Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.991
Peer-review started: October 20, 2023
First decision: December 5, 2023
Revised: December 21, 2023
Accepted: January 19, 2024
Article in press: January 19, 2024
Published online: March 15, 2024
Processing time: 144 Days and 6.4 Hours
The precise role of mitochondrial carrier homolog 2 (MTCH2) in promoting malignancy in gastric mucosal cells and its involvement in gastric cancer cell metastasis have not been fully elucidated.
To determine the role of MTCH2 in gastric cancer.
We collected 65 samples of poorly differentiated gastric cancer tissue and adjacent tissues, constructed MTCH2-overexpressing and MTCH2-knockdown cell models, and evaluated the proliferation, migration, and invasion of human gastric epithelial cells (GES-1) and human gastric cancer cells (AGS) cells. The mito
The expression of MTCH2 and ATP2A2 in gastric cancer tissues was significantly greater than that in adjacent tissues. Overexpression of MTCH2 promoted colony formation, invasion, migration, MMP expression and ATP production in GES-1 and AGS cells while upregulating ATP2A2 expression and inhibiting cell apoptosis; knockdown of MTCH2 had the opposite effect, promoting overactivation of the mPTP and promoting apoptosis.
MTCH2 can increase the malignant phenotype of GES-1 cells and promote the proliferation, invasion, and migration of gastric cancer cells by regulating mitochondrial function, providing a basis for targeted therapy for gastric cancer cells.
Core Tip: Mitochondrial carrier homolog 2 (MTCH2) increases malignant phenotype of human gastric epithelial cells. MTCH2 promotes progression of gastric cancer cells. Mitochondrial permeability transformation pore opening and ATP synthase play important roles in gastric cancer.
- Citation: Zhang JW, Huang LY, Li YN, Tian Y, Yu J, Wang XF. Mitochondrial carrier homolog 2 increases malignant phenotype of human gastric epithelial cells and promotes proliferation, invasion, and migration of gastric cancer cells. World J Gastrointest Oncol 2024; 16(3): 991-1005
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/991.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.991
Changes in mitochondrial bioenergetics, biosynthesis and signal transduction are necessary conditions for tumorigenesis[1]. Mitochondria are regarded as pivotal organelles responsible for ATP generation and are crucial for maintaining cellular energy metabolism. Mitochondrial outer and inner membrane-related proteins are involved in tumor biological behavior by regulating mtDNA replication and energy metabolism[2]. The role of mitochondrial outer membrane proteins in mitochondrial energy metabolism is particularly important, as they play a key role in the communication between mitochondria and other organelles[3].
Mitochondrial carrier homolog 2 (MTCH2), a member of the solute carrier 25 (SLC25) family, is an insertion enzyme of α-helical mitochondrial outer membrane proteins, and its main function is to transport metabolites to the mitochondrial matrix. It is also a membrane channel for proteins to enter mitochondria, and the loss of MTCH2 is associated with mitochondrial fusion, energy metabolism, lipid homeostasis, and apoptosis[4]. MTCH2 was found to be an important gene driving the progression of colon cancer through whole exome sequencing analysis of human colorectal cancer tissues[5]. In a metabolomics analysis of breast cancer, by exa mining the changes in proteomic metabolism during epithelial–mesenchymal transition (EMT), a relationship between MTCH2 expression and the occurrence of EMT was found[6]. A study involving whole-transcriptome RNA sequencing of myofibroma samples indicated that the fusion transcripts of MTCH2-for min binding protein promoted the malignant progression of myofibroma[7].
MTCH2 plays a crucial role not only in cellular productivity and energy consumption processes but also in the regulation of mitochondrial permeability transformation pore (mPTP) opening[8]. Under normal circumstances, the mPTP is typically maintained in a closed state. However, when stimulated by factors such as oxidation, excessive reactive oxygen species (ROS), or increased Ca2+ levels, stress can be triggered or promoted, allowing for free exchange of proteins[9]. According to noncancer studies, abnormal opening of the mPTP, which results in impaired mitochondrial function, is thought to be the central event in inflammation[10]. According to cancer research, abnormal opening of the mPTP is thought to mediate cancer cell death by regulating apoptotic signaling[11]. Notably, abnormal opening of the mPTP promotes the enhancement of mitochondrial permeability, resulting in the loss of mitochondrial membrane potential (MMP) and a reduction in mitochondrial ATP, which induces the activation of apoptosis and enhances the therapeutic efficacy of chemotherapeutic drugs[12]. Studies have shown that ADT1, ATP5H, ATPA, and ATPB are the most abundant mPTP proteins[13]. ATP2A2 is a hub in protein-protein interaction networks[14]. During the process of EMT in breast cancer cells, ATP2A2 and MTCH2 have been found to be involved in breast cancer progression[6].
In this study, we detected differences in the expression of MTCH2 between gastric cancer tissues and adjacent tissues and found that MTCH2 was overexpressed in gastric cancer tissues, suggesting that MTCH2 may be a new target for the diagnosis and treatment of gastric cancer. Using in vitro experiments, we proposed that MTCH2 can affect the proliferation and metastasis of human gastric epithelial cells (GES-1) and human gastric cancer cells (AGS) cells by regulating mPTP opening, the MMP and ATP production, providing a reference for cancer therapy involving the targeting of mitochondria.
Tissue specimens of gastric cancer and paracancer tissues of 65 patients with low differentiated gastric cancer were obtained from North China University of Technology Affiliated Hospital. Sixty-five patients in this group had no previous history of radiotherapy and chemotherapy.
GES-1 and AGS were purchased from National Collection of Authenticated Cell Cultural, China (https://www.cellbank.org.cn/). GES-1 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/L D-glucose containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (p/s). AGS cells were maintained in F-12K Medium containing 10% FBS and 1% p/s. All cell lines were cultured in incubator at 37 °C and 5% CO2.
Clone vector (pcDNA3.1) of MTCH2 overexpression (MTCH2-OE, ZB02427, cloning site: Nhei/Bamhi.) were purchased from company Shanghai Sangon (SangonBiotech, China, https://www.sangon.com/). Three siRNA sequences against MTCH2 and/or ATP2A2 (MTCH2-siRNA1, MTCH2-siRNA2, and ATP2A2 siRNA) and one negative control (siRNA-NC) were designed. The sequences were as follows: MTCH2-siRNA1 (5’-3’): Sense: UCCUCCAACAAUAGGACGAAATT; antisense: UUUCGUCCUAUUGUUGGAGGATT. MTCH2-siRNA2 (5’-3’): Sense: GCCUACCUCGUCAAUACCUAUTT; antisense: AUAGGUAUUGACGAGGUAGGCTT. ATP2A2 siRNA (5’-3’): Sense: CCUGGUGAUAUUGUAGAAAUUTT, antisense: AAUUUCUACAAUAUCACCAGGTT. siRNA-NC (5’-3’): Sense: UUCUCCGAACGUGUCACGUTT; antisense: ACGUGACACGUUCGGAGAATT. After 24 h of cultivation, cells were transfected with MTCH2-OE, MTCH2-siRNA1, MTCH2-siRNA2, ATP2A2 siRNA, and siRNA-NC using Lipo8000TM transfection reagent (C0533-0.5 mL, Beyotime, China). After 24 h of transfection, cells were collected for Western blotting.
Following 24 h culture in DMEM or F-12K without serum, cells were collected and diluted to cell concentration of 1 × 105/mL using serum-free medium. Cell suspension (200 μL) was seeded to the upper chamber and 600 μL DMEM or F-12K medium supplemented with 10% FBS was added to the lower chamber. After incubation for 48 h, serum-free medium was removed from the upper chamber. Cells on the undersurface of the upper chamber were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Cells remaining on the upper surface of the upper chamber were wiped with cotton swabs. Five fields were randomly selected under the microscope to count the number of cells that migrated.
Cells were seeded in a 6-well plate and cultured for 24 h in DMEM or F-12K medium supplemented with 10% FBS. Two parallel scratches were made within each well using 200 μL pipette tips. Cells were continued to be cultured in the corresponding medium for 24 h and photographed under the microscope at 0 h and 24 h after the scratches were drawn.
The cells were seeded into a 6-well plate and cultivated for 24 h. After another 24 h of transfection, 25 μL pcMV-AT1.03 plasmid (D2604, Beyotime, China), 4 μL Lipo8000TM transfection reagent and 125 μL serum-free medium were added into each well for mitochondrial ATP fluorescence probe transfection. After 24 h transfection, a fluorescence microscope was used to observe fluorescence intensity.
Cells were seeded into Petri dishes at a density of 200 cells/ well. After the intervention, cells were cultivated at 37 °C, 5% CO2 for 2-3 wk until visible colonies formed. During this time, the cell medium was changed every 2 d. Cells were washed with PBS for twice followed by fixing with 4% paraformaldehyde for 15 min. Cell colonies were then stained with 0.1% crystal violet for 20 min. The number of colonies were counted for analysis.
After dewaxing, rehydration and antigen retrieval, paraffin-embedded tissue sections were incubated with 3% hydrogen peroxide for 15 min and then with 10% goat serum for 30 min both in a 37 °C water bath. Anti-MTCH2 (1:200, 16888-1-AP, Proteintech, China) and anti-ATP2A2 (1:200, 67248-1-lg, Proteintech, China) were used as primary antibody in an incubation at 4 °C overnight. On the second day, the sections were first incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (PV-6000; ZSGB-BIO, China) for 2 h and then stained with DAB, counterstained with hematoxylin successively. Sections were finally sealed with neutral gum. Photos were taken under an inverted optical microscope.
After the intervention, JC-1 solution (M8650, Solarbio) was added to the 6-well plates. Thoroughly mixed and incubated in the cell incubator at 37 °C for 20 min. Then cells were washed with JC-1 staining buffer for twice. Added 2 mL cell culture medium to each well to observe fluorescence intensity under a fluorescence microscope.
Cells were cultured in a 12-well plate. mPTP opening degree was detected through mPTP Assay Kit (C2009S, Beyotime, China). After the intervention, cells were washed with PBS for twice, added 500 μL Calcein AM staining solution and 500 μL Ionomycin control respectively in the first two wells of untreated cells. Fluorescence quenching solution (500 μL) was added to each well of NC-OE, NC-siRNA, MTCH2-OE and MTCH2 siRNA group. Incubated at 37 °C for 45 min. The reagents were then replaced with pre-heated culture medium at 37 °C and incubated for 30 min away from light. After washed with PBS for twice, Assay buffer was added to each well for fluorescence intensity observation under a fluorescence microscope.
Cell protein was extracted through whole cell lysis assay kit (KGP2100, KeyGEN BioTECH, China). Cells were first oscillatory mixed in Lysis Buffer then centrifuged at 12000 g for 5 min. The supernatant is the protein extract. BCA Protein Assay Kit (PT0001, LEAGENE, China) was used to detected the concentration of cell protein. Afterwards, protein was separated with 10% SDS-PAGE and transferred onto PVDF membranes. The membranes were then incubated with 5% skimmed milk powder at room temperature for 2 h and primary antibodies: Anti-MTCH2 (1:5000, 16888-1-AP, proteintech, China), anti-Bax (1:4000, 50599-2-lg, proteintech, China), anti-Bcl-2 (1:5000, 68103-1-lg, proteintech, China), anti-Cytochrome c (1:4000, 10993-1-AP, proteintech, China), anti-ATP2A2 (1:5000, 67248-1-lg, proteintech, China), anti-Vimentin (1:1000, bs-8533R, Bioss, China), anti-β-catenin (1:1000, bs-1165R, Bioss, China), anti-N-cadherin (1:1000, bs-1172R, Bioss, China), anti-E-cadherin (1:1000, 60335-1-lg, proteintech, China), and anti-b-actin (1:2000, 20536-1-AP, proteintech, China) at 4 °C overnight. On the second day, the membranes were incubated with HRP-conjugated secondary antibody (1:5000, ZB-2301; ZB-2305, ZSGB-BIO, China) at room temperature for 1 h and visualized by enhanced chemilu minescence detection reagents (P1050, Applygen Technologies Inc., China). ImageJ software was used for the quantification of results.
SPSS 21.0 software and GraphPad Prism 7 software were used for statistical analysis. One-way or two-way analysis of variance (ANOVA) was used to analyze the significance of differences between groups. All values were displayed as mean ± SEM, representing three independent experiments, P < 0.05 was considered statistically significant.
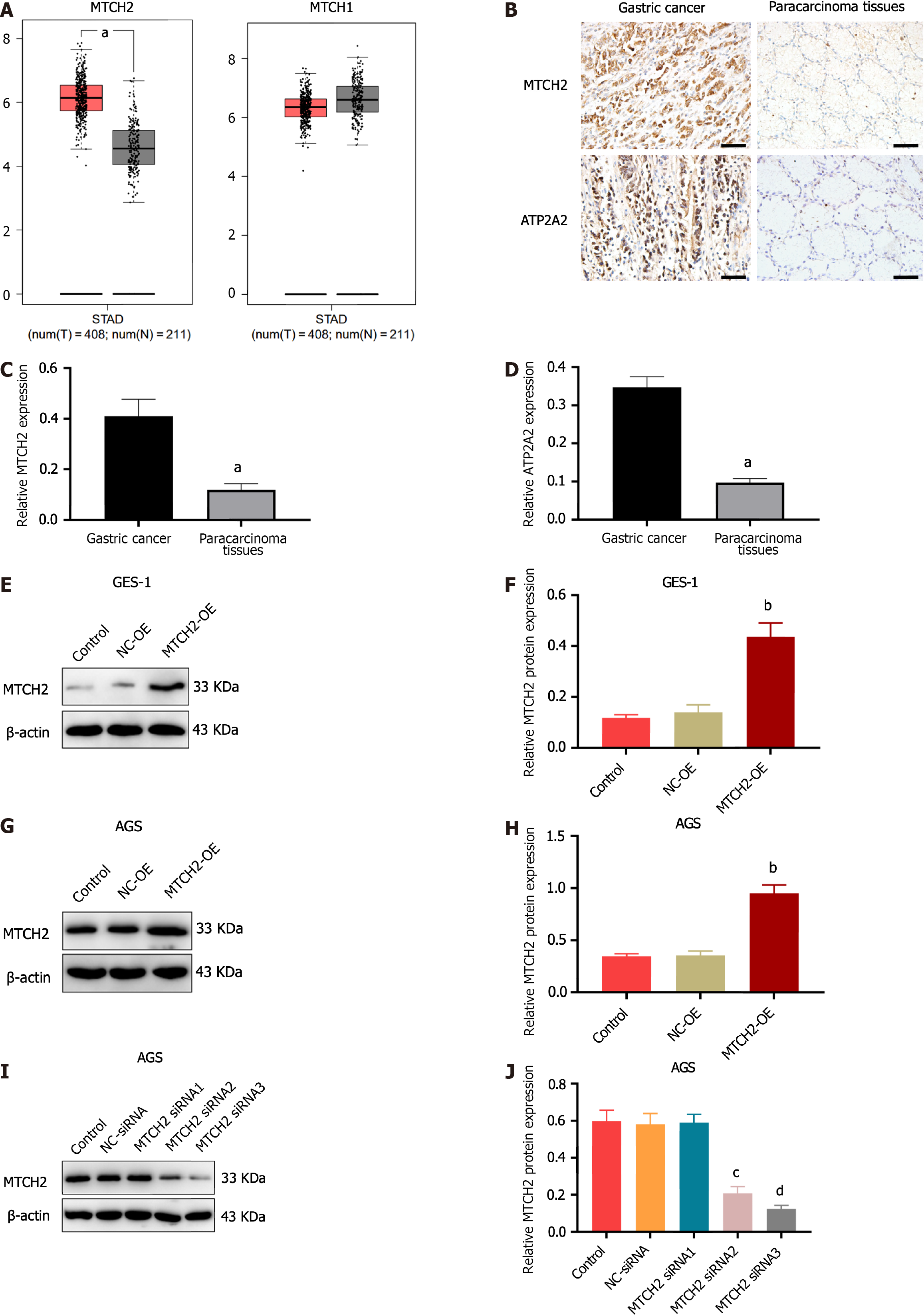
The results of Gene Expression Profiling Interactive Analysis (GEPIA) database analysis showed that MTCH2 was overexpressed in gastric cancer. Compared with normal tissue; There was no difference in MTCH1 expression (Figure 1A). To deter mine how MTCH2 and ATP2A2 were expressed differently, we took tissue samples from 65 cases of poorly differentiated gastric cancer and para-carcinoma tissue. The results showed that MTCH2 was mainly expressed in cytoplasm and mitochondria, while ATP2A2 was mainly expressed in cytoplasm, and the expressions of both were significantly higher in gastric cancer tissues than in adjacent tissues (Figure 1B-D, Table 1), the expression of the two was positively correlated (Table 2). In this study, GES-1 and AGS were used. We constructed cell models of MTCH2 overexpression (MTCH2-OE) and MTCH2 silencing (MTCH2-siRNA), and screened sequences with significant transfection effect for expansion experiments (Figure 1E-J).
| Clinical pathological features | n | MTCH2 expression | χ2 | P value | |
| Positive (n = 48) | Negative (n = 17) | ||||
| Gender | |||||
| Male | 41 | 27 | 14 | 3.673 | 0.055 |
| Female | 24 | 21 | 3 | ||
| Age (yr) | |||||
| < 60 | 33 | 25 | 8 | 0.127 | 0.722 |
| ≥ 60 | 32 | 23 | 9 | ||
| Infiltration depth | |||||
| T1 + T2 | 29 | 16 | 13 | 9.454 | 0.002b |
| T3 + T4 | 36 | 32 | 4 | ||
| TNM stage | |||||
| I + II | 34 | 21 | 13 | 5.388 | 0.020a |
| III + IV | 31 | 27 | 4 | ||
| Lymph node | |||||
| Metastasis | |||||
| Yes | 45 | 37 | 8 | 5.313 | 0.021a |
| No | 20 | 11 | 9 | ||
| Greater omentum metastasis | |||||
| Yes | 37 | 31 | 6 | 4.392 | 0.036a |
| No | 28 | 17 | 11 | ||
| Survival | |||||
| Yes | 44 | 29 | 15 | 4.442 | 0.035a |
| No | 21 | 19 | 2 | ||
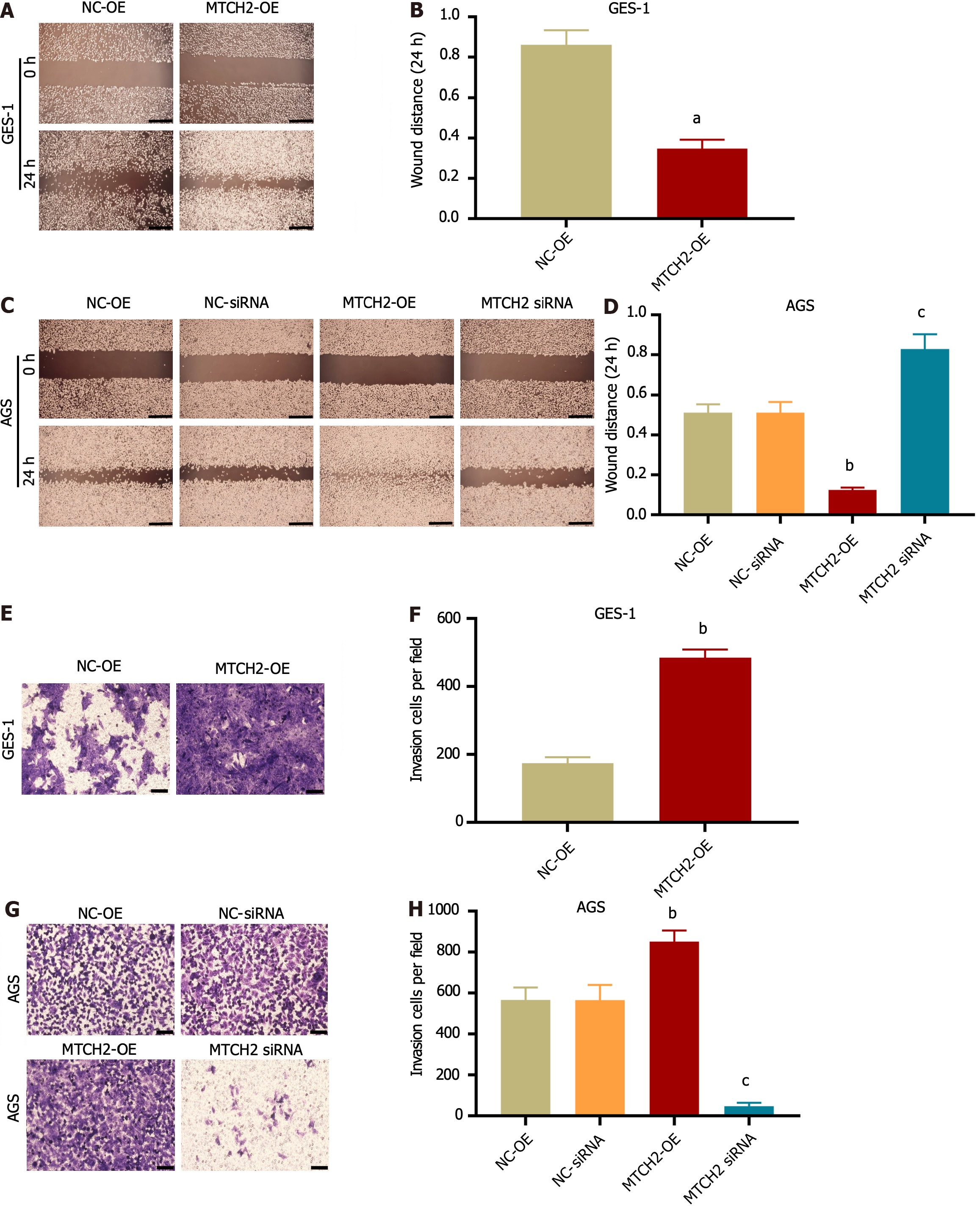
We observed the effects of MTCH2 on two types of cell invasion and migration. Wound-healing scratch assay (Figure 2A-D) and Transwell assay (Figure 2E-H) showed that MTCH2 overexpression promoted the invasion and migration of GES-1 and AGS cells, while MTCH2-siRNA had the opposite effect. The results indicated that MTCH2 regulated the ability of GES-1 and AGS cells to transfer.
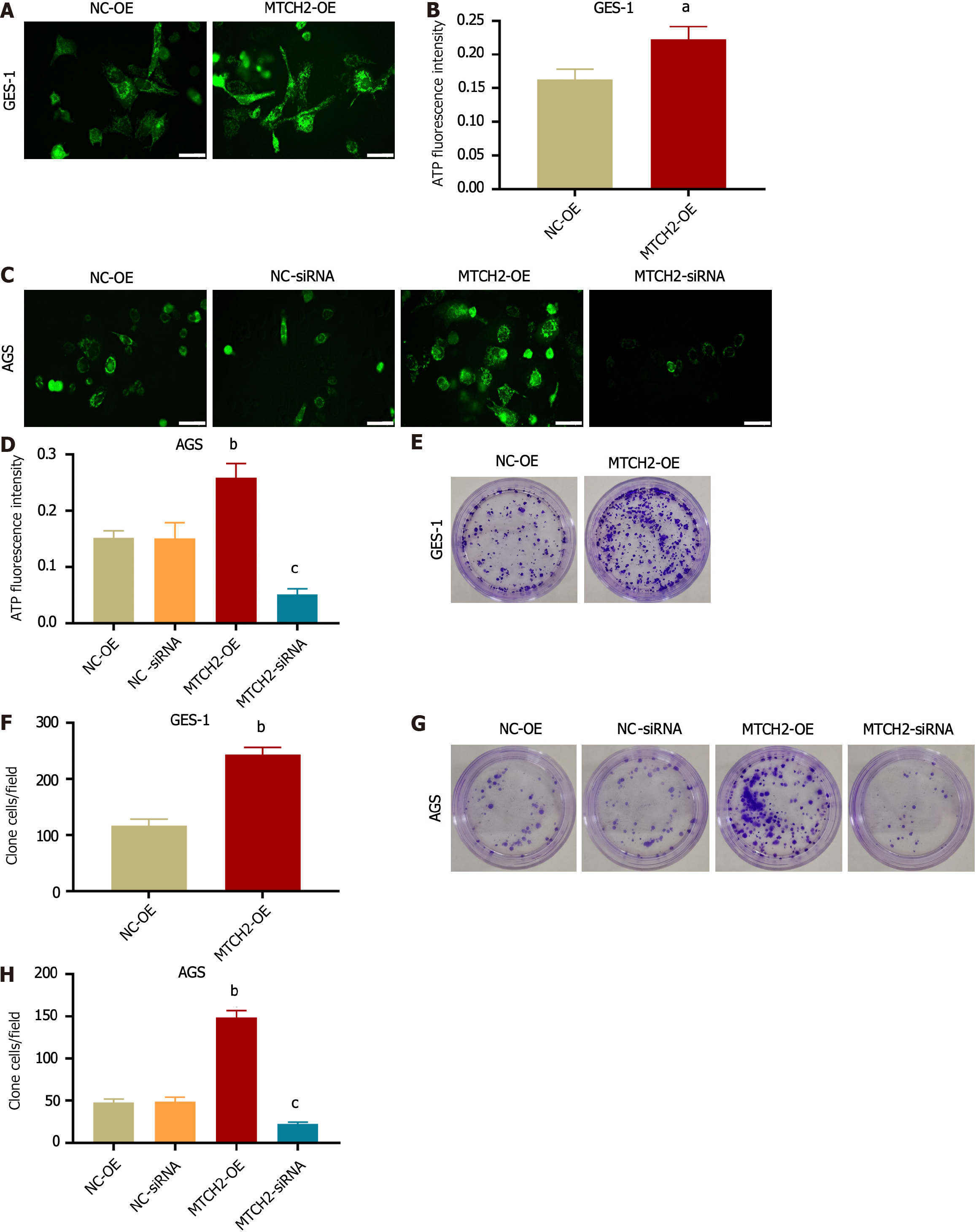
In GES-1 and AGS cells, MTCH2 overexpression stimulated an increase in mitochondrial ATP synthesis, which supplied energy for the malignant phenotype of GES-1 cells and improved the capacity of AGS metastasis. To verify this hypothesis, fluorescent probes were used to detect mitochondrial ATP production (Figure 3A-D), and colony formation was observed through cloning experiments (Figure 3E-H). Overexpression of MTCH2 can increase ATP synthesis and promote proliferation in GES-1 and AGS cells. After MTCH2 knockdown, ATP production and cell proliferation of AGS cells were impaired. Therefore, MTCH2 has carcinogenic and pro-carcinogenic effects in the progression of gastric cancer cells.
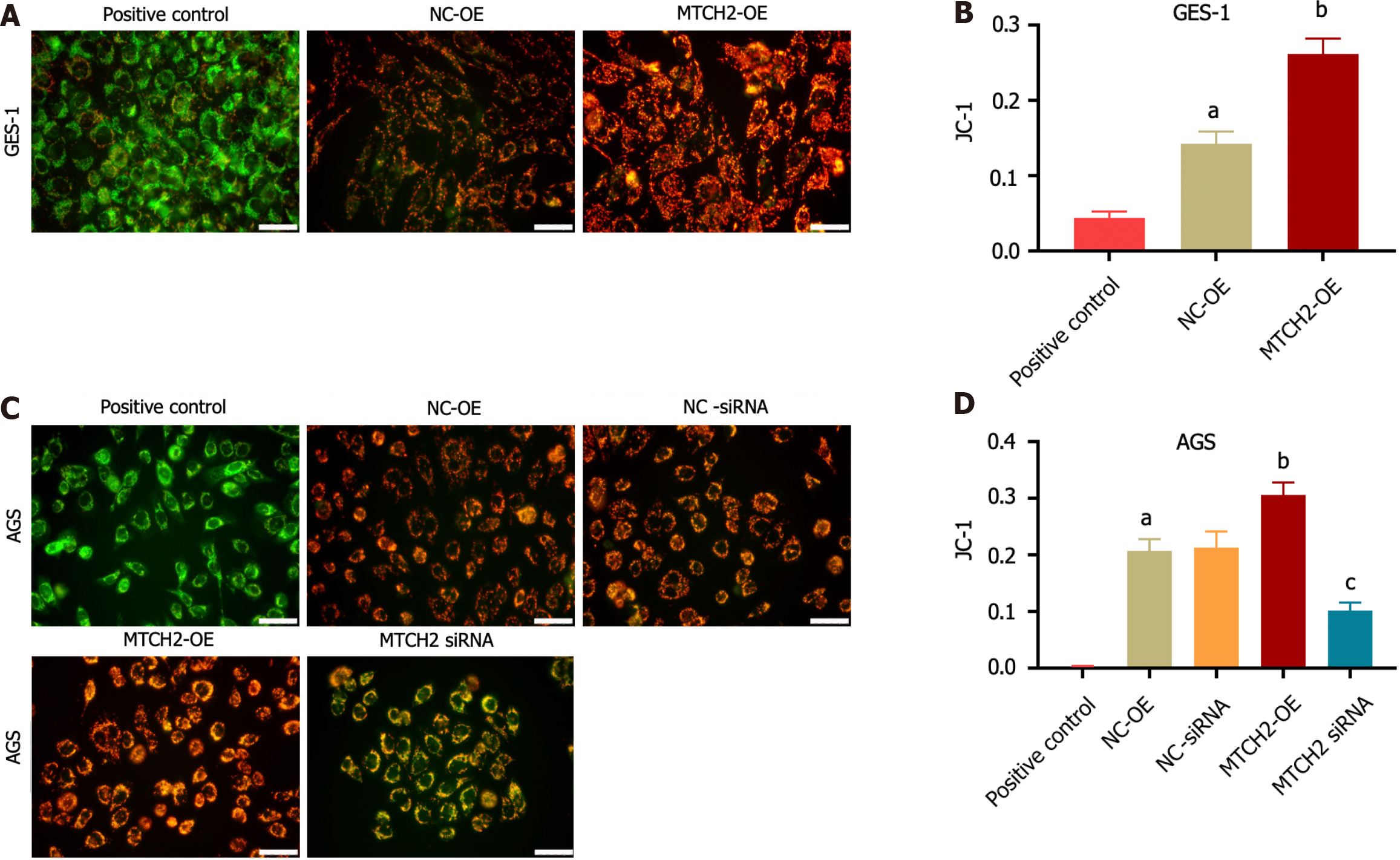
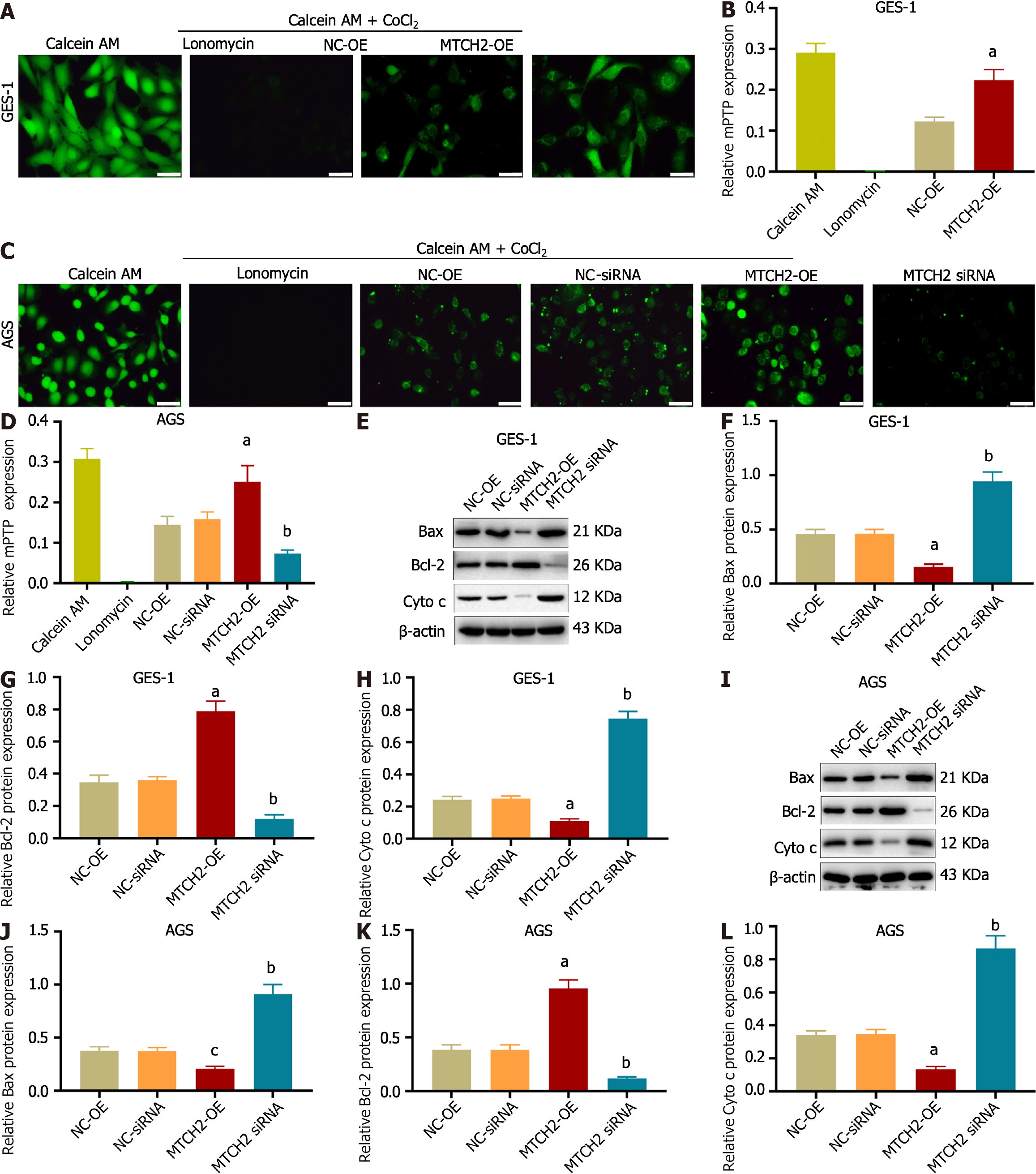
We confirmed the effect of MTCH2 on MMP of GES-1 and AGS cells by JC-1 staining. The results showed that overexpression of MTCH2 could up-regulate the red fluorescence of JC-1, accompanied by a decrease in the intensity of green fluorescence, resulting in an increase in MMP. Knocking down MTCH2 down-regulated the red fluorescence of JC-1, increased the green fluorescence intensity, and lead to the decrease of MMP. These results indicated that MTCH2 can affect mitochondrial function by regulating MMP. As a baseline for experimental outcomes, we employed the elimination of cell mitochondrial JC-1 as a positive control (Figure 4).
MMP is complementary to the opening of mPTP, and the conformational changes of mPTP structural proteins affect mitochondrial function. As a result, we used Calcein AM to detect the degree of mPTP openness. Compared with the NC-siRNA group, the green fluorescence was eliminated by knocking down MTCH2, indicating that mPTP had been opened. Compared with the NC-OE group, the green fluorescence was enhanced when MTCH2 was overexpressed, indicating that mPTP remaining off (Figure 5A-D). Calcein AM is used as a metal complexation indicator and Lonomycin is used as an mPTP open indicator.
We found that overexpression of MTCH2 can decrease the expression of Bax, cytochrome c (Cyto c), and promote the expression of Bcl-2; knocking down MTCH2 significantly decreased the expression of Bcl-2, and upregulated the expression of Bax and Cyto c (Figure 5E-L). These results indicated that MTCH2 can influence apoptosis of cells by regulating mitochondrial function.
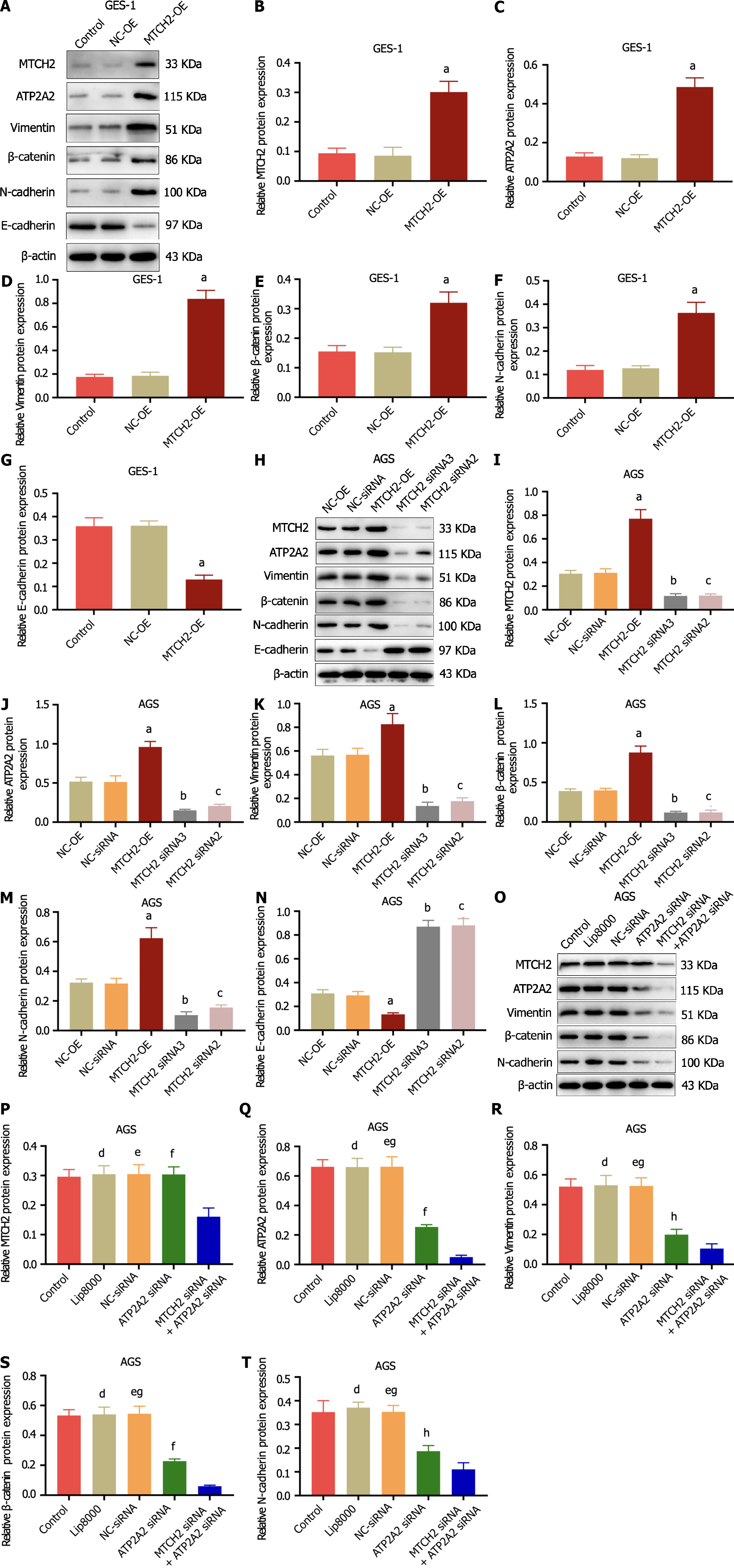
Overexpression of MTCH2 can up-regulate the expression of MTCH2, ATP2A2, N-cadherin, Vimentin, β-catenin, and decrease the expression of E-cadherin in GES-1 cells, promoting the expression of factors related to malignant phenotypic markers (Figure 6A-G). The same effect was also observed in AGS cells. Importantly, in AGS cells, knocking down MTCH2 played the opposite role (Figure 6H-N). This fully indicates that MTCH2 can promote the malignant phenotype of GES-1 and enhance the malignant characteristics of AGS cells.
In order to further explore the mechanism of MTCH2 and ATP2A2 in gastric cancer cell metastasis, we simultaneously knocked down the expression of MTCH2 and ATP2A2. In AGS cells, the combination of MTCH2-siRNA and ATP2A2-siRNA can significantly down-regulate the expression of MTCH2, ATP2A2, N-cadherin, Vimentin, and β-catenin proteins, which is more significant than that of ATP2A2-siRNA alone (Figure 6O-T). These results indicate that MTCH2 and ATP2A2 play a role in the progression of gastric cancer, which is involved in the malignant phenotype of gastric mucosa cells and the metastasis of gastric cancer cells.
For a considerable period, mitochondrial functional proteins have been recognized as pivotal factors influencing tumor progression. Mitochondria, crucial in both cell culture and xenografts, are indispensable for initiating and sustaining tumor cell growth[15]. Throughout cancer cell progression, mitochondria play a vital role by supplying the necessary energy through energy conversion and biosynthesis[16]. Mitochondria maintain oxidative phosphorylation through the membrane potential gradient generated by the electron transport chain, which drives ATP synthesis[17]. MTCH2 plays an important role in mitochondrial energy metabolism[18]. Our study showed that MTCH2 is overexpressed in gastric cancer tissues and is closely related to the progression of gastric cancer. In vitro experiments, the up-regulation of MTCH2 expression promoted the malignant biological behavior of GES-1 and enhanced the metastasis ability of AGS cells. We have further found that MTCH2 influences mitochondrial function and apoptosis of GES-1 cells and gastric cancer cells by regulating MMP, mPTP opening and ATP production, indicating the value of MTCH2 in the study of malignant phenotype of gastric mucosa cells and metastasis of gastric cancer cells.
MTCH2 is intricately linked to numerous cellular processes, including cancer and diseases such as Alzheimer’s, acting as a crucial “gateway” for various proteins to enter the mitochondrial[19,20]. Notably, MTCH2 can integrate with a key protein involved in programmed cell death, presenting a promising target for cancer therapy and potentially enhancing the sensitivity of cancer treatment[21]. Moreover, the overexpression of MTCH2 holds significant clinical value in predicting the risk of endometrial cancer[22]. In the context of breast cancer, targeting MTCH2 expression can effectively impede disease progression, underscoring its research potential in both the diagnosis and treatment of malignant tumors[21]. We have shown that MTCH2 overexpression in human gastric cancer tissues is related to invasion, metastasis, and survival rate, reflecting the clinical value of MTCH2 in the diagnosis and treatment of gastric cancer. In cancer research, the upregulation of MTCH1 has been observed to promote hepatocellular carcinoma cell proliferation, invasion, and migration[23]. Through the search of GEPIA database, MTCH2 is more valuable than MTCH1 in the study of gastric adenocarcinoma. Importantly, the results of this study suggest that overexpression of MTCH2 can promote the invasion and migration of GES-1 and AGS cells. Overexpression of MTCH2 can regulate the production of ATP and the formation of mitochondrial ATP synthase, and also regulate the expression of markers of malignancy in cytoplasm. We are consistent with the above views, if an excess of anti-cancer factors, pro-apoptotic factors, or mitochondria-targeting anti-cancer drugs is present within the cell, the action of MTCH2 as a membrane-inserting protein can be harnessed to achieve therapeutic effects. Obviously, gastric mucosa cells and gastric cancer cells have stronger metastasis ability after MTCH2 overexpression, and we speculate that the overexpression of MTCH2 could lead to the activation of more pro-cancer factors’ insertions, as there might be a lack of additional anti-cancer factors. Based on the results obtained from the ATP fluorescence probe, it appears that the overexpression of MTCH2 induces an “excited” state in mitochondrial energy metabolism, thus providing the necessary energy supply for the progression of gastric cancer. These results enrich our new understanding of MTCH2 in the occurrence and development of gastric cancer.
Mitochondrial ATP synthase plays a pivotal role in cancer cell energy metabolism, with ATP2A2 being one of the crucial sarcoplasmic (endoplasmic) reticulum calcium transporter ATPases responsible for shuttling cytoplasmic calcium ions into the endoplasmic reticulum. This process is involved in the development of various types of tumors[24]. ATP2A2, functioning as an ATP synthase-associated protein, plays a critical role in mitochondrial ion exchange and energy transmission, which significantly influences mitochondrial function[25]. Furthermore, ATP2A2 has been proven to be instrumental in regulating linc00221-mediated acute lymphoblastic leukemia cell proliferation and apoptosis[26]. Notably, overexpressed ATP2A2 has also been observed to promote the proliferation of colon cancer and prostate cancer cells[27,28]. In this study, overexpression of MTCH2 can up-regulate ATP2A2 expression, indicating that MTCH2 can regulate ATP synthase synthesis. However, this effect is inhibited upon downregulating MTCH2. This is likely attributed to the abnormal opening of mPTP. Research has revealed that an imbalance in ion proportions within mitochondria can lead to conformational changes in mitochondrial calcium uptake and ATP synthase dimers. This alteration prompts the opening of mPTP, causing decrease in MMP and ultimately cul minating in cell death[29]. The opening of mPTP allows unrestricted movement of ions and small molecules, resulting in mitochondrial depolarization and ATP consumption, thereby triggering cell death[30]. Notably, abnormal mPTP opening has been shown to impact tumor growth in lung cancer cells (A549)[31]. Moreover, further investigations have indicated that mPTP activity in solid tumors is typically found to be in a closed state[32]. In our experiments, we observed that knocking down MTCH2 promotes mPTP opening, leading to a decrease in MMP and ATP depletion. Additionally, it suppresses the expression of the cell proliferation protein (Bcl-2) and enhances the expression of apoptotic factors (Bax, Cyt c). These findings align with the results of D’Orsi et al[8] and provide further insight into how MTCH2 influences cell proliferation and apoptosis by regulating mPTP opening status. Once again, this underscores the potential therapeutic significance of targeting MTCH2 for cancer treatment.
MTCH is a founding member of a unique class of membrane protein insertion enzymes that utilize SLC25 transporter folding[4]. MTCH2 and ATP synthase (ATP2A2) expression were correlated in human gastric cancer tissues. We constructed GES-1 and AGS cell models and suggested that MTCH2 regulates ATP2A2 expression and is associated with the activation of mPTP opening. We speculate that it may be related to the regulation mechanism of calcium homeostasis, and in the future, further in-depth research is warranted. Importantly, MTCH2 can promote the malignant phenotype of gastric mucosa cells and enhance the invasion, migration, and cell proliferation of gastric cancer cells. This has provided novel insights into the role of MTCH2 in gastric cancer research.
MTCH2 can mediate ATP synthesis and ATP synthase synthesis by regulating mPTP of cancer cells, increase the malignant phenotype of GES-1 cells, promote the proliferation, invasion, and migration of gastric cancer cells, providing a basis for the targeted therapy of gastric cancer cells.
Mitochondrial carrier homolog 2 (MTCH2), as an insertion enzyme of mitochondrial outer membrane protein, plays an important role in cellular production, energy expenditure and apoptosis induced by mitochondrial permeability transformation pore (mPTP) opening.
MTCH2 has been poorly studied in gastric cancer. The addition of MTCH2 research will provide a broader idea for the treatment of gastric cancer.
To investigate the precise role of MTCH2 in gastric cancer will providing a basis for the targeted therapy of gastric cancer.
Sixty-five samples of poorly differentiated gastric cancer tissue and adjacent tissues were collected for MTCH2 and ATP2A2 expression detection. JC-1, mPTP, and ATP fluorescence probe were used for mitochondrial function detection. Wound healing, transwell, and colony formation assay were used for cell migration and proliferation evaluation. Western blotting experiments were conducted to detect the changes in the expression levels of related proteins.
Both MTCH2 and ATP2A2 are highly expressed in gastric cancer. MTCH2 promotes proliferation and migration of gastric cancer cells, enhances mitochondrial activity, and inhibits apoptosis.
MTCH2 plays an important role in cellular production, energy expenditure and apoptosis in gastric cancer cells.
We speculate that the regulation of MTCH2 opening to mPTP may be related to the regulatory mechanism of calcium homeostasis, which will be further studied in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, France S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Zhao Y, Zhou L, Li H, Sun T, Wen X, Li X, Meng Y, Li Y, Liu M, Liu S, Kim SJ, Xiao J, Li L, Zhang S, Li W, Cohen P, Hoffman AR, Hu JF, Cui J. Nuclear-Encoded lncRNA MALAT1 Epigenetically Controls Metabolic Reprogramming in HCC Cells through the Mitophagy Pathway. Mol Ther Nucleic Acids. 2021;23:264-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Zhuang H, He X, Li H, Chen Y, Wu T, Jiang X, Zhang H, Zhao P, Wang Y, Chen J, Zhang J, Liu Y, Bu W. MnS Nanocapsule Mediates Mitochondrial Membrane Permeability Transition for Tumor Ion-Interference Therapy. ACS Nano. 2023;17:13872-13884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Popgeorgiev N, Gil C, Berthenet K, Bertolin G, Ichim G. Shedding light on mitochondrial outer-membrane permeabilization and membrane potential: State of the art methods and biosensors. Semin Cell Dev Biol. 2024;156:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Guna A, Stevens TA, Inglis AJ, Replogle JM, Esantsi TK, Muthukumar G, Shaffer KCL, Wang ML, Pogson AN, Jones JJ, Lomenick B, Chou TF, Weissman JS, Voorhees RM. MTCH2 is a mitochondrial outer membrane protein insertase. Science. 2022;378:317-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 5. | Peng L, Zhao M, Liu T, Chen J, Gao P, Chen L, Xing P, Wang Z, Di J, Xu Q, Qu H, Jiang B, Su X. A stop-gain mutation in GXYLT1 promotes metastasis of colorectal cancer via the MAPK pathway. Cell Death Dis. 2022;13:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Wang Q, Karvelsson ST, Johannsson F, Vilhjalmsson AI, Hagen L, de Miranda Fonseca D, Sharma A, Slupphaug G, Rolfsson O. UDP-glucose dehydrogenase expression is upregulated following EMT and differentially affects intracellular glycerophosphocholine and acetylaspartate levels in breast mesenchymal cell lines. Mol Oncol. 2022;16:1816-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Dachy G, Fraitag S, Boulouadnine B, Cordi S, Demoulin JB. Novel COL4A1-VEGFD gene fusion in myofibroma. J Cell Mol Med. 2021;25:4387-4394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | D'Orsi B, Niewidok N, Düssmann H, Prehn JHM. Mitochondrial Carrier Homolog 2 Functionally Co-operates With BH3 Interacting-Domain Death Agonist in Promoting Ca(2+)-Induced Neuronal Injury. Front Cell Dev Biol. 2021;9:750100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Leem YH, Park JS, Park JE, Kim DY, Kim HS. Suppression of neuroinflammation and α-synuclein oligomerization by rotarod walking exercise in subacute MPTP model of Parkinson's disease. Neurochem Int. 2023;165:105519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Hu Z, Wang D, Gong J, Li Y, Ma Z, Luo T, Jia X, Shi Y, Song Z. MSCs Deliver Hypoxia-Treated Mitochondria Reprogramming Acinar Metabolism to Alleviate Severe Acute Pancreatitis Injury. Adv Sci (Weinh). 2023;10:e2207691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 11. | Waseem M, Wang BD. Promising Strategy of mPTP Modulation in Cancer Therapy: An Emerging Progress and Future Insight. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Qin X, Xu Y, Peng S, Qian S, Zhang X, Shen S, Yang J, Ye J. Sodium butyrate opens mitochondrial permeability transition pore (MPTP) to induce a proton leak in induction of cell apoptosis. Biochem Biophys Res Commun. 2020;527:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Andelova N, Waczulikova I, Talian I, Sykora M, Ferko M. mPTP Proteins Regulated by Streptozotocin-Induced Diabetes Mellitus Are Effectively Involved in the Processes of Maintaining Myocardial Metabolic Adaptation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | de Freitas Germano J, Sharma A, Stastna M, Huang C, Aniag M, Aceves A, Van Eyk JE, Mentzer RM Jr, Piplani H, Andres AM, Gottlieb RA. Proteomics of Mouse Heart Ventricles Reveals Mitochondria and Metabolism as Major Targets of a Post-Infarction Short-Acting GLP1Ra-Therapy. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Ma D, Pignanelli C, Tarade D, Gilbert T, Noel M, Mansour F, Adams S, Dowhayko A, Stokes K, Vshyvenko S, Hudlicky T, McNulty J, Pandey S. Cancer Cell Mitochondria Targeting by Pancratistatin Analogs is Dependent on Functional Complex II and III. Sci Rep. 2017;7:42957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Liu Y, Chen C, Wang X, Sun Y, Zhang J, Chen J, Shi Y. An Epigenetic Role of Mitochondria in Cancer. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 17. | Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 757] [Article Influence: 151.4] [Reference Citation Analysis (0)] |
| 18. | Aloni E, Ruggiero A, Gross A, Segal M. Learning Deficits in Adult Mitochondria Carrier Homolog 2 Forebrain Knockout Mouse. Neuroscience. 2018;394:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ruggiero A, Aloni E, Korkotian E, Zaltsman Y, Oni-Biton E, Kuperman Y, Tsoory M, Shachnai L, Levin-Zaidman S, Brenner O, Segal M, Gross A. Loss of forebrain MTCH2 decreases mitochondria motility and calcium handling and impairs hippocampal-dependent cognitive functions. Sci Rep. 2017;7:44401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Yuan Q, Yang W, Zhang S, Li T, Zuo M, Zhou X, Li J, Li M, Xia X, Chen M, Liu Y. Inhibition of mitochondrial carrier homolog 2 (MTCH2) suppresses tumor invasion and enhances sensitivity to temozolomide in malignant glioma. Mol Med. 2021;27:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Katz C, Zaltsman-Amir Y, Mostizky Y, Kollet N, Gross A, Friedler A. Molecular basis of the interaction between proapoptotic truncated BID (tBID) protein and mitochondrial carrier homologue 2 (MTCH2) protein: key players in mitochondrial death pathway. J Biol Chem. 2012;287:15016-15023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Delahanty RJ, Beeghly-Fadiel A, Xiang YB, Long J, Cai Q, Wen W, Xu WH, Cai H, He J, Gao YT, Zheng W, Shu XO. Association of obesity-related genetic variants with endometrial cancer risk: a report from the Shanghai Endometrial Cancer Genetics Study. Am J Epidemiol. 2011;174:1115-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Chen G, Mo S, Yuan D. Upregulation Mitochondrial Carrier 1 (MTCH1) Is Associated with Cell Proliferation, Invasion, and Migration of Liver Hepatocellular Carcinoma. Biomed Res Int. 2021;2021:9911784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Zarain-Herzberg Á, Izquierdo-Torres E, Hernández-Oliveras A, Rodríguez G, Lozano-Arriaga D. Sarcoplasmic reticulum Ca(2+) ATPases genes differential expression in breast cancer cells. Gac Med Mex. 2021;157:343-349. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Papp B, Brouland JP. Altered Endoplasmic Reticulum Calcium Pump Expression during Breast Tumorigenesis. Breast Cancer (Auckl). 2011;5:163-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Huang M, Zheng J, Ren Y, Zhu J, Kou L, Nie J. LINC00221 suppresses the malignancy of children acute lymphoblastic leukemia. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Crépin A, Bidaux G, Vanden-Abeele F, Dewailly E, Goffin V, Prevarskaya N, Slomianny C. Prolactin stimulates prostate cell proliferation by increasing endoplasmic reticulum content due to SERCA 2b over-expression. Biochem J. 2007;401:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Fan L, Li A, Li W, Cai P, Yang B, Zhang M, Gu Y, Shu Y, Sun Y, Shen Y, Wu X, Hu G, Xu Q. Novel role of Sarco/endoplasmic reticulum calcium ATPase 2 in development of colorectal cancer and its regulation by F36, a curcumin analog. Biomed Pharmacother. 2014;68:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Monteiro J, Marks CA, Braga PC, Bernardino RL, Alves MG, Lobo-da-Cunha A, Videira A, Pereira F. The role of ion homeostasis imbalance due to citrate accumulation in fluoroacetic acid (FAA) toxicity in Neurospora crassa. Comp Biochem Physiol C Toxicol Pharmacol. 2023;271:109661. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Elustondo PA, Nichols M, Negoda A, Thirumaran A, Zakharian E, Robertson GS, Pavlov EV. Mitochondrial permeability transition pore induction is linked to formation of the complex of ATPase C-subunit, polyhydroxybutyrate and inorganic polyphosphate. Cell Death Discov. 2016;2:16070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Zhang R, Li G, Zhang Q, Tang Q, Huang J, Hu C, Liu Y, Wang Q, Liu W, Gao N, Zhou S. Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells. Cell Death Dis. 2018;9:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Bonora M, Pinton P. The mitochondrial permeability transition pore and cancer: molecular mechanisms involved in cell death. Front Oncol. 2014;4:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |