Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1361
Peer-review started: December 21, 2023
First decision: December 28, 2023
Revised: January 12, 2024
Accepted: February 7, 2024
Article in press: February 7, 2024
Published online: April 15, 2024
Processing time: 112 Days and 1.9 Hours
Colorectal cancer (CRC) is among the most prevalent and life-threatening malignancies worldwide. Syndecan-2 methylation (mSDC2) testing has emerged as a widely used biomarker for early detection of CRC in stool and serum samples. Cancer (CRC) is among the most prevalent and life-threatening malignancies worldwide. mSDC2 testing has emerged as a widely used biomarker for early detection of CRC in stool and serum samples.
To validate the effectiveness of fecal DNA mSDC2 testing in the detection of CRC among a high-risk Chinese population to provide evidence-based data for the development of diagnostic and/or screening guidelines for CRC in China.
A high-risk Chinese cohort consisting of 1130 individuals aged 40-79 years was selected for evaluation via fecal mSDC2 testing. Sensitivity and specificity for CRC, advanced adenoma (AA) and advanced colorectal neoplasia (ACN) were determined. High-risk factors for the incidence of colorectal lesions were determined and a logistic regression model was constructed to reflect the efficacy of the test.
A total of 1035 high-risk individuals were included in this study according to established criteria. Among them, 16 suffered from CRC (1.55%), 65 from AA (6.28%) and 189 from non-AAs (18.26%); 150 patients were diagnosed with polyps (14.49%). Diagnoses were established based upon colonoscopic and pathological examinations. Sensitivities of the mSDC2 test for CRC and AA were 87.50% and 40.00%, respectively; specificities were 95.61% for other groups. Positive predictive values of the mSDC2 test for CRC, AA and ACN were 16.09%, 29.89% and 45.98%, respectively; the negative predictive value for CRC was 99.79%. After adjusting for other high-risk covariates, mSDC2 test positivity was found to be a significant risk factor for the occurrence of ACN (P < 0.001).
Our findings confirmed that offering fecal mSDC2 testing and colonoscopy in combination for CRC screening is effective for earlier detection of malignant colorectal lesions in a high-risk Chinese population.
Core Tip: A high-risk Chinese cohort composed of 1130 individuals 40-79 years of age was selected for evaluation using the fecal syndecan-2 methylation (mSDC2) test. Sensitivity and specificity to colorectal cancer (CRC), advanced adenoma and advanced colorectal neoplasia were quantified. High-risk factors for the incidence of colorectal lesions were analyzed; a logistic regression model was subsequently constructed to better reflect the efficacy of fecal mSDC2 testing. The results of this CRC screening study revealed that offering patients a combination of fecal mSDC2 testing and colonoscopy is ideal for facilitating early detection of CRC among a high-risk Chinese population.
- Citation: Luo WF, Jiao YT, Lin XL, Zhao Y, Wang SB, Shen J, Deng J, Ye YF, Han ZP, Xie FM, He JH, Wan Y. Effectiveness of fecal DNA syndecan-2 methylation testing for detection of colorectal cancer in a high-risk Chinese population. World J Gastrointest Oncol 2024; 16(4): 1361-1373
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1361.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1361
Colorectal cancer (CRC) is a life-threatening malignancy that is highly prevalent in China. Indeed, its incidence and mortality rates have steadily increased. According to data from the International Agency for Research on Cancer, in 2020 alone, China witnessed 555.5 thousand new cases and 283.8 thousand deaths from CRC, ranking second and fifth, respectively, among malignancies[1]. Although CRC incidence is relatively low among individuals aged less than 50 years, incidence markedly increases with age. Importantly, recent data has suggested a rise in early-onset CRC cases[2-4]. Progression from colorectal adenoma to CRC takes approximately a decade on average[5]. Consequently, implementation of screening programs to detect precancerous lesions among average-risk populations is a highly effective strategy for reducing the incidence of CRC.
Currently, several screening methods for CRC include colonoscopy, fecal immunochemical testing (FIT) and serum biomarker evaluation. However, each approach has its limitations. Although colonoscopy remains the gold standard for CRC diagnosis, its invasiveness, complicated intestinal preparation and poor compliance rates have hindered widespread adoption[6]. Importantly, colonoscopy may miss certain lesions[7]. While FIT offers an efficient, non-invasive screening option for the average-risk population, it has modest sensitivity in detecting CRC[7,8]. Levels of certain serum proteins such as carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) have been utilized as pan-cancer biomarkers for various malignancies such as those of the pancreas, breast and colon[9-12]. However, their specificity for detecting CRC is limited. Such limitations highlight the need for superior alternative screening methods to improve rates of CRC detection and diagnosis.
Due to advancements in biotechnology, molecular diagnostics have attracted rapidly increasing interest among in vitro techniques. Importantly, such methods outperform biochemical and immunological diagnostics in terms of accuracy and prognostic worth. Stool-based DNA testing is an emerging CRC screening method that detects tumors by identifying relevant biomarkers in stool samples. Aberrant genetic and epigenetic alterations in the setting of CRC may be tracked by extracting DNA from exfoliated cells[13-15]. In the setting of carcinogenesis, DNA methylation is also highly relevant and considered to be a good biomarker of malignancy[16-18]. Genes such as Vimentin, NDRG4, BMP3 and TFP12 have been employed as molecular markers to develop sensitive and simple screening approaches for evaluation of DNA methylation[19,20]. Due to its non-invasive, convenient and sensitive characteristics, stool-based DNA testing is predicted to become extensively employed in large-scale screening among average-risk populations. Cologuard® (Exact Sciences, Madison WI) was the first such commercial product authorized for clinical use by the United States Food and Drug Administration. It incorporates fecal hemoglobin immunoassays and quantitative molecular detection of methylated BMP3, methylated NDRG4 and mutant KRAS[21]. Furthermore, the United States Preventive Services Task Force initially recommended stool DNA testing as a screening approach for CRC in 2016[22]. In particular, syndecan-2 methylation (mSDC2) testing has emerged as a tool for early detection of CRC in stool and serum samples[23,24]. The SDC2 gene is a transmembrane heparan sulfate proteoglycan that is a member of the SDC proteoglycan family. It is relevant in cell proliferation, angiogenesis and cell migration, and is widely expressed in colonic mesenchymal cells. Zhang et al[25] reported that levels of SDC2 regulatory region methylation are significantly higher in colorectal tumor specimens as compared to paired adjacent non-malignant tissues. Zhao et al[26] showed that the methylation level of SDC2 in tumor tissues surpasses that in corresponding non-cancerous tissues and exceeds levels observed in polyps. As mSDC2 can be detected in both adenomas as well as early carcinogenesis, its methylation is considered to be a gradual process. Relative to normal colorectal tissues and polyps, SDC2 was found to be significantly more methylated in different stages of CRC patho
Here, we aimed to evaluate the efficacy of mSDC2 testing as a screening tool for CRC in a high-risk population. Our primary objective was to assess both sensitivity and specificity of mSDC2 testing for detection of CRC and advanced adenoma (AA). Furthermore, we study aimed to evaluate clinical usefulness of mSDC2 testing as an adjunctive tool for CRC and precancerous lesion identification.
From April 2020 to May 2022, CRC screening with stool-based mSDC2 testing was performed at the Panyu Center Hospital of Guangzhou (Guangdong, China) as a primary screening method. Over 1000 high-risk participants were enrolled in this study. Fresh stool was collected from every participant for mSDC2 testing prior to initiating bowel preparation for colonoscopy; subjects subsequently underwent colonoscopy for further diagnostic evaluation. This study was approved by the institutional review board, IRB approval number: No. [2019]62, and written, informed consent was obtained from all study participants.
Individual outpatients 40-79 years old completed a high-risk factor questionnaire (HRFQ). A positive HRFQ was defined as: (1) A family history of CRC in first-degree relatives; (2) A history of polyps; and/or (3) A history of two or more of the following: (1) Chronic diarrhea; (2) Chronic constipation; (3) Mucoid bloody feces; (4) Chronic appendicitis or appendectomy; (5) Chronic cholecystitis or cholecystectomy; and (6) Psychiatric trauma (e.g., divorce, death of first-degree relatives)[29-31].
Individuals unsuitable for colonoscopic examination such as those suffering severe cardiac or chronic renal conditions or pregnant patients were excluded from analyses. Participants previously diagnosed with any malignancies, those who had undergone chemo- or immuno-therapy treatments or subjects who were unable or unwilling to provide written, informed consent were also excluded from analyses.
Specimen collection and processing: Each participant was required to provide about 4.5 g of fresh stool for mSDC2 testing using a stool collection device (Creative Biosciences Co. Ltd., Guangzhou, China) at home. Samples stored in preservation buffer were sent to the designated clinical laboratory within two days of collection. Stool specimens were immediately homogenized and centrifuged upon receipt. Supernatants were then aliquoted and frozen at -80 °C until further use. Frozen aliquots were subsequently tested in batches by experienced laboratory technicians using Colosafe® testing reagent kits (Creative Biosciences).
mSDC2 testing: For blind testing, each stool collection device was labeled with a unique code and no other identifying information. Target and control genes [SDC2 and β-actin (ACTB)] were extracted from stool supernatant after centrifugation and subsequently enriched and purified via sequence-specific capture technology. Positive and negative controls SDC2 gene controls were also tested in parallel. Real-time quantitative methylation-specific polymerase chain reaction was employed to quantitatively detect SDC2 and ACTB methylation status in stool samples using a LightCycler 480 II machine (Roche, Basel, Switzerland). ACTB was amplified as a reference for DNA input. Primers, probes and amplification conditions in this study were all as previously described[32].
Positive criteria: Stool samples with CT values of SDC2 ≤ 38 were classified as positive while those with CT values of SDC2 > 38 were classified as negative under the precondition that CT values of ACTB ≤ 36. Stool samples with CT values of ACTB > 36 were considered invalid.
After mSDC2 testing, all participants were required to undergo colonoscopy. Colonoscopic examinations were performed by gastroenterology specialist sat the endoscopy center of Panyu Center Hospital. After standard bowel preparation, endoscopy with a minimum withdrawal time of 6 min was performed. All lesions noted were measured with opened biopsy forceps and recorded based on size, morphology and localization. Neoplastic lesions observed on colonoscopy were immediately removed and/or biopsied for histologic diagnosis. Individuals suspected of having CRC or polyps that could not be removed endoscopically were referred for surgery. If more than one polyp was noted, the most advanced pathological lesion or largest lesion was considered in analyses.
CRC was staged according to eight edition guidelines of the American Joint Committee on Cancer[33]. AA was defined as an adenomatous lesion with either a diameter of ≥ 10 mm, of ≥ 25% villous character or characterized by high-grade dysplasia[29,30]. Advanced colorectal neoplasia (ACN) was defined as evident CRC and AA lesions. Non-adenomatous polyps (NAP) included inflammatory, hyperplastic and juvenile polyps[29]. Other findings of diagnostic evaluation included completely normal appearance as well as the presence of colitis and colonic diverticulum.
All data were represented as numbers and relevant detection rates (DR). Pearson chi-squared or Fisher’s exact tests were used, as deemed appropriate, to compare detection data and patient clinicopathological characteristics. A logistic regression model was constructed to compare high-risk factors for colorectal lesions as well as odds ratios and relevant 95%CI to determine exposure risk. Additionally, P values of logistic model data were adjusted via false discover rate methods to ensure reliability of significance testing. Data analyses were performed using SPSS software version 23.0 (IBM, Armonk, United States). Receiver operating characteristic (ROC) curve and predictive value [sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), area under the curve and relevant 95%CI] analyses were performed using GraphPad Prism version 8.0. P < 0.05 was considered statistically significant at α = 0.05.
