Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1309
Peer-review started: October 30, 2023
First decision: December 5, 2023
Revised: December 18, 2023
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: April 15, 2024
Processing time: 163 Days and 18.3 Hours
Despite continuous changes in treatment methods, the survival rate for advanced hepatocellular carcinoma (HCC) patients remains low, highlighting the importance of diagnostic methods for HCC.
To explore the efficacy of texture analysis based on multi-parametric magnetic resonance (MR) imaging (MRI) in predicting microvascular invasion (MVI) in preoperative HCC.
This study included 105 patients with pathologically confirmed HCC, categorized into MVI-positive and MVI-negative groups. We employed Original Data Analysis, Principal Component Analysis, Linear Discriminant Analysis (LDA), and Non-LDA (NDA) for texture analysis using multi-parametric MR images to predict preoperative MVI. The effectiveness of texture analysis was determined using the B11 program of the MaZda4.6 software, with results expressed as the misjudgment rate (MCR).
Texture analysis using multi-parametric MRI, particularly the MI + PA + F dimensionality reduction method combined with NDA discrimination, demonstrated the most effective prediction of MVI in HCC. Prediction accuracy in the pulse and equilibrium phases was 83.81%. MCRs for the combination of T2-weighted imaging (T2WI), arterial phase, portal venous phase, and equilibrium phase were 22.86%, 16.19%, 20.95%, and 20.95%, respectively. The area under the curve for predicting MVI positivity was 0.844, with a sensitivity of 77.19% and specificity of 91.67%.
Texture analysis of arterial phase images demonstrated superior predictive efficacy for MVI in HCC compared to T2WI, portal venous, and equilibrium phases. This study provides an objective, non-invasive method for preoperative prediction of MVI, offering a theoretical foundation for the selection of clinical therapy.
Core Tip: Texture analysis using arterial phase images provides superior predictive efficacy for microvascular invasion (MVI) in hepatocellular carcinoma (HCC) compared to T2-weighted imaging, portal venous, and equilibrium phases. The texture analysis of liver magnetic resonance images holds significant value for the preoperative prediction of MVI in HCC patients.
- Citation: Nong HY, Cen YY, Qin M, Qin WQ, Xie YX, Li L, Liu MR, Ding K. Application of texture signatures based on multiparameter-magnetic resonance imaging for predicting microvascular invasion in hepatocellular carcinoma: Retrospective study. World J Gastrointest Oncol 2024; 16(4): 1309-1318
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1309.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1309
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers worldwide[1]. Currently, the optimal treatment for liver cancer remains radical resection. However, many patients miss the opportunity for curative surgery at the time of diagnosis. Despite the availability of treatments for advanced, unresectable HCC, such as transcatheter arterial chemoembolization, radiofrequency thermal ablation, and molecular targeted drugs (including tyrosine kinase inhibitors like sorafenib and lovatinib), their efficacy remains limited[2]. Thus, there is an urgent need for more effective diagnostic methods to detect the disease status of patients early and enhance their survival rates.
Microvascular invasion (MVI) is a crucial risk factor influencing tumor relapse post-surgery[3-5]. Accurate pre-surgery prediction of MVI influences the selection of tumor treatment plans. Currently, the assessment of HCC MVI primarily depends on postoperative pathological biopsies; however, this method is invasive and carries risks like bleeding, tumor implantation, and metastasis. Image texture analysis offers an objective and quantitative means of image description. The local characteristics of image pixel gray values, their changing patterns, and distribution, as quantitatively analyzed, can reflect the physiological heterogeneity within the region of interest (ROI). Recently, it has been applied in a range of medical imaging techniques to aid in disease diagnosis and treatment[6-9]. Previous studies have indicated that computed tomography (CT) imaging is the principal method for texture analysis in predicting MVI in HCC[10,11], with most studies opting for 2D ROIs. Compared to 3D ROIs, these studies lacked comprehensive assessment of tumor heterogeneity[12-14]. Furthermore, the software, analysis, and discrimination methods used in medical imaging texture analysis vary. Texture analysis is widely used to predict disease progression, including aggressiveness, disease-free survival, and overall survival in patients with pancreatic ductal adenocarcinoma[15]. Recent studies have also demonstrated that multiparametric magnetic resonance (MR) imaging (MRI) as a standard of pre-biopsy care has increased the prostate cancer detection rate from 26% to 38%[16]. Medical image texture analysis is utilized to quantitatively extract features not discernible to the naked eye, further reflecting the distribution pattern and changes in pixel gray values in the ROI, aiding in the identification of potential heterogeneity in tumor lesions[17].
In this study, our goal is to acquire quantitative features from the ROI using a high-throughput method and forecast MVI in HCC through texture analysis based on MRI. We aim to offer an objective auxiliary method for noninvasive preoperative prediction of MVI in HCC, potentially guiding clinical decisions.
From June 2016 to March 2022, 105 eligible HCC patients from our hospital were enrolled in this study. Patients were categorized into the MVI-positive group (57 cases) and the MVI-negative group (48 cases) based on postoperative pathological results. Inclusion criteria for the study were: (1) Pathologically confirmed HCC; (2) age ≥ 18 years; (3) plain liver MRI scan and three-phase enhanced scan conducted 2 wk before surgery; and (4) completion of at least two cycles of chemotherapy. Exclusion criteria included: (1) Non-primary central nervous system lymphoma; (2) previous treatment with other BTK inhibitors; and (3) a single HCC lesion. Additionally, none of the patients had undergone any tumor-related treatment prior to surgery.
All procedures involving human participants were in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of The Third Affiliated Hospital of Guangxi Medical University. All patients provided written informed consent for their participation.
Following the methodology of Rodríguez-Perálvarez et al[18], patients were grouped into MVI-positive and MVI-negative categories based on their postoperative pathological diagnosis of MVI. The diagnostic criteria for this study were: The presence of tumor emboli in the portal vein, hepatic vein, or large blood vessels, as opposed to tumors in the small bile ducts of the surrounding liver tissue.
Preparation before MRI scan and enhanced examination: Patients were required to fast for 4 h before the examination to ensure an empty stomach.
Prior to the examination, patients were instructed on the breath-holding process to minimize breathing motion artifacts during the scan, and were asked to remove all metal objects. Patients with no contraindications for MRI examinations had an indwelling needle placed in their forearm by a nurse.
Liver MRI routine sequence and parameter settings: SIEMENS Verio 3.0T was used with the following parameters: Axial T1 VIBE FSTRA P2 HB 320 (TR/TE 3.92/1.39 ms), slice thickness 4 mm; Axial T2 BLADE TRA FS P2 TRIG 320 (TR/TE 4185.31/89.00 ms), layer thickness 6 mm. A bolus injection of gadopentetate dimeglumine (Gd-DTPA; 0.2 mmol/kg) was administered for enhanced scanning via the cubital vein using a dual-barrel high-pressure syringe at a preset flow rate of 2.0 mL/s. Sterile saline (20 mL of 0.9% solution) was injected to flush the tubes. The arterial, portal venous, and equilibrium phases were scanned at 15-20 s, 50-55 s, and 85-90 s after the contrast agent injection, respectively. The selected MR images were exported from the PACS system in BMP format.
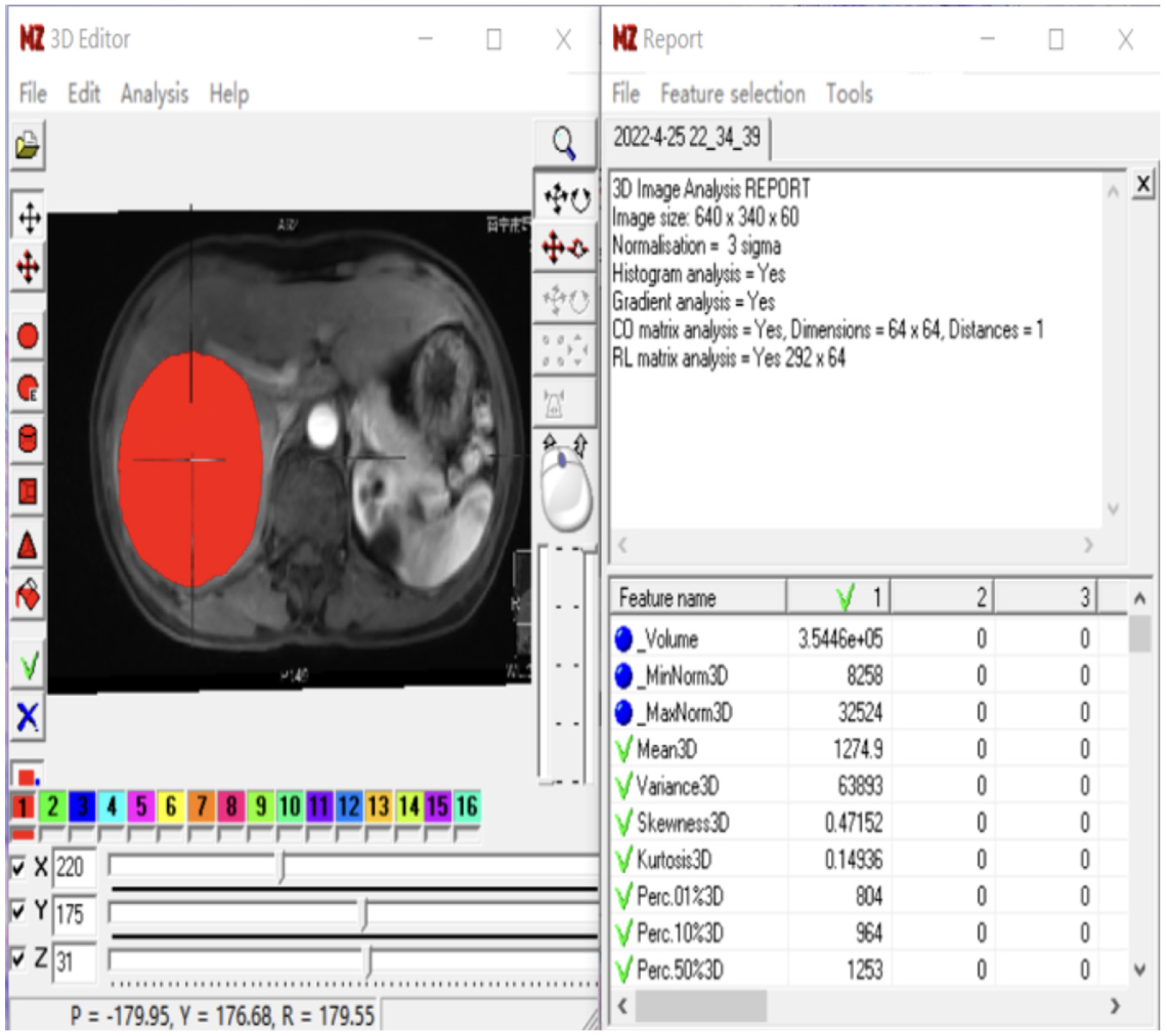
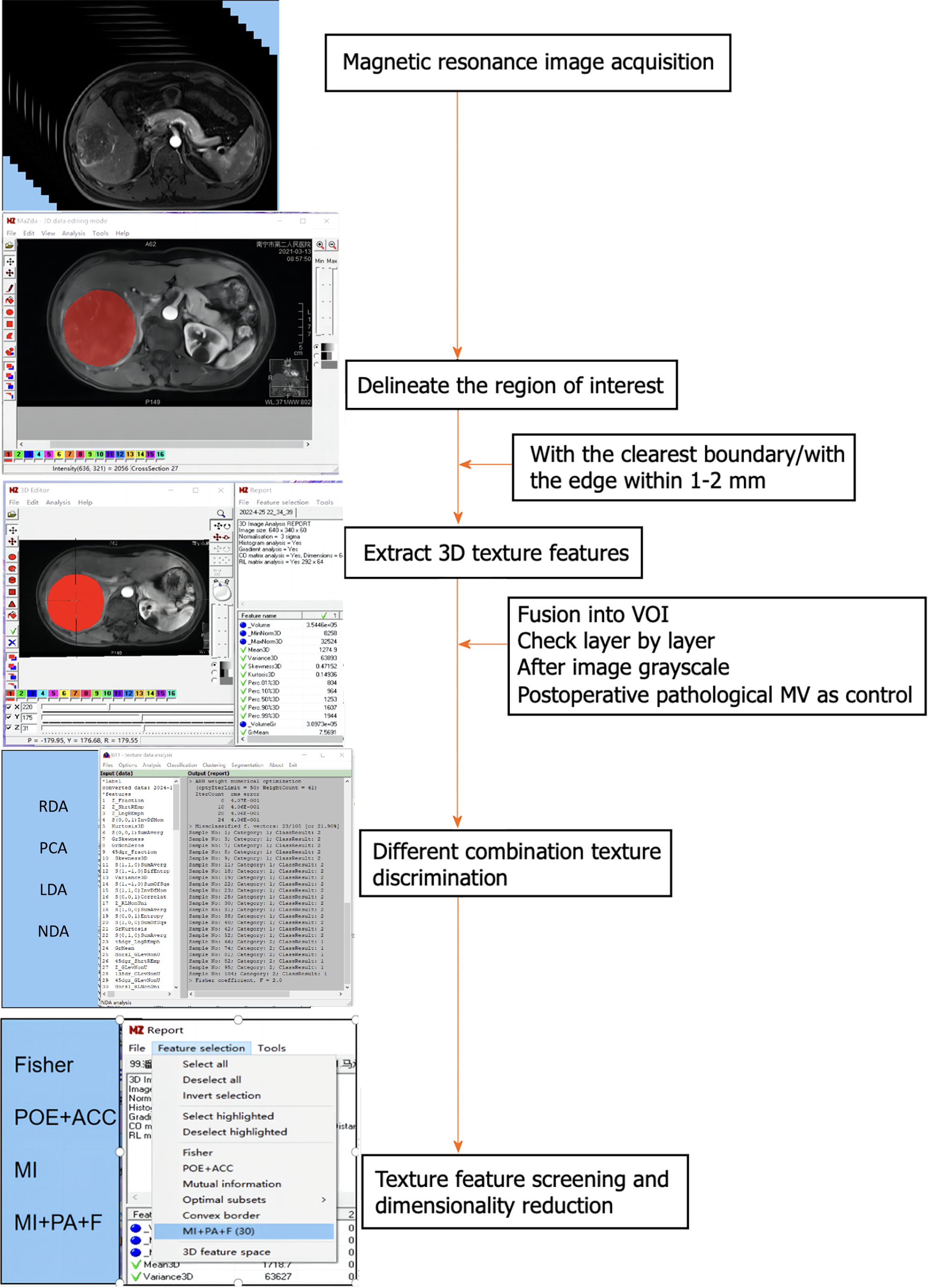
MaZda4.6 software (http://www.eletel.p.lod14z.pl/mazda/) was utilized as follows: Firstly, a radiologist identified the contrast-enhanced scan phase with the clearest lesion boundary in each HCC patient, manually drawing it layer by layer and merging it into the volume area of interest (VOI), which was then copied onto the MR image of the enhanced scanning sequence. Secondly, the ROI was manually outlined layer by layer and merged into the VOI, accounting for differences in image layer thickness and enhanced images in the T2-weighted imaging (T2WI) sequence. Finally, a senior radiologist verified the placement of all ROIs layer by layer. If there were any issues with the ROI delineation or VOI registration, the lesions were re-delineated or registered until consensus was reached. To minimize the impact of the local volume effect, the ROI was manually outlined within 1-2 mm of the tumor on all slices. Additionally, grayscale normalization was applied to all included MR images using MaZda4.6 to minimize image errors due to patient-specific conditions or variations in scanning personnel before extracting texture features[14].
To avoid excessive redundancy in texture features, the 10 most significant texture features were identified using Fisher, POE + ACC, MI, and MI + PA + F through texture feature screening (feature dimension reduction), by MaZda4.6 software. The PA + F feature dimensionality reduction method identified the top 30 texture features with the most significance for subsequent automatic classification and discrimination by a computer. Subsequently, the classification methods Redundancy Analysis, Principal Component Analysis, Linear Discriminant Analysis (LDA), and Non-LDA (NDA), included in the MaZda4.6 software's B11 program, were employed to automatically distinguish HCC in different MVI groups and calculate the misjudgment rate (MCR) based on these optimal discriminant features. MCR (%) = (total number of misjudged cases/total number of cases identified in different groups) × 100 (The VOI generated from the texture analysis based on the MR image, along with the extracted 3D texture features, are illustrated in Figure 1. and the workflow of texture analysis based on MR images was depicted in Figure 2).
Meta-analyses were performed using SPSS 19.0 and MaZda4.6 software. Results are presented as mean ± SD. Textural parameters between the two groups were compared using the independent sample Mann–Whitney U-test. Receiver operating characteristic (ROC) analyses were conducted to evaluate the diagnosis of MVI. A P value < 0.05 was considered statistically significant.
Between June 2016 and March 2022, 105 eligible HCC patients were included in this study. The cohort consisted of 13 females and 92 males, with a median age of 52.97 years (range 29-80 years). Patients were categorized into MVI-positive [57 cases; 50 males and 7 females; median age 51.35 ± 12.565 years (range 29-80)] and MVI-negative [48 cases; 42 males and 6 females; median age 54.90 ± 10.896 years (range 35-79)] groups based on postoperative pathological results (details showed in Table 1).
| MVI negative group (n = 48) | MVI positive group (n = 57) | T/χ2 | P value | |
| Age (yr) | 54.90 ± 10.896 | 51.35 ± 12.565 | -1.529 | 0.129 |
| Gender | ||||
| Male | 42 | 50 | 0.001 | 0.973 |
| Female | 6 | 7 |
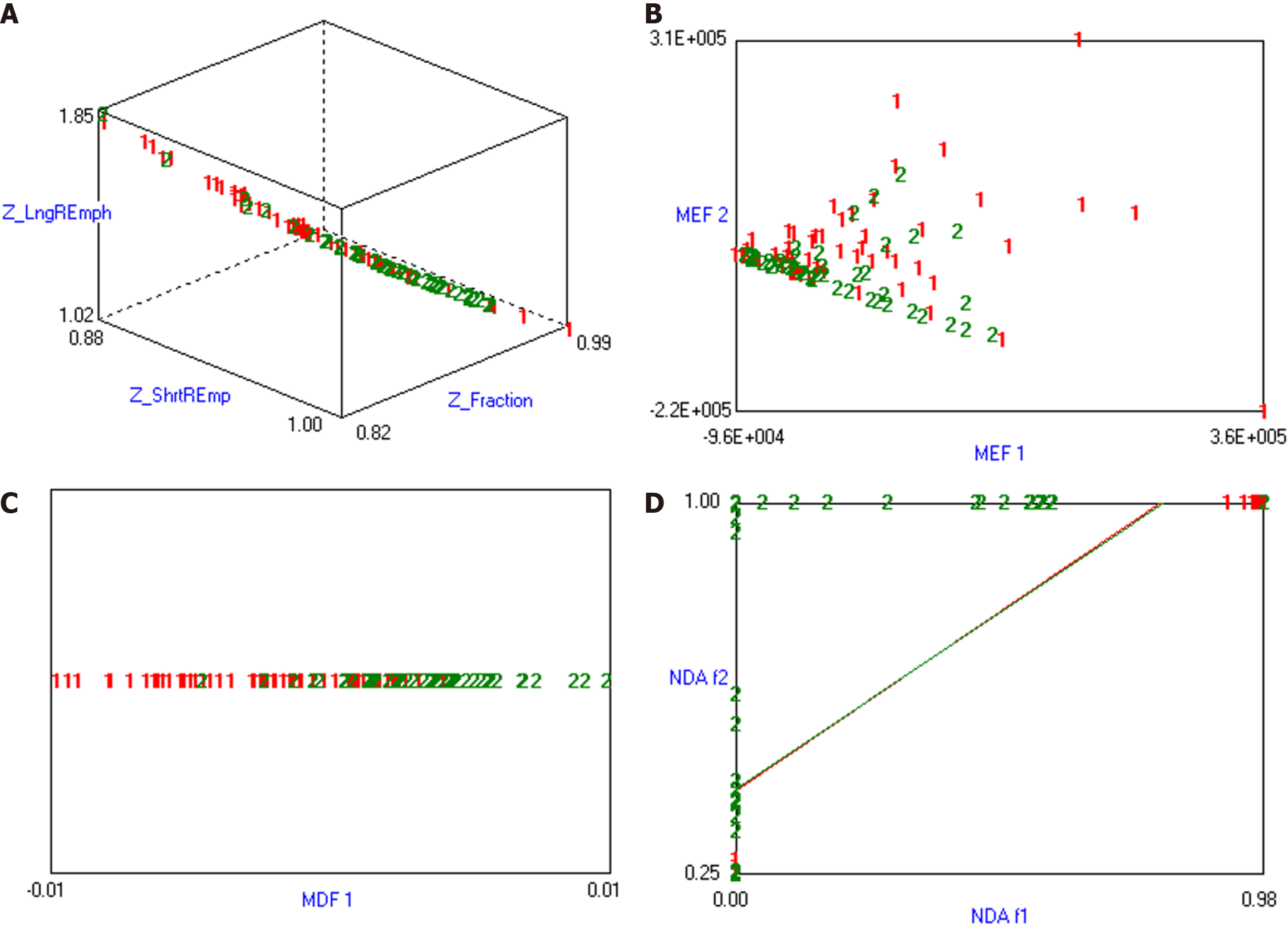
The combination of MI + PA + F and NDA in texture analysis based on MR images demonstrated the most effective prediction for HCC MVI. The MCRs were 22.86%, 16.19%, 20.95%, and 20.95%, respectively (details were presented in Table 2, and the visualization of the predicted results based on MR arterial phase image analysis was shown in Figure 3).
| Extraction method | Parameters |
| Grayscale histogram (9) | Mean, variance, skewness, kurtosis, and percentiles (1%, 10%, 50%, 90%, and 99%) |
| Grayscale co-occurrence matrix (55) | Angular second order distance, correlation, contrast, entropy, entropy sum, square sum, mean sum, mean difference, inverse distance, entropy difference, standard deviation |
| Grayscale gradient (5) | Average gradient, gradient variance, gradient skewness, gradient kurtosis, non-zero gradient |
| Long run matrices (25) | (Horizontal, vertical, 0°, 45°, 135°) Run length non-uniformity, gray value non-uniformity, long run enhancement, short run enhancement, fractional run |
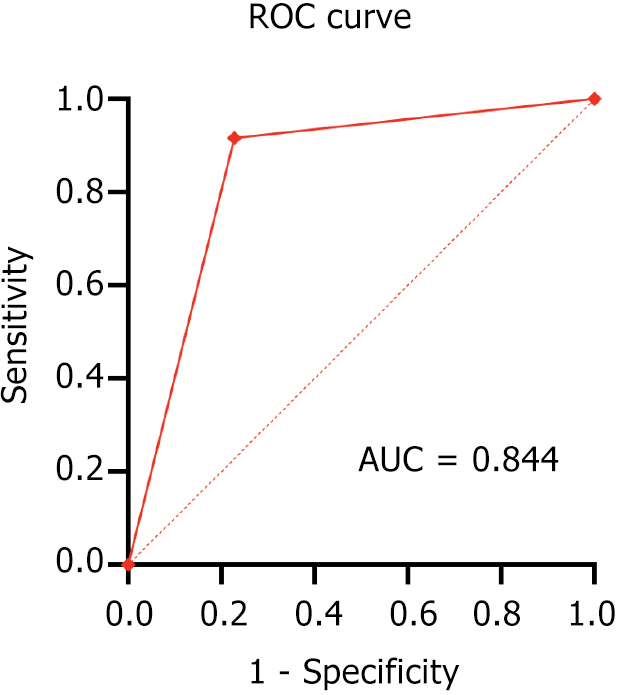
The ROC curve depicting the positive prediction of MVI in HCC using the texture analysis combination of MI + PA + F and NDA, specifically based on arterial phase MR images, was shown in Figure 4. This method also demonstrated high accuracy (lower MCR) in predicting MVI in HCC, particularly when based on arterial phase images. The prediction accuracy was 83.81%, with an area under the curve (AUC) for predicting MVI in HCC of 0.844, a sensitivity of 77.19%, and a specificity of 91.67% (Table 3 and Figure 2).
| MR image | Project | Fisher | POE + ACC | MI | MI + PA + F |
| T2WI | RDA | 57/105 (or 54.29) | 57/105 (or 54.29) | 59/105 (or 56.19) | 50/105 (or 47.62) |
| PCA | 52/105 (or 49.52) | 57/105 (or54.29) | 57/105 (or 54.29) | 58/105 (or 55.24) | |
| LDA | 46/105 (or 43.81) | 46/105 (or43.81) | 47/105 (or 44.76) | 34/105 (or 32.38) | |
| NDA | 26/105 (or 24.76) | 27/105 (or25.71) | 29/105 (or 27.62) | 24/105 (or 22.86) | |
| Arterial phase | RDA | 44/105 (or 41.90) | 52/105 (or49.52) | 38/105 (or 36.19) | 49/105 (or 46.67) |
| PCA | 46/105 (or 43.81) | 52/105 (or49.52) | 39/105 (or 37.14) | 49/105 (or 46.67) | |
| LDA | 41/105 (or 39.05) | 36/105 (or34.29) | 46/105 (or 43.81) | 34/105 (or 32.38) | |
| NDA | 22/105 (or 20.95) | 23/105 (or21.90) | 25/105 (or 23.81) | 17/105(or16.19)) | |
| Portal phase | RDA | 41/105 (or 39.05) | 46/105 (or43.81) | 48/105 (or 45.71) | 38/105 (or 36.19) |
| PCA | 41/105 (or 39.05) | 41/105 (or39.05) | 48/105 (or 45.71) | 38/105 (or 36.19) | |
| LDA | 45/105 (or 42.86) | 48/105 (or45.71) | 42/105 (or 40.00) | 32/105 (or 30.48) | |
| NDA | 32/105 (or 30.48) | 28/105 (or26.67) | 33/105 (or 31.43) | 22/105 (or 20.95) | |
| Equilibrium period | RDA | 43/105 (or 40.95) | 56/105 (or53.33) | 42/105 (or 40.00) | 48/105 (or 45.71) |
| PCA | 42/105 (or 40.00) | 55/105 (or52.38) | 45/105 (or 42.86) | 42/105 (or 40.00) | |
| LDA | 49/105 (or 46.67) | 42/105 (or40.00) | 45/105 (or 42.86) | 34/105 (or 32.38) | |
| NDA | 28/105 (or 26.67) | 23/105 (or21.90) | 27/105 (or 25.71) | 22/105 (or 20.95) |
MVI is a crucial risk factor for tumor recurrence and metastasis following HCC surgery. The occurrence of MVI is common across all stages of HCC, resulting from the combined action of multiple factors[19]. Preoperative prediction of MVI in HCC plays a significant role in treatment selection and prognosis evaluation[3,4,20]. Currently, the preoperative assessment of HCC MVI primarily relies on semantic features of multiphase CT and enhanced MRI. These include smoothness of the tumor margin, larger tumor size (> 5 cm in diameter), peritumoral arterial enhancement, lack of enhanced capsule, low tumor signal, all of which possess a degree of subjectivity and lack objective and repeatable measurement indices[21-24]. The predictive capabilities of functional imaging modalities, such as imaging diffusion kurtosis, diffusion-weighted imaging, and positron emission tomography-CT, require improvement due to their instability and lack of reproducibility[25-27]. Liver texture analysis has been reported to aid in predicting the nature of intrahepatic lesions, classifying HCC lesions, and forecasting treatment prognosis[28]. Dynamic contrast-enhanced MRI image texture analysis technology is beneficial for preoperative prediction of MVI, showing higher prediction accuracy[29]. Consequently, it is essential to further explore objective quantitative methods for more accurate preoperative of MVI prediction in HCC.
In this study, we expanded our analysis to include multi-parametric MR-enhanced images for texture analysis, encompassing images of MR-T2WI, arterial phase, portal venous phase, and equilibrium phase. The MCRs were 22.86%, 16.19%, 20.95%, and 20.95%, respectively, demonstrating high accuracy (lower MCRs) in predicting MVI of HCC, particularly the arterial phase images. The prediction accuracy was 83.81%, with an AUC of 0.844, a sensitivity of 77.19%, and a specificity of 91.67%. These results were similar to those reported by Liu et al[30], suggesting the texture quantification based on MR images was a viable method for predicting MVI in HCC[30]. Liu et al[30] developed a binary logistic regression prediction model based on MR arterial phase texture features combined with imaging features. The AUC, sensitivity, and specificity for predicting MVI in HCC were 0.810, 0.811, and 0.790, respectively. This study determined that the combination of MI + PA + F and NDA texture analysis, specifically based on MR arterial phase images, had the most effective prediction for HCC MVI.
The contribution of the hepatic arterial system to tumor supply becomes increasingly apparent and gradually dominates the development and progression of HCC. Consequently, the texture features of arterial phase images can more accurately characterize tumor progression. When MVI exists in HCC, the local hemodynamics around the tumor are more pronounced compared to the venous phase. Moreover, this study showed better performance using arterial and equilibrium phase images compared to MR-T2WI and portal venous phase, aligning with the findings of Zhu et al[31]. However, this study deviates from previous ones[32,33], where some have indicated that radiomic sores of the portal venous phase surpass those of the arterial or equilibrium phases. This could be attributed to selection bias and inclusion criteria. Nonetheless, texture analysis based on enhanced CT and MRI requires further validation for predicting HCC progression. Currently, there are limited studies comparing the efficacy of texture analysis based on CT and MR images for predicting MVI in HCC, necessitating further research.
This study had several limitations. Firstly, the images utilized were acquired by multiple scanning technicians. To mitigate the impact of the diversity in MR scanning personnel, the variability in patient conditions, and the lack of standardization in MRI, all images underwent grayscales normalization before texture feature extraction. However, future applications and development of artificial intelligence in this field will need to address the challenges posed by multi-center equipment. Thus, optimizing algorithms and conducting multicenter’s research and analysis are imperative for future advancements. Secondly, this study focused solely on comparing the identification capabilities of texture analysis based on MRI-T2WI and three-phase enhanced scanning MRI, without a comprehensive examination and comparison of clinically relevant data and imaging manifestations. The employed high-degree evaluation method aligns with the requirements for a comprehensive clinical assessment. Future research in this area should involve expanding the sample size to achieve higher predictive accuracy and identify more practical methods. Thirdly, the use of a 3D ROI may introduce some subjectivity due to manual delineation. Lastly, retrospective studies are more susceptible to bias and confounding variables, which can lead to incorrect conclusions. Future studies should focus on utilizing automatic delineation and larger datasets to mitigate these issues.
This study demonstrated that textural features based on multi-parametric MR images are reliable predictors of vascular invasion in HCC. Specifically, the MI + PA + F of arterial phase images, in conjunction with NDA texture analysis, might aid in selecting optimal choice of therapeutic approaches. However, further large-scale studies are necessary to validate these findings.
The study aims to provide an objective, non-invasive method for the hepatocellular carcinoma (HCC) prediction, addressing the limitations of current assessment methods that are invasive and carry risks. It seeks to validate the use of texture analysis in magnetic resonance (MR) imaging (MRI) as a reliable predictive tool for microvascular invasion (MVI), potentially guiding clinical decisions and improving poor patient outcomes in HCC.
The primary motivation is to enhance preoperative MVI prediction in HCC, which is crucial for selecting appropriate treatment plans.
The main objective of the study was to assess the effectiveness of texture analysis based on multi-parametric MR images in predicting MVI of HCC. The goal was to provide a non-invasive, objective method to aid in the preoperative prediction of MVI, thereby valuable information for treatment planning and prognosis evaluation in HCC patients.
The study employed a retrospective analysis approach, including 105 patients with pathologically confirmed HCC. It used texture analysis methods such as original data analysis, principal component analysis, linear discriminant analysis (LDA), and non-LDA on multi-parametric MR images. The effectiveness of these methods was evaluated using the misjudgment rate derived from the MaZda4.6 software. This approach allowes for a detailed quantitative analysis of the MR images, offering novel insights into the potential of texture analysis in medical imaging.
The study found that texture analysis of arterial phase images from multi-parametric MRI was highly effective in predicting MVI in HCC. The combination of MI + PA + F dimensionality reduction method and nonlinear discriminant analysis showed the highest prediction accuracy. These results contribute significantly to the field by offering a non-invasive, objective predictive tool for MVI in HCC, potentially improving treatment decisions. However, the need for larger, prospective studies to validate these findings, highlighting a key area for future research in this domain.
This study introduced new methods for predicting MVI in HCC using texture analysis of multi-parametric MR images. It proposed the combination of MI + PA + F dimensionality reduction and nonlinear discriminant analysis as a novel and effective approach. This methodology represents a significant advancement in non-invasive, objective diagnostic tool in the field of HCC management.
Future research should focus on validating the efficacy of texture analysis in larger, multicenter studies, exploring its integration with other diagnostic modalities to enhance MVI prediction accuracy in HCC. Additionally, investigating the applicability of this method in other types of cancer could broaden its clinical significance and utility.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosmane NS, United States S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, Park JW. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol. 2021;74:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 2. | Chen M, Shu G, Lv X, Xu X, Lu C, Qiao E, Fang S, Shen L, Zhang N, Wang J, Chen C, Song J, Liu Z, Du Y, Ji J. HIF-2α-targeted interventional chemoembolization multifunctional microspheres for effective elimination of hepatocellular carcinoma. Biomaterials. 2022;284:121512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Expert consensus on multidisciplinary diagnosis and treatment of precancerous lesions of hepatocellular carcinoma (2020 edition). Zhonghua Gan Zang Bing Za Zhi. 2020;28:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Nitta H, Allard MA, Sebagh M, Ciacio O, Pittau G, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Guettier C, Lewin M, Samuel D, Baba H, Adam R. Prognostic Value and Prediction of Extratumoral Microvascular Invasion for Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26:2568-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 5. | Chen ZH, Zhang XP, Wang H, Chai ZT, Sun JX, Guo WX, Shi J, Cheng SQ. Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB (Oxford). 2019;21:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 6. | Sun R, Zhao S, Jiang H, Dai Y, Zhang C, Wang S. Imaging Tool for Predicting Renal Clear Cell Carcinoma Fuhrman Grade: Comparing R.E.N.A.L. Nephrometry Score and CT Texture Analysis. Biomed Res Int. 2021;2021:1821876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 7. | Feng M, Zhang M, Liu Y, Jiang N, Meng Q, Wang J, Yao Z, Gan W, Dai H. Texture analysis of MR images to identify the differentiated degree in hepatocellular carcinoma: a retrospective study. BMC Cancer. 2020;20:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 8. | López-Gómez C, Ortiz-Ramón R, Mollá-Olmos E, Moratal D; Alzheimer’s Disease Neuroimaging Initiative. ALTEA: A Software Tool for the Evaluation of New Biomarkers for Alzheimer's Disease by Means of Textures Analysis on Magnetic Resonance Images. Diagnostics (Basel). 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 9. | Giganti F, Antunes S, Salerno A, Ambrosi A, Marra P, Nicoletti R, Orsenigo E, Chiari D, Albarello L, Staudacher C, Esposito A, Del Maschio A, De Cobelli F. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol. 2017;27:1831-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 10. | Li Y, Xu X, Weng S, Yan C, Chen J, Ye R. CT Image-Based Texture Analysis to Predict Microvascular Invasion in Primary Hepatocellular Carcinoma. J Digit Imaging. 2020;33:1365-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 11. | Bakr S, Echegaray S, Shah R, Kamaya A, Louie J, Napel S, Kothary N, Gevaert O. Noninvasive radiomics signature based on quantitative analysis of computed tomography images as a surrogate for microvascular invasion in hepatocellular carcinoma: a pilot study. J Med Imaging (Bellingham). 2017;4:041303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Ni M, Zhou X, Lv Q, Li Z, Gao Y, Tan Y, Liu J, Liu F, Yu H, Jiao L, Wang G. Radiomics models for diagnosing microvascular invasion in hepatocellular carcinoma: which model is the best model? Cancer Imaging. 2019;19:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 13. | Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 14. | Wieczorek-Pastusiak J, Kociński M, Raźniewski M, Strzelecki M, Stefańczyk L, Majos A. An attempt toward objective assessment of brain tumor vascularization using susceptibility weighted imaging and dedicated computer program - a preliminary study. Pol J Radiol. 2013;78:50-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Gao J, Huang X, Meng H, Zhang M, Zhang X, Lin X, Li B. Performance of Multiparametric Functional Imaging and Texture Analysis in Predicting Synchronous Metastatic Disease in Pancreatic Ductal Adenocarcinoma Patients by Hybrid PET/MR: Initial Experience. Front Oncol. 2020;10:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Patel N, Henry A, Scarsbrook A. The value of MR textural analysis in prostate cancer. Clin Radiol. 2019;74:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5550] [Article Influence: 616.7] [Reference Citation Analysis (3)] |
| 18. | Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 19. | Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular Carcinoma with Macrovascular Invasion: Defining the Optimal Treatment Strategy. Liver Cancer. 2017;6:360-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1107] [Article Influence: 100.6] [Reference Citation Analysis (1)] |
| 21. | Ryu T, Takami Y, Wada Y, Tateishi M, Hara T, Yoshitomi M, Momosaki S, Yasumori K, Saitsu H, Okuda K. A Clinical Scoring System for Predicting Microvascular Invasion in Patients with Hepatocellular Carcinoma Within the Milan Criteria. J Gastrointest Surg. 2019;23:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Kim AY, Sinn DH, Jeong WK, Kim YK, Kang TW, Ha SY, Park CK, Choi GS, Kim JM, Kwon CHD, Joh JW, Kim MJ, Sohn I, Jung SH, Paik SW, Lee WJ. Hepatobiliary MRI as novel selection criteria in liver transplantation for hepatocellular carcinoma. J Hepatol. 2018;68:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Huang M, Liao B, Xu P, Cai H, Huang K, Dong Z, Xu L, Peng Z, Luo Y, Zheng K, Peng B, Li ZP, Feng ST. Prediction of Microvascular Invasion in Hepatocellular Carcinoma: Preoperative Gd-EOB-DTPA-Dynamic Enhanced MRI and Histopathological Correlation. Contrast Media Mol Imaging. 2018;2018:9674565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 24. | Ariizumi S, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, Yamamoto M. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Bakr S, Gevaert O, Patel B, Kesselman A, Shah R, Napel S, Kothary N. Interreader Variability in Semantic Annotation of Microvascular Invasion in Hepatocellular Carcinoma on Contrast-enhanced Triphasic CT Images. Radiol Imaging Cancer. 2020;2:e190062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, Han JK, Choi BI. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015;40:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol. 2014;29:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin Cancer Res. 2018;24:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 588] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 29. | Ma XH, Zhu YJ, Wang S, Feng B, Wang Q, Kong Y, Zhao XM. Value of contrast-enhanced MRI texture analysis in predicting microvascular invasion of primary hepatocellular carcinoma before operation. Zhonghua Fangshexue Zazhi. 2018;52:327-332. [DOI] [Full Text] |
| 30. | Liu JP, Cheng DL, Liao YT, Zhang X, Lin SQ, Chen HW, Huang HM, Gao MY. To Explore the Preliminary Value of Predicting Microvascular Invasion of Hepatocellular Carcinama Based on MR-T2WI Texture Analysis. Linchuang Fangshexue Zazhi. 2021;40:1625-1628. |
| 31. | Zhu YJ, Feng B, Wang S, Wang LM, Wu JF, Ma XH, Zhao XM. Model-based three-dimensional texture analysis of contrast-enhanced magnetic resonance imaging as a potential tool for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Oncol Lett. 2019;18:720-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Yao W, Yang S, Ge Y, Fan W, Xiang L, Wan Y, Gu K, Zhao Y, Zha R, Bu J. Computed Tomography Radiomics-Based Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Front Med (Lausanne). 2022;9:819670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Zhang W, Yang R, Liang F, Liu G, Chen A, Wu H, Lai S, Ding W, Wei X, Zhen X, Jiang X. Prediction of Microvascular Invasion in Hepatocellular Carcinoma With a Multi-Disciplinary Team-Like Radiomics Fusion Model on Dynamic Contrast-Enhanced Computed Tomography. Front Oncol. 2021;11:660629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |