Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.750
Peer-review started: October 26, 2023
First decision: December 5, 2023
Revised: January 2, 2024
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 15, 2024
Processing time: 137 Days and 21.3 Hours
Lipid metabolism reprogramming is suspected to exist in pre-cancerous lesions, including colorectal adenoma. Screening colonoscopy frequently reveals chicken skin mucosa (CSM; white or yellow-white speckled mucosa) surrounding colo
To highlight the clinical significance of CSM surrounding colorectal polyps and clarify the associated treatment for endoscopists.
This retrospective cohort study included 177 patients with CSM-positive colorectal polyps diagnosed using endoscopy. All patient-related information was extracted from the Goldisc soft-clinic DICOM system or electronic medical record system. Based on the pathological results, patients were classified as non-neoplastic polyps (five juvenile polyps), neoplastic polyps, non-invasive high-grade neoplasia (NHGN), or submucosal invasive carcinoma (SM stage cancer). We analyzed and compared the clinical features, suspected risk factors for malignant transformation of neoplastic polyps, and early infiltration of sub
The diameters of NHGN and SM polyps were much smaller than those of neoplastic polyps. Most NHGN polyps had a deeper red mucosal color. On logistic regression analyses, diameter and deeper red mucosal color were independent risk factors for malignant transformation of neoplastic polyps. Type 1 CSM was more common in high-grade intraepithelial neoplasia and SM; type 2 CSM was more common in neoplastic polyps. Logistic regression analyses revealed no significant differences in the malignant transformation of neoplastic polyps or early submucosal invasion of CSM-positive colorectal cancer. Changes in the CSM mucosa surrounding neoplastic polyps and submucosal invasion of colorectal cancer disappeared within 12 months. No tumor recurrence was found during either partial or complete endoscopic resection of the CSM.
CSM-positive colorectal polyps > 1 cm in diameter or with deeper red mucosa may be related to NHGN. Resection of CSM surrounding colorectal adenomas did not affect tumor recurrence.
Core tip: Chicken skin mucosa (CSM) has emerged as a critical feature of early colorectal cancer or pre-cancerous lesions. We performed further risk stratification analysis of CSM-positive colorectal polyps under white light endoscopy. Possible risk factors of malignant transformation and submucosal infiltration of CSM-positive colorectal polyps were proposed. Cold snare polypectomy was determined to be inadequate. CSM type was not associated with malignant transformation of neoplastic polyps or early submucosal invasion. We also confirmed that partial or complete resection of the CSM around colorectal adenomas did not affect tumor recurrence. The CSM disappeared within 12 months after polypectomy.
- Citation: Zhang YJ, Yuan MX, Wen W, Li F, Jian Y, Zhang CM, Yang Y, Chen FL. Mucosa color and size may indicate malignant transformation of chicken skin mucosa-positive colorectal neoplastic polyps. World J Gastrointest Oncol 2024; 16(3): 750-760
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/750.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.750
The abnormality of fat metabolism in tumors is becoming a research focus. Tumor cells are believed to undergo lipid metabolism reprogramming to adapt to the hypoxic, nutrient-poor microenvironment[1]. Lipid metabolism repro
CSM around the base of colorectal polyps, caused by macrophages engulfing and accumulating the lipids decomposed by colon cells or adjacent tumors, is characterized by prolific pale or yellow-spotted mucosal changes observed under conventional white light endoscopy[3-5]. CSM was first reported in 1998 by Shatz et al.[6]. Subsequently, several studies have shown that CSM is associated with juvenile polyps in both adults and children[5,7]. As the expression of Ki-67 or p53 in the CSM around juvenile polyps does not increase, they are not believed to be preneoplastic[8,9]. However, in recent studies, CSM was demonstrated to be associated with colorectal neoplastic polyps[4], malignant transformation of neoplastic polyps, and early submucosal invasion of colorectal cancer[3,10,11]. CSM-positive colorectal polyps are involved in a wide range of diseases, pathological types, and prognoses; therefore, treatment options should be carefully considered and further analyzed.
The CSM adjacent to juvenile polyps was previously thought to be non-preneoplastic because of the lack of related markers for proliferation and malignant transformation[9] and almost disappeared within a month following polypectomy in most cases. However, in contrast to juvenile polyps, some studies have suggested that the Ki-67 index in the CSM adjacent to colorectal adenomas is significantly increased, which may be a type of primary precancerous lesion. Researchers suggested that it should be resected together with the endoscopic resection of colorectal adenomas to reduce the risk of local tumor recurrence[12].
At present, it is unclear whether CSM surrounding colorectal adenomas should be resected at the same time as endoscopic treatment. In the present study, the endoscopic and pathological features of 183 cases of CSM-positive colorectal polyps were recorded and analyzed retrospectively, including the anatomical location, morphological features, characteristics of the polyps under white light endoscopy, type of CSM, different CSM resections, polyp resections, and follow-up surveillance colonoscopies. The purpose of this study was to improve endoscopists' understanding of colorectal polyps with CSM positivity to avoid misdiagnosis and the adoption of inappropriate treatment and improve patient prognosis.
We reviewed the endoscopic characteristics of patients (18–85 years old) who underwent health screening, surveillance in high-risk populations, or diagnostic colonoscopy at the Department of Endoscopic Medicine of the Second People’s Hospital of Chengdu during a 6-month period (from January 2021 to June 2022). The presence of any of the following conditions meant exclusion from the study: (1) A history of clinically or pathologically diagnosed malignancy; (2) inflammatory bowel disease; (3) familial adenomatous polyposis; and (4) a bowel cleanliness score (Boston score) of < 6.
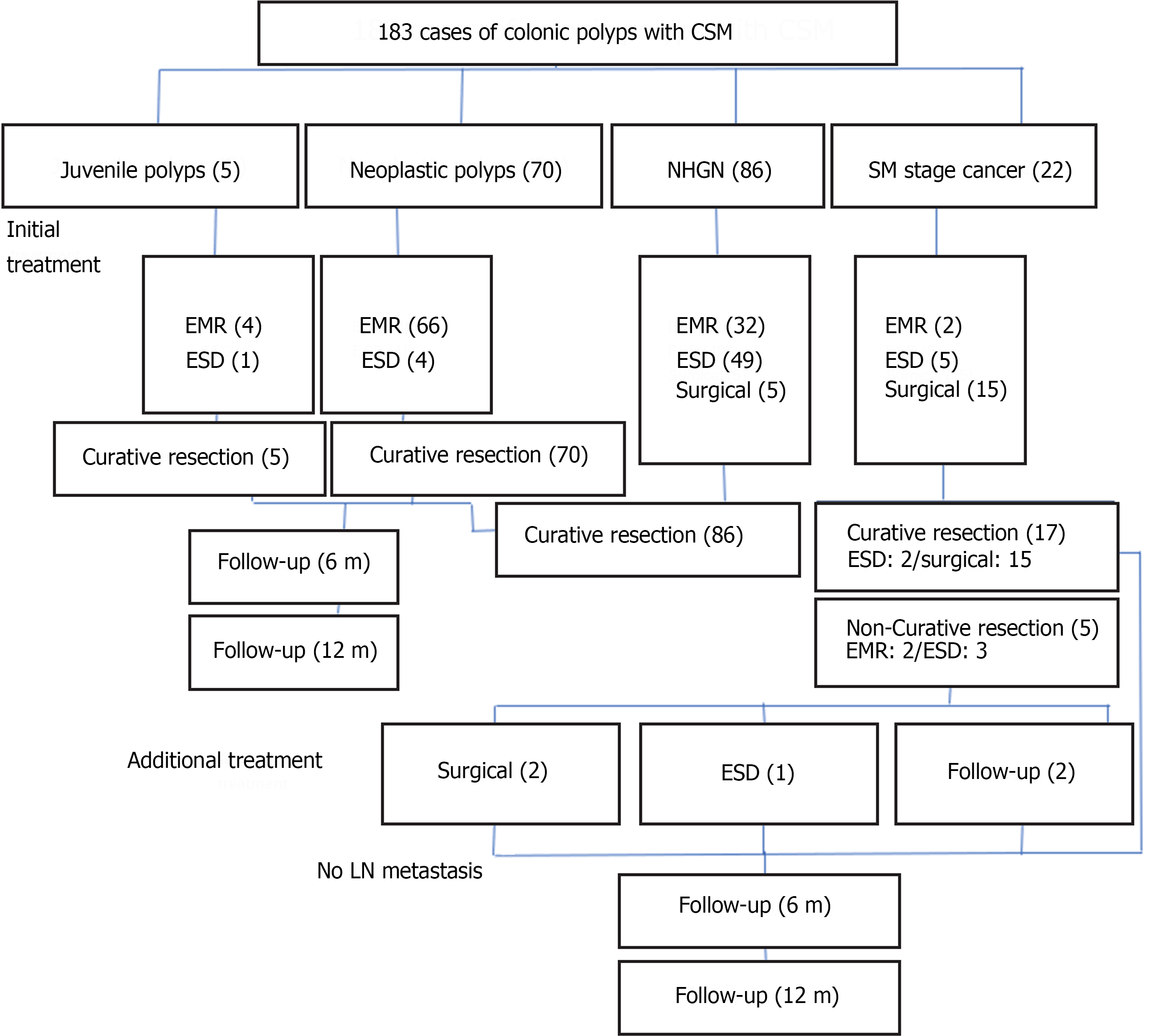
A total of 177 patients (183 CSM-positive colorectal polyps) who underwent endoscopic or surgical treatment were enrolled (Figure 1). We recorded the basic information of the patients, the anatomical location, and characteristics of the polyps under white light endoscopy; the type of CSM, the method of CSM resection, and the manner of polyp resection; and the follow-up of the pathological results of the polyps to investigate the influence of different CSM resection methods on local tumor recurrence.
This study was approved by the Ethics Committee of Chengdu Second People's Hospital, No. 2021018. The req
Based on the pathological results, patients were classified as non-neoplastic polyps (five juvenile polyps), neoplastic polyps, non-invasive high-grade neoplasia (NHGN), or submucosal invasive carcinoma (SM stage cancer). According to the content of villous architecture in the neoplastic polyps, they are classified into villous (> 50% villous architecture), tubulovillous (20%–50% villous architecture), or tubular (< 20% villous architecture)[12].
The definition of NHGN included carcinoma in situ, intramucosal carcinoma, high-grade dysplasia, and high-grade intraepithelial neoplasia (HGIN). This stage of the tumor has a common feature in that it does not invade the large intestine muscularis mucosa; therefore, is considered to have a low risk of metastasis. It is classified as pTis or Stage 0 according to the TNM staging system. If the lesion is defined as a carcinoma in situ, high-grade dysplasia, or HGIN according to the Vienna classification, it indicates that the tumor is confined to the epithelial layer without invading the lamina propria[13,14].
Compared with NHGN, SM stage cancer refers to a lesion in which the tumor has advanced such that atypical cells have penetrated the muscularis mucosae and infiltrated the submucosa[15]. At this stage, the risk of distant metastasis and local lymph node metastasis significantly increases. It is classified as T1 and corresponds to stage I in the TNM staging system[14].
All endoscopic imaging data were reviewed and analyzed, including anatomical location, diameter, morphological classification, CSM resection method (partial resection or complete resection), polyp resection method, local tumor recurrence, and CSM regression during the follow-up surveillance colonoscopy. When our record or description was not clear or controversial, it was reconfirmed by a senior and experienced doctor.
Anatomical location was classified into the right (including the cecum, hepatic curvature, ascending colon, and right side of the transverse colon) or left colon (including the left side of the transverse colon, splenic curvature, descending colon, and sigmoid colon), and rectum.
Based on the endoscopic morphological features and the Paris classification[16], there are three main types of morphological classification: Prominent (0–I), flat (0–II), and depressed (0–III) types. The prominent type was classified as pedunculated, sessile-pedunculated, or sessile lesions, depending on whether the lesions had a pedicle, which was described as a polypoid (0–Ip, 0–Isp, or 0–Is). Flat (0–II) and depressed (0–III) subtypes were described as non-polypoids according to the Paris classification[17]. In our study, non-polypoids (0–III) were observed.
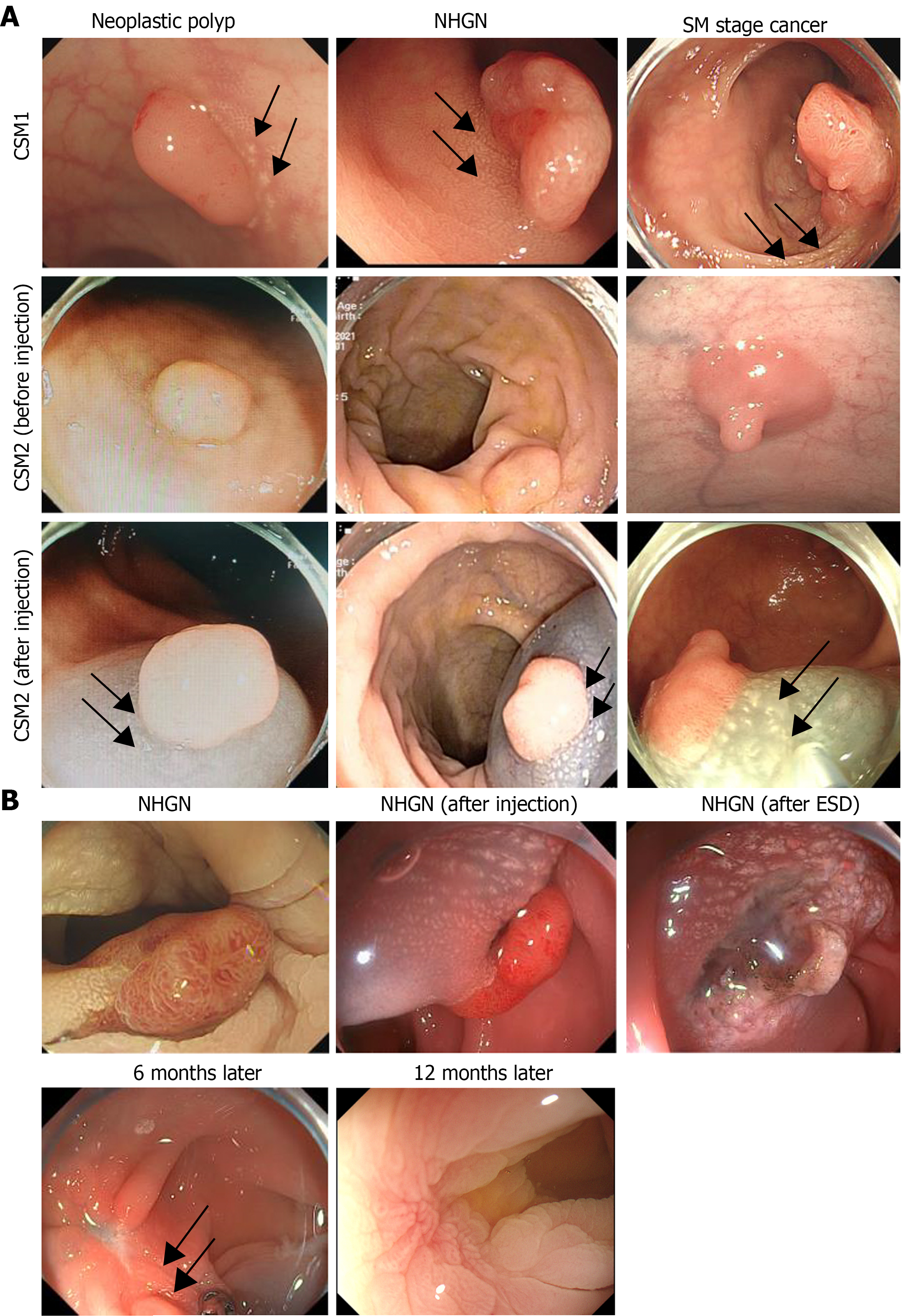
CSM-positive lesions are classified into two types based on their endoscopic features[3]. Type 1 CSM lesions are evident and do not require additional assistance under white light endoscopy; they are dense plaques or yellow-white speckles adjacent to colorectal polyps. Their appearance under a white-light endoscope is similar to the white spots reported by Iwai et al[18], which are associated with invasive cancer and are believed to play a role in inhibiting the progression of malignant tumors[18]. Type 2 CSM are tiny yellow–white speckles adjacent to colorectal polyps. They are easily overlooked by endoscopists under white-light endoscopy; however, spots or irregular reticular white stripes can be seen after submucosal injection. An expansive mucosal and blue mucosal background (normal saline with methylene blue) facilitates the observation of the spots or reticular white stripes (Figure 2A).
En-bloc resection and R0 resection are ideal for all polyps, particularly malignant polyps. It is well known that the polyp resection method can influence the risk of recurrence. Endoscopic submucosal dissection (ESD) can be used to resect sessile or flat adenomatous lesions, as well as complete resection of large (> 2 cm) colonic lesions and early submucosal invasive carcinoma (within the SM1), which is unrivaled by other techniques[19]. Surgical resection is recommended for the treatment of difficult-to-resect lesions and neoplastic polyps associated with deep mucosa carcinoma (the penetration depth of carcinoma to the inferior margin of mucous muscle is beyond 1000 μm)[7].
The type of CSM resection includes partial resection or complete resection. In partial resection, CSM mucosal changes are still found in the cut edge of the polyps and the residual mucosa around the base. However, in complete resection, all CSM mucosa are resected together with the polyps. The residual mucosa around the cut edge and basal mucosa are normal, and no CSM mucosal changes are observed.
The requirements for curative resection are as follows: (1) Intramucosal carcinoma or invasion submucosa carcinoma with a penetration depth within 0.1 cm of the residual muscularis mucosa, or a penetration depth of pedunculated submucosa carcinoma < 0.3 cm from the Haggitt’s level II; (2) no lymphatic or vascular invasion in the postoperative pathology; (3) well-differentiated or moderately differentiated adenocarcinoma; and (4) R0 resection. Curative resection is achieved only if all the aforementioned conditions are met simultaneously, according to the postoperative pathology[9].
Regarding the follow-up surveillance colonoscopy, the main focus of SM stage cancer and NHGN was to identify three study-defined follow-up periods: Short (6 months), intermediate (1 year), and long (3 years); however, for neoplastic polyps, it was 1, 3, and 5 years. Local tumor recurrence and CSM persistence were recorded.
A retrospective statistical analysis was performed by a biomedical statistician using IBM SPSS Statistics, Version 25.0, depending on the pathological results. Measurement data are expressed as mean ± SD.
The enumeration data are expressed as cases. The Student’s t-test, χ2 test, and Fisher’s exact test were used to analyze the patients’ basic characteristics and the CSM type differences in the subgroups. Multivariate analysis of the risk factors for malignant transformation of neoplastic polyps and early submucosal invasion of CSM-positive colorectal cancer was performed using logistic regression. The data that showed a level of significance at P < 0.05 or slightly larger than 0.05 in the bivariate analysis were included as independent variables. The statistical methods used in this study were reviewed by Ye Yang and Feng-Lin Chen from the Department of the Chengdu Medical College of Statistics. Statistical significance was set at P < 0.05.
From January 2021 to June 2022, 183 CSM-positive colonic polyps (177 patients) were found under endoscopy and underwent surgical (20 cases) or endoscopic (163 cases) treatment in our hospital, including 98 males and 79 females. Among the patients who were initially treated with endoscopic therapy, five patients were pathologically proven to have submucosal invasion of cancer cells exceeding SM1 and failed to achieve curative resection. After communicating with them, two patients underwent additional surgery, one patient underwent additional ESD (initial treatment: Endoscopic mucosal resection), and the other two were followed up. The oldest patient was 85 years old, and the youngest was 18 years old. There were five cases of sporadic juvenile polyps (age range: 18–38 years), 70 cases of neoplastic polyps, 86 cases of NHGN, and 22 cases of SM carcinoma. The mean age of the enrolled patients with sporadic juvenile polyps was 26.66 ± 8.17 years, that of patients with neoplastic polyps was 58.69 ± 9.15 years, and that of patients with NHGN and SM carcinoma was much older at 61.26 ± 11.12 and 64.4 ± 8.73 years, respectively (P < 0.001; Table 1). However, sporadic juvenile polyps were not the focus of this study.
| Juvenile polyps (n = 5) | Neoplastic polyps (n = 70) | NHGN (n = 86) | SM stage cancer (n = 22) | P value | ||
| Age (mean SD, yr) (n = 177) | 26.66 8.17 | 58.68 9.15 | 61.26 11.12 | 64.4 8.73 | < 0.001 | |
| diameter (cm) (n = 183) | 1.57 0.34 | 0.88 0.37 | 1.61 0.52 | 1.93 0.53 | < 0.001 | |
| Sex (n = 177) | Male | 4 | 33 | 46 | 15 | 0.34 |
| Female | 1 | 31 | 40 | 7 | ||
| Location (n = 183) | Left colon | 2 | 48 | 50 | 12 | 0.38 |
| Rectum | 3 | 22 | 36 | 10 | ||
| Morphology (n = 183) | Polypoid (Is, Isp, Ip) | 5 | 57 | 75 | 18 | 0.63 |
| Nonpolypoid (IIa, LST) | 0 | 13 | 11 | 4 | ||
| CSM | Type 1 | 5 | 25 | 59 | 17 | < 0.001 |
| Type 2 | 0 | 45 | 27 | 5 | ||
All lesions were located in the rectum or left colon (60% vs 40%, 31.43% vs 68.57%, 41.86% vs 58.14%, 45.45% vs 54.55%, P = 0.38). The diameter of neoplastic polyps was 0.88 ± 0.37 cm, and that of juvenile polyps and NHGN was much larger, reaching (1.57 ± 0.34 cm and 1.61 ± 0.52 cm, respectively. The diameter of all SM carcinoma was the largest, reaching 1.93 ± 0.53 cm, with a statistically significant difference observed (P < 0.001). The distribution of polypoid and non-polypoid polyps was not significantly different in the different subgroups of CSM-positive colonic polyps (Table 1). Both neoplastic polyps and NHGN may be multilobulated or have a granulated surface; however, no statistical differences were observed between them. The majority (n = 54; 77.14%) of neoplastic polyps were generally round and smooth; however, most (n = 54; 62.79%) of the NHGN polyps had a deeper red mucosal color, with a statistical difference observed between them (Table 2). A minority (n = 3; 3.49%) of NHGN polyps had a demarcated depressed area, but that of SM carcinomas was much higher, reaching 14 (63.64%); there was a statistical difference observed between them (Table 3).
| Endoscopic features | Neoplastic polyps (n = 70) | NHGN (n = 86) | P value |
| Multilobulated | 20 | 40 | 0.03 |
| Round and smooth | 54 | 21 | 0.00 |
| Deeper red mucosal color | 11 | 54 | 0.00 |
| Granulated surface | 2 | 6 | 0.55 |
| Endoscopic features | NHGN (n = 86) | SM stage cancer (n = 22) | P value |
| Ulceration or errhysis (all) | 2 | 3 | 0.08 |
| Demarcated depressed area (all) | 3 | 14 | < 0.001 |
| Deeper red mucosal color (all) | 54 | 16 | 0.25 |
| Stalk swelling (P) | 7 | 3 | 0.65 |
| Fullness (P) | 12 | 4 | 0.81 |
Type 1 CSM was more common in HGIN and SM, and type 2 was more common in neoplastic polyps; however, CSM1 was found in sporadic juvenile polyps, and a statistically significant difference was observed (P < 0.001; Table 1). In further logistic regression analyses, the diameter of colorectal polyps and deeper red mucosal color were found to be independent risk factors for the malignant transformation of neoplastic polyps (Table 4). In addition, a demarcated depressed area was an independent risk factor for early submucosal invasion in CSM-positive colorectal cancer (Table 5).
| Variable | OR (95%CI) | P value |
| Diameter (≥ 10/< 10 mm) | 15.84 (5.01–50) | 0.00 |
| CSM (type ½) | 0.56 (0.23–1.36) | 0.20 |
| Multilobulated | 0.4 (0.13–1.22) | 0.11 |
| Deeper red mucosal color | 3.5 (1.25–9.82) | 0.02 |
| Variable | OR (95%CI) | P value |
| CSM (type ½) | 1.63 (0.37–7.14) | 0.52 |
| Ulcer bleeding | 1.68 (0.08–37.06) | 0.74 |
| Demarcated depressed area | 78.03 (13.04–466.94) | 0.00 |
| Diameter (≥ 10/< 10 mm) | 1.28 (0.14–11.67) | 0.83 |
Among the 183 lesions enrolled in the study, 20 cases from the SM subgroup and five cases from the HGIN subgroup had endoscopic suspicion of submucosal invasion during preoperative evaluation and surgical resection, while the remaining 163 cases all chose endoscopic treatment, including ESD or endoscopic mucosal dissection (Table 6). Except for the other patients described, only three patients from the NHGN subgroup with partial resection of the CSM had CSM persistence 6 months later (Figure 2B); however, they disappeared on the follow-up colonoscopy after 6 months. There was no tumor recurrence among the subgroups for either partial or complete resection of the CSM during follow-up colonoscopies 6 and 12 months later.
| Juvenile polyps (n = 5) | Neoplastic polyps (n = 70) | NHGN (n = 81) | SM stage cancer (n = 7) | P value | |
| Complete resection | 0 | 30 | 26 | 2 | 0.194 |
| Partial resection | 5 | 40 | 55 | 5 |
Statistically, compared to all malignant tumors, the incidence and mortality rates of CRC rank third and second, respectively. Hyperplastic polyps and adenomas are the two most common types of polyps in adults. About 95% of colorectal cancers arise from adenomas; adenoma detection and resection can reduce CRC carcinoma mortality. Hyperplastic polyps are non-neoplastic lesions, causing little harm. A non-neoplastic polypectomy does not reduce the risk of colorectal cancer, increases the burden of pathological investigations and procedural costs, and increases the risk of complications.
It is widely acknowledged that early detection and diagnosis are important for improving the prognosis and quality of life of patients with colorectal cancer. Endoscopic characteristics play a crucial role in endoscopic diagnosis and treatment. Therefore, before performing colonic polypectomy, it is important to understand the nature of polyps, distinguishing neoplastic lesions from non-neoplastic lesions, distinguishing HGIN from neoplastic lesions, and distinguishing SM carcinoma from HGIN. The opening of the glandular ducts in the mucosa of the large intestine can be observed by combining narrowband imaging and magnifying endoscopy, which can distinguish neoplastic from non-neoplastic lesions, predicting the pathological diagnosis and depth of invasion by assessing different types of pit patterns in many institutes[20]; however, it is not a routine choice.
In addition to pits, some features under white light endoscopy can still be used to identify tumors that may have submucosal invasion, such as CSM, which is a plaque or yellow-white speckled mucosal change seen under endoscopy adjacent to colorectal polyps located in the colon or rectum, characterized by fat accumulation in the macrophages of the lamina propria. CSM-positive colorectal polyps are frequently found during routine screening colonoscopies, with a prevalence ranging from 29.5% to 31.3%[2,4,10]. According to the related literature[2-5,7,10,11], CSM is involved in a wide range of diseases, including juvenile polyps, neoplastic polyps, advanced colorectal adenoma, submucosal invasion, colorectal cancer, and de novo colorectal cancer. Endoscopists usually make treatment choices based on the endoscopic features of the lesion[13]. A total of five patients (5/177) had a solitary juvenile polyp; their age range was 18 to 38 years old, and the median diameter of the polyps was 1.57 ± 0.34 cm. All were polypoid polyps with a reddish and erosional surface, and CSM was observed adjacent to the polyps located in the left colon and rectum. These findings are consistent with those of previous studies[5,21]. However, it is worth noting that juvenile polyps are a type of hamartoma. These polyps are most common in children but are rare in adults. The incidence of juvenile polyps in children and adolescents is approximately 2%, which accounts for the majority (about 80%–90%) of polyps in pediatric patients. However, less than 1% of juvenile polyps occur in adults, and few studies have been conducted on juvenile polyps in adults. Juvenile polyposis syndrome is generally characterized by multiple hamartomatous polyps throughout the gastrointestinal tract, and it is considered an autosomal dominant disorder. The expression of Ki-67 or p53 in the CSM around juvenile polyps does not increase; they are not believed to be preneoplastic[8,9]. They are often solitary and rarely undergo malignant transformation.
The average diameter of the neoplastic polyps was the smallest, at 0.88 ± 0.37 cm, followed by NHGN polyps, at 1.61 ± 0.52 cm, and SM carcinoma, which were the largest at 1.93 ± 0.53 cm (P < 0.001). Neoplastic polyps often exhibited round and smooth morphology, whereas NHGN could be multilobulated, with statistical differences observed between them.
Most of the neoplastic polyps (77.14%) were round and smooth, and most of the NHGN polyps (62.79%) had a deeper red mucosal color, which may be related to the different degrees of tumor cell proliferation and dysplasia, and even the resulting changes in tumor growth vessels (such as increased number and diameter thickening). Therefore, large neoplastic polyps or NHGN may be multilobulated, whereas small neoplastic polyps often appear round and smooth. Most of the neoplastic polyps in this study were < 1 cm in diameter; therefore, they appeared smooth and round on white light endoscopy. With the increase in tumor volume and deep infiltration, the growth rate of tumor cells exceeds the growth rate of blood vessels; tumor cells far from the blood vessels lack an adequate supply of oxygen and nutrients, leading to differing extents of necrosis and depressions of the tumor surface. Ulcer bleeding is also a common symptom. At present, an increasing number of countries and regions prefer cold snare polypectomy when they encounter polyps < 1.0 cm in diameter without malignant transformation or signs of submucosal invasion. Furthermore, some recent studies have expanded the indication to colorectal adenomas with a diameter 1.0–1.4 cm, with no severe adverse events occurring. However, the histologically complete resection rate was only 63.8%, which is hardly a satisfactory result, and the resection rate of the submucosa was only 25%, which deserves careful consideration[22]. It is obvious that this treatment is not effective enough for CSM-positive colonic polyps > 1.0 cm.
Thus far, the specific reasons for the formation of CSM are still unclear. It is regarded as a compensatory response to polyp growth in the previous literature, which included colonic metaplasia, toxic factors of damaged intraluminal mucosa or previous mild damage. The formation of CSM may be caused by macrophages engulfing and accumulating the lipids decomposed by colon cells or adjacent tumors. CSM positivity was classified into two types depending on the appearance and the need for injection under white light endoscopy in related research[3]. However, whether the appearance of the CSM affects the malignant transformation of neoplastic polyps or early submucosal invasion of CSM-positive colorectal cancer remains unknown. Our study offers some insight into solving this problem.
Previous studies have confirmed that CSM adjacent to benign juvenile polyps is not preneoplastic[9] and almost disappeared within a 1-month period following polypectomy. However, it has also been reported that the expression of Ki-67 and COX2 in CSM is increased, indicating that CSM is associated with malignant transformation[23]. In contrast to juvenile polyps, research led by Lv et al[24] suggested that the Ki-67 index in CSM adjacent to colorectal adenoma was significantly increased, which may be a type of primary precancerous lesion; they suggested that it should be resected together with the endoscopic resection of colorectal adenomas to reduce the risk of local tumor recurrence[24]. However, in our study, tumor recurrence was not associated with the type of CSM resection (partial or complete). The residual CSM disappeared after a certain period (up to 12 months), and no tumor recurrence was observed after tumor resection, regardless of whether partial or complete resection was performed.
Our study had several unavoidable limitations. First, this was a retrospective, single-center study, and the limitations of sample size and bias are unavoidable. The cases of colorectal carcinoma and submucosal invasion colorectal cancer enrolled in our study all belonged to the carcinoma in adenoma type; we did not encounter or recognize de novo colorectal cancer and polyps (Paris morphology: 0–III). Second, due to the limited number of colorectal cancer cases with submucosal invasion, we did not further measure the specific diameter of the demarcated depressed area, which is considered to be associated with the depth of cancer invasion. Third, when the lesion is suspected of submucosal invasion under white light endoscopy, it is essential to evaluate the invasion depth in further detail by different methods, for example, magnified endoscopy, endoscopic ultrasound, and computed tomography imaging. Although we had completed these studies, the related data were not included in this study.
In our future research, we plan to focus on changes in cellular lipid metabolism in different digestive tract pathologies and the associated changes on endoscopic findings.
CSM has emerged as a critical feature of early colorectal cancer or pre-cancerous lesions. We found that a diameter of > 1 cm or a deeper red mucosa may be independent risk factors for the malignant transformation of CSM-positive colorectal neoplastic polyps. Partial or complete resection of the CSM around colorectal adenomas did not affect tumor recurrence, and the CSM disappeared within 12 months after polypectomy. The ability to diagnose the nature of CSM-positive colorectal polyps using white light endoscopy is beneficial in the decision to provide further treatment.
Lipid metabolism reprogramming is suspected to exist in pre-cancerous lesions, including colorectal adenoma. Chicken skin mucosa (CSM) surrounding colorectal polyps is frequently detected during screening colonoscopy. CSM is the pathological accumulation of fat in the macrophages of the lamina propria.
CSM-positive colorectal polyps are associated with various diseases; however, their clinical significance is not yet clear, and they are often not properly treated. We conducted this study to highlight the clinical significance of CSM surrounding colorectal polyps and to clarify the associated treatment for endoscopists.
We performed risk stratification analysis of CSM-positive colorectal polyps under white light endoscopy to identify possible risk factors for malignant transformation and submucosal infiltration of CSM-positive colorectal polyps.
This study retrospectively recorded the endoscopic appearance, clinical features, and pathological findings of patients with CSM-positive colorectal polyps. The Student’s t-test, χ2 test, and logistic regression were used to analyze and compare the clinical features, suspected risk factors for malignant transformation of neoplastic polyps, and early infiltration of submucosal carcinoma.
We found that a diameter of > 1 cm or a deeper red mucosa may be independent risk factors for the malignant transformation of CSM-positive colorectal neoplastic polyps. Partial or complete resection of the CSM around colorectal adenomas did not affect tumor recurrence, and the CSM disappeared within 12 months after polypectomy.
CSM has emerged as a critical feature of early colorectal cancer or pre-cancerous lesions. We found that a diameter of > 1 cm or a deeper red mucosa may be independent risk factors for the malignant transformation of CSM-positive colorectal neoplastic polyps. Endoscopic mucosal resection may be the best treatment option for these lesions. Partial or complete resection of the CSM around colorectal adenomas did not affect tumor recurrence. Abnormal CSM should not be considered when resecting CSM-positive colorectal adenomas endoscopically.
We should pay more attention to the endoscopic features of different types of lipid metabolic abnormalities and attempt to discover the relationship between these characteristics and pathological changes.
We would like to thank Professor Hu Bing of West China Hospital Sichuan University gave scientific guidance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Šarenac TM, Serbia S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 2. | Jin HR, Wang J, Wang ZJ, Xi MJ, Xia BH, Deng K, Yang JL. Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics. J Hematol Oncol. 2023;16:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 158] [Article Influence: 79.0] [Reference Citation Analysis (1)] |
| 3. | Lee YM, Song KH, Koo HS, Lee CS, Ko I, Lee SH, Huh KC. Colonic Chicken Skin Mucosa Surrounding Colon Polyps Is an Endoscopic Predictive Marker for Colonic Neoplastic Polyps. Gut Liver. 2022;16:754-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Chung EJ, Lee JY, Choe J, Chang HS, Kim J, Yang DH, Ye BD, Byeon JS, Kim KJ, Yang SK, Kim JH, Myung SJ. Colonic Chicken Skin Mucosa is an Independent Endoscopic Predictor of Advanced Colorectal Adenoma. Intest Res. 2015;13:318-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Dong J, Ma TS, Xu YH, Li P, Chen WY, Tu JF, Chen YW. Characteristics and potential malignancy of colorectal juvenile polyps in adults: a single-center retrospective study in China. BMC Gastroenterol. 2022;22:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 6. | Shatz BA, Weinstock LB, Thyssen EP, Mujeeb I, DeSchryver K. Colonic chicken skin mucosa: an endoscopic and histological abnormality adjacent to colonic neoplasms. Am J Gastroenterol. 1998;93:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Sun XG, Han Y, Song MQ, Shan TD. A solitary rectal juvenile polyp with chicken skinlike changes in the surrounding mucosa in an adult: A case report. Exp Ther Med. 2023;25:185. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | El-Hodhod MA, Soliman AA, Hamdy AM, Abdel-Rahim AA, Abdel-Hamid FK. Fate and ultra-structural features of chicken skin mucosa around juvenile polyps. Acta Gastroenterol Belg. 2011;74:17-21. [PubMed] |
| 9. | Nowicki MJ, Bishop PR, Subramony C, Wyatt-Ashmead J, May W, Crawford M. Colonic chicken-skin mucosa in children with polyps is not a preneoplastic lesion. J Pediatr Gastroenterol Nutr. 2005;41:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Zhang YJ, Wen W, Li F, Jian Y, Zhang CM, Yuan MX, Yang Y, Chen FL. Chicken skin mucosa surrounding small colorectal cancer could be an endoscopic predictive marker of submucosal invasion. World J Gastrointest Oncol. 2023;15:1062-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 11. | Li SY, Yang MQ, Liu YM, Sun MJ, Zhang HJ. Endoscopic and pathological characteristics of de novo colorectal cancer: Retrospective cohort study. World J Gastroenterol. 2023;29:2836-2849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Castells A, Marzo-Castillejo M, Mascort JJ, Amador FJ, Andreu M, Bellas B, Ferrández A, Ferrándiz J, Giráldez M, Gonzalo V, Jover R, Quintero E, Alonso-Coello P, Bonfill X, Lanas A, Piñol V, Piqué J. [Clinical practice guideline. Prevention of colorectal cancer. 2009 update. Asociación Española de Gastroenterología]. Gastroenterol Hepatol. 2009;32:717.e1-717.58. [PubMed] |
| 13. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1547] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 14. | Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol. 2010;16:3103-3111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 15. | Liu S, Linghu E; Colorectal Group, Digestive Endoscopy Branch of Chinese Medical Association. [Expert consensus on endoscopic diagnosis and treatment for colorectal cancer and precancerous lesions in China (2023, Guangzhou)]. Zhonghua Xiaohua Neijing Zazhi. 2023;40:505-520. [DOI] [Full Text] |
| 16. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Takiuchi H, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 17. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1325] [Article Influence: 60.2] [Reference Citation Analysis (4)] |
| 18. | Iwai T, Imai K, Hotta K, Ito S, Yamaguchi Y, Kawata N, Tanaka M, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Ono H. Endoscopic prediction of advanced histology in diminutive and small colorectal polyps. J Gastroenterol Hepatol. 2019;34:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Dumoulin FL, Hildenbrand R. Endoscopic resection techniques for colorectal neoplasia: Current developments. World J Gastroenterol. 2019;25:300-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Glover B, Patel N, Ashrafian H, Teare J. Diagnostic accuracy of i-scan image enhancement for real-time endoscopic diagnosis of small colorectal polyps: a meta-analysis. Therap Adv Gastroenterol. 2018;11:1756284818814948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Brosens LA, Langeveld D, van Hattem WA, Giardiello FM, Offerhaus GJ. Juvenile polyposis syndrome. World J Gastroenterol. 2011;17:4839-4844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Yabuuchi Y, Imai K, Hotta K, Ito S, Kishida Y, Yoshida M, Kawata N, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Aizawa D, Oishi T, Imai T, Ono H. Efficacy and safety of cold-snare endoscopic mucosal resection for colorectal adenomas 10 to 14 mm in size: a prospective observational study. Gastrointest Endosc. 2020;92:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Guan J, Zhao R, Zhang X, Cheng Y, Guo Y, Wang L, Mi L, Liu F, Ma X, Li B. Chicken skin mucosa surrounding adult colorectal adenomas is a risk factor for carcinogenesis. Am J Clin Oncol. 2012;35:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lv L, Han B, Guan J, Zhao C. [The expression and significance of Survivin, COX-2 and Ki-67 proteinum in colorectal chicken skin mucosa beside adenoma]. Shiyong Zhouliuxue Zazhi. 2010;5:422-424. [DOI] [Full Text] |