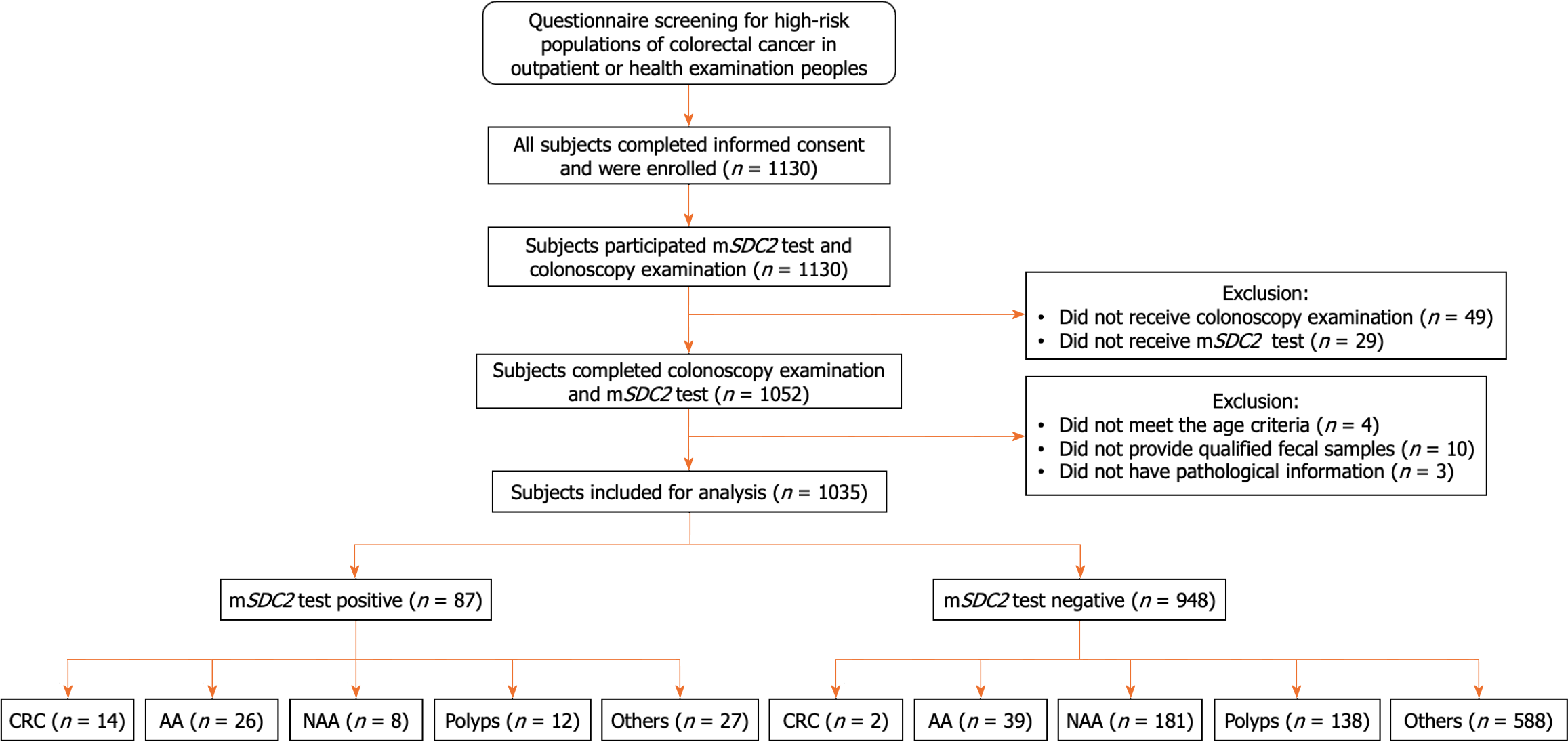
This study enrolled a total of 1130 high-risk subjects who were either outpatients at our hospital’s department of gastroenterology or attending well-adult examination sat the health examination center. After excluding 49 persons with inadequate colonoscopy findings and 29 persons who did not complete mSDC2 testing, a total of 1052 subjects were effectively screened and the pass rate was 93.10% (1052/1130). After excluding three patients who lacked pathological data, 10 subjects without qualifying stool samples and four persons who did not meet the age requirement, 1035 cases were finally evaluated in this study (Figure 1). Our studied sample included 502 males and 533 females 40-79 years of age (median age: 52). Most subjects were 50-59 years of age (39.22%); few were 70-79 years of age (4.35%). Statistical data are shown in Table 1. A total of 87 subjects (87/1035, 8.41%) were positive on mSDC2 testing, including 52 males (52/502, 10.36%) and 35 females (35/533, 6.57%); data significantly different among the two groups (χ2 = 4.828, P = 0.028). Furthermore, rates of positivity for different age groups (i.e., 40-49, 50-59, 60-69, 70-79) were 4.02% (15/373), 9.61% (39/406), 11.85% (25/211), and 17.78% (8/45), respectively. Rates of positivity on mSDC2 testing increased with age; rates of positivity significantly differed among age groups (χ2 = 18.454, P < 0.001).
| Characteristics | Cases (N%) | mSDC2 (N%) | P value | |
| (+) n = 87 | (-) n = 948 | |||
| Gender | 0.028a | |||
| Male | 502 (48.50) | 52 (5.02) | 450 (43.48) | |
| Female | 533 (51.50) | 35 (3.38) | 498 (48.12) | |
| Age | < 0.001b | |||
| 40-49 | 373 (36.04) | 15 (1.45) | 358 (34.59) | |
| 50-59 | 406 (39.22) | 39 (3.77) | 367 (35.46) | |
| 60-69 | 211 (20.39) | 25 (2.42) | 186 (17.97) | |
| 70-79 | 45 (4.35) | 8 (0.77) | 37 (3.57) | |
| Family history of CRC | < 0.001b | |||
| Yes | 99 (9.57) | 18 (1.74) | 81 (7.83) | |
| No | 936 (90.43) | 69 (6.67) | 867 (83.77) | |
| History of colorectal polyps | 0.406 | |||
| Yes | 252 (24.35) | 18 (1.74) | 234 (22.61) | |
| No | 783 (75.65) | 69 (6.67) | 714 (68.99) | |
| History of chronic diarrhea | 0.108 | |||
| Yes | 342 (33.04) | 22 (2.13) | 320 (30.92) | |
| No | 693 (66.96) | 65 (6.28) | 628 (60.68) | |
| History of chronic constipation | 0.747 | |||
| Yes | 493 (47.63) | 40 (3.86) | 453 (43.77) | |
| No | 542 (52.37) | 47 (4.54) | 495 (47.83) | |
| History of hematochezia | 0.692 | |||
| Yes | 580 (56.04) | 47 (4.54) | 533 (51.50) | |
| No | 455 (43.96) | 40 (3.86) | 415 (40.10) | |
| History of appendicitis | 0.243 | |||
| Yes | 66 (6.38) | 3 (0.29) | 63 (6.09) | |
| No | 969 (93.62) | 84 (8.12) | 885 (85.51) | |
| History of cholecystitis | 0.836 | |||
| Yes | 66 (6.38) | 6 (0.58) | 60 (5.80) | |
| No | 969 (93.62) | 81 (7.83) | 888 (85.80) | |
| History of psychiatric trauma | 0.890 | |||
| Yes | 63 (6.09) | 5 (0.48) | 58 (5.60) | |
| No | 972 (93.91) | 82 (7.92) | 890 (85.99) | |
| Pathological classification | < 0.001b | |||
| CRC | 16 (1.55) | 14 (1.35) | 2 (0.19) | |
| AA | 65 (6.28) | 26 (2.51) | 39 (3.77) | |
| NAA | 189 (18.26) | 8 (0.77) | 181 (17.49) | |
| Polyps | 150 (14.49) | 12 (1.16) | 138 (13.33) | |
| Others | 615 (59.42) | 27 (2.61) | 588 (56.81) | |
As shown in Figure 1 and Table 1, a total of 420 patients (420/1035, 40.58%) with intestinal lesions of different severity and 615 others (e.g., normal findings on colonoscopy, colitis, colonic diverticulum, etc.; 615/1035, 59.42%) were evaluated on colonoscopy. Among all intestinal lesions, 16 cases of CRC diverticulum (16/1035, 1.55%), 65 cases of AA (65/1035, 6.28%), 189 cases of non-AA (189/1035, 18.26%), and 150 cases of polyps (150/1035, 14.49%) were diagnosed. All lesions were verified on histopathology. The DR of intestinal lesions in males was significantly higher than that in females (47.41% vs 34.15%, χ2 = 18.862, P < 0.001; Table 2). DR markedly increased with age (χ2 = 55.920, P < 0.001) with the highest rate of positivity among individuals 70-79 years of age (28/45, 62.22%). Pathological CRC characteristics of lesions detected in this study are detailed in Table 3.
| Characteristics | Intestinal lesions (N%) | Others (N%) | P value | |||
| CRC | AA | NAA | Polyps | |||
| Gender | < 0.001a | |||||
| Male | 9 (0.87) | 43 (4.15) | 109 (10.53) | 77 (7.44) | 264 (25.51) | |
| Female | 7 (0.68) | 22 (2.13) | 80 (7.73) | 73 (7.05) | 351 (33.91) | |
| Age (yr) | < 0.001a | |||||
| 40-49 | 3 (0.29) | 8 (0.77) | 48(4.64) | 46 (4.44) | 268 (25.89) | |
| 50-59 | 5 (0.48) | 30 (2.90) | 73 (7.05) | 59 (5.70) | 239 (23.09) | |
| 60-69 | 7 (0.68) | 18 (1.74) | 57 (5.51) | 38 (3.67) | 91 (8.79) | |
| 70-79 | 1 (0.10) | 9 (0.87) | 11 (1.06) | 7 (0.68) | 17 (1.64) | |
| mSDC2 | < 0.001a | |||||
| Positive | 14 (1.35) | 26 (2.51) | 8 (0.77) | 12 (1.16) | 27 (2.61) | |
| Negative | 2 (0.19) | 39 (3.77) | 181 (17.49) | 138 (13.33) | 588 (56.81) | |
| Characteristics | Colonoscopy (N = 16) | mSDC2 (N%) | |
| Positive (n = 14) | Negative (n = 2) | ||
| Gender | |||
| Male | 9 (56.25) | 8 (50.00) | 1 (6.25) |
| Female | 7 (43.75) | 6 (37.50) | 1 (6.25) |
| Age (yr) | |||
| 40-49 | 3 (18.75) | 2 (12.50) | 1 (6.25) |
| 50-59 | 5 (31.25) | 5 (31.25) | 0 (0) |
| 60-69 | 7 (43.75) | 7 (43.75) | 0 (0) |
| 70-79 | 1 (6.25) | 0 (0) | 1 (6.25) |
| TNM stage | |||
| 0/I/II | 8 (50.00) | 6 (43.7) | 2 (12.50) |
| III/IV | 5 (31.25) | 5 (31.25) | 0 (0) |
| Unknown | 3 (18.75) | 3 (18.75) | 0 (0) |
| Tumor location | |||
| Proximal | 3 (18.75) | 3 (18.75) | 0 (0) |
| Distal | 13 (81.25) | 11 (68.75) | 2 (12.50) |
| Tumor size (mm) | |||
| ≤ 30 | 9 (56.25) | 7 (43.75) | 2 (12.50) |
| > 30 | 7 (43.75) | 7 (43.75) | 0 (0) |
| Dysplasia | |||
| Low | 0 (0) | 0 (0) | 0 (0) |
| Median | 12 (75.00) | 12 (75.00) | 0 (0) |
| High | 4 (25.00) | 2 (12.50) | 2 (12.50) |
Importantly, mSDC2 test positivity, sex, age, a family history of CRC and a history of polyps were all significant risk factors for the development of ACN (i.e., CRC and AA). Otherwise, chronic diarrhea, constipation, appendicitis and cholecystitis, as well as hematochezia and a history of psychiatric trauma, were not significant risk factors for CRC (Table 4).
| Characteristics | B | SE | Wald | df | P value | OR | 95%CI | |
| Lower limit | Upper limit | |||||||
| Gender | ||||||||
| Male | -0.768 | 0.294 | 6.816 | 1 | 0.009b | 0.464 | 0.261 | 0.826 |
| Age | ||||||||
| 40-49 | 22.792 | 3 | < 0.001c | |||||
| 50-59 | -2.627 | 0.567 | 21.459 | 1 | < 0.001c | 0.072 | 0.024 | 0.220 |
| 60-69 | -1.612 | 0.499 | 10.422 | 1 | 0.001c | 0.200 | 0.075 | 0.531 |
| 70-79 | -1.266 | 0.516 | 6.010 | 1 | 0.014a | 0.282 | 0.102 | 0.776 |
| mSDC2 | ||||||||
| Positive | -2.798 | 0.320 | 76.308 | 1 | < 0.001c | 0.061 | 0.033 | 0.114 |
| Family history of CRC | ||||||||
| Yes | -1.674 | 0.530 | 9.958 | 1 | 0.002b | 0.188 | 0.066 | 0.530 |
| History of colorectal polyps | ||||||||
| Yes | 2.055 | 0.580 | 12.560 | 1 | < 0.001c | 7.809 | 2.506 | 24.335 |
| History of chronic diarrhea | ||||||||
| Yes | 0.077 | 0.405 | 0.036 | 1 | 0.850 | 1.080 | 0.488 | 2.388 |
| History of chronic constipation | ||||||||
| Yes | 0.417 | 0.399 | 1.090 | 1 | 0.296 | 1.518 | 0.694 | 3.320 |
| History of hematochezia | ||||||||
| Yes | 0.322 | 0.442 | 0.529 | 1 | 0.467 | 1.380 | 0.580 | 3.284 |
| History of appendicitis | ||||||||
| Yes | 0.860 | 0.818 | 1.105 | 1 | 0.293 | 2.362 | 0.476 | 11.729 |
| History of cholecystitis | ||||||||
| Yes | -0.306 | 0.555 | 0.305 | 1 | 0.581 | 0.736 | 0.248 | 2.183 |
| History of psychiatric trauma | ||||||||
| Yes | 1.287 | 0.912 | 1.991 | 1 | 0.158 | 3.621 | 0.606 | 21.633 |
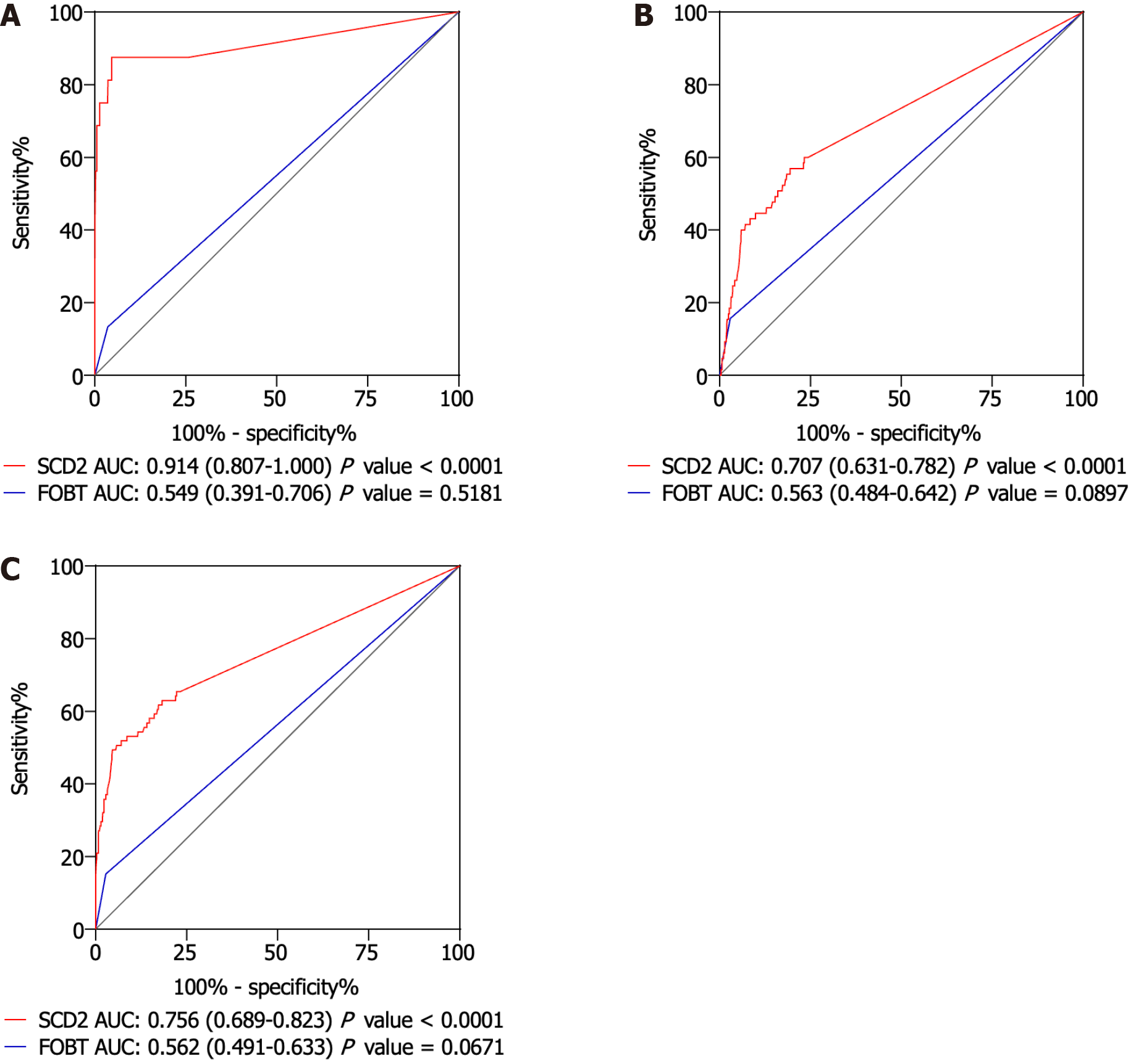
To assess the performance of the mSDC2 test in identifying intestinal malignancies, an ROC curve was constructed (Figure 2). The value of the area under the ROC curve (AUC) in regards to detecting SDC2 gene methylation in CRC cases was 0.914 (95%CI: 0.807-1.000), highlighting the capacity of this test to accurately distinguish CRC lesions from non-advanced or benign tissue (Figure 2A). For AAs, the AUC value decreased to 0.707 (95%CI: 0.631-0.782), indicating relatively standard test efficacy in detection of precancerous lesions (Figure 2B). For ACN, the AUC value was 0.756 (95%CI: 0.689-0.823; Figure 2C). Furthermore, compared with fecal occult blood testing, mSDC2 testing exhibited significantly improved diagnostic sensitivity, specificity and AUC values for CRC, AA and ACN, suggesting that mSDC2 is effective for use in the early diagnosis of malignancy. Although the price of mSDC2 testing far exceeds that of fecal occult blood testing, early detection of CRC has the potential to save lives and direct more effective clinical intervention. As such, the long-term benefits of mSDC2 testing warrant its use in clinic.
In this study, as shown in Tables 1, 2 and 5, the mSDC2 test was able to identify 14 out of 16 CRC cases with a sensitivity of 87.50% (95%CI: 60.41-97.80). For AA cases, sensitivity was 40.00% (95%CI: 28.28-52.90). The specificity of the mSDC2 test for detecting other conditions was 95.61% (95%CI: 93.59-97.03) among 615 individuals. For 954 subjects who were diagnosed with conditions other than AA or CRC, mSDC2 test specificity was 95.07% (95%CI: 93.45-96.32).
| Category | Colonoscopy (N = 1035) | mSDC2 test (N = 1035) | PPV % (95%CI) | NPV % (95%CI) | |
| Positive (n = 87) | Sensitivity % (95%CI) | ||||
| Colorectal cancer | 16 | 14 | 87.50 (60.41-97.80) | 16.09 (9.38-25.87) | 99.79 (99.15-99.96) |
| Advanced adenoma | 65 | 26 | 40.00 (28.28-52.90) | 29.89 (20.78-40.79) | 95.89 (94.37-97.02) |
| Advanced colorectal neoplasia | 81 | 40 | 49.38 (38.19-60.64) | 45.98 (35.36-56.96) | 95.68 (94.13-96.84) |
| Negative (n = 948) | Specificity % (95%CI) | ||||
| Others | 615 | 588 | 95.61 (93.59-97.03) | ||
| Others, polyps and NAA | 954 | 907 | 95.07 (93.45-96.32) | ||
| Normal | 552 | 527 | 95.47 (93.29-96.99) | ||
The PPVs of the mSDC2 test for CRC, AA and ACN were 16.09% (14/87) (95%CI: 9.38-25.87), 29.89% (26/87) (95%CI: 20.78-40.79) and 45.98% (40/87) (95%CI: 35.36-56.96), respectively. The NPVs of this test for CRC, AA, and ACN were 99.79% (946/948) (95%CI: 99.15-99.96), 95.89% (909/948) (95%CI: 94.37-97.02) and 95.68% (907/948) (95%CI: 94.13-96.84), respectively.
The risk factors questionnaire is a cost-effective and easily administered tool, but its high rate of false positivity and subjectivity have resulted in low compliance with colonoscopy among patients it identifies as indicated to undergo the procedure. Consequently, the effectiveness of large-scale population screening remains effectively compromised. To address such limitations, this study evaluated the efficacy, specificity and sensitivity of mSDC2 testing for detection of intestinal malignancies. Our findings indicate that mSDC2 testing is a valuable complementary approach for large-scale population screening. Furthermore, mSDC2 testing can enhance patient compliance with colonoscopy, more precisely identify patients indicated to undergo colonoscopy, conserve medical resources, and improve screening efficiency.
Importantly, mSDC2 testing can also miss diagnoses. Based on our study as well as previous literature, the rate of missed diagnosis for CRC was estimated to be 10%-15%. Missed diagnoses can occur for several reasons. Firstly, as this is a self-sampling product, test accuracy may be influenced by factors such as adherence to proper sampling technique by subjects and timely sample transportation to the laboratory. Secondly, while mSDC2 is a reliable marker for CRC detection, there may be a small number of patients who do not exhibit abnormal mSDC2. To minimize missed diagnoses, it is beneficial to combine mSDC2 testing with other methods such as questionnaires and fecal occult blood tests to enhance overall disease detection. Additionally, regular and repeated testing can also effectively reduce missed diagnoses.
Despite the excellent efficacy of mSDC2 testing, its price currently remains high, thus limiting its accessibility and popularity among the general public. Many individuals are unaware of this product or even lack knowledge concerning the importance of CRC screening in general. As such, there is an urgent need to raise awareness among the general patient population. Continuous product optimization is also essential, focusing on enhancing sampling success and simplifying laboratory testing. Such improvements are bound to contribute towards expanding mSDC2 utilization worldwide.
Usually, CRC develops from adenomas and certain genes relevant for CRC pathogenesis undergo alterations in methylation levels during the adenomatous stage. Most gene promoters associated with CRC contain CpG islands; abnormal methylation of cytosine within these sequences can result in gene inactivation. As disease progresses, gene methylation increases and the number of malignant cells with methylation mutations increases. One such relevant gene is SDC2, which encodes a transmembrane proteoglycan that influences CRC cell proliferation, migration and invasive capabilities. Methylation of SDC2 leads to the silencing of DNA transcription, disrupting cell growth and differentiation, promoting tumor cell proliferation and enhancing invasive and metastatic characteristics. Interestingly, levels of mSDC2 in CRC are higher as compared to AA tissue.
Recently, enteroscopy was reported to not be a suitable method for large-scale population screening[34]. Considering China’s large population as well as an uneven distribution of medical technology across different geographic regions, comprehensive colorectal screening poses significant challenges. As such, optimization of CRC screening methods and efficiency is urgently warranted.
Here, we found that men and older individuals were more likely to possess intestinal lesions as compared to women and younger individuals, respectively. Based on known data, most specialists recommend that individuals over the age of 40 undergo screening for CRC. If no precancerous lesions such as polyps are detected during initial screening, subsequent screening should be performed five years later.
It is important to note that the sample used in this study was primarily native to Guangzhou, China, which naturally imposes geographic and ethnic limitations. In the future, large-scale studies that clinically validate mSDC2 while considering possible ethnic and geographic differences within a studied population will be necessary. Finally, combinations of stool mSDC2 methylation analysis with other tumor markers, such as combined mSDC2 and serum CEA detection[35], stool mSDC2 and TFPI2 methylation detection[36], or stool mSDC2 and NDRG4 methylation detection[37], significantly enhance both sensitivity and diagnostic accuracy of CRC. Further investigation relevant to this topic is urgently warranted to develop novel simple, accurate and efficacious CRC screening methods.
In summary, stool mSDC2 testing carries a sensitivity of 87.50% for the detection of CRC among high-risk populations, underscoring its clinical value in early cancer screening. Importantly, mSDC2 serves as a reliable biomarker for CRC diagnosis. Use of stool samples for analyses offers a simpler and less invasive approach, minimizing patient discomfort and promoting patient compliance.
Here, we solely focused on evaluating SDC2 from the perspective of a diagnostic biomarker; as such, our study was not without its limitations. Multi-target fecal DNA testing, which includes markers such as WIF, NAP, PENK, SETP9, is described in literature as relevant to the diagnosis of CRC. Use of multiple fecal DNA biomarkers in combination was reported to enhance CRC DR, better identify early intestinal lesions and enable patients to receive treatment earlier, thereby reducing the case fatality rate. Thus, future research should consider incorporating multi-target fecal DNA testing in analyses to improve CRC screening accuracy and efficiency.
Colorectal cancer (CRC) is a prevalent and life-threatening malignant tumor affecting the digestive system globally. Testing for syndecan-2 methylation (mSDC2) has emerged as a widely used screening tool for early detection of CRC in stool and serum samples.
Our findings provide evidence-based data concerning diagnostic and screening methods relevant to a Chinese population at high-risk population for CRC.
To validate the effectiveness of fecal mSDC2 testing in the detection of CRC among a high-risk Chinese population.
A high-risk Chinese cohort composed of 1130 individuals 40-79 years of age was selected for evaluation using the fecal mSDC2 test. Sensitivity and specificity to CRC, advanced adenoma (AA) and advanced colorectal neoplasia (ACN) were quantified. High-risk factors for the incidence of colorectal lesions were analyzed; a logistic regression model was subsequently constructed to better reflect the efficacy of fecal mSDC2 testing.
According to criteria previously established, 1035 high-risk individuals were included in analyses. Among them, 16 CRC cases (1.55%), 65 AA cases (6.28%), 189 non-advanced adenoma cases (18.26%), and 150 cases of polyps (14.49%) were successfully identified on colonoscopy and pathological examination. The sensitivities of mSDC2 testing for CRC and AA were 87.50% and 40.00%, respectively; the specificity for subjects in the “others” group was 95.61%. The positive predictive values of mSDC2 testing for CRC, AA, and ACN were 16.09%, 29.89% and 45.98%, respectively. In addition, negative predictive value of mSDC2 testing for CRC was 99.79%. Positivity on mSDC2 testing is a significant risk factor for the development of ACN (P < 0.001) after adjusting for other high-risk covariates.
The results of this CRC screening study revealed that offering patients a combination of fecal mSDC2 testing and colonoscopy is ideal for facilitating early detection of CRC among a high-risk Chinese population. CRC screening study revealed that offering patients a combination of fecal mSDC2 testing and colonoscopy is ideal for facilitating early detection of CRC among a high-risk Chinese population.
Detection of stool mSDC2 offers great promise for early and effective CRC screening.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miyoshi E, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Wu CW, Lui RN. Early-onset colorectal cancer: Current insights and future directions. World J Gastrointest Oncol. 2022;14:230-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 3. | Saad El Din K, Loree JM, Sayre EC, Gill S, Brown CJ, Dau H, De Vera MA. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 4. | Siegel RL, Medhanie GA, Fedewa SA, Jemal A. State Variation in Early-Onset Colorectal Cancer in the United States, 1995-2015. J Natl Cancer Inst. 2019;111:1104-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, Lim YC, Kibria KMK, Mohiuddin AKM, Ming LC, Goh KW, Hadi MA. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 417] [Article Influence: 139.0] [Reference Citation Analysis (1)] |
| 6. | Liao C, Chen X, Fu Y, Salivary. analysis: An emerging paradigm for non-invasive healthcare diagnosis and monitoring. Interdiscip Med. 2023;. [DOI] [Full Text] |
| 7. | Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for Colorectal Cancer Screening. Gastroenterology. 2020;158:418-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 415] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 8. | Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis. Ann Intern Med. 2019;170:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 9. | Hata T, Chiba K, Mizuma M, Masuda K, Ohtsuka H, Nakagawa K, Morikawa T, Hayashi H, Motoi F, Unno M. Levels of tumor markers CEA/CA 19-9 in serum and peritoneal lavage predict postoperative recurrence in patients with pancreatic cancer. Ann Gastroenterol Surg. 2022;6:862-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Kato H, Kishiwada M, Hayasaki A, Chipaila J, Maeda K, Noguchi D, Gyoten K, Fujii T, Iizawa Y, Tanemura A, Murata Y, Kuriyama N, Usui M, Sakurai H, Isaji S, Mizuno S. Role of Serum Carcinoma Embryonic Antigen (CEA) Level in Localized Pancreatic Adenocarcinoma: CEA Level Before Operation is a Significant Prognostic Indicator in Patients With Locally Advanced Pancreatic Cancer Treated With Neoadjuvant Therapy Followed by Surgical Resection: A Retrospective Analysis. Ann Surg. 2022;275:e698-e707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Chen R, Jiang C, Zhu Q, You S, Li Y, Li S, Ding L, Meng H, Yang Y, Zha X, Wang J. Combining the tumor abnormal protein test with tests for carcinoembryonic antigens, cancer antigen 15-3, and/or cancer antigen 125 significantly increased their diagnostic sensitivity for breast cancer. Medicine (Baltimore). 2020;99:e21231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Lakemeyer L, Sander S, Wittau M, Henne-Bruns D, Kornmann M, Lemke J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Ryan L, Wong Y, Dwyer KM, Clarke D, Kyprian L, Craig JM. Coprocytobiology: A Technical Review of Cytological Colorectal Cancer Screening in Fecal Samples. SLAS Technol. 2021;26:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Zhang W, Yang C, Wang S, Xiang Z, Dou R, Lin Z, Zheng J, Xiong B. SDC2 and TFPI2 Methylation in Stool Samples as an Integrated Biomarker for Early Detection of Colorectal Cancer. Cancer Manag Res. 2021;13:3601-3617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Ansa BE, Lewis N, Hoffman Z, Datta B, Johnson JA. Evaluation of Blood Stool Test Utilization for Colorectal Cancer Screening in Georgia, USA. Healthcare (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 16. | Grady WM, Yu M, Markowitz SD. Epigenetic Alterations in the Gastrointestinal Tract: Current and Emerging Use for Biomarkers of Cancer. Gastroenterology. 2021;160:690-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 17. | Joo JE, Clendenning M, Wong EM, Rosty C, Mahmood K, Georgeson P, Winship IM, Preston SG, Win AK, Dugué PA, Jayasekara H, English D, Macrae FA, Hopper JL, Jenkins MA, Milne RL, Giles GG, Southey MC, Buchanan DD. DNA Methylation Signatures and the Contribution of Age-Associated Methylomic Drift to Carcinogenesis in Early-Onset Colorectal Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Hong J, Rhee JK. Genomic Effect of DNA Methylation on Gene Expression in Colorectal Cancer. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 19. | Koch A, Joosten SC, Feng Z, de Ruijter TC, Draht MX, Melotte V, Smits KM, Veeck J, Herman JG, Van Neste L, Van Criekinge W, De Meyer T, van Engeland M. Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol. 2018;15:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 20. | Chen J, Sun H, Tang W, Zhou L, Xie X, Qu Z, Chen M, Wang S, Yang T, Dai Y, Wang Y, Gao T, Zhou Q, Song Z, Liao M, Liu W. DNA methylation biomarkers in stool for early screening of colorectal cancer. J Cancer. 2019;10:5264-5271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1241] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 22. | US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1387] [Article Influence: 154.1] [Reference Citation Analysis (1)] |
| 23. | Yue C, Zhang Y, Wang Y, Zhang Z, Zhang M, Wang H, Chen W, Shang Z, Xin Y, Zhang X. The Application Value of Syndecan-2 Gene Methylation for Colorectal Cancer Diagnosis: A Clinical Study and Meta-Analyses. Front Med (Lausanne). 2022;9:753545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Wang L, Liu Y, Zhang D, Xiong X, Hao T, Zhong L, Zhao Y. Diagnostic accuracy of DNA-based SDC2 methylation test in colorectal cancer screening: a meta-analysis. BMC Gastroenterol. 2022;22:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Dong L, Lu C, Huang W, Yang C, Wang Q, Lei R, Sun R, Wan K, Li T, Sun F, Gan T, Lin J, Yin L. Methylation of SDC2/TFPI2 and Its Diagnostic Value in Colorectal Tumorous Lesions. Front Mol Biosci. 2021;8:706754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Zhao G, Li H, Yang Z, Wang Z, Xu M, Xiong S, Li S, Wu X, Liu X, Zhu Y, Ma Y, Fei S, Zheng M. Multiplex methylated DNA testing in plasma with high sensitivity and specificity for colorectal cancer screening. Cancer Med. 2019;8:5619-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Kim JH, Park SC. Syndecan-2 Methylation as a New Biomarker for Early Detection of Colorectal Neoplasm. Gut Liver. 2018;12:479-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ryu HY, Lee J, Yang S, Park H, Choi S, Jung KC, Lee ST, Seong JK, Han IO, Oh ES. Syndecan-2 functions as a docking receptor for pro-matrix metalloproteinase-7 in human colon cancer cells. J Biol Chem. 2009;284:35692-35701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Meng W, Cai SR, Zhou L, Dong Q, Zheng S, Zhang SZ. Performance value of high risk factors in colorectal cancer screening in China. World J Gastroenterol. 2009;15:6111-6116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Lin G, Feng Z, Liu H, Li Y, Nie Y, Liang Y, Li K. Mass screening for colorectal cancer in a population of two million older adults in Guangzhou, China. Sci Rep. 2019;9:10424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | The Chinese Society of Digestive Endoscopy, Tumor Endoscopy Committee of China Anti-Cancer Association. [China Guideline for the Early Colorectal Cancer Screening and Treatment by Endoscopology (2014, Beijing)]. Chinese Journal of Degistive Endoscopology. 2015;32:341-360. [DOI] [Full Text] |
| 32. | Wang J, Liu S, Wang H, Zheng L, Zhou C, Li G, Huang R, Li C, Fan X, Fu X, Wang X, Guo H, Guan J, Sun Y, Song X, Li Z, Mu D, Sun J, Liu X, Qi Y, Niu F, Chen C, Wu X, Zou H. Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: a multicenter clinical study. Clin Epigenetics. 2020;12:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Amin MB, Gress DM, Meyer Vega LR, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Compton CC. AJCC Cancer Staging Manual 8th edition. New York: Springer, 2017: 252-274. |
| 34. | Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, Rupinski M, Dekker E, Spaander M, Bugajski M, Holme Ø, Zauber AG, Pilonis ND, Mroz A, Kuipers EJ, Shi J, Hernán MA, Adami HO, Regula J, Hoff G, Kaminski MF; NordICC Study Group. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 410] [Article Influence: 136.7] [Reference Citation Analysis (1)] |
| 35. | Li J, Sun Z. [Application of noninvasive feces SDC2 methylation combined with serum CEA in the early diagnosis of colorectal cancer and the value assessment]. Jilin Med J. 2021;42:1582-1584. [DOI] [Full Text] |
| 36. | Wu H, Fang H, Deng Y, He B. [Analysis on diagnostic efficacy of combined detection of fecal genes SDC2 andTFPI2 methylation in diagnosing colorectal cancer and precancerous lesions]. Modern Med J China. 2023;25:7-12. |
| 37. | Jin W, Zhang X, Liu L, Zhou Q, Miao Y, Tan X, Tan L. [Application of SDC2 and NDRG4 gene methylation detection in colorectal cancer screening]. Journal of Hunan Normal University (Medical Science). 2021;18:88-92. [DOI] [Full Text] |