Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1236
Peer-review started: October 4, 2023
First decision: December 18, 2023
Revised: December 29, 2023
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: April 15, 2024
Processing time: 189 Days and 12.9 Hours
The efficacy and safety of transarterial chemoembolization (TACE) combined with lenvatinib plus programmed cell death protein-1 (PD-1) for unresectable hepatocellular carcinoma (HCC) have rarely been evaluated and it is unknown which factors are related to efficacy.
To evaluate the efficacy and independent predictive factors of TACE combined with lenvatinib plus PD-1 inhibitors for unresectable HCC.
This study retrospectively enrolled patients with unresectable HCC who received TACE/lenvatinib/PD-1 treatment between March 2019 and April 2022. Overall survival (OS) and progression-free survival (PFS) were determined. The objective response rate (ORR) and disease control rate (DCR) were evaluated in accordance with the modified Response Evaluation Criteria in Solid Tumors. Additionally, the prognostic factors affecting the clinical outcome were assessed.
One hundred and two patients were enrolled with a median follow-up duration of 12.63 months. The median OS was 26.43 months (95%CI: 17.00-35.87), and the median PFS was 10.07 months (95%CI: 8.50-11.65). The ORR and DCR were 61.76% and 81.37%, respectively. The patients with Barcelona Clinic Liver Cancer Classification (BCLC) B stage, early neutrophil-to-lymphocyte ratio (NLR) response (decrease), or early alpha-fetoprotein (AFP) response (decrease > 20%) had superior OS and PFS than their counterparts.
This study showed that TACE/lenvatinib/PD-1 treatment was well tolerated with encouraging efficacy in patients with unresectable HCC. The patients with BCLC B-stage disease with early NLR response (decrease) and early AFP response (decrease > 20%) may achieve better clinical outcomes with this triple therapy.
Core Tip: Transarterial chemoembolization/lenvatinib/programmed cell death protein-1 combined treatment was well tolerated with encouraging efficacy in unresectable hepatocellular carcinoma patients. The patients with Barcelona Clinic Liver Cancer Classification (BCLC) B, with early neutrophil-to-lymphocyte ratio (NLR) response (decrease) and early alpha fetoprotein (AFP) response (decrease > 20%) might achieve better clinical outcomes with this triple therapy. It is advisable that BCLC stage, NLR, and AFP should be considered at clinical decision-making in order to obtain better prognosis.
- Citation: Ma KP, Fu JX, Duan F, Wang MQ. Efficacy and predictive factors of transarterial chemoembolization combined with lenvatinib plus programmed cell death protein-1 inhibition for unresectable hepatocellular carcinoma. World J Gastrointest Oncol 2024; 16(4): 1236-1247
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1236.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1236
Globally, liver cancer is estimated to be the sixth most prevalent cancer and ranks third in terms of mortality[1]. Early-stage hepatocellular carcinoma (HCC) is usually latent in onset, which results in rapid progression without immediate awareness, and makes curative surgical resection non-feasible. In such cases of unresectable HCC, developing methods that provide a prolonged survival benefit to patients is a priority. Transarterial chemoembolization (TACE) is the standard locoregional therapy considered in such cases[2]. TACE induces necrosis and apoptosis of tumor cells, which are deprived of nutrient supply and are locally surrounded by chemotherapeutic agents. However, not every patient can reap the benefits of TACE, and there is also a relatively high post-TACE recurrence[3].
A primary underlying reason is that hypoxia in tumor tissues are aggravated after feeding vessels are eradicated by TACE, which further promotes hypoxia-induced tumor angiogenesis. This leads to tumor regrowth and even residual tumor cell metastasis. In addition, sorafenib and lenvatinib have recently been used as first-line systemic therapy per guideline recommendations[4]. Lenvatinib (Eisai Co., Ltd., Japan) can target the vascular endothelial growth factor receptor, efficiently block neovascularization required for tumor growth, and concurrently target multiple proteins related to tumor growth. The combination of lenvatinib with TACE for HCC treatment has obtained satisfactory efficacy by impeding tumor angiogenesis post-embolization and eliminating residual tumor cells[5,6].
Due to TACE-induced necrosis, tumor cells release large volumes of debris as tumor antigens, which alters the local tumor immune microenvironment. This can cause antigen-presenting cell maturation, promote T lymphocyte infiltration, and eventually activate systemic anti-tumor immunity. Focused on immune regulation, immune checkpoint inhibitors (ICIs) have shown promising results for unresectable HCC[7,8]. In patients with advanced disease with a high intrahepatic tumor burden or extrahepatic metastases, concurrent ICI treatment and TACE could facilitate tumor elimination[9]. Programmed cell death protein-1 (PD-1) inhibitors such as nivolumab and pembrolizumab have been recommended as the first-line systemic therapy for advanced HCC[4]. This has revolutionized the current landscape of systemic therapy. Basic research also showed that tyrosine kinase inhibitors could facilitate current immune therapies, and this combined treatment has synergistic and consolidated effects[10-12]. Clinical trials also consistently showed that 46% of patients with unresectable HCC achieved stable objective radiographic responses after receiving lenvatinib plus pembrolizumab as first-line therapy[13]. However, considering that patients with unresectable HCC usually face severe situations such as vascular invasion or distant metastasis, the sequential therapeutic regimen after TACE remains challenging.
Based on the findings from these studies, we assumed that TACE in combination with lenvatinib plus PD-1 inhibitors may achieve a better prognosis for patients with unresectable HCC. However, the efficacy and safety of this combined therapy have rarely been evaluated, and the factors related to efficacy have yet to be identified. In this study, we first assessed the efficacy and safety of TACE-lenvatinib-PD1 therapy for patients with unresectable HCC to explore the predictive factors of clinical outcomes.
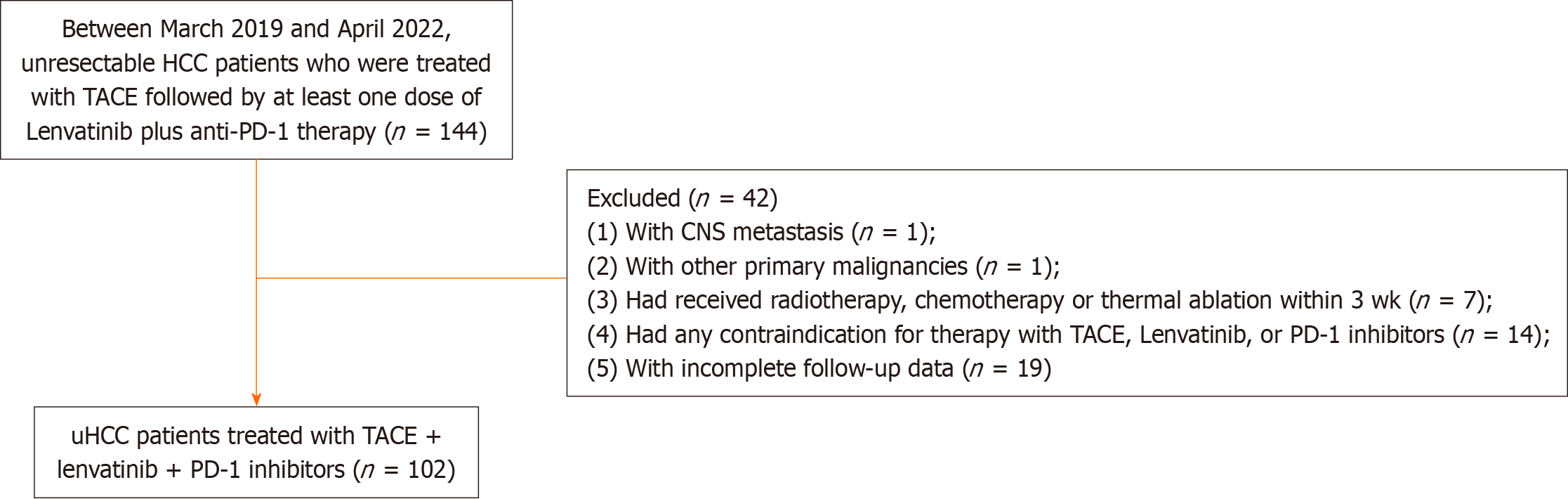
This was a single-center retrospective analysis approved by the Institutional Review Board of the Chinese People's Liberation Army General Hospital (Beijing, China). The study complied with the Declaration of Helsinki. Between March 2019 and April 2022, patients with unresectable HCC who were initially treated with TACE combined with at least one dose of anti-PD-1 therapy plus lenvatinib were included for analysis.
The inclusion criteria were as follows: (1) Age more than 18 years; (2) Radiologically or pathologically diagnosed with HCC; (3) Barcelona Clinic Liver Cancer Classification (BCLC) stage B or C; and (4) Eastern Cooperative Oncology Group (ECOG) score 0-1. The exclusion criteria were as follows: (1) Poor patient compliance (such as failure to visit the clinic per schedule, leading to incomplete follow-up data); (2) Presence of medical contraindications, including severe cardiac, pulmonary, renal, or coagulation dysfunction; (3) Presence of central nervous system metastasis or other primary malignancies; (4) Previous treatment with other targeted drugs or PD-1 immunotherapy; and (5) Previous treatment with radiotherapy, chemotherapy or thermal ablation within 3 wk.
TACE was initiated before the administration of lenvatinib or PD-1 inhibitors. TACE was performed by two interventional radiologists with 25 (MQ.W) and 15 years (F.D) of vascular and interventional radiology experience, respectively. In the TACE procedure, a 4F catheter was first introduced via the femoral artery, and angiography was performed to assess the tumor and the tumor-feeding arteries. Next, chemotherapeutic agents [epirubicin (Pfizer, United States), 40-50 mg; oxaliplatin (Sanofi, United States), 100-150 mg; 5-fluorouracil (Tianjin Jinyao Co., Ltd., China), 500-750 mg; calcium folinate (Jiangsu Hengrui Pharmaceuticals Co., Ltd., China), 200-300 mg] were infused through the hepatic artery at distinct doses. Embolization was conducted using a microcatheter (2.7F, Terumo Medical, Japan; or 2.8F, Boston Scientific, United States; or 2.6F/1.98F, Asahi Intecc, Japan) either selectively or super-selectively using a conventional lipiodol based technique. Following the administration of 4-20 mL of lipiodol (Lipiodol, Laboratoire Guerbet, Roissy, France), a gelatin sponge or polyvinyl alcohol embolic microspheres were injected as supplements if stasis was not achieved. If there were few blood vessels or incomplete tumor staining, the inferior phrenic artery, intercostal artery, internal thoracic artery branches, and omental branches were examined with precision. When these vessels were found to feed the tumor, the collateral arteries were super-selected and embolized.
Based on the restored liver function and the patient’s general status, lenvatinib or PD-1 inhibitors were subsequently administered in accordance with the instructions for use. Anti-PD-1 antibodies [sintilimab (Innovent Biologics Co., Ltd., China), 200 mg administered every 3 wk/nivolumab (Bristol-Myers Squibb Company, United States) 3 mg/kg every 2 wk/camrelizumab (Jiangsu Hengrui Pharmaceuticals Co., Ltd., China), 200 mg every 2 wk/pembrolizumab (Merck & Co., Inc., United States), 200 mg every 3 wk/toripalimab (Shanghai Junshi Biosciences Co., Ltd., China), 3 mg/kg every 2 wk] were administered intravenously. The types of PD-1 antibodies depended on the patients’ choices based on the offered guideline recommendations and individual financial conditions, among other factors. In addition, lenvatinib (body weight ≥ 60 kg, 12 mg/d; body weight < 60 kg, 8 mg/d) was administered orally. Discontinuation of the therapeutic regimen or changes to the same were considered based on disease progression, unacceptable adverse events (AEs), patient refusal, or clinician decision.
Before each treatment (TACE and PD-1 inhibition) or contrast-enhanced computed tomography/magnetic resonance imaging at a 4-8-wk interval, we assessed the patients for tumor responses and AEs. The follow-up was routinely performed until death or the end of the study (April 30, 2022). During the imaging follow-up, “on-demand” TACE procedures were repeated based on the presence of viable tumors or intrahepatic recurrences. If these patients had sufficient liver function, repeated TACE was performed. The AFP level was assessed every 4 wk. In addition, lenvatinib/PD-1 inhibitor treatment was discontinued due to disease progression.
Tumor responses were assessed by a physician (MQ.W) with 25 years’ experience using the modified Response Evaluation Criteria in Solid Tumors. Tumor responses were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The objective response rate (ORR) was defined as the proportion of patients achieving CR and PR. The disease control rate (DCR) was defined as the proportion of patients with CR, PR, and SD. The tumor responses of all patients were confirmed no less than 4 wk after the initial observation. PFS was defined as the time interval between TACE and the time of disease progression owing to any cause. Overall survival (OS) was defined as the period from TACE to the time of death or the last follow-up date.
All AEs during the combination therapy were recorded and evaluated based on the Common Terminology Criteria for Adverse Events Version 5.0 and standard laboratory examinations. TACE-related transient AEs such as fever, nausea, vomiting, abdominal pain, and elevated liver transaminase were not included. The neutrophil-to-lymphocyte ratio (NLR) was calculated using the neutrophil and lymphocyte percentages of whole blood cell counts.
We evaluated the prognostic factors correlated with survival and disease progression using the variables gender, age, ECOG PS (0 vs 1), BCLC stage (B vs C), etiology [hepatitis B virus (HBV) vs others], maximum tumor diameter (≤ 6.8 cm vs > 6.8 cm), number of tumors (≤ 3 vs > 3), portal vein invasion, extrahepatic metastasis, Child-Pugh class (A vs B), alpha-fetoprotein (AFP), Des-gamma-carboxyprothrombin (DCP), NLR, and lactate dehydrogenase (LDH). Subgroup analysis for each factor was further conducted to evaluate its potential contribution to predicting treatment responses.
Continuous variables are presented as mean with standard error, and categorical variables are presented as counts with percentages. Survival analysis was conducted using the Kaplan-Meier method, and survival comparison was conducted using the log-rank test. The Cox proportional hazards regression method was used to identify factors associated with the OS and PFS, and multivariate analysis was conducted using variables with P < 0.05 obtained in the univariate analysis. Statistical analyses were performed using IBM SPSS software (version 25.0 SPSS Inc., Chicago, IL, United States).
Between March 30, 2019, and April 30, 2022, 102 patients with HCC [mean age, 58 years (range, 34-91 years)] who receiv
| Variables | Total (n = 102) |
| Age, mean ± SD (range), yr | 57.64 ± 10.37 (34-91) |
| Male, n (%) | 89 (87.25) |
| ECOG PS, n (%) | |
| 0 | 53 (51.96) |
| 1 | 49 (48.04) |
| Etiology, HBV/others, n (%) | 80/22 (78.43/21.57) |
| BCLC stage, B/C, n (%) | 48/54 (47.06/52.94) |
| Maximum tumor diameter, mean ± SD, cm | 6.80 ± 3.74 |
| Number of tumors > 3, n (%) | 51 (50.00) |
| Portal vein invasion, n (%) | |
| Yes | 29 (28.43) |
| Extrahepatic metastasis, n (%) | |
| Yes | 42 (41.18) |
| Extrahepatic metastatic sites, n (%) | |
| Lung | 27 (26.47) |
| Bone | 7 (6.86) |
| Lymph nodes | 13 (12.75) |
| Abdominal cavity | 7 (6.86) |
| PD-1 antibody class, n (%) | |
| Sintilimab | 52 (50.98) |
| Nivolumab | 20 (19.61) |
| Camrelizumab | 17 (16.67) |
| Pembrolizumab | 7 (6.86) |
| Toripalimab | 6 (5.88) |
| Child-Pugh class, n (%) | |
| A | 93 (91.18) |
| B | 9 (8.82) |
| AFP level, n (%) | |
| > 400 ng/mL | 49 (48.04) |
| ≤ 400 ng/mL | 53 (51.96) |
| DCP level, n (%) | |
| > 40 mAU/mL | 89 (87.25) |
| ≤ 40 mAU/mL | 13 (12.75) |
| NLR, mean ± SD | 2.46 ± 1.59 |
| LDH, mean ± SD, U/L | 198.52 ± 102.05 |
Twenty-nine patients (28.43%) showed portal vein tumor thrombosis, and 42 patients (42/102, 41.18%) showed distal metastasis, most commonly in the lung (27/102, 26.47%). Forty-nine patients (48.04%) had an AFP level > 400 ng/mL, and 87.25% patients had a DCP level > 40 mAU/mL at enrollment. The mean baseline NLR and LDH were 2.46 ± 1.59 and 198.52 ± 102.05 U/L, respectively.
No patient died due to AEs. Treatment-related AEs for the combination therapy are recorded in Supplementary Table 1. Ninety-five (95/102, 93.13%) patients experienced AEs, with most being grade 1-2 (62/102, 60.78%), which did not warrant medical intervention. The most common clinical toxicity was grade 1-2 asthenia detected in 44 patients (43.14%). Twenty-eight patients (27.45%) reported grade 1-2 hand-foot syndrome. Hypertension (7/102, 6.86%) and rash (6/102, 5.88%) were the most common grade 3-4 AEs.
With respect to the cutoff, 77.45% (79/102) of patients were alive. One hundred and two response-evaluable patients were included in the efficacy analysis with a median follow-up of 12.63 months. PD-1 inhibition was performed with a median number of cycles of 6.5 (IQR: 3.75-12.25). Clinical responses are summarized in Table 2. Overall, CR was achieved in ten patients, PR was achieved in 53 patients, and SD and PD were achieved in 20 and 19 patients, respectively. The confirmed ORR was 61.76% (63/102), and the DCR was 81.37% (83/102). The median PFS was 10.07 months (95%CI: 8.50-11.65), and the median OS was 26.43 months (95%CI: 17.00-35.87).
| Variables | Total (n = 102) |
| Best overall response | |
| CR | 10 |
| PR | 53 |
| SD | 20 |
| PD | 19 |
| Objective response rate | 61.76% |
| Disease control rate | 81.37% |
| Median PFS | 10.07 months (95%CI: 8.50-11.65) |
| 6-month tumor PFS | 70.82% (95%CI: 60.80-78.72) |
| 12-month tumor PFS | 36.11% (95%CI: 26.49-45.79) |
| Median OS | 26.43 months (95%CI: 17.00-35.87) |
| 6-month survival | 92.63% (95%CI: 85.14-96.42) |
| 12-month survival | 84.15% (95%CI: 74.05-90.56) |
The independent factors that were predictive of clinical outcomes based on the results of univariate and multivariate Cox regression analyses are summarized in Tables 3 and 4. In the univariate analysis, gender, ECOG PS, BCLC stage, NLR, LDH, early NLR response, early AFP response, and early DCP response were found to be significantly associated with OS. In the multivariate analysis, baseline factors of BCLC C [vs B; hazard ratio (HR) = 3.10, 95%CI: 1.18-8.13; P = 0.021], LDH ≤ 198.52 (vs > 198.52; HR = 0.22, 95%CI: 0.08-0.56; P = 0.002), post-treatment factor of early NLR decrease (vs increase; HR = 0.31, 95%CI: 0.11-0.89; P = 0.030), early AFP decrease ≤ 20% (vs > 20%; HR = 3.90, 95%CI: 1.42-10.69; P = 0.008) were independent factors predictive of OS.
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (≤ 58 yr vs > 58 yr) | 0.65 (0.29-1.48) | 0.2900 | ||
| Gender (male vs female) | 0.40 (0.11-1.38) | 0.0412 | ||
| ECOG PS (0 vs 1) | 0.44 (0.20-0.98) | 0.0392 | ||
| Etiology (HBV vs others) | 0.61 (0.21-1.78) | 0.2843 | ||
| BCLC stage (C vs B) | 3.11 (1.36-7.11) | 0.0068 | 3.10 (1.18-8.13) | 0.021 |
| Maximum tumor diameter (≤ 6.8 vs > 6.8) | 0.66 (0.29-1.49) | 0.3100 | ||
| Number of tumors (≤ 3 vs > 3) | 0.67 (0.26-1.40) | 0.2131 | ||
| Portal vein invasion (absent vs presence) | 0.56 (0.22-1.41) | 0.1545 | ||
| Extrahepatic metastasis (absent vs presence) | 0.69 (0.30-1.59) | 0.3531 | ||
| Child-Pugh class (A vs B) | 1.29 (0.35-4.78) | 0.7266 | ||
| Baseline AFP (≤ 400 vs > 400) | 0.90 (0.39-2.03) | 0.7799 | ||
| Baseline DCP (≤ 40 vs > 40) | 1.78 (0.47-6.67) | 0.2890 | ||
| NLR (≤ 3 vs > 3) | 0.42 (0.16-1.09) | 0.0306 | ||
| LDH (≤ 198.52 vs > 198.52) | 0.43 (0.17-1.06) | 0.0347 | 0.22 (0.08-0.56) | 0.002 |
| Early NLR response (decrease vs increase) | 0.37 (0.16-0.89) | 0.0100 | 0.31 (0.11-0.89) | 0.030 |
| Early AFP response (decrease ≤ 20% vs > 20%) | 3.11 (1.31-7.39) | 0.0043 | 3.90 (1.42-10.69) | 0.008 |
| Early DCP response (decrease ≤ 20% vs > 20%) | 2.42 (0.78-7.51) | 0.0407 | ||
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (≤ 58 vs > 58 yr) | 0.98 (0.64-1.49) | 0.9279 | ||
| Gender (male vs female) | 0.64 (0.31-1.32) | 0.1413 | ||
| ECOG PS (0 vs 1) | 0.90 (0.59-1.37) | 0.6087 | ||
| Etiology (HBV vs others) | 0.78 (0.45-1.36) | 0.3417 | ||
| BCLC stage (C vs B) | 1.82 (1.18-2.80) | 0.0036 | 1.75 (1.12-2.74) | 0.014 |
| Maximum tumor diameter (≤ 6.8 vs > 6.8) | 0.99 (0.65-1.52) | 0.9933 | ||
| Number of tumors (≤ 3 vs > 3) | 0.81 (0.53-1.23) | 0.3099 | ||
| Portal vein invasion (absent vs presence) | 0.77 (0.47-1.26) | 0.2597 | ||
| Extrahepatic metastasis (absent vs presence) | 0.73 (0.47-1.14) | 0.1408 | ||
| Child-Pugh class (A vs B) | 1.26 (0.67-2.36) | 0.5094 | ||
| Baseline AFP (≤ 400 vs > 400) | 1.00 (0.65-1.53) | 0.9992 | ||
| Baseline DCP (≤ 40 vs > 40) | 1.27 (0.64-2.55) | 0.4486 | ||
| NLR (≤ 3 vs > 3) | 0.93 (0.56-1.54) | 0.7707 | ||
| LDH (≤ 198.52 vs > 198.52) | 0.85 (0.53-1.35) | 0.4605 | ||
| Early NLR response (decrease vs increase) | 0.54 (0.34-0.86) | 0.0025 | 0.56 (0.35-0.90) | 0.016 |
| Early AFP response (decrease ≤ 20% vs > 20%) | 1.70 (1.08-2.66) | 0.0116 | 1.73 (1.12-2.66) | 0.013 |
| Early DCP response (decrease ≤ 20% vs > 20%) | 1.73 (0.97-3.09) | 0.0250 | ||
In the univariate analysis, BCLC stage, early NLR response, early AFP response, and early DCP response were significantly associated with PFS. In the multivariate analysis, BCLC C (vs B; HR = 1.75, 95%CI: 1.12-2.74; P = 0.014), early NLR decrease (vs increase; HR = 0.56, 95%CI: 0.35-0.90; P = 0.016), and early AFP decrease ≤ 20% (vs > 20%; HR = 1.73, 95%CI: 1.12-2.66; P = 0.013) were independent factors predictive of PFS.
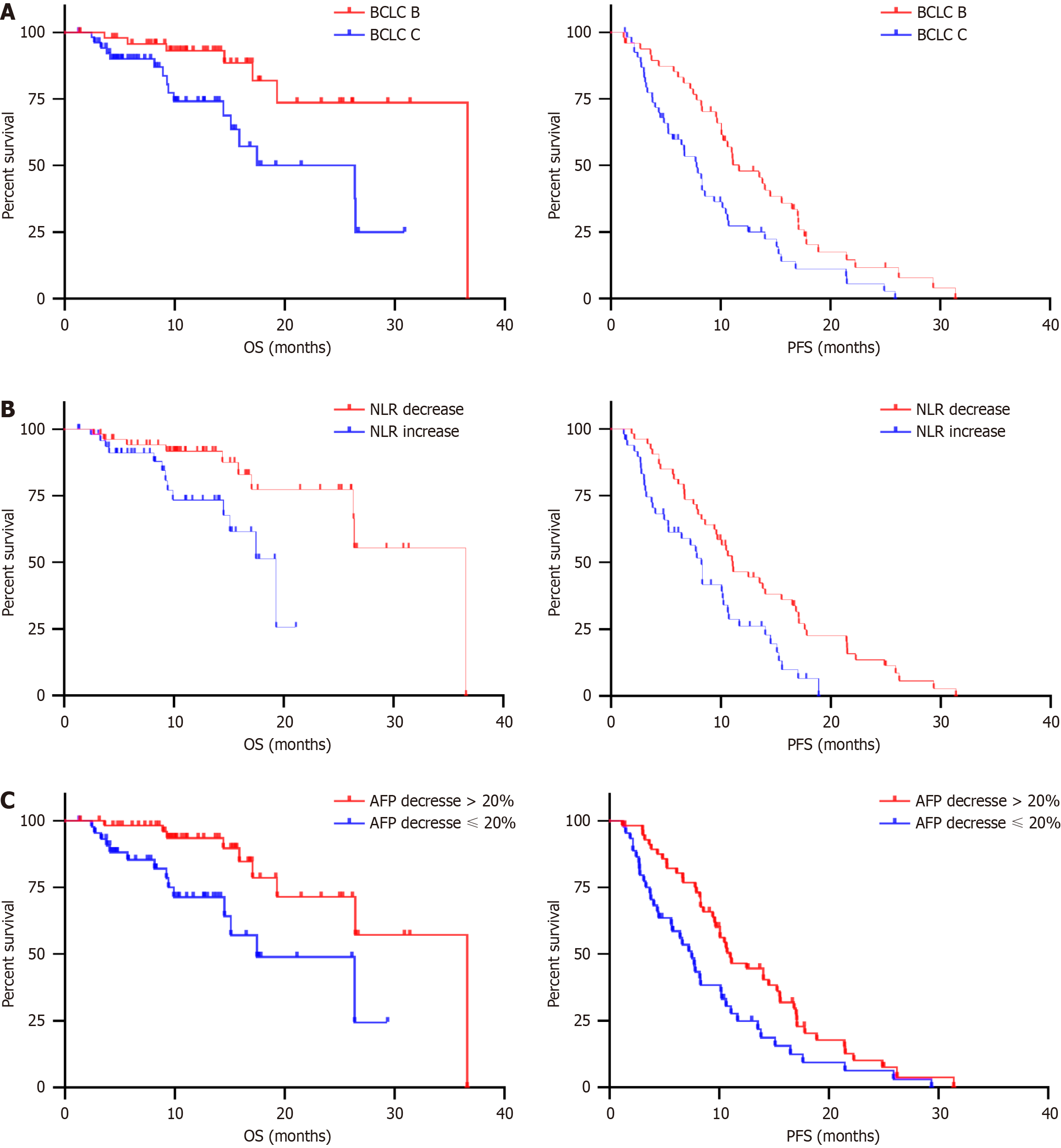
Thus, the BCLC stage, early NLR and early AFP responses were independent predictors of both OS and PFS. We further compared the survival and disease progression stratified by the BCLC stage (C vs B), early NLR response (decrease vs increase), and early AFP response (decrease ≤ 20% vs > 20%). Patients with BCLC B had a superior median OS and PFS (36.60 months, 11.67 months) than patients with BCLC C (26.37 months, P = 0.0068; 7.80 months, P = 0.0036; Figure 2A). Patients with early NLR decrease had a longer median OS and PFS (36.60, 11.07 months) than patients with NLR increase (19.33 months, P = 0.0100; 8.23 months, P = 0.0025; Figure 2B). Patients with an early AFP response (decrease ≤ 20%) also exhibited a shorter median OS and PFS (36.60, 11.0 months) than their counterparts (17.47 months, P = 0.0043; 7.50 months, P = 0.0116; Figure 2C).
In this study, TACE-lenvatinib-PD1 therapy showed a favorable efficacy and an acceptable safety profile in patients with unresectable HCC. The ORR was 61.76%, and the DCR was 81.37%, as assessed in 102 response-evaluable patients. The median PFS was 10.7 months, and the median OS was 26.43 months. Most AEs were acceptable with proper medical management. BCLC B, early NLR response (decrease), and early AFP response (decrease > 20%) were identified as independent predictors of clinical outcomes.
Our study investigated the toxicity of combined therapy. The incidences of treatment-related AEs were consistent with those previously reported[14,15]. Most were grade 1-2 AEs and could be managed without life-threatening events. In grade 1-2 AEs, asthenia and hand-foot syndrome were the most frequent AEs and occurred in 43.14% and 27.45% of patients, respectively. Hypertension (6.86%) and rash (5.88%) were the most frequent grade 3-4 AEs. In all, the toxicity profile of this combination therapy was manageable under close monitoring.
In previously reported TACE-sorafenib-PD1 combined therapy, the ORR was 54.6%-60.6%[16-18], which was lower than the ORR in our study (61.76%). Reportedly, as an antiangiogenic agent, lenvatinib showed better efficacy than sorafenib, especially in HBV-positive Chinese patients[19]. Similarly, in a real-world study, lenvatinib-PD1-TACE triple therapy showed encouraging efficacy and manageable safety in patients with unresectable HCC, with a higher ORR of 69.3%[20].
Compared with the previously reported median OS of 12.3 to 23.9 months[16-18,20-25], the median OS of 26.43 months recorded by us was the longest. The excellent survival benefit could be attributed to the use of a more precise microcatheter with a small diameter in TACE for super-selection and the complete embolization of collateral vessels. This could not only eliminate the primary lesion but also prevent potential tumor metastasis originating from the lesions feeding vessels in advance, thus significantly improving patient survival. Ten patients received conversion therapy with positive clinical outcomes (eight for hepatectomy and two for liver transplantation). Another contributing factor is that even though disease progression occurred, many patients underwent various subsequent treatments to improve OS instead of discontinuing treatment (Supplementary Table 2). For instance, processes for local tumor ablation, such as microwave ablation or radiofrequency ablation, are micro-invasive and help reduce the tumor burden[26]. Owing to subsequent treatment, our patients showed long OS after progression.
We found that TACE combined with lenvatinib treatment plus PD-1 inhibition was a superior treatment option for patients with intermediate- or advanced-stage HCC. This could be explained by the following reasons: (1) TACE can trigger tumor necrosis after feeding vessel embolization and the release of tumor antigens, which can induce the maturation of antigen-presenting cells. Subsequently, tumor-specific immune responses can be initialized, followed by the generation of large amounts of cytokines and the activation of adaptive antitumor immunity. In addition, as the local tumor environment changes substantially, the immunosuppressive cells can also be downregulated, eventually leading to favorable survival prognosis in patients; and (2) Lenvatinib may reduce post-TACE hypoxia-induced angiogenesis[27], modulate VEGF-mediated immunosuppression in the tumor microenvironment, and promote cytotoxic T-cell infiltration. Therefore, TACE, lenvatinib, and PD-1 inhibition exert a synergistic antitumor effect and improve clinical benefits for patients with unresectable HCC.
In the present study, BCLC B, early NLR response (decrease), and early AFP response (decrease > 20%) were identified as independent predictors of OS and PFS. With respect to the BCLC stage, patients with stage C disease had a higher tumor burden at baseline than patients with stage B disease. Early AFP response indicates a reduced tumor burden after combined therapy, indicating a direct and effective tumor-killing capability. Thus, it is not difficult to understand that patients with a low tumor burden either at baseline or in response to treatment effects had better clinical prognoses.
NLR is an indicator of tumor-related inflammation and helps predict tumor prognosis[28,29]. In nivolumab-treated patients with HCC, dynamic changes in the NLR (at week 4) are effective prognostic indicators and may facilitate patient selection and subsequent clinical strategies for immunotherapies[30]. This is consistent with our finding that patients with a decreased NLR had superior median OS and PFS than their counterparts. To be specific, peripheral neutrophils partially reflect the immunosuppressive cell population (tumor-associated neutrophils), indicating immunosuppression and a poor response to immunomodulation therapy. Besides, peripheral lymphocytes indicate the cytotoxic T-cell response. A higher proportion of lymphocytes indicates an enhanced anti-tumor immune response. Therefore, a low NLR is correlated with reduced systemic inflammation and enhanced adaptive anti-tumor immunity.
There were several limitations in this study. First, this was a retrospective single-center study with a small sample size and a short follow-up period. However, our results still indicated high efficacy of the treatment method for patients with unresectable HCC. Further prospective studies with larger sample sizes are necessary. Second, we included more than one type of PD-1 inhibitor, which may have affected the consistency of the results of immunotherapies. Subgroup analyses are necessary to identify further unknown differences attributed to each agent.
In summary, our findings demonstrated that TACE-lenvatinib-PD1 therapy is well-tolerated and has promising efficacy in patients with unresectable HCC. Patients with BCLC B-stage disease, early NLR response (decrease), and early AFP response (decrease > 20%) may achieve better clinical outcomes with the proposed triple therapy.
Transarterial chemoembolization (TACE) is the standard locoregional therapy for unresectable hepatocellular carcinoma (HCC), but not every patient can benefit from TACE, and there is also relatively high post-TACE recurrence. Triple therapy with TACE combined with lenvatinib plus PD-1 inhibitors, may result in a better prognosis for HCC patients.
The efficacy and safety of this triple therapy have been rarely evaluated and it is unknown which factors are related to efficacy. By solving this problem, this will aid clinical decision-making.
In this study, we aimed to first assess the efficacy and safety of TACE-lenvatinib-PD1 therapy for unresectable HCC patients and to explore the predictive factors of clinical outcomes.
During follow-up, tumor responses were assessed based on the modified Response Evaluation Criteria in Solid Tumors and categorized as complete response, partial response, stable disease, or progression disease. The objective response rate (ORR), disease control rate (DCR), overall survival (OS), and progression-free survival (PFS) were also calculated. The Cox proportional hazards regression method was used to identify the factors associated with OS and PFS.
The confirmed ORR was 61.76% (63/102), and the DCR was 81.37% (83/102). The median PFS was 10.07 months (95%CI: 8.50-11.65), and the median OS was 26.43 months (95%CI: 17.00-35.87). Barcelona Clinic Liver Cancer Classification (BCLC) B stage, early neutrophil-to-lymphocyte ratio (NLR) response (decrease) and early AFP response (decrease > 20%) were identified as the independent predictors of clinical outcomes.
This study showed that TACE-lenvatinib-PD-1 treatment was well tolerated with encouraging efficacy in unresectable HCC patients. The patients with BCLC B, with early NLR response (decrease) and early AFP response (decrease > 20%) might achieve better clinical outcomes with this triple therapy.
Further prospective studies with larger sample sizes are necessary. In addition, subgroup analyses are needed to determine the unknown differences attributing to each agent.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsayed MOK, United Kingdom; Gajanan G, India S-Editor: Zhang H L-Editor: Webster JR P-Editor:Zheng XM
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2593] [Article Influence: 864.3] [Reference Citation Analysis (59)] |
| 3. | Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T; Liver Cancer Study Group of Japan. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87 Suppl 1:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 574] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 5. | Mawatari S, Tamai T, Kumagai K, Saisyoji A, Muromachi K, Toyodome A, Taniyama O, Sakae H, Ijuin S, Tabu K, Oda K, Hiramine Y, Moriuchi A, Sakurai K, Kanmura S, Ido A. Clinical Effect of Lenvatinib Re-Administration after Transcatheter Arterial Chemoembolization in Patients with Intermediate Stage Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, Zhuang W, Chen X, Chen H, Xu B, Lai J, Guo W. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2022;148:2115-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 7. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3305] [Article Influence: 413.1] [Reference Citation Analysis (1)] |
| 8. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1893] [Article Influence: 270.4] [Reference Citation Analysis (0)] |
| 9. | Marinelli B, Kim E, D'Alessio A, Cedillo M, Sinha I, Debnath N, Kudo M, Nishida N, Saeed A, Hildebrand H, Kaseb AO, Abugabal YI, Pillai A, Huang YH, Khan U, Muzaffar M, Naqash AR, Patel R, Fischman A, Bishay V, Bettinger D, Sung M, Ang C, Schwartz M, Pinato DJ, Marron T. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 10. | Long J, Chen P, Yang X, Bian J, Wang A, Lin Y, Wang H, Sang X, Zhao H. Co-expression of receptor tyrosine kinases and CD8 T-lymphocyte genes is associated with distinct prognoses, immune cell infiltration patterns and immunogenicity in cancers. Transl Res. 2023;256:14-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Talbot T, D'Alessio A, Pinter M, Balcar L, Scheiner B, Marron TU, Jun T, Dharmapuri S, Ang C, Saeed A, Hildebrand H, Muzaffar M, Fulgenzi CAM, Amara S, Naqash AR, Gampa A, Pillai A, Wang Y, Khan U, Lee PC, Huang YH, Bengsch B, Bettinger D, Mohamed YI, Kaseb A, Pressiani T, Personeni N, Rimassa L, Nishida N, Kudo M, Weinmann A, Galle PR, Muhammed A, Cortellini A, Vogel A, Pinato DJ. Progression patterns and therapeutic sequencing following immune checkpoint inhibition for hepatocellular carcinoma: An international observational study. Liver Int. 2023;43:695-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, Chen L, Shi WK, Li ML, Zhu JJ, Tan CJ, Tang ZY, Zhou J, Fan J, Sun HC. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer. 2021;10:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 13. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 874] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 14. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 501] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 15. | Ye SL, Yang J, Bie P, Zhang S, Chen X, Liu F, Liu L, Zhou J, Dou K, Hao C, Shao G, Xia Q, Chen Y, Deng X, Liu Y, Yuan Y, Fu Z, Nakajima K, Lv Z. Safety assessment of sorafenib in Chinese patients with unresectable hepatocellular carcinoma: subgroup analysis of the GIDEON study. BMC Cancer. 2018;18:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, Wu X, Song J, Zhao Z, Ji J. Efficacy and Safety of TACE Combined With Sorafenib Plus Immune Checkpoint Inhibitors for the Treatment of Intermediate and Advanced TACE-Refractory Hepatocellular Carcinoma: A Retrospective Study. Front Mol Biosci. 2020;7:609322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 17. | Qin J, Huang Y, Zhou H, Yi S. Efficacy of Sorafenib Combined With Immunotherapy Following Transarterial Chemoembolization for Advanced Hepatocellular Carcinoma: A Propensity Score Analysis. Front Oncol. 2022;12:807102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 18. | Yang XG, Sun YY, Wang HQ, Li DS, Xu GH, Huang XQ. Efficacy and safety of transarterial chemoembolization combining sorafenib with or without immune checkpoint inhibitors in previously treated patients with advanced hepatocellular carcinoma: A propensity score matching analysis. Front Oncol. 2022;12:914385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Choi NR, Kim JY, Hong JH, Hur MH, Cho H, Park MK, Kim J, Lee YB, Cho EJ, Lee JH, Yu SJ, Yoon JH, Kim YJ. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol. 2022;22:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 20. | Li X, Fu Z, Chen X, Cao K, Zhong J, Liu L, Ding N, Zhang X, Zhai J, Qu Z. Efficacy and Safety of Lenvatinib Combined With PD-1 Inhibitors Plus TACE for Unresectable Hepatocellular Carcinoma Patients in China Real-World. Front Oncol. 2022;12:950266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 21. | Zhang JX, Chen YX, Zhou CG, Liu J, Liu S, Shi HB, Zu QQ. Efficacy and Safety of the Combination of Transarterial Chemoembolization with Camrelizumab plus Apatinib for Advanced Hepatocellular Carcinoma: A Retrospective Study of 38 Patients from a Single Center. Can J Gastroenterol Hepatol. 2022;2022:7982118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Wang J, Zhao M, Han G, Han X, Shi J, Mi L, Li N, Yin X, Duan X, Hou J, Yin F. Transarterial Chemoembolization Combined With PD-1 Inhibitors Plus Lenvatinib Showed Improved Efficacy for Treatment of Unresectable Hepatocellular Carcinoma Compared With PD-1 Inhibitors Plus Lenvatinib. Technol Cancer Res Treat. 2023;22:15330338231166765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Zou X, Xu Q, You R, Yin G. Correlation and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor in the treatment of hepatocellular carcinoma with portal vein tumor thrombus based on immunological features. Cancer Med. 2023;12:11315-11333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 24. | Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhu K. Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front Immunol. 2022;13:848387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 25. | Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, Hu L, Zhang J, Wang R, Wei L, Wu D. Efficacy and Safety of TACE Combined With Lenvatinib Plus PD-1 Inhibitors Compared With TACE Alone for Unresectable Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Front Oncol. 2022;12:874473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6048] [Article Influence: 864.0] [Reference Citation Analysis (3)] |
| 27. | Lencioni R. Chemoembolization in patients with hepatocellular carcinoma. Liver Cancer. 2012;1:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Fiore M, Ljevar S, Pasquali S, Morelli D, Callegaro D, Sanfilippo R, Barisella M, Sangalli C, Miceli R, Gronchi A. Preoperative Neutrophil-to-Lymphocyte Ratio and a New Inflammatory Biomarkers Prognostic Index for Primary Retroperitoneal Sarcomas: Retrospective Monocentric Study. Clin Cancer Res. 2023;29:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | El Asmar A, Delcourt M, Kamden L, Khaled C, Bohlok A, Moreau M, Sclafani F, Donckier V, Liberale G. Prognostic Value of Preoperative Serological Biomarkers in Patients Undergoing Curative-Intent Cytoreductive Surgery for Colorectal Cancer Peritoneal Metastases. Ann Surg Oncol. 2023;30:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Choi WM, Kim JY, Choi J, Lee D, Shim JH, Lim YS, Lee HC, Yoo C, Ryu MH, Ryoo BY, Kim KM. Kinetics of the neutrophil-lymphocyte ratio during PD-1 inhibition as a prognostic factor in advanced hepatocellular carcinoma. Liver Int. 2021;41:2189-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |