Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.907
Peer-review started: December 2, 2023
First decision: December 14, 2023
Revised: January 5, 2024
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: March 15, 2024
Processing time: 101 Days and 6.8 Hours
Duodenal neuroendocrine tumours (DNETs) are rare neoplasms. However, the incidence of DNETs has been increasing in recent years, especially as an incidental finding during endoscopic studies. Regrettably, there is no consensus regarding the ideal treatment of DNETs. Even there are few studies on the clinical features and survival analysis of DNETs.
To analyze the clinical characteristics and prognostic factors of patients with duodenal neuroendocrine tumours.
The clinical data of DNETs diagnosed in the First Affiliated Hospital of Air Force Military Medical University from June 2011 to July 2022 were collected. Neuroendocrine tumours located in the ampulla area of the duodenum were divided into the ampullary region group; neuroendocrine tumours in any part of the duo
Twenty-nine DNET patients were screened. The male to female ratio was 1:1.9, and females comprised the majority. The ampullary region group accounted for 24.1% (7/29), while the nonampullary region group accounted for 75.9% (22/29). When diagnosed, the clinical symptoms of the ampullary region group were mainly abdominal pain (85.7%), while those of the nonampullary region groups were mainly abdominal distension (59.1%). There were differences in the composition of staging of tumours between the two groups (Fisher's exact probability method, P = 0.001), with nonampullary stage II tumours (68.2%) being the main stage (P < 0.05). After the diagnosis of DNETs, the survival rate of the ampullary region group was 14.3% (1/7), which was lower than that of 72.7% (16/22) in the nonampullary region group (Fisher's exact probability method, P = 0.011). The survival time of the ampullary region group was shorter than that of the nonampullary region group (P < 0.000). The median survival time of the ampullary region group was 10.0 months and that of the nonampullary region group was 451.0 months. Multivariate analysis showed that tumours in the ampulla region and no surgical treatment after diagnosis were independent risk factors for the survival of DNET patients (HR = 0.029, 95%CI 0.004-0.199, P < 0.000; HR = 12.609, 95%CI: 2.889-55.037, P = 0.001). Further analysis of nonampullary DNET patients showed that the survival time of patients with a tumour diameter < 2 cm was longer than that of patients with a tumour diameter ≥ 2 cm (t = 7.243, P = 0.048). As of follow-up, 6 patients who died of nonampullary DNETs had a tumour diameter that was ≥ 2 cm, and 3 patients in stage IV had liver metastasis. Patients with a tumour diameter < 2 cm underwent surgical treatment, and all survived after surgery.
Surgical treatment is a protective factor for prolonging the survival of DNET patients. Compared to DNETs in the ampullary region, patients in the nonampullary region group had a longer survival period. The liver is the organ most susceptible to distant metastasis of nonampullary DNETs.
Core Tip: A retrospective study of the clinical features and prognostic factors of the ampullary and nonampullary of the duodenum neuroendocrine tumours. This study comprehensively analyses the basic characteristics, clinical symptoms, tumour characteristics, histological grading and classification, tumour clinical staging, treatment, and factors affecting the survival prognosis of patients with duodenal neuroendocrine tumours (DNETs). We found that surgical treatment is a protective factor for prolonging the survival of DNET patients. Compared to DNETs in the ampullary region, patients in the nonampullary region had a longer survival period. The liver is the organ most susceptible to distant metastasis of nonampullary DNETs.
- Citation: Fang S, Shi YP, Wang L, Han S, Shi YQ. Clinical features and prognostic factors of duodenal neuroendocrine tumours: A comparative study of ampullary and nonampullary regions. World J Gastrointest Oncol 2024; 16(3): 907-918
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/907.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.907
Duodenal neuroendocrine tumours (DNETs) are rare tumours that account for 1%-3% of primary duodenal tumours and 5%-8% of all gastrointestinal neuroendocrine tumours[1]. Most DNETs are located in the first or second part of the duodenum, with only 20% occurring in the periampullary area[2]. The vater ampulla is composed of a common channel of the common bile duct, pancreatic duct, and duodenal papilla, which is the intersection of the intestinal, pancreatic, and biliary epithelium[3,4]. The ampulla area of the duodenum refers to the area with a diameter of 2 cm centred around the opening of the duodenal papilla. DNETs in the ampulla region are usually considered independent entities with strong invasiveness, high risk of local and distant metastasis, and poor prognosis. Their clinical behaviour is more similar to that of pancreatic tumours[5]. The volume of nonampullary DNETs is mostly less than 2 cm, with an average tumour size of 1.2-1.5 cm. After surgical treatment, it usually has a good survival prognosis of 5-10[6].
Previously, DNET tissue types were divided into five subtypes[7]: gastrin tumours, somatostatin tumours, nonfunctional tumours, neuroendocrine carcinoma (NEC), and ganglion cell paraganglioma. Vanoli’s research[7] suggests that NEC is mainly located in the ampullary area. Compared with neuroendocrine tumours (NETs), NECs are more prone to lymphatic vessel invasion, duodenal wall infiltration, local lymph node metastasis, and distant metastasis. According to the World Health Organization (WHO)'s 2019 histological classification and grading standards for tumours[8], DNETs are classified into two categories: NETs and NECs. In de Jorge Huerta’s data on DNETs, NET-G1 is the most common, while NECs are extremely rare (≤ 3%)[9].
Most DNETs produce hormones that can be detected in serum or tumour cells through immunohistochemistry, but only a few hormones can cause clinical symptoms. According to clinical symptoms and hormone secretion, DNETs are classified as functional and nonfunctional. The secreted hormones leading to corresponding clinical symptoms are functional DNETs, while nonfunctional DNETs are those where no specific hormones are detected or the secreted hormones do not cause corresponding clinical symptoms. 90% of DNETs are nonfunctional neuroendocrine tumours, and only 10% are functional DNETs[9].
At present, there is no consensus on the treatment of DNETs, which mainly depends on the size and location of the tumour, histopathology classification and grading, staging, and tumour type[10]. Surgical removal of DNETs is currently a recommended treatment method[5]. For patients with a tumour diameter ≥ 2 cm or local/distant metastasis, surgical surgery is preferred[11]. For patients with nonampullary DNETs with a diameter of < 1 cm, no function, G1/G2 grade, no lymph nodes and distant metastasis, endoscopic resection and follow-up are recommended[12,13]. When DNETs in the ampulla region are diagnosed, they often invade the intrinsic muscle layer and metastasize to the lymph node. Even if the diameter is less than 1 cm, surgical resection and lymph node dissection should be performed[14]. Due to the high probability of metastasis in nonampullary DNETs with a thickness of 1-2 cm, there is still controversy over whether to perform endoscopic resection or surgical resection. It is recommended to use endoscopic ultrasound examination to determine the depth of tumor infiltration, local lymph node metastasis, and puncture biopsy before making a definitive choice[15].
There are few studies on the survival prognosis analysis of DNETs, and some studies[5-7,11] suggest that the prognosis of DNETs is related to the tumour region (ampullary/nonampullary), function, classification and grading, staging, treatment, etc. However, there are no articles that comprehensively analyse the impact of these factors on the survival of DNETs. Due to the rarity of DNETs and insufficient knowledge of their natural history, their disease characteristics and prognostic factors are currently not well understood[5]. At present, there are few prognostic analysis data on DNETs in China. This study comprehensively analyses the basic characteristics, clinical symptoms, tumour characteristics, histological grading and classification, tumour clinical staging, treatment, and factors affecting the survival prognosis of patients with DNETs diagnosed at the First Affiliated Hospital of Air Force Military Medical University to enrich the understanding of the clinical characteristics and prognostic factors of DNETs.
The clinical data of patients with DNETs diagnosed at the First Affiliated Hospital of Air Force Military Medical University from June 2011 to July 2022 were retrospectively included in the study. Inclusion criteria: According to the "China Anti-Cancer Association guidelines for the diagnosis and treatment of neuroendocrine neoplasms (2022 Edition)"[14], patients diagnosed with neuroendocrine tumors in duodenal tissue pathology are diagnosed with DNETs. Exclusion criteria: Incomplete clinical and pathological data. A total of 29 DNETs were screened out. Neuroendocrine tumours located in the ampulla area of the duodenum were divided into the ampullary region group, and neuroendocrine tumours in any part of the duodenum outside the ampullary area were divided into the nonampullary region group.
We recorded in detail the basic information and clinical data of all DNET patients, including patient sex, age at diagnosis, symptoms, reason for endoscopy (physical examination or not), endoscopic data, imaging data, histo
Tumour diameter at diagnosis is the largest diameter measured by endoscopy or imaging. The histological classification and grading of tumours[8] adopts the standards released by the WHO in 2019 to classify DNETs into two categories: NETs and NECs. NET classification is based on mitotic cell count and/or Ki-67 proliferation index: mitotic cell count [< 2/10 HPF, ≥ (2-20)/10 HPF, > 20/10 HPF] and/or Ki-67 index (< 3%, 3%-20%, > 20%), classified as corresponding G1, G2, G3. NEC are classified into small cell NEC and large cell NEC based on the morphology of tumour cells, including the size of the sex nucleus, the characteristics of chromatin, and the amount of cytoplasm. The American Joint Committee on Cancer 8th edition staging system was adopted for tumour staging[14].
Date of diagnosis was defined as the date the tumor was first diagnosed through tissue pathology. Length of follow-up was calculated from the date of diagnosis to the date of the doctor's last phone contact, or the date of death. Follow up termination event refers to the end of follow-up or death caused by tumor recurrence and metastasis. The survival status was followed up by phone, and the deadline was November 1, 2022. The study protocol was approved by the local Clinical Research Ethics Committee.
Diagnostic criteria for gastrinoma (ZES): Patients with Zollinger Ellison syndrome signs and symptoms (recurrent peptic ulcer, gastroesophageal reflux, and diarrhoea) are suspected of having ZES[16]. Suspected ZES patients with gastric pH < 2 and serum gastrin concentration > 10 times the normal upper limit can be diagnosed with ZES. If the gastric pH is < 2 and serum gastrin concentration is < 10 times the normal upper limit, if the pancreatic gastrin test is positive (the gastrin concentration increases by > 120 pg/mL compared to the fasting baseline) or if the basal gastric acid secretion increases (> 15 mmol/h)[5], ZES can also be diagnosed.
Statistical analysis was conducted using SPSS26.0 software. The measurement data with normal distribution is represented by mean ± SD. The comparison between the two groups is performed by independent sample t test. The measurement data with non normal distribution are represented by the median (lower quartile, upper quartile), and the rank sum test is used for inter group comparison. Counting data is expressed in terms of examples and percentages, and comparisons between groups are conducted using the χ2 test or Fisher exact probability method. The Kaplan Meier method was used for survival analysis, and the log rank test was used for comparison between group survival analyses. Cox regression model was used for prognostic risk factor analysis, and factors with P < 0.1 in univariate analysis were included in Cox multivariate analysis. P < 0.05 indicates a statistically significant difference.
Twenty-nine patients were confirmed to have DNETs during the study period, including 7 patients (24.1%) with tumours in the ampullary region and 22 patients (75.9%) with tumours in the nonampullary region. Among them, there were 10 males (34.5%) and 19 females (65.5%). The DNET patients were diagnosed at an age of 55.7 ± 10.3 years old. At the time of diagnosis, the main clinical symptoms were abdominal pain (51.7%), followed by abdominal distension (17.2%), acid reflux (17.2%), nausea (13.8%), black stools (7.0%), poor appetite (7.0%), and vomiting (3.4%). When diagnosed with DNETs, 65.5% of tumours had a diameter greater than 2 cm, 96.6% were nonfunctional DNETs, the histological classification and grading were mainly NET-G1 (48.3%) and NET-G2 (44.8%), and the main stage was stage II (55.2%). After the diagnosis of DNETs, 86.2% of patients received surgical treatment, and 20.7% received postoperative chemotherapy. The total mortality rate of DNET patients was 41.4% (12/29), the mortality rate in the ampullary region group was 85.7% (6/7), and the mortality rate in the nonampullary region group was 27.3% (6/22) (Table 1).
| Clinical features | Duodenal neuroendocrine tumours (n = 29) |
| Gender | |
| Males | 34.5 (10/29) |
| Females | 65.5 (19/29) |
| Tumour site | |
| Ampullary region | 24.1 (7/29) |
| Nonampullary region | 75.9 (22/29) |
| Age at diagnosis | |
| Median age (yr) | 55.7 ± 10.3 |
| 30-39 | 7.0 (2/29) |
| 40-49 | 24.1 (7/29) |
| 50-59 | 34.5 (10/29) |
| 60-69 | 24.1 (7/29) |
| ≥ 70 | 10.3 (3/29) |
| Symptoms | |
| Abdominal pain | 51.7 (15/29) |
| Abdominal distension | 17.2 (5/29) |
| Acid reflux | 17.2 (5/29) |
| Nausea | 13.8 (4/29) |
| Vomiting | 3.4 (1/29) |
| Black stools | 7.0 (2/29) |
| Poor appetite | 7.0 (2/29) |
| No symptoms | 20.7 (6/29) |
| Tumour size | 2.6 ± 1.6 cm |
| ≤ 1 cm | 13.8 (4/29) |
| 1-2 cm | 20.7 (6/29) |
| ≥ 2 cm | 65.5 (19/29) |
| Histological classification and grading | |
| NET-G1 | 48.3 (14/29) |
| NET-G2 | 44.8 (13/29) |
| NET-G3 | 3.4 (1/29) |
| NEC | 3.4 (1/29) |
| Tumour staging | |
| I | 13.8 (4/29) |
| II | 55.2 (16/29) |
| III | 13.8 (4/29) |
| IV | 17.2 (5/29) |
| Tumour type | |
| Nonfunction | 96.6 (28/29) |
| Function(gastrinoma) | 3.4 (1/29) |
| Surgery | |
| Yes | 86.2 (25/29) |
| No | 13.8 (4/29) |
| Postoperative chemotherapy | |
| Yes | 20.7 (6/29) |
| No | 79.3 (23/29) |
| Survival status | |
| Death | 41.4 (12/29) |
| Survival | 58.6 (17/29) |
After diagnosis, the survival time of the ampullary region group was shorter than that of the nonampullary region group (P < 0.000). There were differences in the histological classification and grading composition of DNET patients between the two groups (Fisher’s exact probability method, P = 0.023). The ampullary region group was mainly composed of NET-G2 (57.1%), while the nonampullary region group was mainly composed of NET-G1 (54.5%). There was also a difference in the composition of stages of DNET patients between the two groups (Fisher’s exact probability method, P = 0.001). The ampullary region group was mainly composed of stage I (28.6%), stage III (28.6%), and stage IV (28.6%) tumours, while the nonampullary region group was mainly composed of stage II tumours (68.2%). The survival rate of patients in the nonampullary region group was higher than that in the ampullary region group, and the difference was statistically significant (Fisher's exact probability method, P = 0.026) (Table 2).
| Clinical features | Ampullary region (n = 7) | Nonampullary region (n = 22) | Statistical value | P value |
| Males/females (n/n) | 1/6 | 9/13 | Fisher exact test | 0.367 |
| Age at diagnosis (yr) | 59.256 ± 11.368 | 54.546 ± 9.99 | t = 1.059 | 0.299 |
| Tumour size (cm) | 2.543 ± 1.316 | 2.696 ± 1.648 | t = -0.223 | 0.826 |
| Survival time (month) | 10 (8, 30) | 71.5 (35, 123) | Z = -3.238 | 0.000 |
| Histological classification and grading, n (%) | Fisher exact probability method = 4.098 | 0.241 | ||
| NET-G1 | 2 (28.6) | 12 (54.5) | ||
| NET-G2 | 4 (57.1) | 9 (41) | ||
| NET-G3 | 1 (14.3) | 0 (0) | ||
| NEC | 0 (0) | 1 (4.5) | ||
| Tumour staging, n (%) | Fisher exact probability method = 6.996 | 0.036 | ||
| I | 2 (28.6) | 2 (9.1) | ||
| II | 11 (14.3) | 15 (68.2) | ||
| III | 2 (28.6) | 2 (9.1) | ||
| IV | 2 (28.6) | 3 (13.6) | ||
| With/without functional tumours (n/n) | 1/6 | 0/22 | Fisher exact test | 0.241 |
| With/without surgery (n/n) | 5/2 | 20/2 | Fisher exact test | 0.238 |
| With/without chemotherapy (n/n) | 1/6 | 5/17 | Fisher exact test | 0.545 |
| Death/survival (n/n) | 6/1 | 6/16 | Fisher exact test | 0.011 |
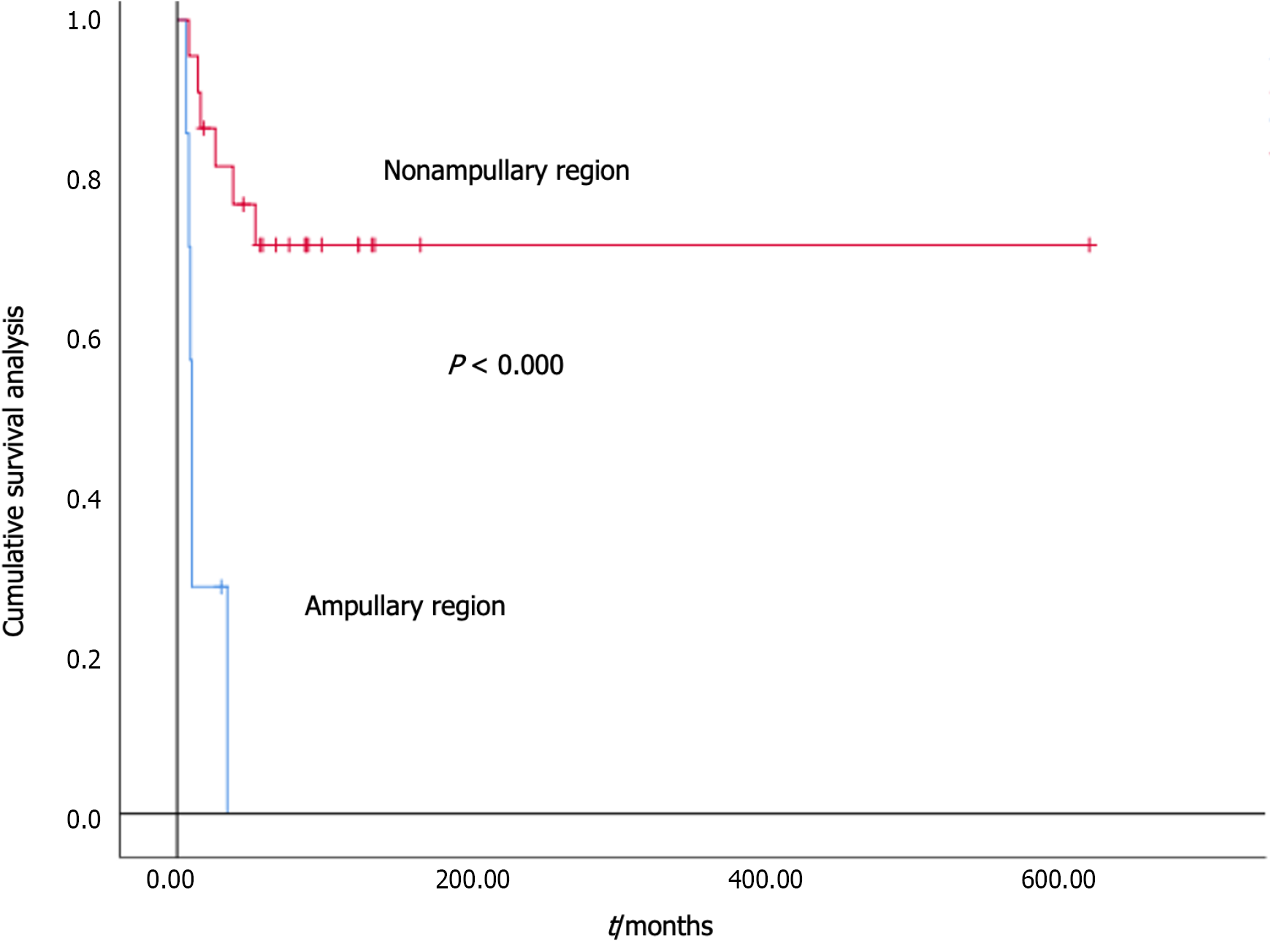
At follow-up, there were a total of 12 deaths (41.4%) among DNET patients, including 6 deaths (85.7%) in the ampullary region group and 6 deaths (27.3%) in the nonampullary region group. The median survival time in the ampullary region group was 10.0 months, while that in the nonampullary region group was 451.0 months. The survival curve is shown in Figure 1. We further analysed the impact of factors such as sex, age at diagnosis, tumour diameter, location, histological classification and grading, tumour staging at diagnosis, surgical status after diagnosis, and postoperative chemotherapy on patient survival. The univariate analysis results of the Cox regression model showed that there were statistically significant differences in tumour staging at diagnosis, postoperative surgery (with/without), and tumour location (ampullary/nonampullary) (P = 0.003, 0.000, 0.000). The above three risk factors were further included in the Cox multivariate analysis, and the results showed that there were statistically significant differences in tumour site (ampullary/nonampullary) and surgery (with/without) after diagnosis (HR = 0.029, 95%CI: 0.004-0.199, P < 0.000; HR = 12.609, 95%CI: 2.889-55.037, P = 0.001) (Table 3).
| Factor | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Tumour staging | 8.000 (5.060-10.940) | 0.003 | - | |
| Surgery (with/without) | 8.000 (6.303-9.697) | 0.000 | 0.029 (0.004-0.199) | 0.000 |
| Tumour site (ampullary/nonampullary region) | 10.000 (8.829-11.171) | 0.000 | 12.609 (2.889-55.037) | 0.001 |
Clinical data of nonampullary DNET patients: Among 22 patients with nonampullary DNETs, there were 9 males (40.9%) and 13 females (59.1%), and all patients had serum gastrin levels below 150 pg/mL. Nonampullary DNET patients had a tumour diameter of 2.696 ± 1.648 cm, with 8 patients (36.4%) having a tumour diameter less than 2 cm and 14 patients (63.6%) having a tumour diameter ≥ 2 cm. Nonampullary DNET patients were diagnosed at an age of 54.5 ± 10.0 years old, with 81.8% (18/22) of patients under 65 years old. At the time of diagnosis, the clinical symptoms were mainly bloating (59.1%), followed by abdominal pain (41.0%), and 27.3% were incidentally detected during asymptomatic physical examinations. When diagnosed with nonampullary DNETs with a tumour diameter of less than 2 cm, half of the patients had no clinical symptoms and were accidentally discovered during physical examinations. When diagnosed with nonampullary DNETs with a tumour diameter of ≥ 2 cm, the clinical symptoms were mainly abdominal distension (85.7%), followed by abdominal pain (57.1%), and a few (14.3%) patients had no clinical symptoms. At the time of diagnosis, only one patient had a pathological indication of small cell NEC, with a tumour diameter of 0.5 cm. The pathology of the remaining patients was NET, and their histological classification and grading were mainly NET-G1 (54.5%) and NET-G2 (41.0%). Most tumours were stage II (68.2%). Three stage IV patients were diagnosed with a tumour diameter ≥ 2 cm, and all had liver metastasis. After diagnosis, 90.9% received surgical treatment, and 22.7% received chemotherapy. The overall survival rate of nonampullary DNET patients was 72.7% (16/22). The survival rate of patients with a tumour diameter < 2 cm was 100.0% (8/8), and the survival rate of patients with a tumour diameter ≥ 2 cm was 57.1% (8/14) (Table 4).
| Clinical features | Tumour size (diameter) | |
| < 2 cm (n = 8) | ≥ 2 cm (n = 14) | |
| Gender | ||
| Males | 12.5 (1/8) | 57.1 (8/14) |
| Females | 87.5 (7/8) | 42.9 (6/14) |
| Age at diagnosis | ||
| Median age (yr) | ||
| < 65 | 75 (6/8) | 85.7 (12/14) |
| ≥ 65 | 25 (2/8) | 14.3 (2/14) |
| Symptoms | ||
| Abdominal pain | 12.5 (1/8) | 57.1 (8/14) |
| Abdominal distension | 12.5 (1/8) | 85.7 (12/14) |
| Acid reflux | 25 (2/8) | 0 (0/14) |
| Nausea | 0 (0/8) | 14.3 (2/14) |
| Vomiting | 0 (0/8) | 7.1 (1/14) |
| Black stools | 0 (0/8) | 14.3 (2/14) |
| Poor appetite | 0 (0/8) | 14.3 (2/14) |
| No symptoms | 50 (4/8) | 14.3 (2/14) |
| Histological classification and grading | ||
| NET-G1 | 62.5 (5/8) | 50 (7/14) |
| NET-G2 | 25 (2/8) | 50 (7/14) |
| NET-G3 | 0 (0/8) | 0 (0/14) |
| NEC | 12.5 (1/8) | 0 (0/14) |
| Tumour staging | ||
| I | 25 (2/8) | 0 (0/14) |
| II | 75 (6/8) | 64.3 (9/14) |
| III | 0 (0/8) | 14.3 (2/14) |
| IV | 0 (0/8) | 21.4 (3/14) |
| Surgery | ||
| Yes | 100 (8/8) | 85.7 (12/14) |
| No | 0 (0/8) | 14.3 (2/14) |
| Postoperative chemotherapy | ||
| Yes | 12.5 (1/8) | 28.6 (4/14) |
| No | 87.5 (7/8) | 71.4 (10/14) |
| Survival status | ||
| Death | 0 (0/8) | 42.9 (6/14) |
| Survival | 100 (8/8) | 57.1 (8/14) |
Clinical characteristics of nonampullary DNET patients: After diagnosis, the survival time of patients with a tumour diameter < 2 cm was longer than that of patients with a tumour diameter ≥ 2 cm, and the difference was statistically significant (t = 7.243, P = 0.048). The survival rate of patients with a tumour diameter < 2 cm (100.0%) was higher than that of patients with a tumour diameter ≥ 2 cm (57.1%), but the difference was not statistically significant (Fisher's exact probability method, P = 0.051). When diagnosed with nonampullary DNETs, there were no statistically significant differences in sex ratio, age, tumour histological classification and grading, staging, surgery, or chemotherapy between the two groups of patients with tumour diameter < 2 cm and tumour diameter ≥ 2 cm (Table 5).
| Clinical features | Tumour size (diameter) | Statistical value | P value | |
| < 2 cm (n = 8) | ≥ 2 cm (n = 14) | |||
| Males/females (n/n) | 1/7 | 8/6 | Fisher exact test | 0.052 |
| Age at diagnosis (yr) | 56.50 ± 12.00 | 53.43 ± 8.97 | t = 0.684 | 0.502 |
| Survival time (month) | 191.25 ± 175.47 | 42.93 ± 30.49 | t = 7.243 | 0.048 |
| Histological classification and grading, n (%) | Fisher exact probability method = 5.090 | 0.120 | ||
| NET-G1 | 5 | 7 | ||
| NET-G2 | 2 | 7 | ||
| NET-G3 | 0 | 0 | ||
| NEC | 1 | 0 | ||
| Tumour staging, n (%) | Fisher exact probability method = 2.459 | 0.363 | ||
| I | 2 | 0 | ||
| II | 6 | 9 | ||
| III | 0 | 2 | ||
| IV | 0 | 3 | ||
| With/without surgery (n/n) | 8/8 | 12/2 | Fisher exact test | 0.515 |
| With/without chemotherapy (n/n) | 1/7 | 4/10 | Fisher exact test | 0.394 |
| Death/survival (n/n) | 0/8 | 8/14 | Fisher exact test | 0.051 |
Survival analysis of nonampullary DNET patients: As of follow-up, there were a total of 6 deaths (27.3%) among nonampullary DNET patients. When the 6 deceased patients were diagnosed, the tumour diameter was ≥ 2 cm, 3 cases were diagnosed with stage IV tumour and all had liver metastasis, 2 cases were diagnosed with stage III tumour, and 1 case was diagnosed with stage II tumour but the patient had concurrent rectal cancer. Patients with a tumour diameter < 2 cm underwent surgical treatment, and all survived after surgery. One patient with small cell NEC had a tumour diameter of 0.5 cm and survived surgical treatment.
DNETs are rare duodenal tumours. The total incidence rate in the United States is 0.19/100000, the overall prevalence rate in the United Kingdom is 0.04/100000, and the overall prevalence rate in Japan is 0.17/100000[1]. There are no epidemiological research data on DNETs in China. In recent years, with the continuous improvement of diagnostic technology, the incidence rate of DNETs has increased[9], but there is still insufficient clinical knowledge and management of DNETs[10]. This study retrospectively analysed the clinical characteristics and prognosis of single centre confirmed DNET patients to help guide disease management.
Sato et al[1] have shown that DNETs are more common in males than in females, and 75% of DNETs have tumours with a diameter less than 2 cm. In this study, women accounted for the majority, with a male to female ratio of 1:1.9, and 65.5% of patients had tumours with a diameter greater than 2 cm. The basic characteristics of tumours are different from those of foreign countries, which may be related to various factors such as disease awareness, geographical environment, and racial differences. The median survival time of NEC in previous studies[7] was 10 months, and its mortality rate was 85.2% (23/27). One case of small cell NEC in this centre was located in the nonampullary region, with the main symptom being burping. This tumour had a diameter of 0.5 cm and underwent surgery. The patient has survived to this day after surgery. Consistent with previous studies[1,9], nonampullary DNETs account for the majority (75.9%), the mortality rate of ampullary DNETs is high (85.7%), and the proportion of nonfunctional DNETs is high (96.6%).
de Jorge Huerta et al[9] shows that the majority of DNETs (81.0%) are usually accidentally discovered during upper gastrointestinal endoscopy for other reasons (digestive disorders are the most common). With increasing awareness of people's health, the proportion of cases found during routine physical examinations has significantly increased. In this study, 20.7% of patients had no clinical symptoms and were accidentally discovered during physical examinations. When DNETs were diagnosed, the main clinical symptoms were abdominal pain (51.7%), followed by abdominal distension, acid reflux, nausea, black stools, fatigue, poor appetite, and vomiting. The proportion of abdominal pain is higher (85.7%) when diagnosed with ampullary DNETs, and the proportion of abdominal distension is higher (59.1%) when diagnosed with nonampullary DNETs. However, for nonampullary DNET patients with a tumour diameter ≥ 2 cm, abdominal distension was the main clinical symptom (85.7%). For patients with unexplained abdominal distension, careful observation of the duodenum should be performed during endoscopic examination, and attention should be given to identifying nonampullary DNETs. To determine whether the clinical symptoms of DNETs are caused by relevant hormones they secrete and to distinguish between functional and nonfunctional DNETs, it is recommended that all patients measure serum gastrin levels and actively seek correct classification of symptoms.
In functional DNETs, ZES accounts for a higher proportion, and somatostatin tumours are rare[1,10]. There is only one case of functional DNETs in this centre, which is a ZES patient in the ampullary region. The clinical symptoms are mainly abdominal pain. When diagnosed, serum gastrin was 1520 pg/mL, the pH value in the stomach was 0.9, the tumour diameter was 1.5 cm, and the clinical stage was III. Surgical treatment and chemotherapy were not performed, and the patient ultimately died. For ZES patients, proton pump inhibitor therapy is usually effective, so the average time from symptom onset to final diagnosis often exceeds 5 years, and 25% of patients experience local or distant metastasis at diagnosis. Patients with combined metastasis have a poor prognosis[5].
There are many differences between ampullary region and nonampullary region DNETs[1,2]. Ampullary region DNETs have higher tumour invasiveness, are diagnosed in later stages of the disease, have poorer overall survival rates, and have different treatment plans. This study compared DNETs in the ampullary region with those in the nonampullary region and found that the main staging at the time of diagnosis was in nonampullary region group II (68.2%). The survival rate of patients in the nonampullary region group (72.7%) was higher than that in the ampullary region group (14.3%) (P = 0.011), which is consistent with previous studies[1]. The median survival time of the ampullary region group in this study was 10.0 months, while that of the nonampullary region group was 451.0 months. The survival time of the ampullary region group was shorter than that of the nonampullary region group (P < 0.000), indicating that the prognosis of nonampullary region DNETs is relatively good[5].
The selection of surgical resection methods for DNETs patients should be based on comprehensive considerations such as tumor differentiation, tumor diameter, tumor location, and tumor staging. DNETs located around the ampulla have a relatively high degree of malignancy when diagnosed. The analysis results of the SEER database show that[2] compared to nonampullary areas, DNENs tumors around the ampulla are often larger, with high-grade pathology and more distant metastasis. The median survival time is significantly shorter than that of the nonampullary area group, but there is no statistically significant difference in the median overall survival time between the two groups after surgical resectionD. Therefore, ENETS suggests that curative resection should be the first choice for DNENs around the ampulla. Milanetto et al[17] analyzed 18 cases of dNENs around the ampulla, and the research results showed that the surgical local resection group had shorter surgical time and less blood loss. During the follow-up period, only 3 G3/NEC patients who underwent pancreaticoduodenectomy experienced recurrence. Therefore, it is recommended to perform local resection for dNENs around the ampulla with a diameter of < 2 cm.
de Jorge Huerta et al[9] showed that 21 patients with sporadic and nonfunctional DNETs only had distant affected organs in the liver (2 cases). In this study, three stage IV patients were diagnosed with a tumour diameter ≥ 2 cm, and all had liver metastases, indicating that the liver may be the organ most susceptible to distant metastasis of nonampullary DNETs. One stage IV patient underwent surgical treatment for primary duodenal tumour and liver metastasis but ultimately died. Previously, it was believed that patients with nonampullary DNETs had a good prognosis[5], but it is a heterogeneous tumour that can exhibit invasive features. In this study, patients with lymph node and organ metastasis all died. Therefore, when diagnosing nonampullary DNET patients, attention should be given to evaluating lymph node and organ metastasis, and selecting effective treatment plans is still a problem that needs to be solved[18].
At present, there is no prognostic model for DNETs. In this study, a Cox regression model was used to analyse prognostic risk factors. Univariate analysis showed that tumour staging, whether surgery was performed after diagnosis, and tumour location (ampullary/nonampullary) affected the survival rate of DNET patients. Further multivariate analysis showed that whether surgery was performed, as well as the location of the tumour (ampullary/nonampullary), affected the overall survival rate of DNET patients, suggesting that surgical treatment is a protective factor for prolonging the survival period of DNET patients. Therefore, for patients with DNETs, it is recommended to fully evaluate the lesion after diagnosis and actively choose surgical treatment[19] to improve the survival rate and prolong survival. Vanoli et al[7] suggested that tumour size > 2 cm and tissue grade G3/NEC were risk factors affecting patient prognosis, but there was no statistically significant impact of tumour size and tissue grade on the survival of DNETs in our data.
This study, as the first clinical study on DNETs in China, can provide some reference for the prevention and treatment of this disease. However, the design of a single centre retrospective study has a limited sample source, and it is difficult to fully reflect the characteristics of the disease. Therefore, large-scale, multicentre research is still needed to further explore the characteristics and risk factors for this disease and provide the best diagnosis and treatment strategies for DNET patients.
Surgical treatment is a protective factor for prolonging the survival of DNET patients. Compared to DNETs in the ampullary region, patients in the nonampullary region group had a longer survival period. The liver is the organ most susceptible to distant metastasis of nonampullary DNETs.
Duodenal neuroendocrine tumours (DNETs) are rare neoplasms. However, the incidence of DNETs has been increasing in recent years, especially as an incidental finding during endoscopic studies. Regrettably, there is no consensus regarding the ideal treatment of DNETs. Even there are few studies on the clinical features and survival analysis of DNETs. Some studies suggest that the prognosis of DNETs is related to the tumour region (ampullary/nonampullary), function, classification and grading, staging, treatment, etc. However, there are no articles that comprehensively analyse the impact of these factors on the survival of DNETs. Due to the rarity of DNETs and insufficient knowledge of their natural history, their disease characteristics and prognostic factors are currently not well understood. At present, there are few prognostic analysis data on DNETs in China.
This study, as the first clinical study on DNETs in China, can provide some reference for the prevention and treatment of this disease.
This study comprehensively analyses the basic characteristics, clinical symptoms, tumour characteristics, histological grading and classification, tumour clinical staging, treatment, and factors affecting the survival prognosis of patients with DNETs. We found that surgical treatment is a protective factor for prolonging the survival of DNET patients. Compared to DNETs in the ampullary region, patients in the nonampullary region had a longer survival period. The liver is the organ most susceptible to distant metastasis of nonampullary DNETs.
A retrospective study of the clinical features and prognostic factors of the ampullary and nonampullary of the duodenum neuroendocrine tumours. Compare the clinical characteristics of patients with ampullary and nonampullary DNETs, and analyze the prognostic factors affecting DNETs. Further research will be conducted on the clinical characteristics and prognosis of nonampullary DNETs. In this study, a Cox regression model was used to analyse prognostic risk factors.
Twenty-nine DNET patients were screened. The male to female ratio was 1:1.9, and females comprised the majority. When diagnosed, the clinical symptoms of the ampullary region group were mainly abdominal pain (85.7%), while those of the nonampullary region groups were mainly abdominal distension (59.1%). The survival time of the ampullary region group was shorter than that of the nonampullary region group (P < 0.000). Multivariate analysis showed that tumours in the ampulla region and no surgical treatment after diagnosis were independent risk factors for the survival of DNET patients. Further analysis of nonampullary DNET patients showed that the survival time of patients with a tumour diameter < 2 cm was longer than that of patients with a tumour diameter ≥ 2 cm (t = 7.243, P = 0.048). This study, as the first clinical study on DNETs in China, can provide some reference for the prevention and treatment of this disease. However, the design of a single centre retrospective study has a limited sample source, and it is difficult to fully reflect the characteristics of the disease. Therefore, large-scale, multicentre research is still needed to further explore the characteristics and risk factors for this disease and provide the best diagnosis and treatment strategies for DNET patients.
In this study, Compare the clinical characteristics of patients with ampullary and nonampullary DNETs, and analyze the prognostic factors affecting DNETs. A Cox regression model was used to analyse prognostic risk factors. Multivariate analysis showed that whether surgery was performed, as well as the location of the tumour (ampullary/nonampullary), affected the overall survival rate of DNET patients, suggesting that surgical treatment is a protective factor for prolonging the survival period of DNET patients.
Due to the high probability of metastasis in nonampullary DNETs with a diameter of 1-2 cm, there is still controversy over whether to perform endoscopic resection or surgical resection. For this group of patients, further research is needed to determine the optimal treatment plan.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gerontology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohammadi S, Iran S-Editor: Gong ZM L-Editor: A P-Editor: Zhang XD
| 1. | Sato Y, Hashimoto S, Mizuno K, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22:6817-6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (5)] |
| 2. | Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg. 2014;18:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Pea A, Riva G, Bernasconi R, Sereni E, Lawlor RT, Scarpa A, Luchini C. Ampulla of Vater carcinoma: Molecular landscape and clinical implications. World J Gastrointest Oncol. 2018;10:370-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 4. | Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Rossi RE, Rausa E, Cavalcoli F, Conte D, Massironi S. Duodenal neuroendocrine neoplasms: a still poorly recognized clinical entity. Scand J Gastroenterol. 2018;53:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Tran CG, Sherman SK, Suraju MO, Nayyar A, Gerke H, Abiad RGE, Chandrasekharan C, Ear PH, O'Dorisio TM, Dillon JS, Bellizzi AM, Howe JR. Management of Duodenal Neuroendocrine Tumors: Surgical versus Endoscopic Mucosal Resection. Ann Surg Oncol. 2022;29:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Vanoli A, La Rosa S, Klersy C, Grillo F, Albarello L, Inzani F, Maragliano R, Manca R, Luinetti O, Milione M, Doglioni C, Rindi G, Capella C, Solcia E. Four Neuroendocrine Tumor Types and Neuroendocrine Carcinoma of the Duodenum: Analysis of 203 Cases. Neuroendocrinology. 2017;104:112-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Division of Digestive Diseases, Chinese Society of Pathology; 2020 Chinese Consensus on the Pathological Diagnosis of Gastrointestinal and Pancreatic Neuroendocrine Neoplasms Experts Group. [Chinese consensus on the pathological diagnosis of gastrointestinal and pancreatic neuroendocrine neoplasms(2020)]. Zhonghua Bing Li Xue Za Zhi. 2021;50:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | de Jorge Huerta L, Solares Fernández I, Sánchez-Moreno B, Males Maldonado D, de Ibarrola Andrés C, Díaz-Simón R. Sporadic, non-functional, gastrin-producing duodenal neuroendocrine tumors: A retrospective study of an infrequent disease. J Dig Dis. 2022;23:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Nakao E, Namikawa K, Hirasawa T, Nakano K, Tokai Y, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Nunobe S, Fujisaki J. Risk factors for lymph node metastasis and indication of local resection in duodenal neuroendocrine tumors. JGH Open. 2022;6:189-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Delle Fave G, Kwekkeboom DJ, Van Cutsem E, Rindi G, Kos-Kudla B, Knigge U, Sasano H, Tomassetti P, Salazar R, Ruszniewski P; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012;95:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Klemm N, Lu-Cleary D, Chahal D, Trasolini R, Lam E, Donnellan F. Endoscopic Management of Diminutive Duodenal Neuroendocrine Tumors. J Gastrointest Cancer. 2021;52:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Nabi Z, Ramchandani M, Asif S, Basha J, Chavan R, Darisetty S, Reddy N. Outcomes of Endoscopic Submucosal Dissection in Duodenal Neuroendocrine Tumors. J Gastrointest Surg. 2022;26:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Society of Neuroendocrine Neoplasm of China Anti-Cancer Association. China Anti-Cancer Association guideline for diagnosis and treatment of neuroendocrine neoplasm (2022 edition). Zhongguo Aizheng Zazhi. 2022;32:545-579. [DOI] [Full Text] |
| 15. | Liang Y, Wu W, Nie Y, Chen J. Interpretation on the Chinese Guideline for Diagnosis and Treatment of Neuroendocrine Neoplasms from The China Anti-Cancer Association (2022). Xiehe Yixue Zazhi. 2023;14:94-100. |
| 16. | Rossi RE, Elvevi A, Citterio D, Coppa J, Invernizzi P, Mazzaferro V, Massironi S. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J Gastroenterol. 2021;27:5890-5907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (11)] |
| 17. | Milanetto AC, Pasquali C, Da Broi M, Brambilla T, Capretti G, Zerbi A. Ampullary neuroendocrine neoplasms: surgical experience of a rare and challenging entity. Langenbecks Arch Surg. 2018;403:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Zhang XF, Wu XN, Tsilimigras DI, Poultsides G, Rocha F, Abbott DE, Fields R, Idrees K, Cho C, Maithel SK, Pawlik TM; other members of the US Neuroendocrine Tumor Study Group. Duodenal neuroendocrine tumors: Impact of tumor size and total number of lymph nodes examined. J Surg Oncol. 2019;120:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Brito HP, Torres IT, Turke KC, Parada AA, Waisberg J, Botelho RV. Comparison of endoscopic resection techniques for duodenal neuroendocrine tumors: systematic review. Endosc Int Open. 2021;9:E1214-E1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |