Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1091
Peer-review started: October 26, 2023
First decision: January 6, 2024
Revised: January 14, 2024
Accepted: February 1, 2024
Article in press:
Published online: April 15, 2024
Processing time: 167 Days and 15.1 Hours
In this editorial, we have analyzed the historical evolution of rectal and breast cancer surgery, focusing on the progressive reduction of demolitive approaches and the increasing use of more conservative strategies, accompanied by a growing emphasis on perioperative treatments aimed at enhancing surgical outcomes. All of these changes have been made possible due to an increased awareness and understanding of oncological diseases and improved perioperative treatments.
Core Tip: Rectal and breast cancer surgeries share similar historical pathways. An enhanced comprehension of oncological diseases has led to a significant shift towards more conservative strategies with the primary objective of enhancing surgical outcomes and the quality of life for patients. Research and ongoing advancements continue to shape the future of cancer surgery.
- Citation: Pesce A, Fabbri N, Iovino D, Feo CV. Parallel pathways: A chronicle of evolution in rectal and breast cancer surgery. World J Gastrointest Oncol 2024; 16(4): 1091-1096
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1091.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1091
If we examine the evolution of rectal surgery, we can observe how its history bears a striking resemblance to that of breast surgery, with a progressive reduction in the demolitive approach and a growing emphasis on perioperative treatments aimed at enhancing surgical outcomes with more conservative approaches. The primary objective of this study was to offer a historical overview of the evolution of surgery for rectal and breast cancer throughout the years, with a focus on highlighting the important milestones.
The history of rectal surgery dates back thousands of years to when ancient Egyptians and Greeks pioneered the initial techniques for treating conditions such as hemorrhoids and anal fistulas. The Ebers and Medical Papyri document various rectal procedures, along with over 40 different medications for rectal ailments. In the 5th century, the Greek historian Herodotus remarked on the extensive body of knowledge related to rectal medicine that he encountered during his studies at the Library of Alexandria.
However, rectal cancer remained considered inoperable for many years until the eighteenth century when Morgagni[1], an Italian anatomist and pathologist first proposed surgical resection of the rectum as a potential treatment for rectal cancer.
In 1776, Henry Pillore, a French surgeon from Rouen, performed the first caecostomy in a patient with a scirrous obstructive rectal cancer[2]. Pillore performed a horizontal incision above the groin, lifted the cecum, made a transverse opening, and secured it to the two edges of the abdominal wound. Unfortunately, the patient did not survive.
Another French surgeon, Jacques LisFranc[3], achieved the first successful excision of the rectum for uncomplicated cancer in 1826 at the Pitié Hospital in Paris. Within seven years, LisFranc[3] performed nine more rectal resections using perineal or posterior approaches. LisFranc's[3] technique was quite primitive, lacking anesthesia or hemostasis. Despite LisFranc's[3] ability to perform rectal resections, initial outcomes were unfavorable, marked by frequent sepsis, and the majority of patients succumbed to recurrent disease. The introduction of anesthesia and asepsis rules led to previously impossible operations.
Theodor Billroth, a German and Austrian surgeon, performed 45 rectal resections from 1860 to 1872 following the surgical technique suggested by Lisfranc. Some years after, Aristide Auguste Stanislas Verneuil, a French surgeon and pupil of Lisfranc, modified his master's technique by performing perineal resections and removing the coccyx for better exposure and more radical excision. In 1868, he became a professor of pathology, and from 1872 onward, he served as a professor of clinical surgery at the Pitié Hospital in Paris.
In 1885, Paul Kraske, a German surgeon, developed his technique for perineal rectum removal. He made an incision from the center of the sacrum to the anus, disarticulated and detached the left side of the coccyx and sacrum, thereby freeing the rectum from its attachments, and performed a posterior resection[4]. Kraske presented this new approach to the Congress of the German Society of Surgery in 1885, receiving great acclaim and rapid adoption.
Vincent Czerny, a German Bohemian surgeon, faced with the challenge of removing a rectal tumor through the sacral approach, repositioned his patient in a lying position and successfully completed surgery via the anterior abdominal route in 1884[5]. This marked the initial attempt at combined abdominal and posterior resection. Vogel conducted a comprehensive review of 1500 rectal resection cases performed by twelve renowned surgeons in those years. He documented a perioperative mortality and relapse rate of 20.9% and 80%, respectively[5]. Recurrence emerged as a frequent and formidable issue, almost deemed inevitable. During the same period in England, Sir William Ernest Miles[6] acknowledged the challenge of local recurrence while evaluating his patients. Despite performing 57 perineal resections, 54 of them (95%) experienced tumor recurrence, mostly within the initial six months. To address this issue, he advocated for a more extensive mesenteric lymphadenectomy as a preventive measure. Miles delineated three zones of spread–downward, lateral, and upward–with upward spread being the most significant. Consequently, he devised a procedure to excise the rectum and the 'upward zone of spread', encompassing the cancer, recto-sigmoid, and associated lymph nodes, utilizing a combined abdominal and perineal approach. Miles coined this approach the radical "Abdomino-Perineal Resection" (APR). In a 1908 published article, Miles documented 12 procedures with high mortality rate (42%)[6]. Miles' procedure adhered to five key principles: (1) Establishing a permanent terminal colostomy; (2) excising the rectum, sigmoid, along with its vascular supply; (3) resecting the pelvic mesocolon; (4) removing the iliac lymph nodes; and (5) conducting a broad perineal resection with the removal of the elevator ani muscle.
John Percy Lockhart-Mummery, an assistant surgeon and rival of Ernest Miles at St Mark's Hospital in London, conducted 200 rectal resections by a perineal approach, achieving a 50% overall survival and an operative mortality of 8.5%[7]. Mummery asserted that Miles' procedure posed heightened risks for patients over 60 years of age or with important co-morbidities, despite its efficacy in reducing local recurrence.
In 1912, an American surgeon Mayo[8] adopted a two-staged approach for the APR. Initially, he established a colostomy and mobilized the rectum, followed by the resection of the rectum some weeks later through a perineal approach, with the belief that this sequence would minimize blood loss.
In 1921, during the 30th Congress of the French Surgical Association, Professor Henri Hartmann, head of the Hotel-Dieu in Paris, presented findings on two patients with occlusive sigmoid cancer. These individuals were managed with proximal colostomy, sigmoid resection, and closure of the rectal stump through an abdominal approach[9]. After the operation, he characteristically said that both cases were as uneventful as an operation for a cold appendix[9].
The abdominal-perineal resection held the position as the most frequently performed procedure until 1948, when Claude F Dixon[10], the surgical chair at the Mayo Clinic, unveiled the outcomes of restorative anterior resection for tumors located in the proximal rectum and distal sigmoid colon during the meeting of the American Surgical Association in Quebec, Canada. Dixon's findings redirected the emphasis in rectal cancer surgery from the radical APR to procedures that spared the sphincter. Indeed, the rectum anterior resection by the abdominal approach became the standard of care for middle and upper rectal tumors. This new approach in the treatment of tumors in the middle to upper rectum has represented a significant milestone in rectal cancer surgery.
A more refined and simple method for lower anterior resection of the rectum was attained through the utilization of the circular mechanical stapler. This technique was pioneered by two American surgeons, Charles Knight and Dean Griffen, and rapidly gained global acceptance. In 1980, Knight and Griffen[11] introduced the double stapling technique for low colorectal anastomoses. This innovative procedure significantly expedited the creation of colorectal anastomoses, even in the confined pelvic region, by overcoming the challenges of connecting bowel segments of different sizes and reducing intraoperative contamination. Between 1980 and 1986, Parks, Lazorthes, and Parc introduced coloanal anastomosis, intersphincteric rectal dissection, and colonic-pouch anal anastomosis, respectively. These approaches aimed at preserving or enhancing sphincter function, especially in cases of low rectal tumors[12,13].
Surely, the introduction of the new concept of Total Mesorectal Excision (TME) by Bill Richard Heald changed the surgical approach to rectal cancer through the knowledge of a new anatomical entity, the so-called mesorectum.
This groundbreaking work was initially documented in 1982[14], marking a pivotal achievement in modern rectal cancer surgery, further supported by the evolution of minimally invasive approaches in the subsequent years. It began to emphasize that the surgery of malignant disease was not solely about removing organs, but also about the precise understanding of the anatomy of lymphatic drainage and more conservative approaches compared to the destructive interventions of the past. The TME aimed to reduce the risk of local recurrence of cancer and improved long-term outcomes for patients.
The findings from the Dutch trial in 2001 also indicated that short-term pre-operative radiotherapy decreased the risk of local recurrence in patients with rectal cancer who underwent standardized TME[15].
In 2013, the transanal endoscopic approach, known as the Cecil approach Ta-TME, proposed by de Lacy et al[16], a Spanish surgeon from Barcelona, represented only a technical evolution in rectal surgery. This approach involves bottom-to-up dissection of the distal rectum in cases of low rectal cancers, narrow pelvis, and male and obese patients without any clear advantages in post-operative oncological outcomes.
The "watch and wait strategy" was initially suggested by Dr. Habr-Gama[17], a Brazilian surgeon, and her group, who have advocated for the non-surgical treatment of locally-advanced rectal cancer for nearly two decades in patients achieving a complete clinical response (cCR) – defined as the absence of clinically-detectable residual tumor – after neoadjuvant therapy. These patients undergo a specific follow-up protocol to ensure effective cancer control, including early identification of recurrent disease. Nevertheless, an ongoing debate surrounds various issues such as patient selection, clinical and radiological criteria for cCR, and optimal follow-up protocols. Furthermore, subsequent studies conducted by different research teams have shown that there are no significant differences in overall survival and local recurrence between patients treated surgically and those undergoing "watch and wait" strategy[18]. This strategy has represented the ultimate expression of conservative treatment in rectal cancer surgery.
However, robust findings from prospective multicenter studies are essential to validate the feasibility of this approach in terms of non-inferiority when compared to surgical treatment.
Immunotherapy represented an important advancement in rectal cancer treatment. It aims to enhance the body's natural immune response to target and destroy cancer cells. While immunotherapy has shown success in certain types of cancer, its role in rectal cancer is still evolving[19]. Immune checkpoint inhibitors, like pembrolizumab and nivolumab, are medications that inhibit specific proteins on cancer cells or immune cells, enabling the immune system to better identify and fight cancer. In recent years, there have been breakthroughs in the application of immunotherapy in locally advanced rectal cancer, introducing a new concept of selective surgery that involves the targeted removal or preservation of lymph nodes[20].
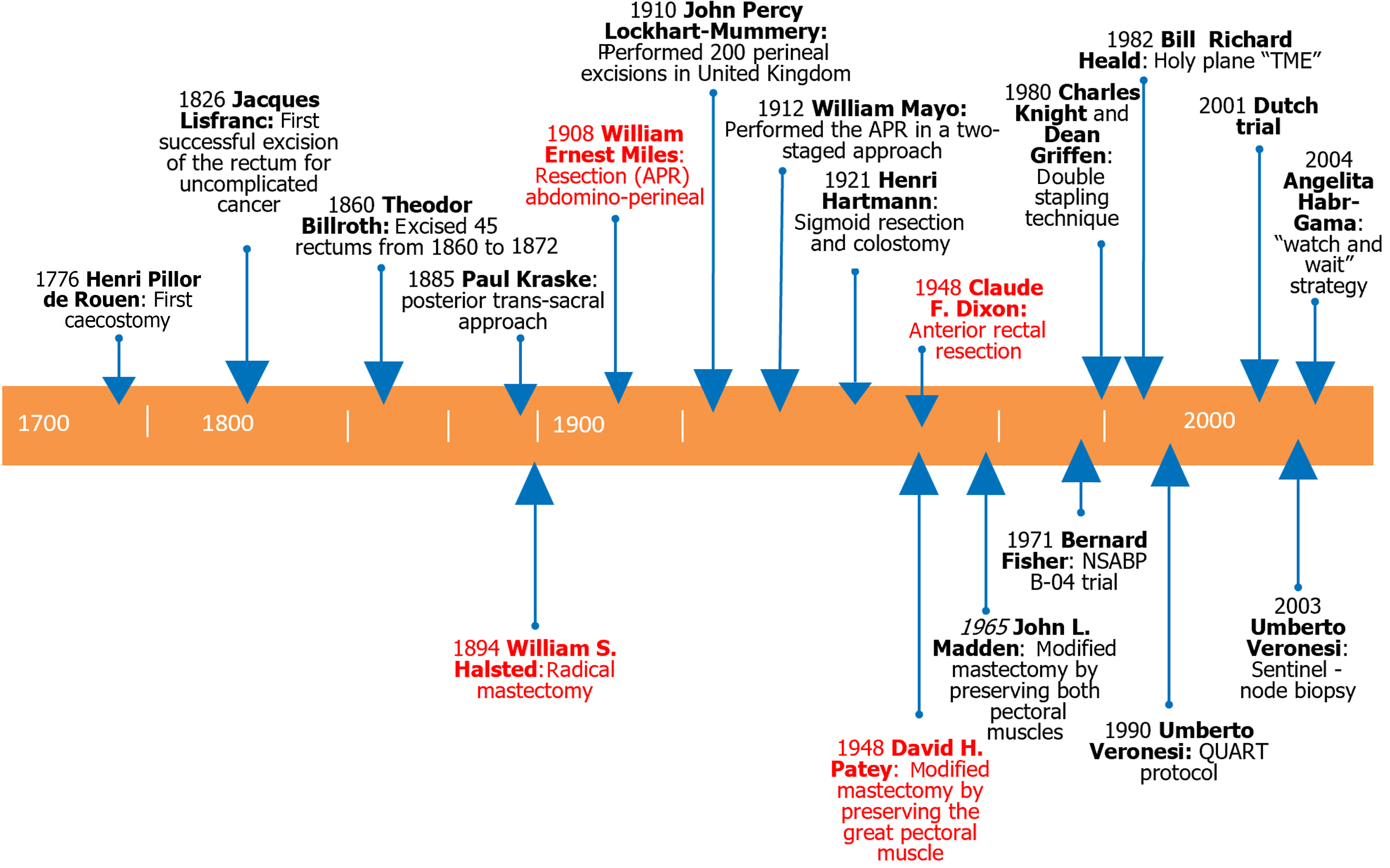
All the important milestones in rectal cancer history are summarized and shown in Figure 1.
In 1894, Halsted[21], Professor of Surgery at Johns Hopkins Hospital in Baltimore, United States, documented the first interventions he carried out, establishing guidelines for radical oncological surgery in breast cancers. In the same years, in Europe Sir William Ernest Miles performed the APR in rectal cancer. The Halsted operation consisted on radical mastectomy and it continued to be performed on more than 90% of patients with breast cancer in the United States until 1948 when Patey and Dyson[22] modified Halsted's operation by preserving the great pectoral muscle. In the same year, Claude Dixon in United States performed the first anterior resection of the rectum. Patey and Dyson’s approach was less traumatic and resulted in fewer post-operative complications. In 1965, another American surgeon at St. Clare's Hospital in New York, Madden[23], described a new technique of modified radical mastectomy, in which he concluded that keeping both pectoral muscles provided the best result. This modified surgical approach represented a first step towards a greater interest in breast pathology, in contrast to the few changes in the early 1900s.
The major obstacle to attempts to treat breast cancer with less mutilating surgical procedures was the high rates of multifocality of breast cancer.
In 1980, Fisher et al[24], a surgeon at the University of Pittsburgh, showed that the Halsted operation did not result in a higher curative rate compared to a simple mastectomy, which spared the muscles and uninvolved lymph nodes. The so-called National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 trial[24] represented a milestone for many surgeons, who had previously considered any surgery less than radical mastectomy to be malpractice.
In order to systematize breast surgery, Berg[25], an American radiotherapist, divided the axillary lymph nodes into three stations based on their position relative to the minor pectoris muscle. Nevertheless, mortality rates for breast cancer have decreased since 1975, attributed to the increased adoption of screening mammography and greater utilization of adjuvant treatments, including radiotherapy[26]. In the 1950s, certain authors proposed that conservative breast surgery followed by the then-standard postoperative radiotherapy regimen might be suitable for a small subgroup of patients.
The results of the initial randomized controlled trial led by Umberto Veronesi et al[27], which compared Halsted mastectomy with wide local excision and quadrantectomy followed by local radiotherapy, demonstrated that, for patients with T1-N0 tumors, the extension of surgery does not significantly impact rates of distant recurrence or patient survival. Over the past four decades, trials conducted by the NSABP have played a crucial role in reducing the scope of surgical procedures and enhancing outcomes for early-stage breast cancer patients. Consistent with earlier findings, the most recent update from the NSABP trial continued to affirm the value of lumpectomy and breast radiation as the preferred treatment for the majority of patients with operable invasive breast cancer, as evidenced by results at 20 years of follow-up[24]. According to the Geneva Tumour Registry[28], the rate of all curative conservative breast surgeries increased from 3% before 1985 to 51% in 1990, and then to 67% since 1998.
In 1990, The Uppsala-Orebro Breast Cancer Study Group conducted a randomized trial comparing sector resection with or without postoperative radiotherapy for Stage I breast cancer[26]. Their conclusion was that sector resection combined with radiotherapy to the breast effectively ensures local tumor control. Although surgery alone yields comparable survival prospects, the likelihood of local recurrence approaches 20% at 5 years[26].
Regarding the treatment of the axillary cavity, as approximately 75% of axillary dissections for breast cancer turned out to be negative, there was a parallel reduction in extensive surgery, similar to what happened with the breast. Already in the 1990s, thanks to the studies of Bostick et al[29] on lymphatic drainage in melanoma the concept of the sentinel lymph node as the "first lymph node draining from the breast tumor" was applied. This node could be identified using a tracer, initially colored and later with a radioguided method, by the Milan group led by Professor Veronesi et al[30].
The evolution of technology, alongside the reduction of surgical trauma, has brought complementary radiotherapy into the operating room. In fact, in the first decade of the 21st century, thanks to ELIOT (intraoperative Electron radiotherapy) and TARGIT (TARGeted Intraoperative radioTherapy) trials, an intraoperative booster dose of radiotherapy, followed by lower postoperative wall breast irradiation, has been proposed for early-stage tumors. This approach yields therapeutic results equivalent to traditional radiotherapy while reducing the toxic effects of radiation[31,32].
Immunotherapy has emerged as a promising therapeutic approach in the treatment of breast cancer by augmenting the host's immune system, particularly in cases of triple-negative breast cancers[33]. However, the major concerns are tumor recurrence and drug resistance[33].
Both rectal and breast cancer surgery have evolved significantly over the years, with a focus on improving outcomes, recurrence rate and survival, reducing the invasiveness of procedures, and tailoring treatments to individual patients' needs and the improvement of their quality of life. Research and ongoing advancements continue to shape the future of cancer surgery. We really think that Anyone must know the past to improve the future, this is our key-message for the younger generations.
We wish to thank Ms. Eleanor Lea for the English editing.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ji Y, China S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Morgagni GB. Founders of Modern Medicine: Giovanni Battista Morgagni. (1682-1771). Med Library Hist J. 1903;1:270-277. [PubMed] |
| 2. | Fong CF, Corman ML. History of right colectomy for cancer. Ann LaparoscEndosc Surg. 2019;4:49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Lisfranc J. Mémoire sur l’excision de la partie inférieure du rectum devenu carcinomateux. Mémoires de l’Académie de Médecine. 1833;III:291-302. |
| 4. | Classic articles in colonic and rectal surgery. Paul Kraske 1851-1930. Extirpation of high carcinomas of the large bowel. Dis Colon Rectum. 1984;27:499-503. [PubMed] |
| 5. | Mathew DAP, Wagh DMS. Abdominoperineal Excision in current era. Cancer Treat Res Commun. 2022;32:100580. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Classic articles in colonic and rectal surgery. John Percy Lockhart-Mummery 1875-1957. Two hundred cases of cancer of the rectum treated by perineal excision. Dis Colon Rectum. 1984;27:208-219. [PubMed] |
| 8. | Mayo WJ. THE RADICAL OPERATION FOR CANCER OF THE RECTUM AND RECTOSIGMOID. Ann Surg. 1916;64:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ronel DN, Hardy MA. Henri Albert Hartmann: labor and discipline. Curr Surg. 2002;59:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Dixon CF. Anterior Resection for Malignant Lesions of the Upper Part of the Rectum and Lower Part of the Sigmoid. Ann Surg. 1948;128:425-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Knight CD, Griffen FD. An improved technique for low anterior resection of the rectum using the EEA stapler. Surgery. 1980;88:710-714. [PubMed] |
| 12. | Lange MM, Rutten HJ, van de Velde CJ. One hundred years of curative surgery for rectal cancer: 1908-2008. Eur J Surg Oncol. 2009;35:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Lirici MM, Hüscher CG. Techniques and technology evolution of rectal cancer surgery: a history of more than a hundred years. Minim Invasive Ther Allied Technol. 2016;25:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 14. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1930] [Article Influence: 44.9] [Reference Citation Analysis (1)] |
| 15. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3110] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 16. | de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, Sylla P, Martínez-Palli G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: "down-to-up" total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27:3165-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-7; discussion 717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1356] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 18. | López-Campos F, Martín-Martín M, Fornell-Pérez R, García-Pérez JC, Die-Trill J, Fuentes-Mateos R, López-Durán S, Domínguez-Rullán J, Ferreiro R, Riquelme-Oliveira A, Hervás-Morón A, Couñago F. Watch and wait approach in rectal cancer: Current controversies and future directions. World J Gastroenterol. 2020;26:4218-4239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (3)] |
| 19. | Shu Y, Zheng S. The current status and prospect of immunotherapy in colorectal cancer. Clin Transl Oncol. 2024;26:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Yao S, Lan H, Han Y, Mao C, Yang M, Zhang X, Jin K. From organ preservation to selective surgery: How immunotherapy changes colorectal surgery? Surg Open Sci. 2023;15:44-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20:497-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 412] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 22. | Patey DH, Dyson WH. The prognosis of carcinoma of the breast in relation to the type of operation performed. Br J Cancer. 1948;2:7-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Madden JL. Modified radical mastectomy. Surg Gynecol Obstet. 1965;121:1221-1230. [PubMed] |
| 24. | Fisher B, Montague E, Redmond C, Deutsch M, Brown GR, Zauber A, Hanson WF, Wong A. Findings from NSABP Protocol No. B-04-comparison of radical mastectomy with alternative treatments for primary breast cancer. I. Radiation compliance and its relation to treatment outcome. Cancer. 1980;46:1-13. [PubMed] [DOI] [Full Text] |
| 25. | BERG JW. The significance of axillary node levels in the study of breast carcinoma. Cancer. 1955;8:776-778. [PubMed] [DOI] [Full Text] |
| 26. | Liljegren G, Holmberg L, Adami HO, Westman G, Graffman S, Bergh J. Sector resection with or without postoperative radiotherapy for stage I breast cancer: five-year results of a randomized trial. Uppsala-Orebro Breast Cancer Study Group. J Natl Cancer Inst. 1994;86:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 244] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Veronesi U, Banfi A, Salvadori B, Luini A, Saccozzi R, Zucali R, Marubini E, Del Vecchio M, Boracchi P, Marchini S. Breast conservation is the treatment of choice in small breast cancer: long-term results of a randomized trial. Eur J Cancer. 1990;26:668-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 348] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Kurtz J; EUSOMA Working Party. The curative role of radiotherapy in the treatment of operable breast cancer. Eur J Cancer. 2002;38:1961-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Bostick P, Essner R, Glass E, Kelley M, Sarantou T, Foshag LJ, Qi K, Morton D. Comparison of blue dye and probe-assisted intraoperative lymphatic mapping in melanoma to identify sentinel nodes in 100 lymphatic basins. Arch Surg. 1999;134:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 1561] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 31. | Sedlmayer F, Reitsamer R, Fussl C, Ziegler I, Zehentmayr F, Deutschmann H, Kopp P, Fastner G. Boost IORT in Breast Cancer: Body of Evidence. Int J Breast Cancer. 2014;2014:472516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, Alvarado M, Flyger HL, Massarut S, Eiermann W, Keshtgar M, Dewar J, Kraus-Tiefenbacher U, Sütterlin M, Esserman L, Holtveg HM, Roncadin M, Pigorsch S, Metaxas M, Falzon M, Matthews A, Corica T, Williams NR, Baum M. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 529] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 33. | Anayyat U, Ahad F, Muluh TA, Zaidi SAA, Usmani F, Yang H, Li M, Hassan HA, Wang X. Immunotherapy: Constructive Approach for Breast Cancer Treatment. Breast Cancer (Dove Med Press). 2023;15:925-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |