Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.875
Peer-review started: November 3, 2023
First decision: December 1, 2023
Revised: December 12, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: March 15, 2024
Processing time: 129 Days and 20.4 Hours
Pancreatic, periampullary/ampullary, and choledochal adenocarcinomas are aggressive malignancies with a poor prognosis. Immune checkpoint blockade is a promising treatment option for several tumor types. H long terminal repeat-associating 2 (HHLA2), which is analogous to programmed death-ligand 1 (PD-L1), is a recently discovered member of the B7/cluster of differentiation 28 family and is expressed in many malignancies.
To analyze the expression of HHLA2 and its association with the pathologic bio
Ninety-two adenocarcinoma cases located in the pancreas, ampulla, and distal common bile duct were identified. This study assessed 106 pancreaticoduode
Patients with high HHLA2 expression had a higher mean age than those with low expression. Low HHLA2 expression was associated with high perineural inva
Evaluation of HHLA2 expression in microsatellite stable and PD-L1-negative tumors may be useful for predicting the response of individuals to immunotherapy and may serve as a novel therapeutic target for immunotherapy in advanced-stage disease.
Core Tip: Pancreatic, periampullary/ampullary, and choledochal adenocarcinomas are aggressive malignancies with recurrences and metastases occurring in a short period of time. Complete resection is possible in only a small proportion of patients; thus, targeted therapies are needed. H long terminal repeat-associating 2 (HHLA2), which is analogous to programmed death-ligand 1 (PD-L1), is expressed in many malignancies. Evaluation of HHLA2 expression in microsatellite stable and PD-L1-negative tumors may be useful for predicting the response of individuals to immunotherapy, and may serve as a novel therapeutic target in patients with advanced disease who do not respond to classical chemotherapy and have unresectable cancer.
- Citation: Aydın AH, Turhan N. Comparison of mismatch repair and immune checkpoint protein profile with histopathological parameters in pancreatic, periampullary/ampullary, and choledochal adenocarcinomas. World J Gastrointest Oncol 2024; 16(3): 875-882
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/875.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.875
Pancreatic, ampullary, and distal common bile duct (DCBD) carcinomas are characterized by late presentation, advanced disease at the time of diagnosis, and the inability to completely resect because of the location. While the 5-year disease-specific survival (DSS) is 42% in resectable DCBD carcinomas, in ampullary adenocarcinoma (AAC), the DSS rate ranges from 20% to 80% according to the histological type, location, and stage[1]. In pancreatic ductal adenocarcinoma (PDAC), the 5-year survival rate is much lower, between 2% and 9%[1]. Complete resection is possible in only a small proportion of patients, and recurrences and metastases occur in a short period of time; thus, targeted therapies are needed for this disease[2].
Immunotherapy has been used to treat patients with advanced stage disease or unresectable tumor to improve cancer survival. However, most patients do not respond to immunotherapy; hence, there is a need for specific biomarkers to improve the selection of patients who will best respond to therapy.
Mismatch repair (MMR) proteins play an important role in the detection and correction of errors that occur during DNA replication and genetic recombination. MMR deficiency can lead to microsatellite instability (MSI). Consequently, the accumulation of tumor mutational burden and generation of neoantigens stimulate the host immune response. MSI-high (MSI-H) solid tumors are more sensitive to immunotherapy agents and associated with a better prognosis[3].
Immunogenic proteins, also called immune checkpoint proteins, are produced by tumor cells. Programmed cell death protein 1 (PD-1) is an immune checkpoint protein that promotes immune evasion and tumor progression via interaction with its ligands [programmed death-ligand (PD-L)1 and PD-L2). Immune checkpoint inhibitors (iCPIs) allow T cells to destroy tumor cells by blocking this interaction[4].
Clinical success with monoclonal antibodies such as pembrolizumab, which blocks the PD-1/PD-L1 axis, has opened new doors in cancer immunology. Pembrolizumab is approved for the treatment of patients with unresectable or me
PD-L1 is expressed in many tumor types. The relationship between cancer and the immune system is not fully elu
Despite the promising antitumor activity of PD-1/PD-L1 inhibitors, only a small group of patients show marked responses to single-agent antibody therapy. The need to elucidate the mechanism underlying the resistance to immune checkpoint blockade in some patients has been the focus of many studies[3,5-7].
The success of PD-1/PD-L1 blockade in cancer immunotherapy has raised the possibility of other members of the B7/cluster of differentiation 28 (CD28) family serving as targets for cancer immunotherapy. H long terminal repeat-asso
Herein, we describe our assessments of the association of histological and clinical parameters [age, sex, histological type, histological grade, tumor-node-metastasis (TNM) stage, presence of tumor infiltrating lymphocytes (TILs), tumor stroma ratio (TSR), tumor budding (TB)] with the expression of MMR, PD-L1, and HHLA2 proteins in patients diagnosed with adenocarcinoma located in the pancreas, periampulla/ampulla, and DCBD.
Ninety-two cases of adenocarcinoma located in the pancreas, periampulla/ampulla, and DCBD were identified. This study assessed pancreaticoduodenectomy and distal/total pancreatectomy samples that were delivered to Ankara City Hospital (Ankara, Turkey) between 2019 and 2021. All protocols were reviewed and approved by the Ethics Committee of Ankara City Hospital.
Demographic information and pathology reports were obtained from the hospital database (HICAMP). Information on sex, age, tumor macroscopic location, presence/absence of lymphovascular invasion (LVI)/perineural invasion (PNI), and lymph node metastasis were obtained from the pathology reports.
All tumor sections stained with hematoxylin and eosin (H&E) were removed from the slide archive and examined under a microscope by two observers. The parameters related to the histological type and grade of the cases were reeva
To evaluate and grade TB, a three-tier system proposed at the 2016 International Tumor Budding Consensus Conference was used. TILs were evaluated separately as stromal and intraepithelial TILs. To evaluate stromal TILs, standardized criteria prepared in 2014 by the International TIL Study Group were used. At a low magnification, tumor slides were scanned and focused on TS to determine the type of inflammatory infiltrate. Mononuclear infiltrate was accepted as stromal TIL, and three groups were formed based on their densities. Stromal TIL rate of 0%-10% was included in group A, 10%-40% was included in group B, and 40%-90% was included in group C. The number of intraepithelial lymphocytes (IELs) were counted and averaged over five high-power fields for each case; two groups were created: < 2 and ≥ 2 IELs. For TSR evaluation, the most invasive area of the tumor was selected among the H&E-stained slides. Then, the slides were digitized with a 40 × objective using the Aperio AT Turbo digital whole slide scanning system (Leica Biosystems Imaging, Vista, CA, United States). The preparations were evaluated using the semi-quantitative image analysis program Virapath-3.4.4 (Virasoft Software, Istanbul, Turkey). Tissue presegmentation analysis was run in the hotspot regions corresponding to the 10 × magnification area in each evaluated case. The tumoral region area was calculated and the ratio was calculated by dividing it by the area of the stroma regions. Two groups (low and high) were formed using the 50% cutoff value according to the literature[12].
Immunohistochemistry was conducted with mouse monoclonal antibodies against the four main MMR proteins: MLH-1 (clone M1), MSH-2 (clone G219-1129), MSH-6 (clone SP93), and PMS-2 (clone A16-4); a mouse monoclonal antibody against PD-L1 (clone 22C3; Dako Products, Santa Clara, CA, United States); and a rabbit polyclonal antibody against HHLA2 (clone ab214327). For MMR and PD-L1, sections were prepared from formalin-fixed paraffin blocks; for HHLA2, sections were prepared from tissue microarray blocks.
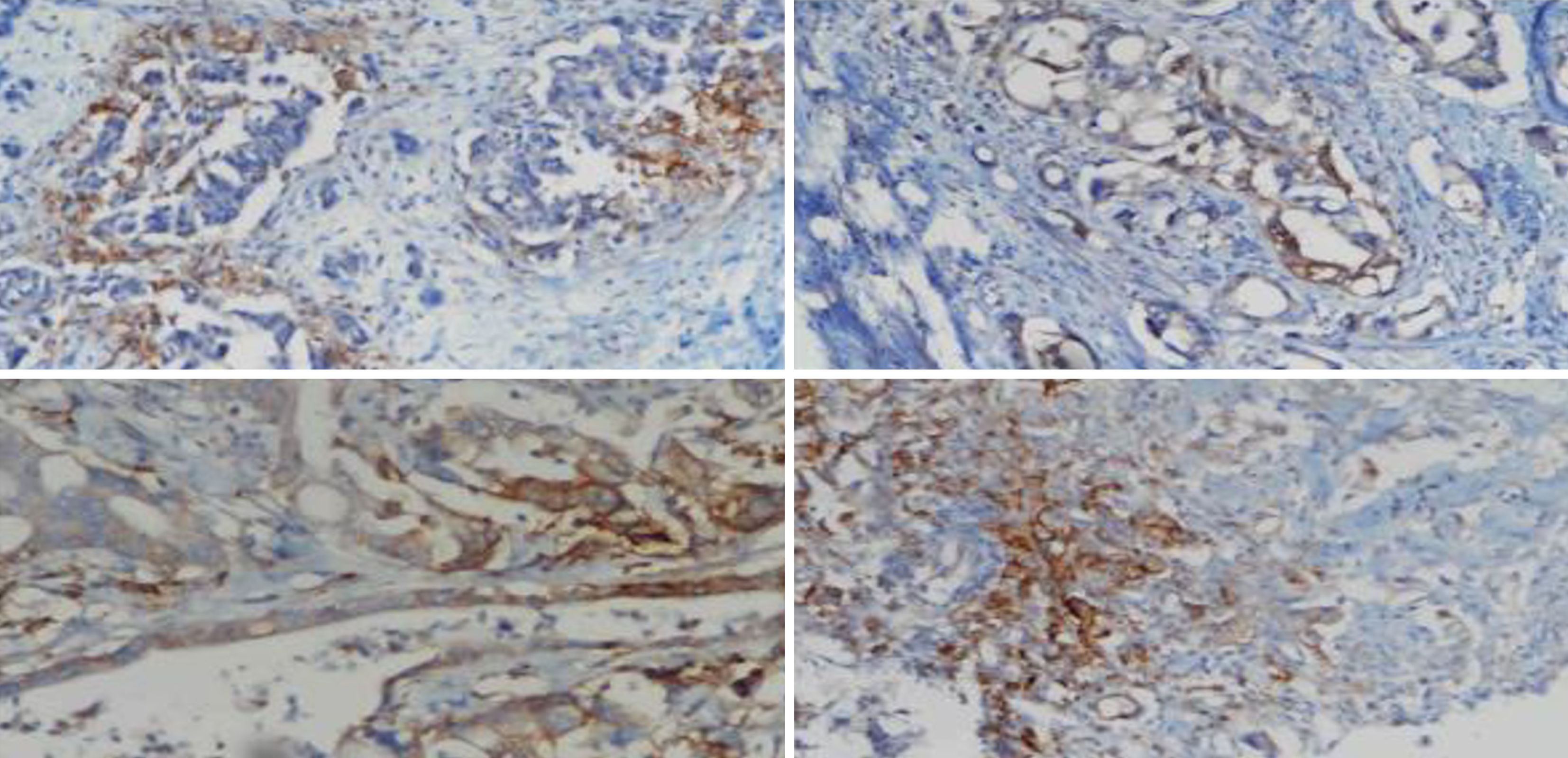
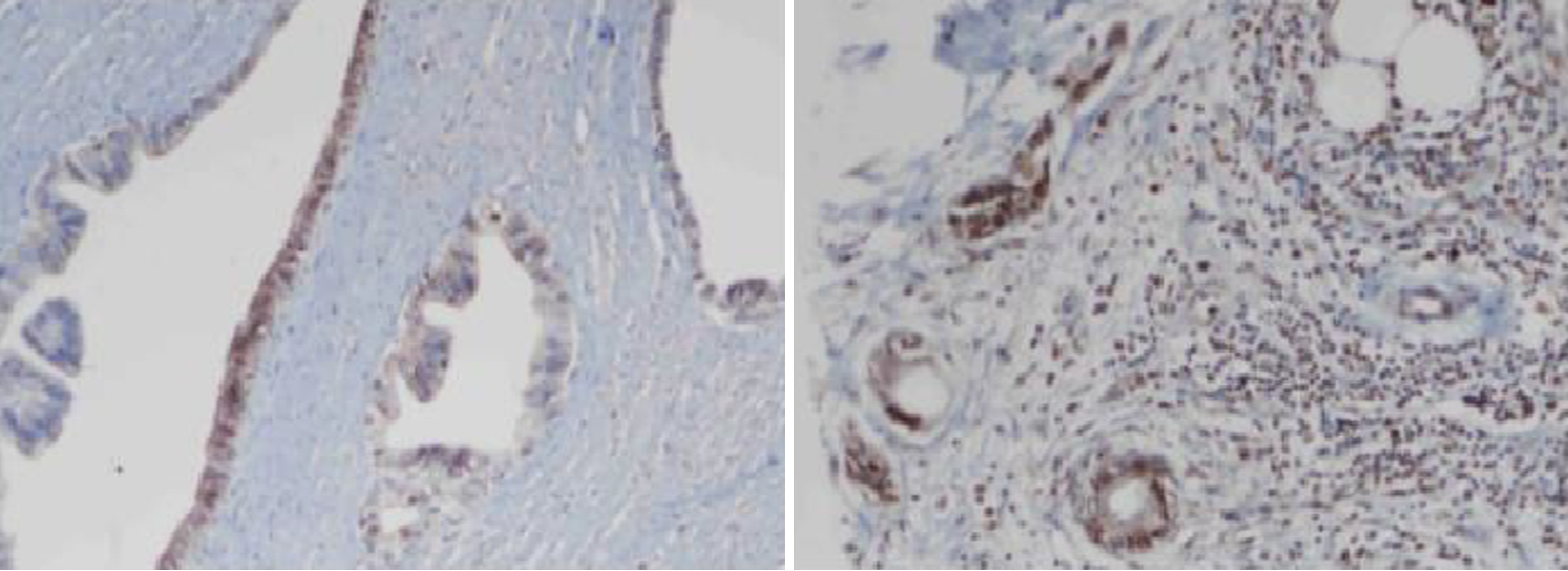
Immunohistochemistry staining of MMR proteins was evaluated for the presence of nuclear staining in the tumor cells. Stromal cells and lymphocytes adjacent to each tumor served as internal controls. In the presence of an optimal internal control, nuclear staining in tumor cells was considered preserved expression. Tumor cells with membranous staining of PD-L1 were considered positive. At least 100 tumor cells were evaluated for each case, and the number of PD-L1-positive tumor cells was divided by the total number of tumor cells. A rate < 1% was considered negative, rates from 1% to 49% were classified as low, and a rate ≥ 50% was classified as high (Figure 1). HHLA2 cytoplasmic staining and the ratio of stained cells were determined for each case. Tumors with no staining were considered negative. The cases with staining were grouped as low and high expression according to the 50% cutoff value (Figure 2).
Statistical analyses were performed using SPSS version 17.0 (IBM, Armonk, NY, United States). The conformity of the variables to a normal distribution was examined by histogram graphs and the Kolmogorov-Smirnov test. Mean, standard deviation, and median values were used to present the descriptive analyses. Categorical variables were compared with Pearson’s χ2 test. In cases where the data were not normally distributed, groups of two were evaluated with the Mann-Whitney U test and groups of more than two were evaluated with the Kruskal-Wallis test. Spearman’s correlation test was used to analyze the measurement data. P < 0.05 was considered statistically significant.
In our study, the mean patient age was 65.51 years, and 64 (69.57%) cases were male and 28 (30.43%) were female. Fifty-eight of the ninety-two cases (63.04%) were located in the pancreas. The location of the other cases was as follows: 21 (22.83%) in the ampulla and 11 (11.96%) in the DCBD. According to histological subtype, 81 (87.79%) cases were ductal adenocarcinoma (DAC), 4 (4.44%) were intraductal papillary mucinous neoplasm-associated invasive carcinoma, 3 (3.33%) were intraductal tubulopapillary neoplasm-associated invasive carcinoma, 3 (3.33%) were classified as adeno
In our study, the cases were classified as < 2 and ≥ 2 according to the presence of IELs. A significant correlation was found between stromal TIL groups and IEL density. It was determined that 52 of the 87 cases with IELs < 2 showed the presence of group B stromal TILs. The rate of group C stromal TILs in cases with IELs ≥ 2 was found to be higher than the rate of group A and B stromal TILs. The cases were divided into two groups (low and high) according to the TSR. The TSR was not significantly associated with histopathological features, TB, or stromal TIL groups. HHLA2 expression was compared with PD-L1 and MMR protein expression. No meaningful correlation was found between the MMR protein expression profile and the expression of PD-L1 and HHLA2. In addition, no significant relationship was found between the absence/presence of HHLA2 expression and TB, stromal TIL groups, IEL density, or TSR. Patients with high HHLA2 expression had a higher mean age than those with low expression. Low HHLA2 expression was associated with high PNI. HHLA2 expression was low in pT3 cases and high in pT1, pT2, and pT4 cases (Table 1).
| Cytoplasmic HHLA2 expression | P value1 | ||||
| Negative, n (%) | Low, n (%) | High, n (%) | |||
| Gender | Male | 7 (63.64) | 48 (75.00) | 9 (52.94) | 0.193 |
| Female | 4 (36.36) | 16 (25.00) | 8 (47.06) | ||
| Pathologic stage of tumor | T1 | 0 (0.00) | 0(.00) | 3(17.65) | 0.011 |
| T2 | 4 (36.36) | 23(35.94) | 8(47.06) | ||
| T3 | 7 (63.64) | 38(59.38) | 5(29.41) | ||
| T4 | 0 (0.00) | 3(4.69) | 1(5.88) | ||
| Lymphovascular invasion | Present | 1 (9.09) | 8(12.50) | 2(11.76) | 0.949 |
| Absent | 10 (90.91) | 56(87.50) | 15(88.24) | ||
| Perineural invasion | Present | 0 (0.00) | 2 (3.13) | 3 (17.65) | 0.044 |
| Absent | 11 (100.00) | 62 (96.88) | 14 (82.35) | ||
| Histologic grade | Poorly | 2 (18.18) | 11 (17.19) | 3 (17.65) | 0.901 |
| Moderately | 7 (63.64) | 41 (64.06) | 9 (52.94) | ||
| Well | 2 (18.18) | 12 (18.75) | 5 (29.41) | ||
| Tumor stroma ratio | Low | 4 (36.36) | 33 (51.56) | 9 (52.94) | 0.625 |
| High | 7 (63.64) | 31 (48.44) | 8 (47.06) | ||
| Diameter of tumor (cm)3 | 3.11 ± 1.25 (3.00) | 3.65 ± 1.24 (4.00) | 2.94 ± 1.03 (3.00) | 0.0882 | |
| Tumor budding degree | 2.45 ± 0.82 (3.00) | 2.48 ± 0.80 (3.00) | 1.88 ± 1.05 (2.00) | 0.0532 | |
| Age4 | 59.91 ± 6.33 (60.00) | 65.38 ± 8.28 (66.00) | 69.65 ± 8.77 (72.00) | 0.0052 | |
Many studies have revealed the relationship between high-grade TB and aggressive histopathologic features (LVI, advan
Corresponding with the studies in the literature, the TB was lower in well-differentiated tumors than in moderately and poorly differentiated tumors in our study. TB was found to be significantly higher in tumors with LVI and PNI. The aggressive nature of tumors is a multifactorial process that is considerably affected by the tumor microenvironment (T
A low TSR has been shown to be associated with poor prognostic parameters such as increased tumor size and ad
The distribution of intratumoral, peritumoral, stromal, and intraepithelial TILs have been investigated in relation to antitumoral immune reactions in the TME[15]. There are few studies in the literature comparing the different localization of TILs in tumors located in the pancreas, ampulla, and DCBD. In a study by Zhang et al[15], the authors assessed the presence of stromal and intraepithelial TILs in PDAC and investigated the effects on prognosis. A significant T cell response due to intratumoral TILs was observed in PDAC tissues, whereas the response due to intraepithelial attack was very low. It has been reported that CD8+ T intraepithelial infiltration is an independent positive prognostic factor in OS and is negatively correlated with vascular invasion. However, high intratumoral CD8+ T cell infiltration without intraepithelial attack is associated with advanced tumor stage and is considered an adverse prognostic factor[14].
In our study, the relationship between the density of IEL and the stromal TIL groups was analyzed. The rate of having group C stromal TILs in cases with IELs ≥ 2 and the rate of being group A and B in cases with IELs < 2 were found to be significantly higher than in group C with IELs < 2. It has been suggested that tumor mutation load is related to the response rate to PD-1 inhibitors[3,17]. Assessment of the presence of MSI in tumors is important for predicting the effectiveness of PD-1/PD-L1 blockade[2]. The median number of somatic mutations is higher in MSI-H tumors than in MSS tumors[18]. MSI is found in 1% of PDAC cases, 9.5% of AAC cases, and approximately 4% of DCBD adenocarcinoma cases[2].
In this study, the expression of MMR proteins was preserved in 90 cases, a loss of MSH6 and PMS2 expression was observed in 1 case each; both cases were pancreatic cancer and belonged to the DAC histomorphologic subtype. A signi
Satisfaction with immunotherapy outcomes has led to investigations into the relationship between PD-L1 expression and prognosis in many solid tumors. Studies have evaluated the expression of PD-L1 in adenocarcinomas located in the pancreas, ampulla, and DCBD and shown that the high expression of PD-L1 is associated with a poor prognosis in PDAC and AAC[19,20]. However expression of PD-L1 in TILs is associated with increased survival[21]. In our study, the expression of PD-L1 was evaluated only in tumor cells according to Tumor Proportion Score. No significant association was found between PD-L1 expression and histopathologic features. HHLA2, which is analogous to PD-1, is a newly discovered member of the B7/CD28 family and is expressed in many malignancies[9].
In a limited number of studies, it has been proposed that the expression of HHLA2 is independent from the other members of the B7/CD28 family, and its high expression is associated with long-term survival[9,10,22]. Nevertheless, Chen et al[22] reported that the high expression of HHLA2 is related to decreased survival. HHLA2 expression has been investigated in many solid tumors[23,24]; however, there has been no study on HHLA2 expression in adenocarcinomas located in the DCBD.
Byers et al[11] detected the decreased expression/Loss of expression of HHLA2 in PDAC compared to normal pan
In our study, patients with high HHLA2 expression had a higher mean age than those with low expression. At the same time, the rate of PNI was lower in cases with high HHLA2 expression. Low HHLA2 expression was correlated with advanced pathological stage. High expression of HHLA2 was observed in pT1, pT2, and pT4 cases, while low expression was found in pT3 cases. Only one pT4 case had high HHLA2 expression, which did not provide sufficient statistics for a meaningful inference.
In various tumors, expression of HHLA2 may be consequence of a complex process that is affected by many histopathologic features. Additional studies are needed to analyze the expression of HHLA2 in solid tumors and investigate the relationship between HHLA2 expression and survival.
In a limited number of studies, it has been proposed that both the expression of HHLA2 and co-expression of HHLA2 and PD-L1 are related to high density of TILs[22]. However, there have been no studies on the co-expression of HHLA2/PD-L1 in adenocarcinomas located in the pancreas, ampulla, and DCBD. In our study, the co-expression of PD-L1 and HHLA2 and its relationship with the presence of TILs were compared and no significant correlation was found. While no statistically significant correlation was found between the MMR and PD-L1 protein expression profiles and histopatho
Our study had some limitations. First, since we designed this research as a retrospective study, the data of some of the patients could not be accessed, so the number of patients included in the study did not reach the desired level. Second, more PDAC cases were included in the study compared to other cancer types and the examples were not homogenous. However, in light of our findings, which need to be supported by larger case studies, HHLA2 may serve as a novel the
The results of this study suggest that HHLA2 expression in MSS and PD-L1-negative tumors may be useful for predicting the response of individuals to immunotherapy, and may serve as a therapeutic target for immunotherapy in patients with advanced-staged disease who do not respond to classical chemotherapy and have unresectable cancer.
Despite advanced techniques in surgical methods and chemotherapy protocols, pancreatic, periampullary/ampullary, and choledochal adenocarcinomas still have high mortality rates. Thus, targeted therapies are needed.
Immunotherapy has opened a new era in cancer treatment. Despite the positive results obtained in treatment, it is nece
To evaluate the relationship of HHLA2 expression with other immunophenotypic markers.
The expression of DNA mismatch repair, programmed death-ligand 1, and HHLA2 proteins was examined by immunohistochemistry. All tumor slides stained with hematoxylin and eosin were screened to evaluate other immunophenotypic features such as intraepithelial tumor-infiltrating lymphocytes.
Low HHLA2 expression was associated with high perineural invasion (PNI). HHLA2 expression was low in pathological stage T3 cases and high in pathological stage T1, T2, and T4 cases. Patients with high HHLA2 expression had a higher mean age than those with low expression.
We found that HHLA2 expression was correlated with age, pathological stage and the presence of PNI irrespective of other immunophenotypic features. Thus, HHLA2 may be a useful biomarker for predicting the response to immunotherapy.
In light of our findings, which will be supported by larger case studies, HHLA2 may serve as a novel therapeutic target for immunotherapy in advanced-stage cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Mei LC, China S-Editor: Lin C L-Editor: A P-Editor: Xu ZH
| 1. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1250] [Article Influence: 178.6] [Reference Citation Analysis (37)] |
| 2. | Qian Y, Gong Y, Fan Z, Luo G, Huang Q, Deng S, Cheng H, Jin K, Ni Q, Yu X, Liu C. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2020;13:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 3. | Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 444] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 4. | Seliger B. Basis of PD1/PD-L1 Therapies. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Liu S, Gӧnen M, Stadler ZK, Weiser MR, Hechtman JF, Vakiani E, Wang T, Vyas M, Joneja U, Al-Bayati M, Segal NH, Smith JJ, King S, Guercio S, Ntiamoah P, Markowitz AJ, Zhang L, Cercek A, Garcia-Aguilar J, Saltz LB, Diaz LA, Klimstra DS, Shia J. Cellular localization of PD-L1 expression in mismatch-repair-deficient and proficient colorectal carcinomas. Mod Pathol. 2019;32:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 7. | Tanigawa M, Naito Y, Akiba J, Kawahara A, Okabe Y, Ishida Y, Ishikawa H, Hisaka T, Fujita F, Yasunaga M, Shigaki T, Sudo T, Mihara Y, Nakayama M, Kondo R, Kusano H, Shimamatsu K, Okuda K, Akagi Y, Yano H. PD-L1 expression in pancreatic adenosquamous carcinoma: PD-L1 expression is limited to the squamous component. Pathol Res Pract. 2018;214:2069-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Wang B, Ran Z, Liu M, Ou Y. Prognostic Significance of Potential Immune Checkpoint Member HHLA2 in Human Tumors: A Comprehensive Analysis. Front Immunol. 2019;10:1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Yan H, Qiu W, Koehne de Gonzalez AK, Wei JS, Tu M, Xi CH, Yang YR, Peng YP, Tsai WY, Remotti HE, Miao Y, Su GH. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett. 2019;442:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Boor PPC, Sideras K, Biermann K, Hosein Aziz M, Levink IJM, Mancham S, Erler NS, Tang X, van Eijck CH, Bruno MJ, Sprengers D, Zang X, Kwekkeboom J. HHLA2 is expressed in pancreatic and ampullary cancers and increased expression is associated with better post-surgical prognosis. Br J Cancer. 2020;122:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Byers JT, Paniccia A, Kaplan J, Koenig M, Kahn N, Wilson L, Chen L, Schulick RD, Edil BH, Zhu Y. Expression of the Novel Costimulatory Molecule B7-H5 in Pancreatic Cancer. Ann Surg Oncol. 2015;22 Suppl 3:S1574-S1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Wu J, Liang C, Chen M, Su W. Association between tumor-stroma ratio and prognosis in solid tumor patients: a systematic review and meta-analysis. Oncotarget. 2016;7:68954-68965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 13. | Karamitopoulou E, Zlobec I, Born D, Kondi-Pafiti A, Lykoudis P, Mellou A, Gennatas K, Gloor B, Lugli A. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer. 2013;49:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Petrova E, Zielinski V, Bolm L, Schreiber C, Knief J, Thorns C, Bronsert P, Timme-Bronsert S, Bausch D, Perner S, Keck T, Wellner U. Tumor budding as a prognostic factor in pancreatic ductal adenocarcinoma. Virchows Arch. 2020;476:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Wang YF, Wu B, Zhong ZX, Wang KX, Yang LQ, Wang YQ, Li YQ, Gao J, Li ZS. Intraepithelial Attack Rather than Intratumorally Infiltration of CD8+T Lymphocytes is a Favorable Prognostic Indicator in Pancreatic Ductal Adenocarcinoma. Curr Mol Med. 2017;17:689-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16:807-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 17. | Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer. 2018;4:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 18. | Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, Edkins S, O'Meara S, Vastrik I, Schmidt EE, Avis T, Barthorpe S, Bhamra G, Buck G, Choudhury B, Clements J, Cole J, Dicks E, Forbes S, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jenkinson A, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, Webb T, West S, Widaa S, Yates A, Cahill DP, Louis DN, Goldstraw P, Nicholson AG, Brasseur F, Looijenga L, Weber BL, Chiew YE, DeFazio A, Greaves MF, Green AR, Campbell P, Birney E, Easton DF, Chenevix-Trench G, Tan MH, Khoo SK, Teh BT, Yuen ST, Leung SY, Wooster R, Futreal PA, Stratton MR. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2512] [Cited by in RCA: 2295] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 19. | Kim MH, Jang M, Kim H, Lee WJ, Kang CM, Choi HJ. Distinct immunological properties of the two histological subtypes of adenocarcinoma of the ampulla of Vater. Cancer Immunol Immunother. 2019;68:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Badalamenti G, Fanale D, Incorvaia L, Barraco N, Listì A, Maragliano R, Vincenzi B, Calò V, Iovanna JL, Bazan V, Russo A. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2019;343:103753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 22. | Chen L, Zhu D, Feng J, Zhou Y, Wang Q, Feng H, Zhang J, Jiang J. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int. 2019;19:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Xiao Y, Freeman GJ. A New B7:CD28 Family Checkpoint Target for Cancer Immunotherapy: HHLA2. Clin Cancer Res. 2015;21:2201-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Zhu Z, Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther. 2018;11:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |