 16(4): 1479-1499
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1479.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1479
In 2023, it is estimated that the number of new cases and deaths of pancreatic ductal adenocarcinoma (PDAC) in China will reach 134374 and 131203 cases, respectively, so PDAC has become a serious public health problem in China[1-3]. Despite significant advances in cancer research, PDAC remains a deadly disease[4-10]. FAM53B is an important member of the tumor necrosis factor superfamily[11-14]. This cytokine is particularly important for reactivating non-reactive T lymphocytes to achieve an optimal CD8 response in CD8+ T cells[15]. Its receptor, a member of the co-stimulatory receptor and tumor necrosis factor receptor superfamily, was initially found on activated T cells and is transiently expressed on activated T cells that encounter homologous antigens[16-20]. The traditional view is that the FAM53B ligand binds to receptors and transmits signals to cells expressing them, that is, positive signal transduction[21]. The mutual recognition of T cells and FAM53B on antigen-presenting cells can lead to receptor aggregation, activation of downstream TRAFS-mediated cascade signaling, and ultimately T cell activation, proliferation, and survival[22-24]. Moreover, FAM53B induces a strong co-stimulatory signal in T cells, which works in tandem with T cell receptor signals to produce interleukin (IL)-2 and interferon-gamma (IFN-γ)[25]. In addition to inducing effector cytokine production, FAM53B co-stimulated T cell memory and effector differentiation and protected T cells from apoptosis[26-28].
PDAC is a highly aggressive tumor with a worrying morbidity-to-mortality ratio[29]. According to the World Health Organization, PDAC is the seventh leading cause of cancer death worldwide. Despite significant advances in cancer research, survival rates for PDAC are relatively low, in part due to its late diagnosis and rapid metastasis. Therefore, an in-depth study of the metastasis mechanism of PDAC is of vital significance for improving patient prognosis. FAM53B is a protein-coding gene whose function has attracted much attention. In recent years, research has shown that FAM53B plays a key role in a variety of cancers, including PDAC. However, the specific role of FAM53B in PDAC and its effect on the regulation of macrophage M2 polarization on PDAC metastasis remain unclear[30]. The PDAC microenvironment has fewer CD8+ T cells than other parts of the body. This means that the positive co-stimulatory signal of FAM53B may not work as well in treating PDAC. Conversely, the reverse signaling of FAM53B can exert anti-tumor effects by reshaping the tumor microenvironment (TME).
In this study, we examined the role of FAM53B in the development of PDAC, focusing on its possible control of macrophage polarization. This study is expected to open up new frontiers in the research and treatment of PDAC and other cancer types.
ASPC-1 and U937 cells (CL-0018; Pricella, Wuhan, China) to be resuscitated were removed from the liquid nitrogen tank, and the cells were quickly shaken and thawed in a pre-heated 37 °C constant temperature water bath. ASPC-1 and U937 cells were cultured with Gibco 1640 medium containing 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, United States). Gibco DMEM high-glucose medium with 10% fetal bovine serum was used to grow COLO357, BXPC-3, and PANC-1 cells. The consumables required for the experiment, including the pasteurized straws, were placed on the ultra-clean operating table and sterilized with ultraviolet light for 30 min. After completion, high glucose DMEM complete culture medium and RPMI 1640 complete culture medium were prepared on the operating table with the ratio of basic culture medium: Serum: Penicillomycin double antibody = 9:1:0.1. The cells to be resuscitated were removed from the liquid nitrogen tank and thawed rapidly in a pre-heated 37 °C constant temperature water bath. The thawed cell suspension was then transferred to a sterile centrifuge tube, and 10 mL of complete medium was added and mixed well. The cells were centrifuged at 1000 rpm for 5 min, the supernatant was discarded, and an appropriate amount of complete medium was added to resuspend the cells. The cell suspension was transferred to a culture bottle and placed in an incubator, cultured at 5% carbon dioxide at 37 °C and observed the next day.
A total of 1 × 105 BXPC-3 and PANC-1 cells (CL-0019; Pricella) were inoculated in per well of a 6-well plate. After 24 h of culture, the cells were washed twice with sterile phosphate-buffered saline (PBS) solution and serum-free medium (10 mL, A2656101; Thermo Fisher Scientific) was added for 48 h.
When U937 cells (CL-0022; Pricella) reached the logarithmic growth phase, the cell suspension was transferred to a centrifuge tube and spun at 800 rpm for 5 min. The pellet was resuspended in complete media and cells were counted under the microscope. A total of 1 × 106 cells in suspension was transferred into a 6 cm petri dish. phorbol 12-myristate 13-acetate (PMA) was added at a concentration of 100 ng/mL. The cells were incubated for 48 h to induce differentiation into macrophage-like U937 cells.
CRISPR/Cas9-based lentiviral infection was used to generate stable FAM53B knockdown pancreatic cancer cell lines. Virus for stable FAM53B knockdown was purchased from Shanghai Jikai Company (Shanghai, China). The lentiviral vector was GV493, which contained GFP and a puromycin resistance cassette. The virus titer was higher than 1E+8TU. The two shRNA sequences were: sh1: 5’-CTACTATGTCTTCTTTCAACT-3’, sh2: 5’-TGGAATACGCCTCTGACGCTT-3'.
The macrophage model was constructed using U937 cells, and macrophage-like U937 cells were transferred to 6-well plates and transfected when the cell density reached 20-40%. Then, under PMA conditions, IL-13 and IL-4 (20 ng/mL each) were added to M0 macrophage culture medium, and the culture was stimulated for 48 h. M0 macrophages were induced to differentiate into M2 macrophages, thus obtaining THP-1 cell-derived M2 macrophages.
Exosomal vesicles derived from U937 cells and macrophages were lysed in RIPA lysis buffer. Antibodies against FAM53B (1:1000, A19236; Abclonal, Wuhan, China), GAPDH (1:2000, AC001; Abclonal) and Tubulin were used. Protein samples were adjusted to 0.375 mg/mL, 0.75 mg/mL, 1.5 mg/mL, 3 mg/mL, and Graphpad software (San Diego, CA, United States) was used to generate a standard protein curve. After washing, the membranes were incubated with peroxidase-labeled goat anti-rabbit secondary antibody (1:2000, AS014; Abclonal) at 37 °C for 1 h. The PKH67 Fluorescent Cell Linker Kit was used to identify and stain exosome suspensions. Diluent C (250 μL) was added to 50 μL of exosomes, followed by 1.5 µL diluent C (1.5 μL). The labeled and diluted exosomes were completely suspended with DMEM, and the exosomes were then added to the U937 cell (DAPI staining) culture supernatant with a pipette. After 24 h of culture, fixation, membrane rupture, and nucleation, laser scanning confocal fluorescence microscopy was used to observe whether U937 cells could take up macrophage-derived exosomes.
The gray value was detected by Image J software (version 1.8.0; National Institutes of Health, Bethesda, MA, United States), and the results were calculated by Graphpad Prism (version 9.0; GraphPad Software, San Diego, CA, United States).
Four-week-old female BALB/c nude mice were fed under standard pathogen-free conditions and divided into two groups of seven mice per group. After 2 wk of feeding, the animals were used for downstream experiments. Firstly, transfected PANC-1 cells were digested, centrifuged, and re-suspended to prepare a 5 × 10/100 μL cell suspension. Next, nude mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (50 mg/kg), placed in the right lying position, and disinfected with 75% alcohol. A longitudinal 1.0 cm-long incision was made on the left side of the abdomen. The spleen was exposed and gently removed from the abdominal cavity. A 1 mL syringe was inserted into the lower pole of the spleen about 1.0 cm and the tumor cell suspension was slowly injected. After injection, the syringe was removed, and the eye of the needle was pressed with a cotton ball of iodophenol for 2 min. The abdomen was closed, and the mice received routine postoperative feeding. After 4 wk of culture, the nude mice were sacrificed by cervical dislocation, and splenic tumor formation, liver metastasis and other metastases were observed anatomically. Splenic graft tumors and liver metastases were collected, and the results were observed by hematoxylin-eosin staining. The experiment was divided into two groups: sh-NC PANC-1 and sh-FAM53B PANC-1.
International council for harmonization: The slices were washed in PBS solution three times for 5 min each. Slices were placed in a dark box, and the excess PBS around the tissue was absorbed by filter paper. An appropriate amount of anti-rabbit and anti-mouse fluorescent secondary antibody was added, and incubated in a 37 °C incubator for 30 min (the subsequent steps were all performed in a dark room). The slices were washed three times in PBS solution for 5 min each. An appropriate amount of DAPI dye was added and stained for 10 min. After the slices were rinsed in PBS solution three times, they were placed in the dark box and 10 μL anti-fluorescence quench agent was added. The number of positively stained cells under 200 times of visual field was counted by fluorescence microscopy, and five visual fields were randomly counted. These data represent the average of the results obtained by two scorers.
Hepatic encephalopathy staining: The slide was soaked in hematoxylin solution for 5 min, washed with running water, then soaked in 1% hydrochloric alcohol for 3 s, and washed again with running water. The slide was dyed in eosin dye for 1 minute and washed with running water. The slide holder was placed in 50% ethanol for 5 min, 75% ethanol for 5 min, 85% ethanol for 5 min, 95% ethanol for 5 min, 100% ethanol for 5 min, and finally placed in two cylinders of xylene solution for 15 min each.
Colony formation assay: PDAC cells were separately plated into culture dishes containing medium. The FAM53B overexpression vector was transfected into one group and the other group was used as a blank control. After culture for a period of time, the cells were fixed with the AGAR flower tumor medium, and the number and size of the colonies were recorded.
Flow cytometry analysis: Macrophages were treated to differentiate into M2 type and divided into different groups (FAM53B overexpression group and control group). Fluorescent markers were used to label surface markers or cytokines, and the proportions of different cell subpopulations and the expression levels of specific markers were detected and analyzed by flow cytometry.
Statistical analysis for this project was conducted using SPSS (version 25.00; IBM Corp., Armonk, NY, United States), including descriptive statistics, t-tests, correlation analysis, multiple regression, and survival analysis.
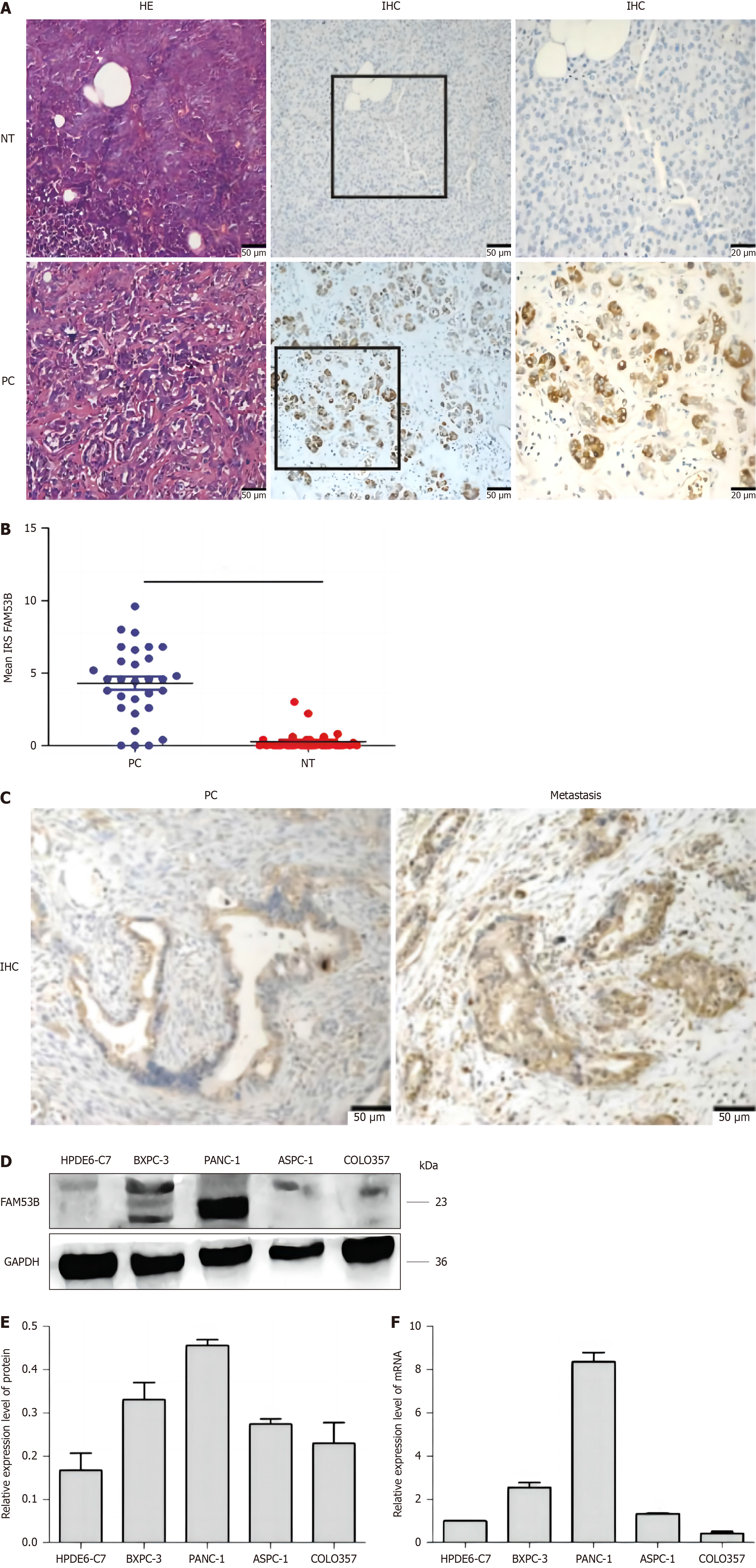
We collected thirty pancreatic cancer tissue samples for this investigation. Additional immunohistochemical staining analyses confirmed that FAM53B expression was substantially higher in pancreatic cancer compared to adjacent non-tumorous tissues (P < 0.001) (Figure 1A-C). We tested FAM53B expression in normal pancreatic cells (HPDE6-C7) and pancreatic cancer cells (ASPC-1, PANC-1, BXPC-3, and COLO357) using quantitative reverse transcriptase PCR (qRT-PCR) and protein immunoblotting. FAM53B protein expression was considerably greater in ASPC-1, PANC-1, and BXPC-3 cells compared to HPDE6-C7 cells (P < 0.05) (Figure 1D). Significant differences in FAM53B expression were observed at the mRNA level between HPDE6-C7 and ASPC-1, PANC-1, and BXPC-3 (P < 0.05); however, FAM53B expression did not differ between COLO357 and HPDE6-C7 cells (Figure 1E and F).
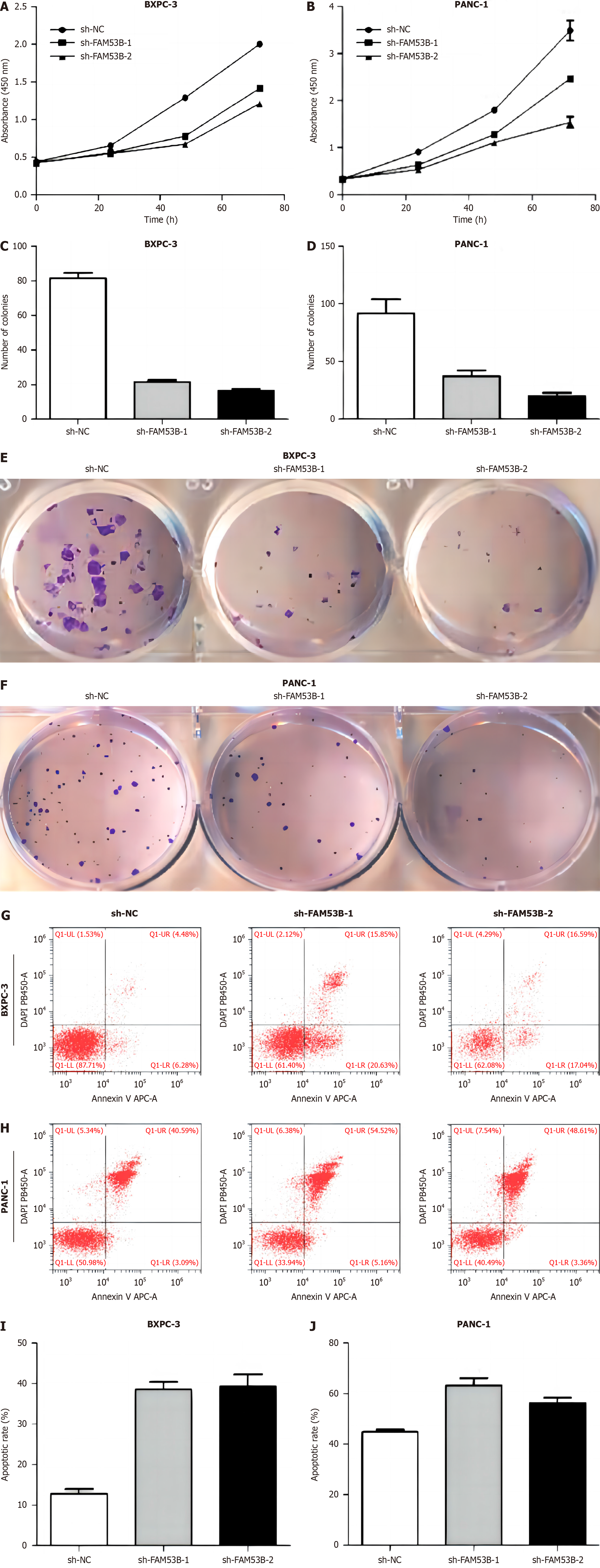
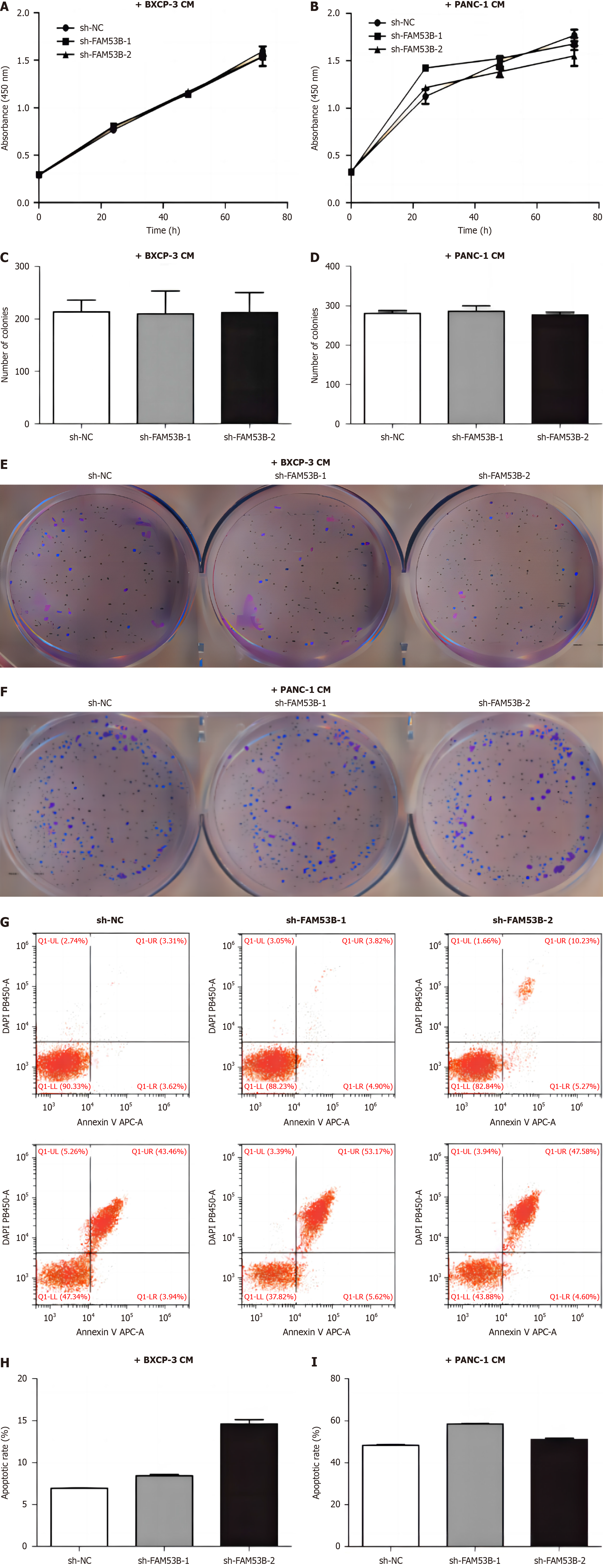
We used cell counting kit-8 (CCK-8) and single-cell cloning to investigate the impact of sh-NC, sh-FAM53B-1, and sh-FAM53B-2 tumor-associated macrophages (TAMs) on pancreatic cancer cell growth. The proliferation of BXPC-3 and PANC-1 cells was unaffected by sh-FAM53B-1 and sh-FAM53B-2 TAMs when co-cultured with sh-NC TAMs (Figure 2A-F). Additionally, FAM53B knockdown macrophages had a higher level of apoptosis in BXPC-3 and PANC-1 compared to those with sh-NCTAMs (P < 0.05) (Figure 2G-J).
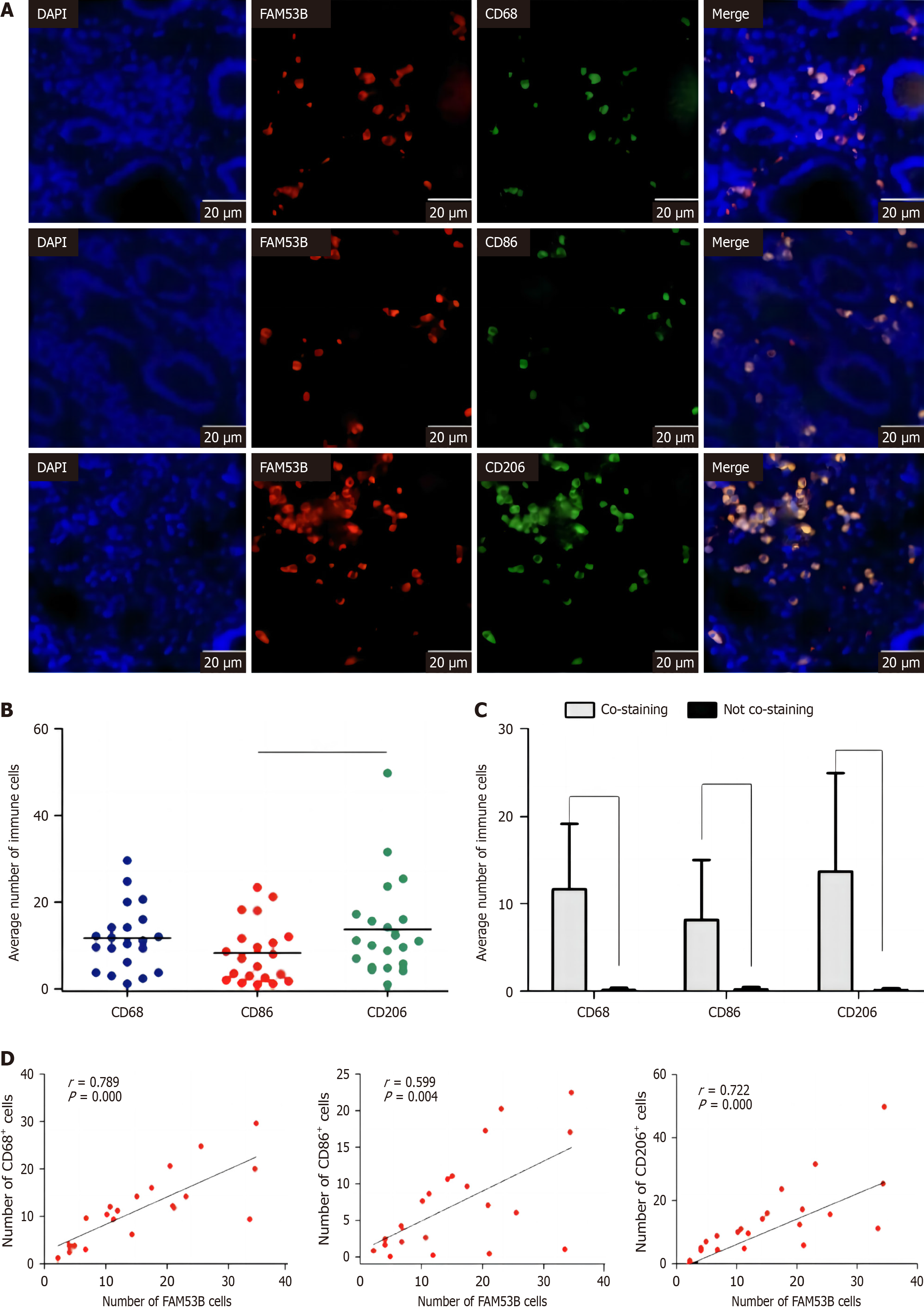
We performed immunofluorescence to examine the relative abundance of M0 macrophages (CD68), M1-type macrophages (CD86), and M2-type macrophages (CD206) in pancreatic cancer tissues. Our findings revealed a statistically significant increase in the number of M2-type macrophages compared to M1-type macrophages (P < 0.01) (Figure 3A and B). Furthermore, fluorescence colocalization analysis revealed that immune cells were the primary source of TNFSF9 expression (P < 0.001) (Figure 3C). Additional analysis revealed a stronger correlation (r = 0.722) between the expression of TNFSF9 and M2 macrophages as opposed to M1 macrophages (r = 0.599) (Figure 3D).
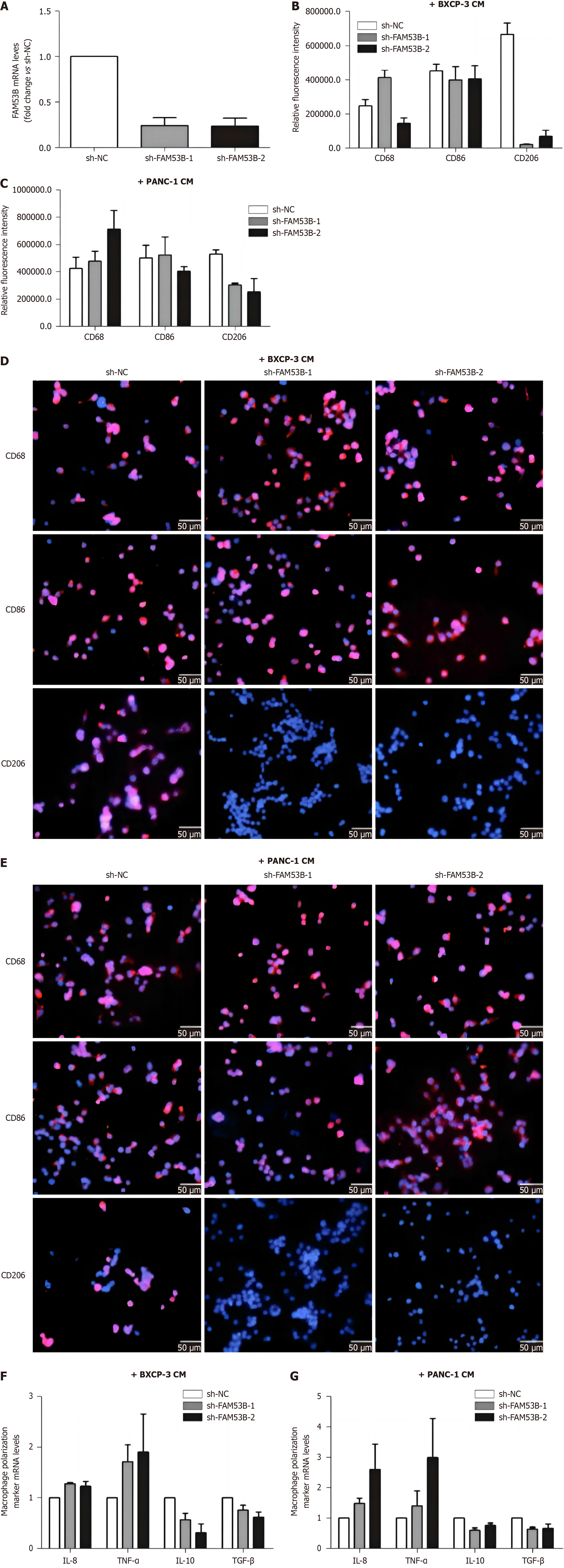
We compared markers of M1 (CD86) and M2 (CD206) macrophages between knockdown FAM53B TAMs (sh-FAM53B-1 TAMs and sh-FAM53B-2 TAMs) and transfected empty vector TAMs (sh-NC TAMs) using immunofluorescence. In comparison to the sh-NC group, both BXPC-3 and PANC-1 cell supernatants produced TAMs. The expression of the M2-type macrophage marker in sh-FAM53B-1 and sh-FAM53B-2 TAMs was considerably decreased (P < 0.05), but the expression of the M1-type macrophage marker was not statistically different from the sh-NC group (Figure 4A-E). We then performed qRT-PCR to determine mRNA expression of M1 markers (IL-8 and tumor necrosis factor-alpha) and M2 markers (IL-10 and transforming growth factor beta) in sh-FAM53B-1TAM, sh-FAM53B-2 TAMs, and sh-NC TAMs. The expression of M1 markers was significantly higher in sh-FAM53B-1 TAMs and sh-FAM53B-2 TAMs in comparison to sh-NC TAMs (Figure 4F-G). Conversely, the expression of M2 markers was found to be significantly lower in both TAMs (all P values < 0.05).
By using CCK-8 and single-cell cloning procedures, researchers were able to investigate the effects of sh-NC, sh-FAM53B-1 TAMs, and sh-FAM53B-2 TAMs on pancreatic cancer cell proliferation. There was no significant alteration in the growth of BXPC-3 and PANC-1 cells when co-cultured with sh-NC, sh-FAM53B-1 or sh-FAM53B-2 TAMs (Figure 5A-F). In comparison to the sh-NC-TAMs group, FAM53B knockdown macrophages induced apoptosis of BXPC-3 and PANC-1 cells (P < 0.05) (Figure 5G-I).
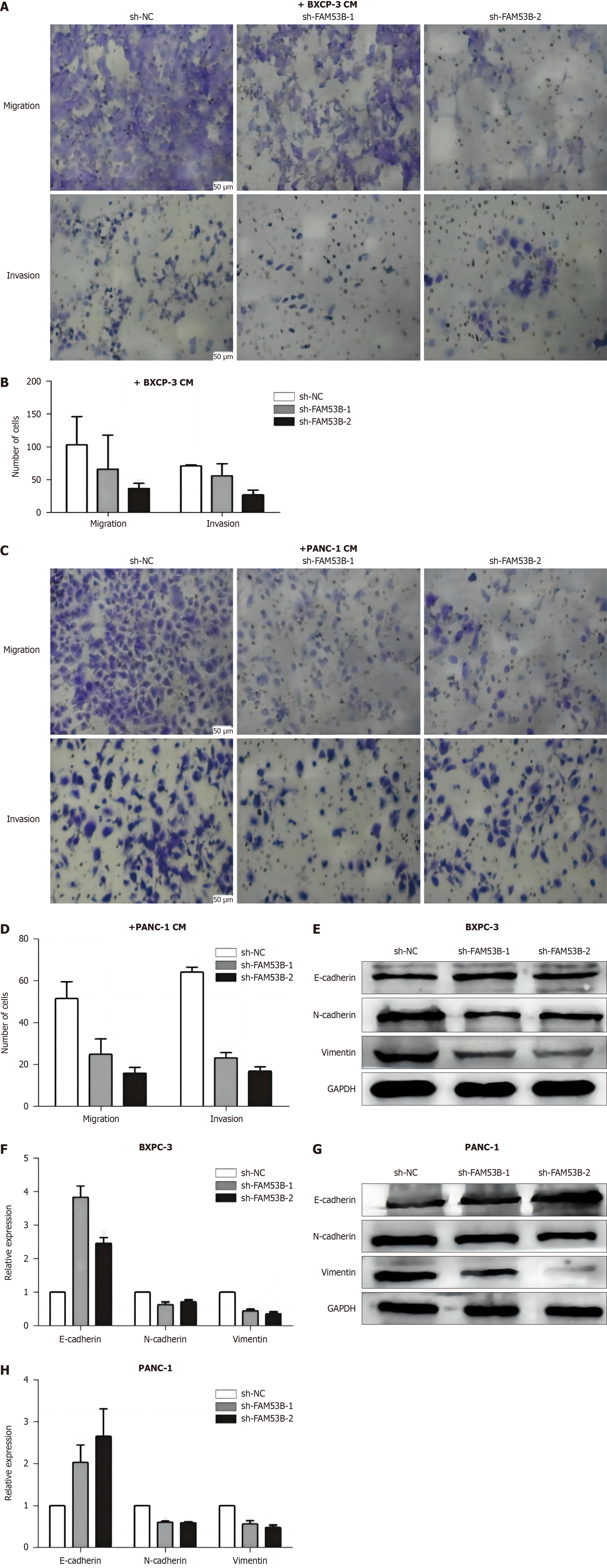
Transwell and matrix gel assays were performed to investigate how down-regulated FAM53B macrophages affect pancreatic cancer cell migration and invasion. A significant reduction in BXPC-3 and PANC-1 cell migration and invasion capacity was observed when treated with sh-FAM53B-1 TAMs and sh-FAM53B-2 TAMs compared to sh-NC TAMs (P < 0.001), as demonstrated in Figure 6A-D. In addition, we performed western blotting to examine the expression of epithelial-mesenchymal-transition-associated proteins (N-cadherin, vimentin, and E-cadherin) in pancreatic cancer cells following Fam53B-knocked macrophage intervention. There was a significant decrease in N-cadherin and vimentin expression in BXPC-3 and PANC-1 cells compared to the sh-NC TAMs group. N-cadherin and vimentin expression was down-regulated by both sh-FAM53B-1 TAMs and sh-FAM53B-2 TAMs. There was a considerable increase in E-cadherin expression (P < 0.05), as shown in Figure 6E-H.
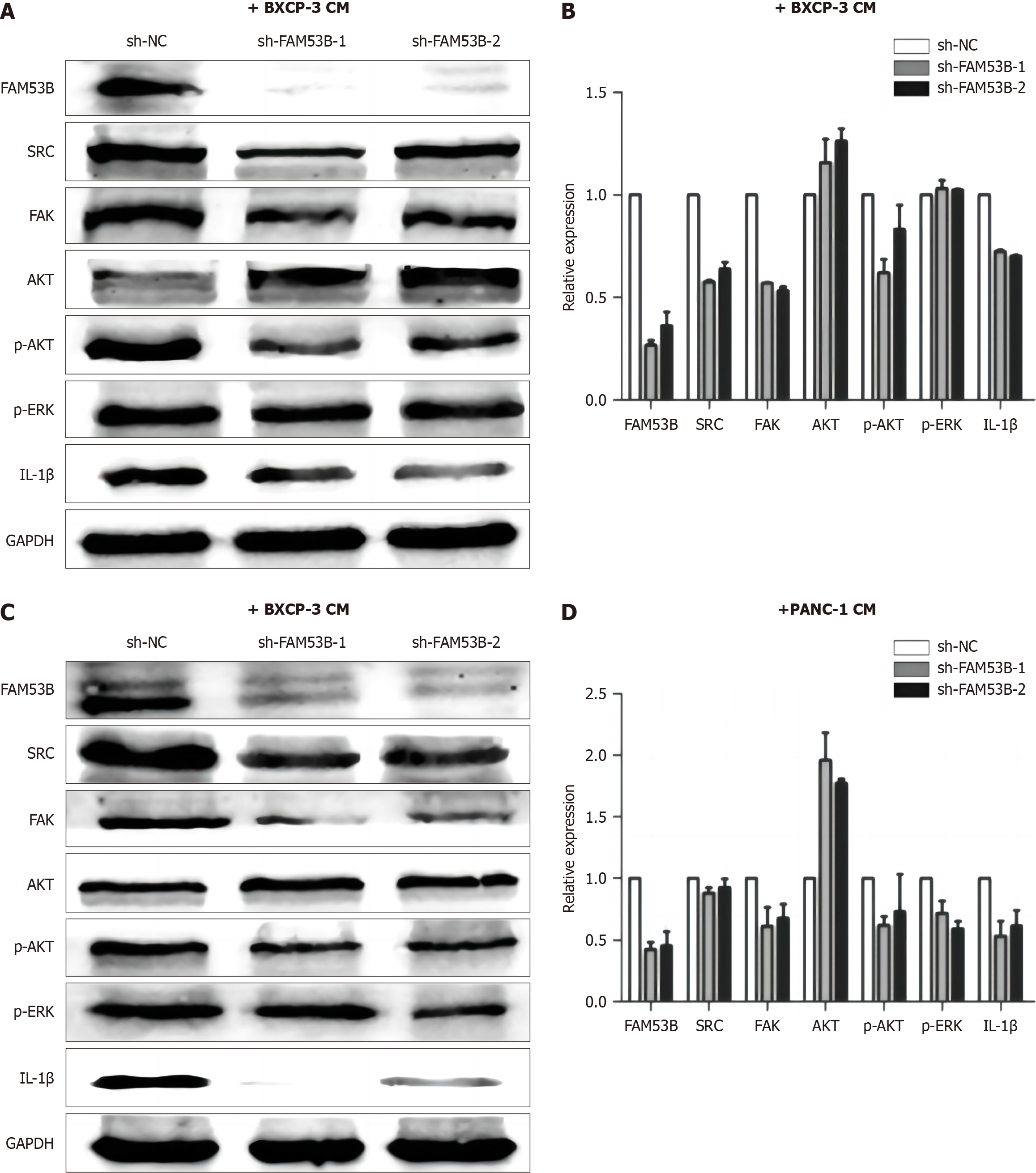
We next tested the function of the Src/FAK/p-Akt/IL-1β signaling pathway in macrophages lacking FAM53B. Compared to sh-NC TAMs, Src, FAK, p-AKT, and IL-1β expression was reduced in sh-FAM53B-1TAMs and sh-FAM53B-2 TAMs in BXPC-3-induced TAMs. AKT expression was significantly elevated (P < 0.05), while P-ERK expression did not exhibit any significant changes (Figure 7A and B). Similarly, after knocking down FAM53B in PANC-1-induced TAMs, the expressions of Src, FAK, P-Akt, and IL-1β in sh-FAM53B-1 TAMs and sh-FAM53B-2TAMS were considerably lower than in the sh-NC TAMs group (P < 0.05). There was a decrease in the expression of P-ERK, while there was a rise in the expression of AKT (P < 0.05) (Figure 7C and D).
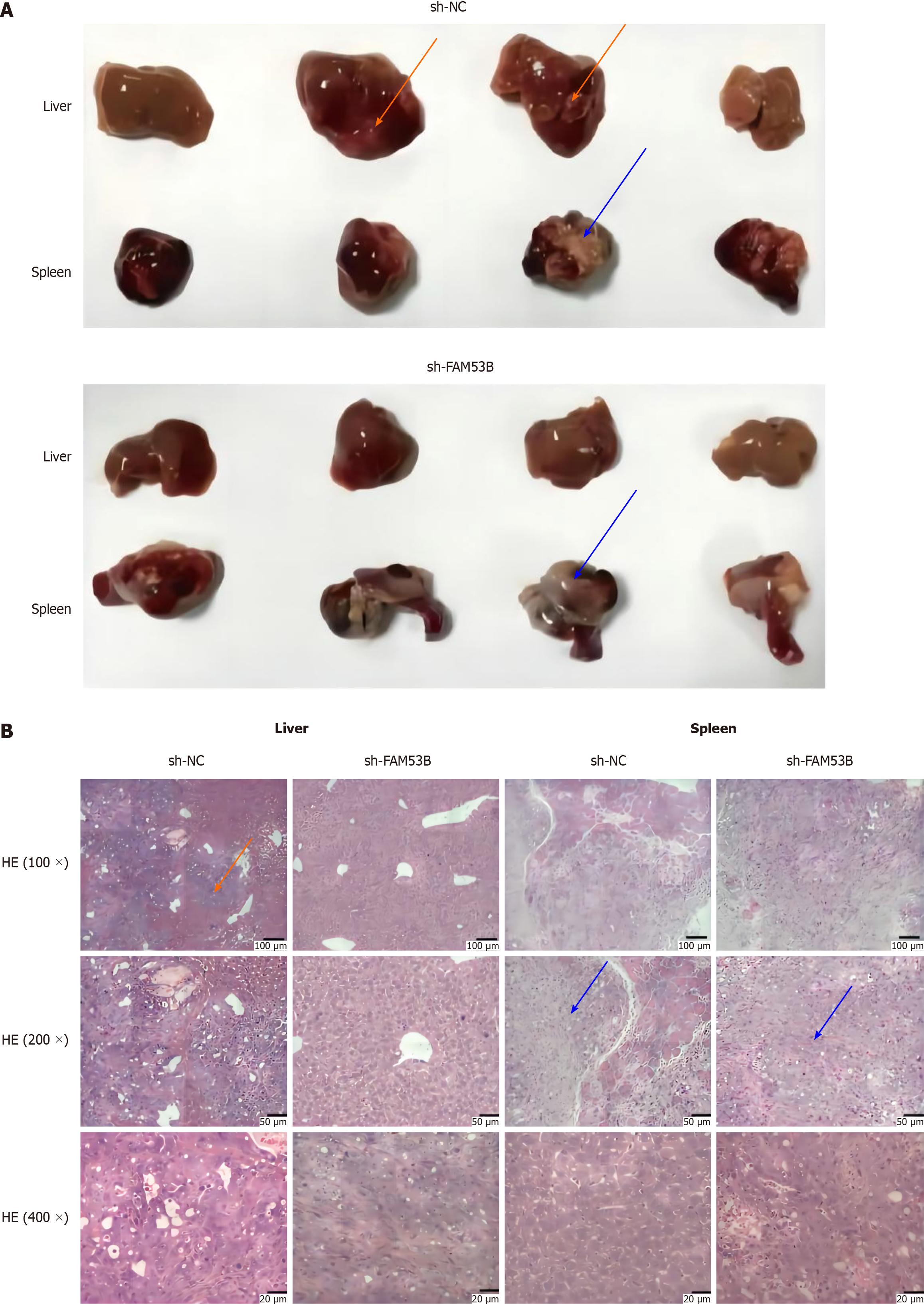
To develop a model of metastasis, we injected FAM53B knockdown PANC-1 cells into the spleens of nude mice. Within the sh-NC group, every mouse showed clear liver metastasis, which indicates that the metastasis rate was one hundred percent. This was manifested on the liver surface as grayish-yellow spots of varying diameters that were dispersed and partially fused into chunks. We determined that all of the tissue seen in the spleen was tumor tissue, as shown in Figure 8A. A considerably reduced metastatic rate of 28.6% was seen in the sh-FAM53B gene deletion group compared to the sh-NC group, and liver metastasis occurred in 2 out of 7 nude mice. There were still obvious signs of normal spleen tissue. To verify the findings, we performed hepatic encephalopathy staining on tissue sections taken from the liver and the spleen (Figure 8B).
As researchers continue to understand the various biological behaviors of cancer and various cancer treatment strategies[31], the survival rate for many cancer types and quality of life of patients have been significantly improved[31-34]. However, the prognosis of PDAC is not optimistic due to its early systemic spread, high vascular invasiveness, difficulty in early diagnosis, and resistance to chemotherapy[35-37]. We focused on exploring whether FAM53B knockdown can change the secretion of tumor cytokines, whether the secreted cytokines can affect macrophage polarization, and whether the macrophages with altered polarization status can affect PDAC cell migration[38-40]. We also found that the activation of the macrophage M2 phenotype can induce stronger migration and invasion in the PDAC metastatic AsPC-1 cell line[41]. TME exosomes transmit messages from one cell to another and reprogram recipient cells, and they are the most important communication medium between various cell types. Tumor cell-derived exosomes can regulate the PTEN/PI3K/AKT pathway, SOCS1/STAT6 pathway and integrin signal STAT1 through the miRNA they carry and further regulate the polarization of macrophages toward M2, creating a microenvironment conducive to tumor survival[42].
Our study looked into the part FAM53B plays in PDAC, specifically how it might affect the metastasis of macrophage M2 polarization. Researchers[43] found that FAM53B was significantly increased in PDAC. They also found that blocking this gene could stop cancer from spreading. We did not find that FAM53B-mediated M2 macrophage polarization directly affected cancer cell growth or death, but that it plays a key role in the metastasis process. This result suggests that FAM53B may be involved in metastasis through other pathways without directly affecting proliferation. Our results support the idea that FAM53B is linked to M2 macrophage polarization, which suggests that it plays a major role in controlling the environment around tumors. However, our study requires more in-depth mechanistic studies to elucidate the exact mode of action of FAM53B during the transfer process. We speculate that FAM53B may play a role by influencing macrophage activity, cell migration, and the TME, but the specific mechanism needs to be further explored.
Our study shows that although FAM53B-mediated polarization of M2 macrophages does not have a direct effect on PDAC cell proliferation and apoptosis, this does not exclude the possibility of its involvement in the metastasis process. FAM53B may primarily affect macrophage activity and polarization, thereby regulating their function in the TME, but not directly affecting the proliferation and apoptosis of pancreatic cancer cells. In addition, this difference may be related to the specificity of cell signaling pathways. As a regulatory factor, FAM53B may be more likely to take part in certain signaling pathways or control certain cell functions without having an effect on cell proliferation and apoptosis. However, it is very important in controlling the metastasis process.
At the same time, exosomes from M2 macrophages can contain certain miRNAs. This specific cargo can make PDAC cells less sensitive to gemcitabine once it gets to the tumor cells, which helps them become resistant to treatment. The study[44] showed that M2 macrophage-derived exosomes re-educate tumor cells by secreting miR-21, greatly reducing the drug sensitivity of tumor cells to cisplatin. In ovarian cancer, M2-type macrophage-derived exosomes modify the TME by regulating the Treg/Th17 cell ratio, resulting in an ovarian cancer TME immune imbalance, thereby promoting tumor cell immune escape. In glioblastoma, M2-type macrophage-derived exosomes can inhibit the proliferation of CD8+ T cells, reduce cytotoxic activity and IFN-γ levels, and promote immune escape of glioma cells by inhibiting PEG3 expression.
In this study, we found that FAM53B expression was elevated in PDAC cells. This result is contrary to previous studies that found that FAM53B inhibited liver cancer, colorectal cancer, and small-cell lung cancer. FAM53B expression is only associated with the prognosis of PDAC among common tumors of the digestive system.
This study revealed the important role of FAM53B in regulating macrophage M2 polarization and promoting the metastasis of PDAC. The experimental results showed that FAM53B promoted the transformation of macrophages into M2 types by influencing specific signaling pathways and played a key regulatory role in PDAC metastasis. Further molecular mechanism studies showed that FAM53B may accelerate the PDAC cell metastasis and invasion by regulating several key genes and cytokines. This finding not only deepens the understanding of PDAC development and metastasis but also provides a potential target for future research and treatment.
Our study investigates the role of FAM53B in regulating macrophage M2 polarization and its potential mechanisms in promoting pancreatic ductal adenocarcinoma (PDAC) metastasis.
The motivation for this study stems from a deep interest in the mechanisms of PDAC development and the critical role of macrophages in the tumor microenvironment. Given the highly aggressive nature of PDAC and its propensity to metastasize, we focused on exploring the underlying molecular mechanisms, with a particular focus on FAM53B's role in regulating macrophage M2 polarization. By further investigating the function of FAM53B, we expect to reveal its specific regulatory mechanisms during PDAC metastasis, providing a new perspective for an in-depth understanding of the development of this cancer.
To explore the role of FAM53B in the regulation of macrophage M2 polarization, further study the molecular mechanism that may be involved in promoting PDAC metastasis, and reveal the influence of FAM53B on the M2 polarization of macrophages, as well as the specific regulatory mechanism in PDAC metastasis.
Various methods were used to investigate the role of FAM53B in regulating macrophage M2 polarization and promoting PDAC metastasis. A macrophage model regulated by FAM53B expression level was constructed by cell culture and gene knockout techniques. Subsequently, immunocytochemistry and coimmunoprecipitation techniques were used to detect M2 macrophage marker expression and the interaction between FAM53B and related proteins. In animal models, the effect of FAM53B on PDAC metastasis was evaluated by transplanting PDAC cell lines and observing tumor growth and metastasis. At the molecular level, transcriptomic and proteomic methods were used to analyze the changes in the FAM53B-regulated signaling pathway and related gene expression.
Our research showed that there was a significant increase in FAM53B levels in PDAC tissues, which was linked to the tumors' cancerous features. Experimental findings indicated that FAM53B can enhance macrophage M2 polarization, leading to an increased release of anti-inflammatory factors. The results from the mouse model further supported the role of FAM53B in PDAC metastasis, as blocking FAM53B stopped the tumor from spreading and invading other tissues.
FAM53B promotes PDAC metastasis by regulating macrophage M2 polarization. This discovery could lead to new ways to treat PDAC.
The results of this study are expected to provide a new molecular mechanism for in-depth understanding of the development and metastasis of PDAC and provide innovative ideas for the development of relevant therapeutic strategies. By revealing the key role of FAM53B in the regulation of macrophage M2 polarization, it can provide a basis for the design of targeted interventions. Further research could focus on developing FAM53B inhibitors or related therapeutic strategies to reduce the aggressiveness of PDAC and improve therapeutic efficacy. In addition, the interrelationships between FAM53B and other signaling pathways will be explored in depth to provide a more comprehensive understanding of the complex regulatory networks in the tumor microenvironment. This will provide important guidance for future clinical transformation and is expected to bring new breakthroughs in the treatment of PDAC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Gondi C, United States; Ozaki T, United States S-Editor: Qu XL L-Editor: Filipodia P-Editor: Zheng XM
| 1. | Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z, Ding X, Bao R, Hong L, Jia W, Fang F, Liu H, Chen L, Zhong J, Zou D, Liu L, Han L, Ginhoux F, Liu Y, Ye Y, Su B. Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat Commun. 2022;13:1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 463] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 2. | He Z, Wang J, Zhu C, Xu J, Chen P, Jiang X, Chen Y, Jiang J, Sun C. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022;548:215751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 3. | Cappellesso F, Orban MP, Shirgaonkar N, Berardi E, Serneels J, Neveu MA, Di Molfetta D, Piccapane F, Caroppo R, Debellis L, Ostyn T, Joudiou N, Mignion L, Richiardone E, Jordan BF, Gallez B, Corbet C, Roskams T, DasGupta R, Tejpar S, Di Matteo M, Taverna D, Reshkin SJ, Topal B, Virga F, Mazzone M. Targeting the bicarbonate transporter SLC4A4 overcomes immunosuppression and immunotherapy resistance in pancreatic cancer. Nat Cancer. 2022;3:1464-1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 4. | Cai H, Wang R, Guo X, Song M, Yan F, Ji B, Liu Y. Combining Gemcitabine-Loaded Macrophage-like Nanoparticles and Erlotinib for Pancreatic Cancer Therapy. Mol Pharm. 2021;18:2495-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Rodriguez E, Boelaars K, Brown K, Eveline Li RJ, Kruijssen L, Bruijns SCM, van Ee T, Schetters STT, Crommentuijn MHW, van der Horst JC, van Grieken NCT, van Vliet SJ, Kazemier G, Giovannetti E, Garcia-Vallejo JJ, van Kooyk Y. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat Commun. 2021;12:1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 6. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Guillot J, Dominici C, Lucchesi A, Nguyen HTT, Puget A, Hocine M, Rangel-Sosa MM, Simic M, Nigri J, Guillaumond F, Bigonnet M, Dusetti N, Perrot J, Lopez J, Etzerodt A, Lawrence T, Pudlo P, Hubert F, Scoazec JY, van de Pavert SA, Tomasini R, Chauvet S, Mann F. Sympathetic axonal sprouting induces changes in macrophage populations and protects against pancreatic cancer. Nat Commun. 2022;13:1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Datta J, Dai X, Bianchi A, De Castro Silva I, Mehra S, Garrido VT, Lamichhane P, Singh SP, Zhou Z, Dosch AR, Messaggio F, Ban Y, Umland O, Hosein PJ, Nagathihalli NS, Merchant NB. Combined MEK and STAT3 Inhibition Uncovers Stromal Plasticity by Enriching for Cancer-Associated Fibroblasts With Mesenchymal Stem Cell-Like Features to Overcome Immunotherapy Resistance in Pancreatic Cancer. Gastroenterology. 2022;163:1593-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (7)] |
| 9. | He Y, Luo W, Liu Y, Wang Y, Ma C, Wu Q, Tian P, He D, Jia Z, Lv X, Ma YS, Yang H, Xu K, Zhang X, Xiao Y, Zhang P, Liang Y, Fu D, Yao F, Hu G. IL-20RB mediates tumoral response to osteoclastic niches and promotes bone metastasis of lung cancer. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 10. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hezaveh K, Shinde RS, Klötgen A, Halaby MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, Grünwald BT, Foerster EG, Chaharlangi D, Guo M, Makhijani P, Zhang X, Pugh TJ, Pinto DM, Co IL, McGuigan AP, Jang GH, Khokha R, Ohashi PS, O'Kane GM, Gallinger S, Navarre WW, Maughan H, Philpott DJ, Brooks DG, McGaha TL. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55:324-340.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 346] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 12. | Wen Y, Cai W, Yang J, Fu X, Putha L, Xia Q, Windsor JA, Phillips AR, Tyndall JDA, Du D, Liu T, Huang W. Targeting Macrophage Migration Inhibitory Factor in Acute Pancreatitis and Pancreatic Cancer. Front Pharmacol. 2021;12:638950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Garcia Garcia CJ, Huang Y, Fuentes NR, Turner MC, Monberg ME, Lin D, Nguyen ND, Fujimoto TN, Zhao J, Lee JJ, Bernard V, Yu M, Delahoussaye AM, Jimenez Sacarello I, Caggiano EG, Phan JL, Deorukhkar A, Molkentine JM, Saur D, Maitra A, Taniguchi CM. Stromal HIF2 Regulates Immune Suppression in the Pancreatic Cancer Microenvironment. Gastroenterology. 2022;162:2018-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 14. | Pratt HG, Steinberger KJ, Mihalik NE, Ott S, Whalley T, Szomolay B, Boone BA, Eubank TD. Macrophage and Neutrophil Interactions in the Pancreatic Tumor Microenvironment Drive the Pathogenesis of Pancreatic Cancer. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X, Zhang K, Teng B, Cao J, Wu W, Cao P, Huang C, Qiu Z. M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2. Mol Ther. 2021;29:1226-1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 16. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 543] [Article Influence: 135.8] [Reference Citation Analysis (0)] |
| 18. | Kemp SB, Steele NG, Carpenter ES, Donahue KL, Bushnell GG, Morris AH, The S, Orbach SM, Sirihorachai VR, Nwosu ZC, Espinoza C, Lima F, Brown K, Girgis AA, Gunchick V, Zhang Y, Lyssiotis CA, Frankel TL, Bednar F, Rao A, Sahai V, Shea LD, Crawford HC, Pasca di Magliano M. Pancreatic cancer is marked by complement-high blood monocytes and tumor-associated macrophages. Life Sci Alliance. 2021;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 19. | Zhang K, Li YJ, Peng LJ, Gao HF, Liu LM, Chen H. M2 macrophage-derived exosomal miR-193b-3p promotes progression and glutamine uptake of pancreatic cancer by targeting TRIM62. Biol Direct. 2023;18:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Shi M, He X, Cao Y, Liu P, Li F, Zou S, Wen C, Zhan Q, Xu Z, Wang J, Sun B, Shen B. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2022;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 21. | Chen YJ, Li GN, Li XJ, Wei LX, Fu MJ, Cheng ZL, Yang Z, Zhu GQ, Wang XD, Zhang C, Zhang JY, Sun YP, Saiyin H, Zhang J, Liu WR, Zhu WW, Guan KL, Xiong Y, Yang Y, Ye D, Chen LL. Targeting IRG1 reverses the immunosuppressive function of tumor-associated macrophages and enhances cancer immunotherapy. Sci Adv. 2023;9:eadg0654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 22. | Boyer S, Lee HJ, Steele N, Zhang L, Sajjakulnukit P, Andren A, Ward MH, Singh R, Basrur V, Zhang Y, Nesvizhskii AI, Pasca di Magliano M, Halbrook CJ, Lyssiotis CA. Multiomic characterization of pancreatic cancer-associated macrophage polarization reveals deregulated metabolic programs driven by the GM-CSF-PI3K pathway. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Li TJ, Jin KZ, Li H, Ye LY, Li PC, Jiang B, Lin X, Liao ZY, Zhang HR, Shi SM, Lin MX, Fei QL, Xiao ZW, Xu HX, Liu L, Yu XJ, Wu WD. SIGLEC15 amplifies immunosuppressive properties of tumor-associated macrophages in pancreatic cancer. Cancer Lett. 2022;530:142-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Melamed JR, Yerneni SS, Arral ML, LoPresti ST, Chaudhary N, Sehrawat A, Muramatsu H, Alameh MG, Pardi N, Weissman D, Gittes GK, Whitehead KA. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci Adv. 2023;9:eade1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 92] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 25. | Ramakrishnan S. HIF-2 in Cancer-Associated Fibroblasts Polarizes Macrophages and Creates an Immunosuppressive Tumor Microenvironment in Pancreatic Cancer. Gastroenterology. 2022;162:1835-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci U S A. 2022;119:e2119168119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 28. | Alonso-Nocelo M, Ruiz-Cañas L, Sancho P, Görgülü K, Alcalá S, Pedrero C, Vallespinos M, López-Gil JC, Ochando M, García-García E, David Trabulo SM, Martinelli P, Sánchez-Tomero P, Sánchez-Palomo C, Gonzalez-Santamaría P, Yuste L, Wörmann SM, Kabacaoğlu D, Earl J, Martin A, Salvador F, Valle S, Martin-Hijano L, Carrato A, Erkan M, García-Bermejo L, Hermann PC, Algül H, Moreno-Bueno G, Heeschen C, Portillo F, Cano A, Sainz B. Macrophages direct cancer cells through a LOXL2-mediated metastatic cascade in pancreatic ductal adenocarcinoma. Gut. 2023;72:345-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 29. | Zhang M, Pan X, Fujiwara K, Jurcak N, Muth S, Zhou J, Xiao Q, Li A, Che X, Li Z, Zheng L. Pancreatic cancer cells render tumor-associated macrophages metabolically reprogrammed by a GARP and DNA methylation-mediated mechanism. Signal Transduct Target Ther. 2021;6:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Geng Y, Fan J, Chen L, Zhang C, Qu C, Qian L, Chen K, Meng Z, Chen Z, Wang P. A Notch-Dependent Inflammatory Feedback Circuit between Macrophages and Cancer Cells Regulates Pancreatic Cancer Metastasis. Cancer Res. 2021;81:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Thibault B, Ramos-Delgado F, Pons-Tostivint E, Therville N, Cintas C, Arcucci S, Cassant-Sourdy S, Reyes-Castellanos G, Tosolini M, Villard AV, Cayron C, Baer R, Bertrand-Michel J, Pagan D, Ferreira Da Mota D, Yan H, Falcomatà C, Muscari F, Bournet B, Delord JP, Aksoy E, Carrier A, Cordelier P, Saur D, Basset C, Guillermet-Guibert J. Pancreatic cancer intrinsic PI3Kα activity accelerates metastasis and rewires macrophage component. EMBO Mol Med. 2021;13:e13502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Bachy S, Wu Z, Gamradt P, Thierry K, Milani P, Chlasta J, Hennino A. βig-h3-structured collagen alters macrophage phenotype and function in pancreatic cancer. iScience. 2022;25:103758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Xiang H, Yang R, Tu J, Xi Y, Yang S, Lv L, Zhai X, Zhu Y, Dong D, Tao X. Metabolic reprogramming of immune cells in pancreatic cancer progression. Biomed Pharmacother. 2023;157:113992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 35. | Chen Y, Wang C, Song J, Xu R, Ruze R, Zhao Y. S100A2 Is a Prognostic Biomarker Involved in Immune Infiltration and Predict Immunotherapy Response in Pancreatic Cancer. Front Immunol. 2021;12:758004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Dong Q, Zhang S, Zhang H, Sun J, Lu J, Wang G, Wang X. MARCO is a potential prognostic and immunotherapy biomarker. Int Immunopharmacol. 2023;116:109783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 37. | Zhang D, Wu S, Pan S, Wang M, Wang Z, He Z, Zhang G, Cui F, Song Y, Li W, Shi X, Huang H, Xu H. Single-cell sequencing reveals heterogeneity between pancreatic adenosquamous carcinoma and pancreatic ductal adenocarcinoma with prognostic value. Front Immunol. 2022;13:972298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Li Y, Yang B, Miao H, Liu L, Wang Z, Jiang C, Yang Y, Qiu S, Li X, Geng Y, Zhang Y, Liu Y. Nicotinamide N -methyltransferase promotes M2 macrophage polarization by IL6 and MDSC conversion by GM-CSF in gallbladder carcinoma. Hepatology. 2023;78:1352-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 39. | Tang HD, Wang Y, Xie P, Tan SY, Li HF, Shen H, Zhang Z, Lei ZQ, Zhou JH. The Crosstalk Between Immune Infiltration, Circulating Tumor Cells, and Metastasis in Pancreatic Cancer: Identification of HMGB3 From a Multiple Omics Analysis. Front Genet. 2022;13:892177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Yang J, Zhang Q, Wang J, Lou Y, Hong Z, Wei S, Sun K, Chen Y, Sheng J, Su W, Bai X, Liang T. Dynamic profiling of immune microenvironment during pancreatic cancer development suggests early intervention and combination strategy of immunotherapy. EBioMedicine. 2022;78:103958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Tichet M, Wullschleger S, Chryplewicz A, Fournier N, Marcone R, Kauzlaric A, Homicsko K, Deak LC, Umaña P, Klein C, Hanahan D. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8(+) T cells and reprogramming macrophages. Immunity. 2023;56:162-179.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 42. | Zuo C, Baer JM, Knolhoff BL, Belle JI, Liu X, Alarcon De La Lastra A, Fu C, Hogg GD, Kingston NL, Breden MA, Dodhiawala PB, Zhou DC, Lander VE, James CA, Ding L, Lim KH, Fields RC, Hawkins WG, Weber JD, Zhao G, DeNardo DG. Stromal and therapy-induced macrophage proliferation promotes PDAC progression and susceptibility to innate immunotherapy. J Exp Med. 2023;220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Yi Q, Wang J, Liu T, Yao Y, Loveless I, Subedi K, Toor J, Adrianto I, Xiao H, Chen B, Crawford HC, Fang D, Zhou L, Mi QS. scRNA-Seq and imaging mass cytometry analyses unveil iNKT cells-mediated anti-tumor immunity in pancreatic cancer liver metastasis. Cancer Lett. 2023;561:216149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Tu M, Klein L, Espinet E, Georgomanolis T, Wegwitz F, Li X, Urbach L, Danieli-Mackay A, Küffer S, Bojarczuk K, Mizi A, Günesdogan U, Chapuy B, Gu Z, Neesse A, Kishore U, Ströbel P, Hessmann E, Hahn SA, Trumpp A, Papantonis A, Ellenrieder V, Singh SK. TNF-α-producing macrophages determine subtype identity and prognosis via AP1 enhancer reprogramming in pancreatic cancer. Nat Cancer. 2021;2:1185-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |