Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.614
Peer-review started: November 9, 2023
First decision: December 18, 2023
Revised: December 28, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: March 15, 2024
Processing time: 124 Days and 7.2 Hours
Solid pseudopapillary tumor of the pancreas (SPTP) is a rare neoplasm predominantly observed in young females. Pathologically, CTNNB1 mutations, β-catenin nuclear accumulation, and subsequent Wnt-signaling pathway activation are the leading molecular features. Accurate preoperative diagnosis often relies on imaging techniques and endoscopic biopsies. Surgical resection remains the mainstay treatment. Risk models, such as the Fudan Prognostic Index, show promise as predictive tools for assessing the prognosis of SPTP. Establishing three types of metachronous liver metastasis can be beneficial in tailoring individualized treatment and follow-up strategies. Despite advancements, challenges persist in understanding its etiology, establishing standardized treatments for unresectable or metastatic diseases, and developing a widely recognized grading system. This comprehensive review aims to elucidate the enigma by consolidating current knowledge on the epidemiology, clinical presentation, pathology, molecular characteristics, diagnostic methods, treatment options, and prognostic factors.
Core Tip: Solid pseudopapillary tumor of the pancreas (SPTP) is a rare neoplasm predominantly affecting young females. Pathologically, CTNNB1 mutations, β-catenin nuclear accumulation, and Wnt signaling pathway activation are key molecular features. Accurate preoperative diagnosis relies on imaging and endoscopic biopsies. Surgical resection is the main treatment, and prognostic models like Fudan Prognostic Index aid in prognosis assessment. Challenges in understanding its etiology, establishing treatments for unresectable/metastatic disease, and developing a standardized grading system persist. This comprehensive review aims to consolidate current knowledge on epidemiology, clinical presentation, pathology, molecular features, and treatment options for SPTP.
- Citation: Xu YC, Fu DL, Yang F. Unraveling the enigma: A comprehensive review of solid pseudopapillary tumor of the pancreas. World J Gastrointest Oncol 2024; 16(3): 614-629
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/614.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.614
Solid pseudopapillary tumor of the pancreas (SPTP), also known as solid pseudopapillary neoplasm (SPN) or solid pseudopapillary epithelial neoplasm (SPEN)[1], is a rare, low-grade malignant neoplasm that primarily affects young females[2,3]. Initially described by Frantz in 1959 as “papillary cystic tumors of the pancreas”[4], the World Health Organization (WHO) later adopted the term “SPTP” in 1996 to better reflect the tumor’s histological characteristics[5]. SPTP has limited malignant potential, and typically results in localized disease. Most cases are asymptomatic and are incidentally detected during routine imaging studies or investigations of other conditions.
Although the exact cause of SPTP is unknown, evidence suggests that it originates from pluripotent cells within the pancreas[6]. It typically presents as a combination of solid and cystic areas, with a central pseudopapillary structure formed by cell accumulation around blood vessels[7]. The disease is associated with a favorable prognosis, with a 5-year survival rate exceeding 95%[8]. Surgical resection is the preferred treatment, and complete tumor removal is curative in most cases[8-13]. In rare situations where the tumor is unresectable or has metastasized, chemotherapy or other anti-cancer therapies may be considered. Predicting the risk of postoperative recurrence can aid clinicians in identifying and closely monitoring patients who may benefit from adjuvant therapy.
In this comprehensive review, we delve into recent advances in understanding SPTP to unravel this enigma.
The epidemiology of SPTP remains poorly defined due to lack of large-scale population-based studies. The exact global incidence varies depending on the region and population studied but is estimated to be less than one in a million per year. SPTP can affect individuals of all races[14]. It is important to note that available data on the geographic and ethnic distribution is limited. Further studies are needed to obtain a more accurate understanding of its distribution. However, due to its rarity, conducting large-scale epidemiological studies is very challenging, and even considered impossible.
There are no established risk factors or predisposing conditions associated with the development of SPTP. The gender and age predilection may be related to sex hormones[6]. Studies have suggested that progesterone may be involved in its pathogenesis[15]. Differential expression of estrogen and androgen receptors have also been observed, although these associations are not definitive[16-18]. Further research is needed to identify the underlying mechanisms.
Several molecular and genetic alterations that contribute to pathogenesis and malignant potential have been identified. The most common genetic alteration is a mutation in exon 3 of CTNNB1[7,19-23], which encodes β-catenin, a crucial component of the Wnt-signaling pathway. Activation of β-catenin has been shown to induce pancreatic tumorigenesis[24]. The Wnt/β-catenin pathway promotes carcinogenesis by influencing gene transcription in a regulated or deregulated manner[25,26]. It also interacts with the Hedgehog and androgen receptor signaling pathways, which trigger epithelial–mesenchymal transition[21]. The mechanism underlying its limited malignant potential is unclear, but may be related to the significant downregulation of BCL9/9L, a crucial transcriptional co-activator of β-catenin[25], and high expression of the cyclin D1 inhibitors p21 and p27[27].
In addition to β-catenin, which has been identified as a positive molecular marker for SPTP[28-30], negative markers such as KRAS, GNAS, RNF43, and chr18 Loss of heterozygosity have also been identified[28]; however, their implications remains poorly understood. Based on these complex molecular mechanisms, SPTP is typically characterized by slow growth and low malignant potential[31]. It is usually well-circumscribed, rarely invades neighboring structures or metastasizes to distant sites, and has a high R0 resection rate. However, an aggressive growth pattern with local infiltration and distant metastasis occurs in approximately 15% of the cases[32].
An analysis of 1,072 cases of SPTP cases from several large cohort studies have shown that abdominal pain and discomfort were the most common symptoms, accounting for 43.5% of cases. More than 40% of patients had no symptoms, making it difficult to detect this tumor. An abdominal mass was the first finding in 14.8% of patients. Other clinical manifestations, with a prevalence of more than 1%, include dyspepsia, nausea, vomiting and back pain. Rare symptoms (< 1%) include obstructive jaundice, anorexia, fever, weight loss, and sinusoidal hypertension[3,9,11-13,32]. It is worth noting that the mechanism by which obstructive jaundice and sinistral portal hypertension occur in SPTP is different from that involved in pancreatic ductal adenocarcinoma. In SPTP, these symptoms are caused by external pressure on the surrounding structures, whereas in pancreatic ductal adenocarcinoma, they result from invasion of the biliary tract and portal vein system[12]. Due to advances in imaging techniques and increased health-consciousness, the proportion of asymptomatic cases has gradually increased in recent years as more people become aware of their health and undergo regular check-ups[3,13]. Nevertheless, the nonspecific symptoms and the absence of specific laboratory tests and tumor markers present difficulties in the accurate diagnosis of SPTP[26].
Ultrasonography is often the initial test used for symptomatic patients and is also used for screening. Ultrasonically, an SPTP appears as a well-defined hypo-echoic cystic mass with few internal flow signals. Contrast-enhanced ultrasonography shows enhanced capsular boundaries with a nonenhanced central area. Additionally, iso/hypo-enhancement can be observed during the early and delayed parenchymal perfusion phases[33,34].
Computed tomography (CT) is the most widely used and sensitive test for the evaluation of pancreatic tumors. Multi-detector CT (MDCT) is recommended as the primary method for detecting SPTP and for assessing its resectability. Li et al[35] classified SPTP into five types based on their solid–cystic ratio. Types III, IV, and V were more common in females. The most prevalent type was type III (29.4%), which appeared as a well-circumscribed mass with mixed solid and cystic components, with no clear boundary between the cystic and solid regions. Interestingly, the solid–cystic ratio may decrease as the SPTP grows. Smaller SPTPs (less than 3 cm) tend to be predominantly solid, while larger SPTPs (more than 3 cm) show more cystic components[36]. On contrast-enhanced CT, noticeable enhancement of the solid area is observed during the arterial and portal venous phases, although it is lower than that of the pancreatic tissue[37]. Peripheral enhancement due to a fibrous pseudo-capsule is also a characteristic feature of SPTP[38]. Enhancement of fibrous components within cystic fluid resembles a “floating cloud” appearance[39]. Hemorrhage, necrosis, and calcification are important features[35].
SPTP exhibits greater heterogeneity on magnetic resonance imaging (MRI). MRI often reveals T2 hyperintensity and T1 hypointensity, as well as heterogeneous enhancement on contrast-enhanced T1-weighted images[35,40]. Although the role of MRI in SPTP has been less extensively studied than that of compared to CT scans, MRI is crucial for its detection due to its non-invasiveness and high diagnostic accuracy[41].
Endoscopic techniques provide methods for the preoperative pathological diagnosis of SPTP. Similar to abdominal ultrasound, SPTP appears as solid, cystic, or solid–cystic on endoscopic ultrasound (EUS)[42]. The application of artificial intelligence, such as the deep learning analysis of EUS images, has the potential to improve the diagnostic value of endoscopic techniques[43]. EUS-guided fine needle aspiration (EUS-FNA) is increasingly utilized in pancreatic tumor diagnosis, with both sensitivity and specificity exceeding 80%[44]. In addition to biopsy, EUS-FNA allows for the minimally-invasive collection of fluid samples, including pancreatic juice and cyst fluid[42,45]. Table 1 summarizes the results of cyst fluid analysis for various pancreatic cystic diseases[45-47]. Considering the predominant solid component in SPTP and the limited diagnostic accuracy of cyst fluid analysis, EUS-guided fine needle biopsy (EUS-FNB) can be a more valuable diagnostic tool. This method often provides a larger tissue sample for possible immunostaining needed for diagnosis and to exclude other tumors with different management such as pancreatic neuroendocrine tumor (PNET). A recent retrospective multi-center study showed an impressive preoperative diagnostic accuracy of 97.2% (103/106) for SPTP using EUS-guided biopsy[48]. However, EUS-guided biopsy demands specialized endoscopic skills and expertise, which may limit its availability on a global scale. Moreover, the learning curve is long, and low cellularity in sometimes encountered, which may limit its clinical utility. Additionally, the probability of biopsy-induced inflammation and needle tract seeding, while extremely low, does exist[42,49-51]. Additionally, EUS-guided needle-based confocal laser endomicroscopy (EUS-nCLE) has emerged as a novel diagnostic method, exhibiting high diagnostic accuracy[52,53]. The typical features of SPTP on EUS-nCLE include tiny round dark cellular clusters with white stroma bands[52,54].
| Type | SCN | MCN | IPMN | SPTP | Pseudocyst |
| Viscosity | Low | High | High | NA | Low |
| Mucin | Low | High | High | NA | Low |
| Amylase | < 250 U/L | < 250 U/L | High | Low | High |
| Cytology | Negative or Glyogen containing cuboid cells | Mucin containing column cells | Papillary clusters of mucin column cells, atypia | Branching papillae cuboid or cylindric cells, high cellularity, myxoid stroma | Dirty material, macrophages, inflammatory cell |
| NGS | VHL; chr3 LOH | CEA | KRAS; GNAS; TP53; PTEN; CEA | CTNNB1 | NA |
Positron emission tomography/CT (PET/CT) complements routine imaging tests and provides insights into the histopathological composition of SPTP. One characteristic finding is the presence of strong focal fluorodeoxyglucose (FDG) uptake, which is indicative of metabolic activity[55]. Recent reports have suggested that fibroblast activation protein inhibitor (FAPI) activity can also be observed in cases where FDG uptake is negative[56]. This highlights the potential of FAPI as an alternative tracer in certain situations. Additionally, the standard uptake value, a quantitative measure of FDG uptake, has been shown to correlate with pathological features such as tumor cellularity, proliferative index, and histological malignancy[57]. PET/MRI is relatively rare used in the evaluation of SPTP. It combines the benefits of both PET and MRI, and provides detailed anatomical and functional information in a single imaging session. Similar to PET/CT, PET/MRI can also show focal FDG uptake, allowing for identification and localization of active tumor regions[58]. Both aid in staging, prognostication, and early detection of recurrence, and assist in treatment planning[59]. However, despite their clinical value, high costs may restrict their widespread application.
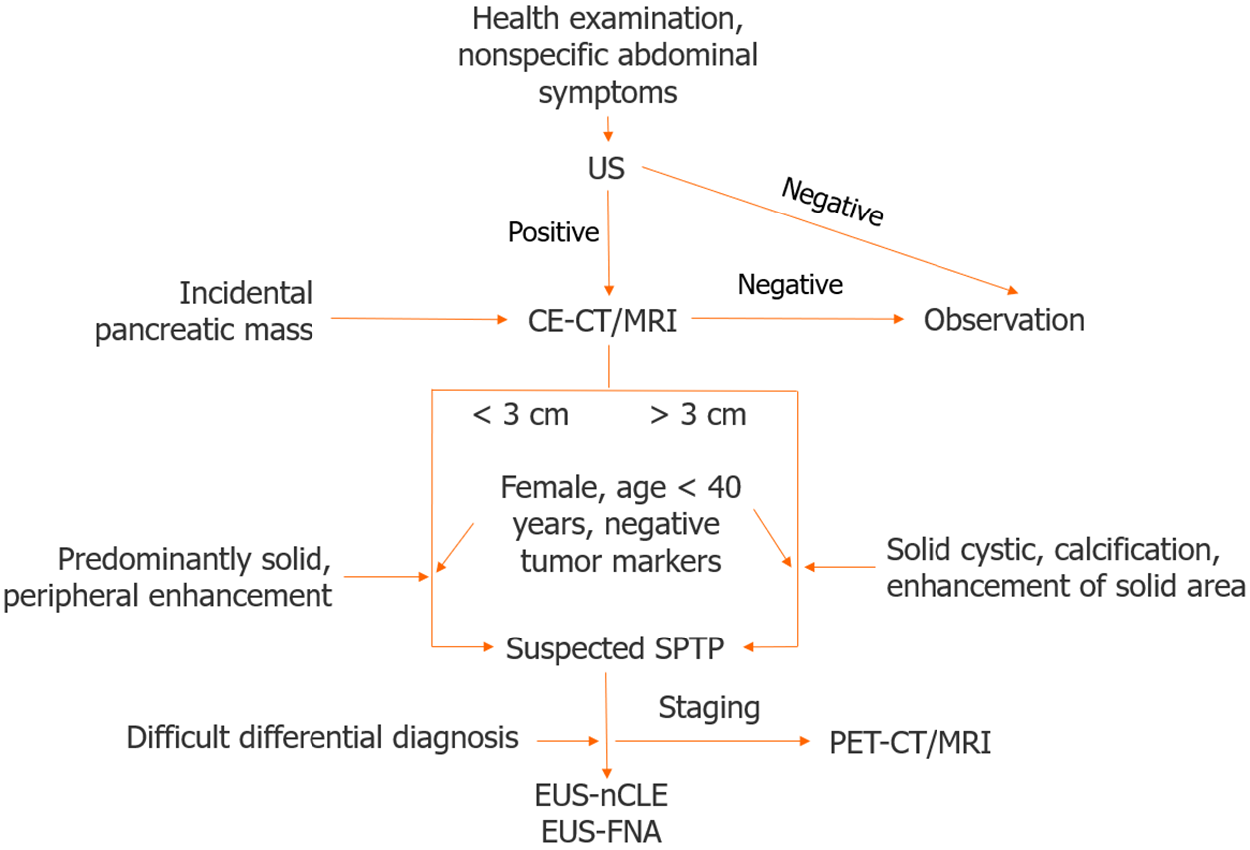
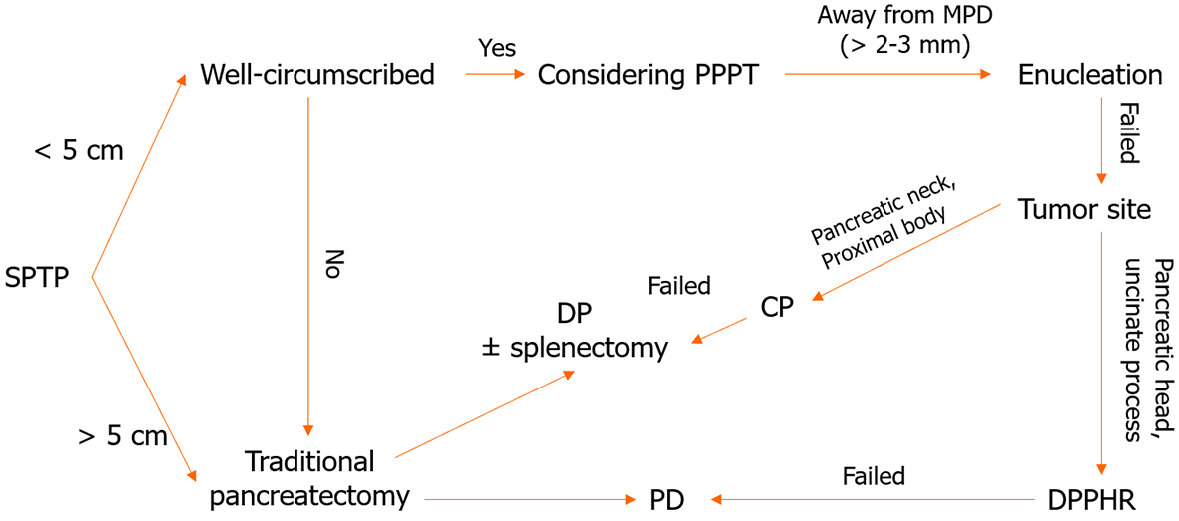
Age and sex are essential factors to consider when diagnosing SPTP. If a solid pancreatic cystic tumor is detected in a young female, SPTP should be considered first. A definitive diagnosis is typically made using a combination of imaging studies. In cases where the differential diagnosis is difficult, a multidisciplinary approach, involving radiologists, gastroenterologists, and surgeons, is usually required to make an accurate diagnosis. Definitive diagnosis before surgery relies on biopsy and histopathological examinations. In the era of guidelines and EUS, the misdiagnosis rate of SPTP is markedly low, at only 6%, making it the lowest among pancreatic cystic neoplasms[60]. A flowchart of the SPTP diagnosis is shown in Figure 1.
Macroscopically, the majority of SPTPs appears as a well-circumscribed mass[61,62]. They often have a fine peripheral capsule, and hemorrhage and necrosis may also be visible[61]. Microscopically, SPTPs typically display characteristic histological features, including pseudopapillary structures, and areas of hemorrhage and necrosis[62]. Tumor cells usually have large round nuclei, with abundant eosinophilic or clear cytoplasm that can be vacuolated[63]. Wang et al[64] identified key cytological features of SPTP, such as myxoid stroma surrounding fibrovascular cores and discohesive epithelioid cells with deficient cytoplasm, pale chromatin, longitudinal nuclear grooves, and small nucleoli closely associated with the nuclear membrane. However, a definitive diagnosis cannot rely solely on morphological features. Immunohistochemistry helps to confirm the diagnosis and differentiate SPTP from other tumor types[63]. Several immunohistochemistry markers, such as CD56, CD10, and β-catenin, have been shown to be commonly expressed in SPTP[65,66]. Table 2[18,26,31,65,66,73-80] provides a list of useful immunohistochemical markers for SPTP. Although SPTP possesses distinct features, it has similarities and overlaps with other tumors, such as non-functional PNET[67] and acinar cell carcinoma[37]. A comprehensive differential diagnosis of SPTP is presented in Table 3[68-72].
| Markers | Positive rate | Mechanisms/implications |
| β-catenin[26,65] | Almost 100% (nuclear) | Activating the Wnt-signaling pathway |
| CD200[73] | 100% (focal) | Marker of stem cell status |
| CD10[65,66,74] | 100% | Marker of SPTP; expressed in immature lymphocyte |
| CD56[66] | 100% | Marker of SPTP |
| AMACR[75] | 96.2% | Marker of SPTP |
| LEF1[18] | 94.7% | Regulating the Wnt-signaling pathway |
| TFE3[76] | 94.7% | Activating and regulating the Wnt-signaling pathway |
| CD99[77] | 78.4% (paranuclear dot-like) | Differentiating from PNET |
| E-cadherin[65,78] | 0% | Differentiating from PNET |
| CgA[26] | 0% | Differentiating from PNET |
| Trypsin[26,79] | 0% | Differentiating from ACC |
| BCL10[26,79] | 0% | Differentiating from ACC |
| Ki-67[31,80] | Mostly 1-2% | Predicting prognosis |
| Disease | Female | Age (yr) | Marker | Clinicopathological features |
| SPTP | 90% | 20-40 | β-catenin | Well-circumscribed; < 3 cm: Mainly solid; > 3 cm: Solid-cystic; myxoid stroma enveloping fibrovascular cores; discohesive epithelioid cells |
| Non-functional PNET | 50% | 50-60 | CgA | Solid: Obviously enhanced with capsule ring-like enhancement; solid-cystic: Mural nodule, uneven wall; high rate of G2 and G3 |
| ACC | < 50% | 60 | AFP | Enhanced solid with large mass having hypodense areas; heterogeneous enhancement; full of large polygonal cells with background necrosis, zymogen-rich and granular cytoplasm, cherry-red nucleoli |
| SCN | 75% | 55-70 | NA | Honeycomb appearance, central scar; stellate scar in the center of the cyst cavity; clear serous fluid |
| MCN | > 95% | 40-60 | NA | Mucin secretion; disconnection from pancreatic duct; ovarian-like stroma; intracellular mucin |
| IPMN | 50% | 60-80 | NA | Communication with pancreatic duct; absence of ovarian-like stroma; mucin |
| Pseudocyst | ≤ 25% | Any | NA | History of pancreatitis or pancreatic trauma; high amylase in pancreatic juice |
| PBL | NA | < 10 | AFP | Hypodense mass; central mass; squamous nest; well-defined margin; heterogeneous; enhanced; circumscribed, plump spindly cell whorls with squamous morules |
Until now, there has been no unified standard for defining malignant SPTP. Certain features, such as cell pleomorphism, prominent necrosis, perineural invasion, and the presence of multiple mitotic figures, may indicate malignant potential[81,82]. The 2010 WHO classification of tumors of the digestive system considered SPTP as a low-grade malignancy[83]. However, the updated system in 2019 introduced the concept of high-grade malignant SPTP, characterized by tumor cells exhibiting high levels of atypia and extensive mitotic figures throughout the tumor[84]. Previous studies defined malignant SPTP based on various criteria including lymph node or distant metastases, cellular atypia, capsule invasion, parenchymal infiltration, perineural or lymphovascular infiltration, or extrapancreatic infiltration in 18.3% of cases[31]. Fleming et al[85] defined malignant SPTP based on the American Joint Committee on Cancer (AJCC) 8th edition staging system, classifying T4 stage, lymph node metastasis, and distant metastasis as malignant, accounting for 13.4%[85]. Further research and collaboration are needed to establish a consensus for diagnosing malignant SPTP, which may require more aggressive treatments.
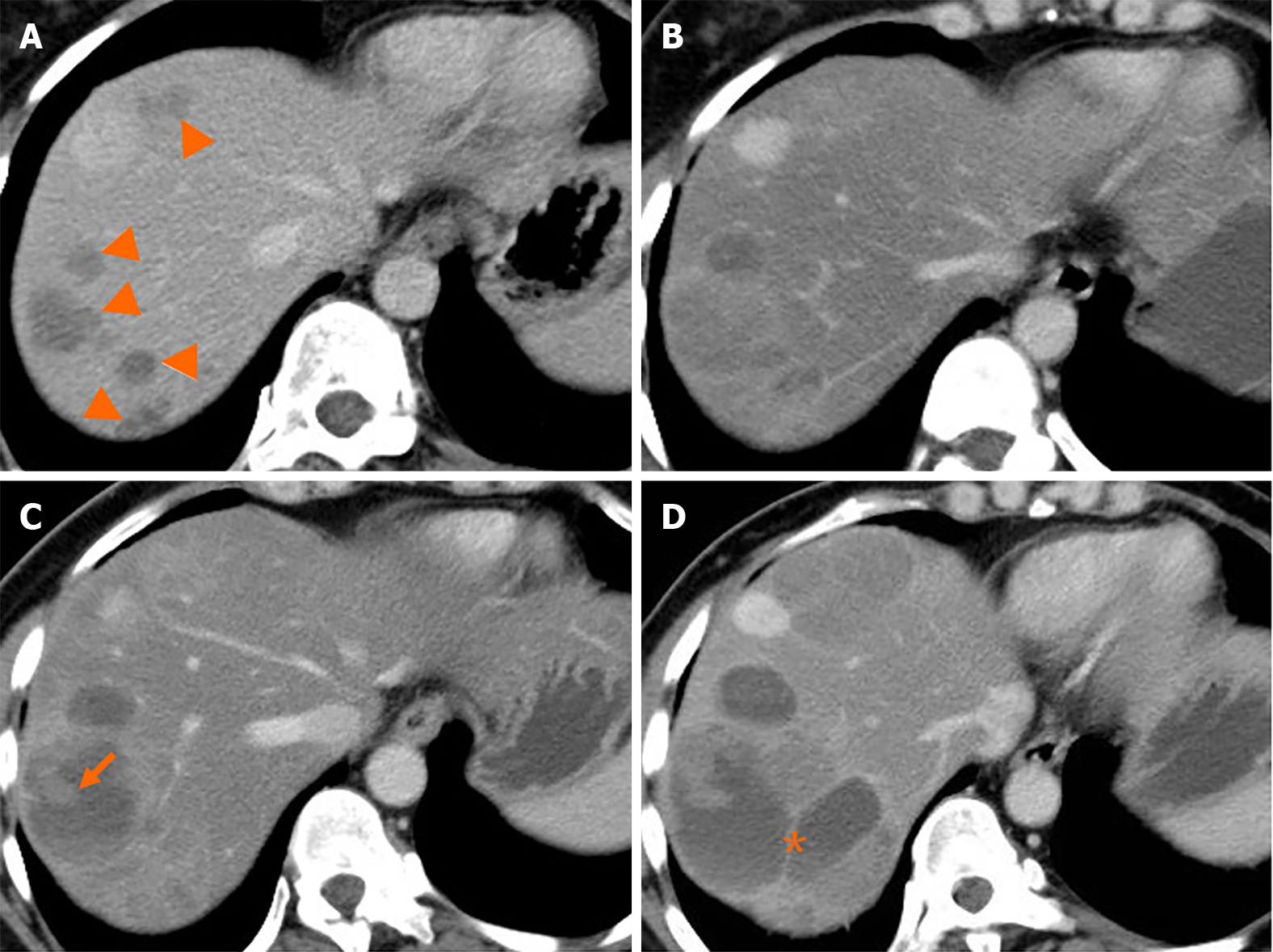
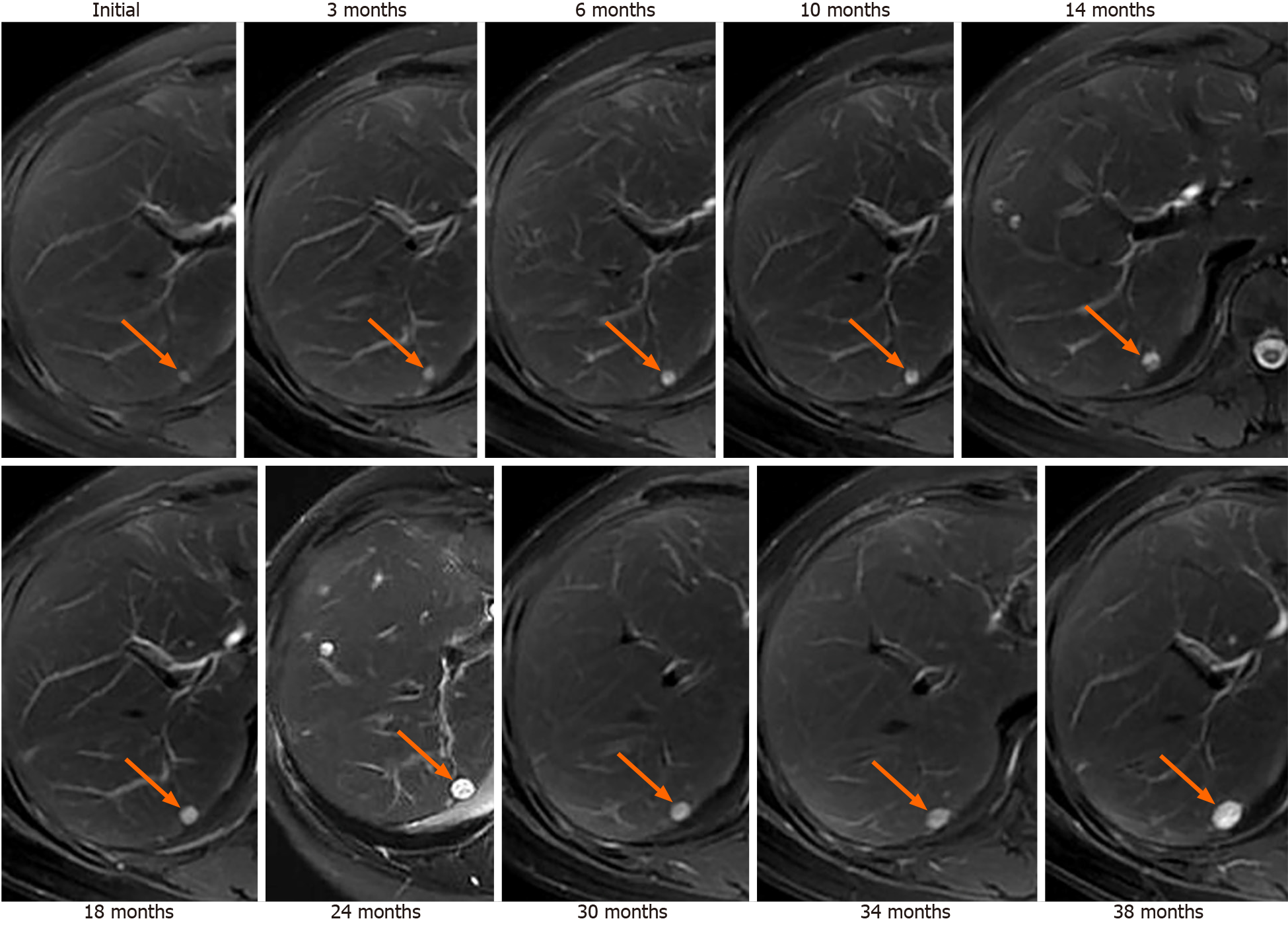
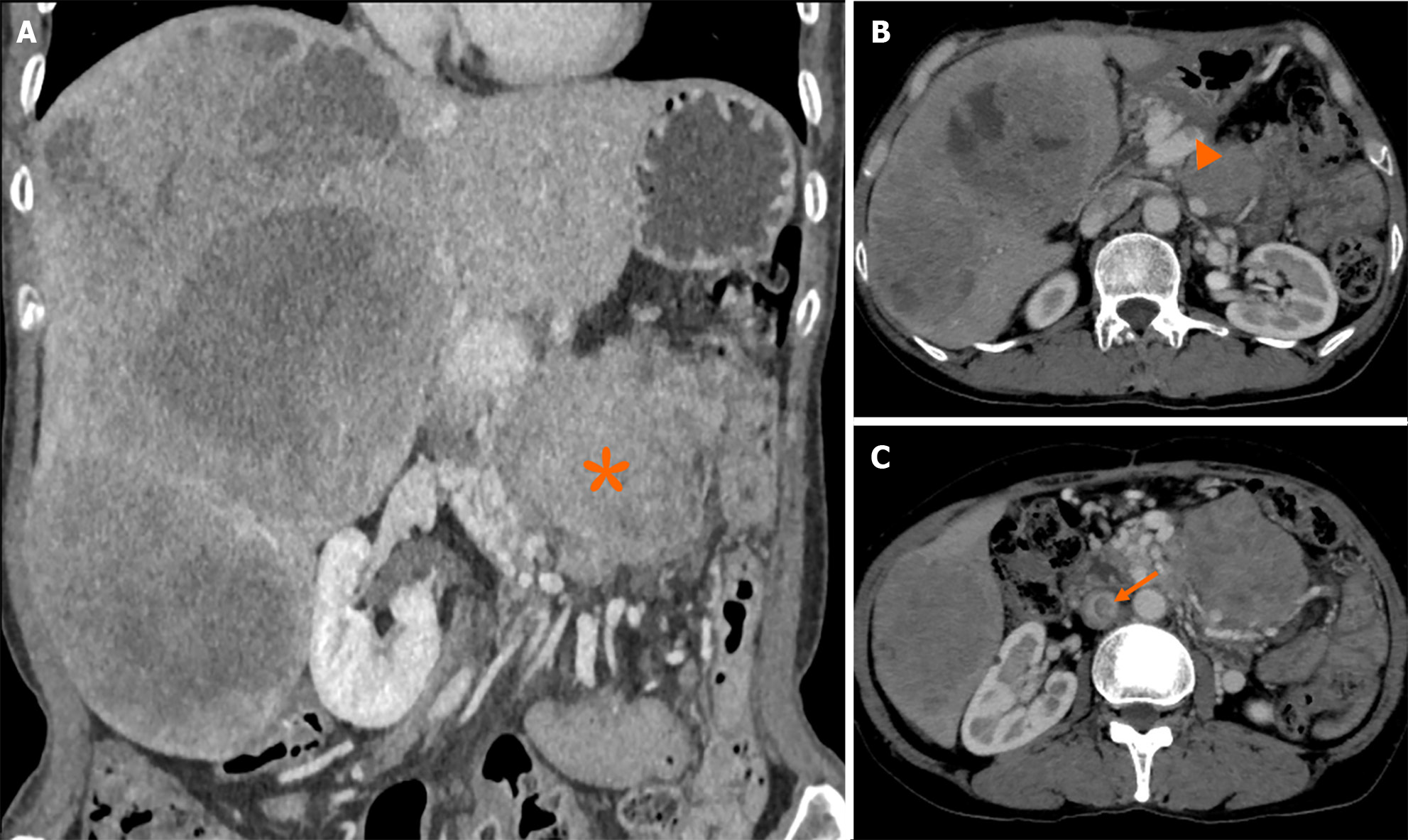
Liver metastasis of SPTP can occur synchronously or metachronously. Chen et al[9] showed that synchronous metastasis rates ranged from 0% to 4.3%, while metachronous metastasis rates ranged from 1.5% to 4.5%. Metachronous liver metastasis can be classified into three types: classical, indolent, and aggressive. In the classical type, metastases grow at a relatively slow rate. Small lesions appear as low-density on CT scan and may be single or multiple. As the lesions increase in size, peripheral enhancement may occur during the arterial and venous phase. With further growth, cystic degeneration and hemorrhagic necrosis may develop. Multiple lesions can even merge to form a larger lesion (Figure 2). The classical type aligns with the natural growth pattern of the primary SPTP lesion[86]. The indolent type exhibits a very slow growth rate, and the lesions are often small (less than 1 cm) when detected. During follow-up, these lesions typically only grow a few millimeters per year (Figure 3). It can be challenging to detect this type, and enhanced MRI has relatively high sensitivity. Treatment options for the indolent type may include observation or radiofrequency ablation (RFA). The aggressive type is usually asymptomatic and discovered incidentally during follow-up. If the metastases are unresectable, they may progress rapidly despite adjuvant therapy. In this condition, surrounding vessels such as splenic vein, portal vein, superior mesenteric vein, and even inferior vena cava can be involved, leading to tumor thrombus formation (Figure 4). The factors contributing to this type are unclear but may be associated with abdominal trauma[87,88]. The prognosis for the aggressive type is generally poor, and surgical intervention is often not possible.
Observation is typically not recommended for SPTP because of its malignant potential[89]. However, a recent study examined 994 cases from the National Cancer Database between 2004 and 2018 and found that the incidence of lymph node metastasis was 0.5% in tumors ≤ 4 cm and 0% in those ≤ 2 cm[90]. This suggests that patients with cT1N0 Lesions should be closely monitored rather than undergoing immediate surgery. The benefit of observation is the avoidance of the morbidity and mortality associated with pancreatic resection[91]. With advancements in interdisciplinary approaches, EUS-guided RFA (EUS-RFA) has emerged as a potential treatment option for pancreatic tumors[92]. In a study by Choi et al[93], two patients with SPTP underwent EUS-RFA without experiencing any procedure-related adverse events, and one patient achieved a complete response. Coupier et al[94] subsequently reported on three SPTP patients who received EUS-RFA, and none of them experienced recurrence during a 2-year follow-up period. However, it should be noted that EUS-RFA is only suitable for individuals who are not eligible for surgical interventions, despite being less invasive. For T1N0 tumors, this treatment option can be discussed, but further data collection is necessary. Currently, R0 resection is the mainstay of treatment for SPTP.
It has been shown that the type of surgery has a limited impact on long-term survival[95]. Generally, for SPTP in the pancreatic head, enucleation, duodenum-preserving pancreatic head resection (DPPHR), and pancreaticoduodenectomy can be performed[96-98]. For tumors in the pancreatic body and tail, enucleation, central pancreatectomy, and distal pancreatectomy (with and without splenectomy) are available[3,31,32]. As depicted in Table 4, parenchyma-preserving pancreatectomies such as enucleation, central pancreatectomy, and DPPHR are increasingly performed[99-104]. They have been reported to reduce the incidence of pancreatic endocrine and exocrine insufficiencies without compromising short- and long-term outcomes[99]. Minimally invasive techniques, including laparoscopic and robotic surgery, may also be considered in both traditional and parenchyma-preserving procedures[32,104-107].
| Ref. | Country | F/M | Median/mean age (year) | Surgery type | Median/mean follow-up (month, range) | R/M |
| Li et al[100] | China | 129/37 | 32.5 (10-68) | 11 EN, 22 CP | 49 (24-102) | 2 |
| Wang et al[103] | China | 84/17 | 31.7 (10-65) | 31 EN | 46.1 (12-101) | 0 |
| Tjaden et al[101] | Germany | 44/8 | 29 (8-71) | 4 EN, 5 CP | 54 (2-230) | 2 |
| Cho et al[102] | Korea | 56/10 | 14.5 ± 5.8 | 15 EN, 4 CP | 24.9 (10-76) | 1 |
| Gao et al[99] | China | 49/13 | 31.76 ± 10.19 | 15 EN, 47 CP | 31 (3-69) | 0 |
| Chen et al[104] | China | 8/2 | 44.6 (32-57) | 10 CP | 22.9 (3-48) | 0 |
| Guo et al[11] | China | 71/16 | 31.3 ± 13.1 | 6 EN, 4 CP | 46 (13-97) | 0 |
| Wang et al[13] | China | 85/12 | 31.6 ± 13.92 | 15 EN, 20 CP, 2 DPPHR | 54 (7-121) | 0 |
To date, no consensus on the optimal approach for the treatment of SPTP metastasis has been established[31,98]. Lymph node metastasis is relatively rare, occurring in approximately 1.0% to 7.9% of cases[8-10,32]. This finding indicates that extended lymphadenectomy may be unnecessary in most patients[108]. Liver-directed therapies, including metastasectomy, RFA, proton beam radiotherapy, chemosaturation, and liver transplantation, can be considered for liver metastasis[109-116]. Although not yet documented, EUS-RFA might prove to be effective for treating small recurrent metastases that are not amenable to surgical resection. It is important to note that the available information regarding therapy for metastasis is predominantly derived from case reports and lacks robust evidence. However, every effort should be made to perform curative resection, even in cases of vascular invasion or distant metastasis. The surgical algorithm that guides the treatment decisions is summarized in Figure 5.
In addition to surgical resection, other treatment options are available for malignant SPTP. Although chemotherapy was shown not to improve overall survival (OS) for both resected and unresected SPTP[85], several reports have shown that oxaliplatin- and gemcitabine-based chemotherapies are applicable for malignant SPTP[117,118]. Other antitumor methods included targeted therapy (mTOR inhibitor)[119-121] and endocrine treatment (tamoxifen)[122], both of which have been reported in individual cases; however, more data are needed to validate these approaches. A multidisciplinary approach should be adopted to develop individualized treatment plans for patients with metastatic SPTP.
The prognosis of SPTP is generally favorable due to its low malignant potential[32]. Previous studies have shown a 10-year recurrence-free survival (RFS) rate of 94.8% and an OS rate of 97.6%[8]. These results are consistent with other large-scale studies and meta-analysis[10-12,32,123]. Even in cases of relapse, the survival rate remains acceptable. In recent years, several risk factors for recurrence have been identified, including male gender, incomplete capsule, young age, high neutrophil-to-lymphocyte ratio, large tumor size, R1 resection, high Ki-67 index, lymphovascular invasion, and synchronous metastasis[9,31,108,124-128].
The traditional TNM staging system has limitations in predicting the prognosis of SPTP[8]. SPTP rarely invades surrounding arteries and has a low incidence of lymph node metastasis, resulting in few cases classified as T4 or N1/N2 tumors. No significant survival differences were reported between stage I and II[8,9]. The European Neuroendocrine Tumor Society staging system shares similar limitations[8]. However, the Fudan Prognostic Index (FPI), which takes into account tumor size and Ki-67 index, has been recently developed[8]. This index categorizes SPTP into three risk groups (Table 5) and shows that each group has a significantly different RFS[8]. The FPI outperforms other staging systems in predicting RFS[8], as demonstrated in both the Huashan and historical cohorts. It represents a groundbreaking study and is the first to report a novel grading system for SPTP[129]. Subsequent studies have further confirmed the value of FPI in predicting the prognosis of SPTP[9,61]. Based on the FPI, the Peking Union Medical College Hospital (PUMCH) risk model was developed by including lymphovascular invasion as an additional factor (Table 5)[9]. This model categorizes patients into low- and high-risk groups, and predicts RFS with an area under the curve of 0.791.
Both models have significantly enhanced clinicians’ understanding of prognosis for SPTP. Nevertheless, there is a difference in the distribution of patients in the intermediate/high risk groups between the two models. In the FPI cohort, only 21.2% of patients were classified as intermediate/high risk, while the PUMCH cohort had 64.1% classified as high-risk patients[8,9]. This suggests that the PUMCH risk model may result in overtreatment and excessive follow-up, potentially burdening patients psychologically. Additionally, the model poses challenges for pathologists due to the heavy workload for assessing lymphovascular invasion[9]. The FPI model allows for more detailed risk stratification. However, both models require further studies for external validation due to the relatively short follow-up time.
With the emergence of radiomics, big data and artificial intelligence, more predictive models are expected to be developed. To achieve this, it is crucial to establish standardized reporting criteria for radiology, histopathology, and immunohistochemistry to ensure accurate identification of predictive factors. Furthermore, large-scale, multicenter, and even multinational studies are necessary, along with the creation of big data cohorts. These efforts will contribute to advancing the development of more precise predictive models for SPTP.
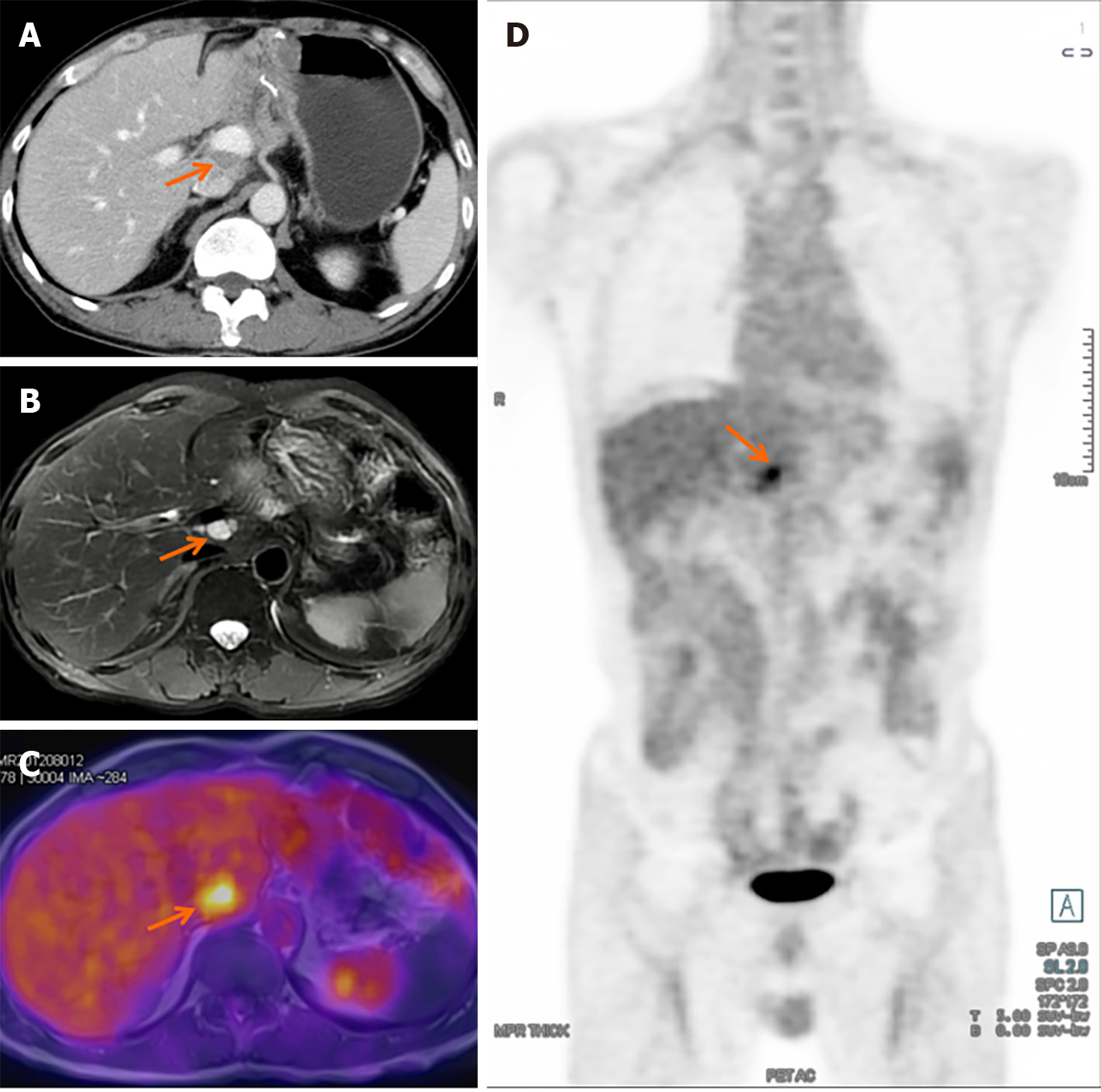
Due to the lack of guidelines, there is currently no consensus for postoperative follow-up for SPTP. Traditionally, patients undergoing SPTP surgery are advised to have regular check-ups every 3 to 6 months for the first two years, followed by 6-month to yearly intervals as necessary. However, with the availability of predictive models, follow-up protocols can be customized based on a patient’s risk profile. For low-risk patients, the follow-up period can be extended to minimize unnecessary use of medical resources. One the other hand, high-risk patients may require a more intensive and personalized follow-up protocol to detect any recurrence. Although there is limited data specifically on SPTP, enhanced CT and MRI scans are considered the optimal methods for identifying recurrence. In cases where routine imaging fails to define lesions, PET-CT or PET-MRI scans may be reasonable alternative options (Figure 6). With the increasing use of parenchyma-preserving pancreatectomy in managing SPTP, it is important to consider the possibility of pancreatic endocrine and exocrine insufficiency after traditional pancreatectomy. Therefore, regular follow-up for these patients should include monitoring of blood glucose level, glycated hemoglobin, and quality of life.
SPTP is characterized by distinct molecular and genetic changes, including mutations in CTNNB1, which activates the Wnt-signaling pathway and promotes tumor growth. Although biomarkers, such as beta-catenin, CD10, and CD56 can assist in diagnosis, they are not specific to SPTP. When evaluating pancreatic tumors, particularly in young women, SPTP should be considered in the differential diagnosis. This tumor is typically associated with favorable long-term outcomes, with low rates of recurrence and metastasis. Surgical resection is the preferred approach, even in patients with recurrence and metastasis. Factors such as large tumor size, high Ki-67 index, and lymphovascular invasion may affect RFS, and patients with these risk factors should undergo more frequent follow-ups. Further research is needed to gain a better understanding of the relationships among clinicopathological, molecular, and genetic factors and their impact on prognosis of patients with SPTP.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Okasha H, Egypt S-Editor: Yan JP L-Editor: A P-Editor: Yu HG
| 1. | Chaudhari VA, Pradeep R, Ramesh H, Bhandare MS, Dhar P, Pal S, Palaniswamy S, Jeswanth S, Menon RN, Singh AN, Sabnis S, Rao GV, Shrikhande SV. Surgery for cystic tumors of pancreas: Report of high-volume, multicenter Indian experience over a decade. Surgery. 2019;166:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | You L, Yang F, Fu DL. Prediction of malignancy and adverse outcome of solid pseudopapillary tumor of the pancreas. World J Gastrointest Oncol. 2018;10:184-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 3. | Yang F, Fu DL, Jin C, Long J, Yu XJ, Xu J, Ni QX. Clinical experiences of solid pseudopapillary tumors of the pancreas in China. J Gastroenterol Hepatol. 2008;23:1847-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Frantz V. Tumors of the pancreas. In: Blumberg CW, editors. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology, 1959: 32-33. |
| 5. | Klöppel DG, Solcia DE, Sobin DLH, Longnecker DS, Capella DC. Histological Typing of Tumours of the Exocrine Pancreas. Proceedings of the World Health Organization International Histological Classification of Tumours, 1996. |
| 6. | Naar L, Spanomichou DA, Mastoraki A, Smyrniotis V, Arkadopoulos N. Solid Pseudopapillary Neoplasms of the Pancreas: A Surgical and Genetic Enigma. World J Surg. 2017;41:1871-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Kao CS, Ulbright TM. A Morphologic and Immunohistochemical Comparison of Nuclear β-Catenin Expressing Testicular Sertoli Cell Tumors and Pancreatic Solid Pseudopapillary Neoplasms Supporting Their Continued Separate Classification. Am J Surg Pathol. 2020;44:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Yang F, Wu W, Wang X, Zhang Q, Bao Y, Zhou Z, Jin C, Ji Y, Windsor JA, Lou W, Fu D. Grading Solid Pseudopapillary Tumors of the Pancreas: the Fudan Prognostic Index. Ann Surg Oncol. 2021;28:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Chen J, Zong L, Wang P, Liu Y, Zhang H, Chang X, Lu Z, Li W, Ma Y, Yu S, Chen J. Solid Pseudopapillary Neoplasms of the Pancreas: Clinicopathologic Analysis and a Predictive Model. Mod Pathol. 2023;36:100141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Chen H, Huang Y, Yang N, Yan W, Yang R, Zhang S, Yang P, Li N, Feng Z. Solid-Pseudopapillary Neoplasm of the Pancreas: A 63-Case Analysis of Clinicopathologic and Immunohistochemical Features and Risk Factors of Malignancy. Cancer Manag Res. 2021;13:3335-3343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Guo T, Wang L, Xie P, Zhang Z, Yu Y. Diagnosis and Surgical Treatment and Pathological Findings of Solid Pseudopapillary Tumor of the Pancreas: A Single-Institution Experience. Cancer Manag Res. 2020;12:581-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kumar NAN, Bhandare MS, Chaudhari V, Sasi SP, Shrikhande SV. Analysis of 50 cases of solid pseudopapillary tumor of pancreas: Aggressive surgical resection provides excellent outcomes. Eur J Surg Oncol. 2019;45:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Wang P, Wei J, Wu J, Xu W, Chen Q, Gao W, Jiang K, Miao Y. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas: A single institution experience with 97 cases. Pancreatology. 2018;18:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Antoniou EA, Damaskos C, Garmpis N, Salakos C, Margonis GA, Kontzoglou K, Lahanis S, Spartalis E, Patsouras D, Kykalos S, Garmpi A, Andreatos N, Pawlik TM, Kouraklis G. Solid Pseudopapillary Tumor of the Pancreas: A Single-center Experience and Review of the Literature. In Vivo. 2017;31:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Zou Y, Huang Y, Hong B, Xiang X, Zhou B, Wei S. Comparison of the clinicopathological features of pancreatic solid pseudopapillary neoplasms between males and females: gender does matter. Histol Histopathol. 2020;35:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Wei G, Luo Q, Fang J, Li X, Shi Y, Li Y, Sun L. The Sex Features of Patients With Solid Pseudopapillary Neoplasms of the Pancreas: A Retrospective Study. Front Oncol. 2022;12:844182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Tognarini I, Tonelli F, Nesi G, Martineti V, Galli G, Gozzini A, Colli E, Zonefrati R, Paglierani M, Marini F, Sorace S, Cavalli T, Cavalli L, Tanini A, Brandi ML. In vitro effects of oestrogens, antioestrogens and SERMs on pancreatic solid pseudopapillary neoplasm-derived primary cell culture. Cell Oncol. 2010;32:331-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | McHugh KE, Stelow EB, Harrison GP, Policarpio-Nicolas MLC. The usefulness of lymphoid enhancer-binding factor 1 and androgen receptor in diagnosing solid pseudopapillary neoplasm of the pancreas on cytopathology. Cancer Cytopathol. 2019;127:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Guo M, Luo G, Jin K, Long J, Cheng H, Lu Y, Wang Z, Yang C, Xu J, Ni Q, Yu X, Liu C. Somatic Genetic Variation in Solid Pseudopapillary Tumor of the Pancreas by Whole Exome Sequencing. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 20. | Selenica P, Raj N, Kumar R, Brown DN, Arqués O, Reidy D, Klimstra D, Snuderl M, Serrano J, Palmer HG, Weigelt B, Reis-Filho JS, Scaltriti M. Solid pseudopapillary neoplasms of the pancreas are dependent on the Wnt pathway. Mol Oncol. 2019;13:1684-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 21. | Park M, Kim M, Hwang D, Park M, Kim WK, Kim SK, Shin J, Park ES, Kang CM, Paik YK, Kim H. Characterization of gene expression and activated signaling pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol. 2014;27:580-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 23. | Kubota Y, Kawakami H, Natsuizaka M, Kawakubo K, Marukawa K, Kudo T, Abe Y, Kubo K, Kuwatani M, Hatanaka Y, Mitsuhashi T, Matsuno Y, Sakamoto N. CTNNB1 mutational analysis of solid-pseudopapillary neoplasms of the pancreas using endoscopic ultrasound-guided fine-needle aspiration and next-generation deep sequencing. J Gastroenterol. 2015;50:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Hallas C, Phillipp J, Domanowsky L, Kah B, Tiemann K. BCL9L expression in pancreatic neoplasia with a focus on SPN: a possible explanation for the enigma of the benign neoplasia. BMC Cancer. 2016;16:648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | La Rosa S, Bongiovanni M. Pancreatic Solid Pseudopapillary Neoplasm: Key Pathologic and Genetic Features. Arch Pathol Lab Med. 2020;144:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 27. | Tiemann K, Heitling U, Kosmahl M, Klöppel G. Solid pseudopapillary neoplasms of the pancreas show an interruption of the Wnt-signaling pathway and express gene products of 11q. Mod Pathol. 2007;20:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 29. | Dinarvand P, Wang WL, Roy-Chowdhuri S. Utility of SOX11 for the diagnosis of solid pseudopapillary neoplasm of the pancreas on cytological preparations. Cytopathology. 2022;33:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Manfrin E, Parisi A, Stefanizzi L, D'Onofrio M, Bernardoni L, Crino SF, Pelosi G, Pancione M, Giordano G, Sina S, Remo A. Bcl-10, trypsin and synaptophysin helps recognize acinar cell and mixed acinar neuroendocrine cell carcinoma of the pancreas on both preoperative cytological samples and needle biopsy specimens. Pathol Res Pract. 2021;226:153593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Yang F, Yu X, Bao Y, Du Z, Jin C, Fu D. Prognostic value of Ki-67 in solid pseudopapillary tumor of the pancreas: Huashan experience and systematic review of the literature. Surgery. 2016;159:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 32. | Liu Q, Dai M, Guo J, Wu H, Wang W, Chen G, Hu Y, Han X, Xu Q, Zhang X, Yang S, Zhang Y, Kleeff J, Liao Q, Wu W, Liang Z, Zhang T, Zhao Y. Long-term Survival, Quality of Life, and Molecular Features of the Patients With Solid Pseudopapillary Neoplasm of the Pancreas: A Retrospective Study of 454 Cases. Ann Surg. 2023;278:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Fan Z, Li Y, Yan K, Wu W, Yin S, Yang W, Xing B, Li X, Zhang X. Application of contrast-enhanced ultrasound in the diagnosis of solid pancreatic lesions--a comparison of conventional ultrasound and contrast-enhanced CT. Eur J Radiol. 2013;82:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Xu M, Xie XY, Liu GJ, Xu HX, Xu ZF, Huang GL, Chen PF, Luo J, Lü MD. The application value of contrast-enhanced ultrasound in the differential diagnosis of pancreatic solid-cystic lesions. Eur J Radiol. 2012;81:1432-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Li DL, Li HS, Xu YK, Wang QS, Chen RY, Zhou F. Solid pseudopapillary tumor of the pancreas: clinical features and imaging findings. Clin Imaging. 2018;48:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | De Robertis R, Marchegiani G, Catania M, Ambrosetti MC, Capelli P, Salvia R, D'Onofrio M. Solid Pseudopapillary Neoplasms of the Pancreas: Clinicopathologic and Radiologic Features According to Size. AJR Am J Roentgenol. 2019;213:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Cai J, Liu Y, Wang C, Yin X. Differentiation between solid pseudopapillary tumor and acinar cell carcinoma of the pancreas based on computed-tomography features. Asian J Surg. 2023;46:1587-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Hu S, Zhang H, Wang X, Sun Z, Ge Y, Yan G, Zhao C, Chen K. Asymptomatic vs symptomatic solid pseudopapillary tumors of the pancreas: clinical and MDCT manifestations. Cancer Imaging. 2019;19:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Maimaijiang A, Wang H, Li W, Wang Y. Diagnosis and treatment of solid pseudopapillary neoplasm of the pancreas in children: A report of 18 cases. Front Pediatr. 2022;10:899965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumor of pancreas: emphasis on magnetic resonance imaging findings. World J Gastroenterol. 2007;13:1811-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (11)] |
| 41. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 42. | De Moura DTH, Coronel M, Ribeiro IB, Farias GFA, Choez MA, Rocha R, Toscano MP, De Moura EGH. The importance of endoscopic ultrasound fine-needle aspiration in the diagnosis of solid pseudopapillary tumor of the pancreas: two case reports. J Med Case Rep. 2018;12:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Kuwahara T, Hara K, Mizuno N, Haba S, Okuno N, Kuraishi Y, Fumihara D, Yanaidani T, Ishikawa S, Yasuda T, Yamada M, Onishi S, Yamada K, Tanaka T, Tajika M, Niwa Y, Yamaguchi R, Shimizu Y. Artificial intelligence using deep learning analysis of endoscopic ultrasonography images for the differential diagnosis of pancreatic masses. Endoscopy. 2023;55:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 44. | Banafea O, Mghanga FP, Zhao J, Zhao R, Zhu L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: a meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016;16:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 45. | Takano S, Fukasawa M, Enomoto N. Molecular assessment of endoscopically collected pancreatic juice and duodenal fluid from patients with pancreatic diseases. Dig Endosc. 2023;35:19-32. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 47. | Singhi AD, Nikiforova MN, Fasanella KE, McGrath KM, Pai RK, Ohori NP, Bartholow TL, Brand RE, Chennat JS, Lu X, Papachristou GI, Slivka A, Zeh HJ, Zureikat AH, Lee KK, Tsung A, Mantha GS, Khalid A. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014;20:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Pawlak KM, Tehami N, Maher B, Asif S, Rawal KK, Balaban DV, Tag-Adeen M, Ghalim F, Abbas WA, Ghoneem E, Ragab K, El-Ansary M, Kadir S, Amin S, Siau K, Wiechowska-Kozlowska A, Mönkemüller K, Abdelfatah D, Abdellatef A, Lakhtakia S, Okasha HH. Role of endoscopic ultrasound in the characterization of solid pseudopapillary neoplasm of the pancreas. World J Gastrointest Endosc. 2023;15:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Rothermel LD, Strosberg C, Centeno BA, Malafa MP. Case Report of Isolated Gastric Metastasis of Pancreatic Cancer From a Diagnostic Biopsy: Management of a Rare Oncologic Entity. Cancer Control. 2020;27:1073274820904042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Dahl S, Mortensen MB. Endoscopic ultrasound-guided fine-needle aspiration can lead to nonresectability of pancreatic cancer due to severe biopsy-induced inflammation. Endoscopy. 2008;40 Suppl 2:E96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 51. | Ngamruengphong S, Xu C, Woodward TA, Raimondo M, Stauffer JA, Asbun HJ, Wallace MB. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy. 2013;45:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Khalyfa AA, Kapur U, Ayub K. The role of confocal endomicroscopy for diagnosis of solid pseudopapillary tumor of the pancreas. Endoscopy. 2022;54:E943-E944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 53. | Machicado JD, Napoleon B, Lennon AM, El-Dika S, Pereira SP, Tan D, Pannala R, Girotra M, Kongkam P, Bertani H, Feng Y, Sijie H, Zhong N, Valantin V, Leblanc S, Hinton A, Krishna SG. Accuracy and agreement of a large panel of endosonographers for endomicroscopy-guided virtual biopsy of pancreatic cystic lesions. Pancreatology. 2022;22:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Okuno N, Hara K, Obata M. Novel method of diagnosing solid pseudopapillary neoplasms of the pancreas: Needle-based confocal laser endomicroscopy. Dig Endosc. 2019;31:461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Schick V, Franzius C, Beyna T, Oei ML, Schnekenburger J, Weckesser M, Domschke W, Schober O, Heindel W, Pohle T, Juergens KU. Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions vs endosonography, endoscopic retrograde cholangio-pancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging. 2008;35:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Liu W, Gong W, Zhang J, Ma J, Zhang C. 68Ga-FAPI-04 PET/CT in the Detection of Non-FDG-Avid Solid Pseudopapillary Neoplasm of the Pancreas. Clin Nucl Med. 2023;48:100-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 57. | Dong A, Wang Y, Dong H, Zhang J, Cheng C, Zuo C. FDG PET/CT findings of solid pseudopapillary tumor of the pancreas with CT and MRI correlation. Clin Nucl Med. 2013;38:e118-e124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Cavaliere A, Giraudo C, Zuliani M, Cecchin D, Quaia E. 18F-FDG PET/MR in an Atypical Pediatric Solid Pseudopapillary Pancreatic Tumor. Clin Nucl Med. 2019;44:e522-e523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 59. | Kim JS, Hao EI, Rho SY, Hwang HK, Lee WJ, Yoon DS, Kang CM. Clinical Pattern of Preoperative Positron Emission Tomography/Computed Tomography (PET/CT) Can Predict the Aggressive Behavior of Resected Solid Pseudopapillary Neoplasm of the Pancreas. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Salvia R, Burelli A, Nepi A, Caravati A, Tomelleri C, Dall'Olio T, Casciani F, Crinò SF, Perri G, Marchegiani G. Pancreatic cystic neoplasms: Still high rates of preoperative misdiagnosis in the guidelines and endoscopic ultrasound era. Surgery. 2023;174:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Yang J, Tan CL, Long D, Liang Y, Zhou L, Liu XB, Chen YH. Analysis of invasiveness and tumor-associated macrophages infiltration in solid pseudopapillary tumors of pancreas. World J Gastroenterol. 2022;28:5047-5057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 62. | Liu BA, Li ZM, Su ZS, She XL. Pathological differential diagnosis of solid-pseudopapillary neoplasm and endocrine tumors of the pancreas. World J Gastroenterol. 2010;16:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Tostes FT, de Carvalho PFDC, Araújo RLC, Ribeiro RC, Apodaca-Torrez FR, Lobo EJ, Gomes DBD, Callegaro-Filho D, Schvartsman G, Moura F, Schraibman V, Goldenberg A, de Lima FT, Segatelli V, Uson Junior PLS. Clinical Course, Genetic, and Immunohistochemical Characterization of Solid Pseudopapillary Tumor of the Pancreas (Frantz Tumors) in a Brazilian Cohort. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 64. | Wang Q, Reid MD. Cytopathology of solid pancreatic neoplasms: An algorithmic approach to diagnosis. Cancer Cytopathol. 2022;130:491-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 65. | Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N. E-cadherin/beta-catenin and CD10: a limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol. 2009;132:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Liu F, Li J, Fang X, Meng Y, Zhang H, Yu J, Feng X, Wang L, Jiang H, Lu J, Bian Y, Shao C. Differentiation of Solid Pseudopapillary Tumor and Non-Functional Neuroendocrine Tumors of the Pancreas Based on CT Delayed Imaging: A Propensity Score Analysis. Acad Radiol. 2022;29:350-357. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Klimstra DS, Wenig BM, Adair CF, Heffess CS. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol. 1995;19:1371-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 133] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Montemarano H, Lonergan GJ, Bulas DI, Selby DM. Pancreatoblastoma: imaging findings in 10 patients and review of the literature. Radiology. 2000;214:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Illyés G, Luczay A, Benyó G, Kálmán A, Borka K, Köves K, Rácz K, Tulassay T, Schaff Z. Cushing's syndrome in a child with pancreatic acinar cell carcinoma. Endocr Pathol. 2007;18:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Orditura M, Petrillo A, Ventriglia J, Diana A, Laterza MM, Fabozzi A, Savastano B, Franzese E, Conzo G, Santini L, Ciardiello F, De Vita F. Pancreatic neuroendocrine tumors: Nosography, management and treatment. Int J Surg. 2016;28 Suppl 1:S156-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Fu M, Yu L, Yang L, Chen Y, Chen X, Hu Q, Sun H. Gender differences in pancreatic neuroendocrine neoplasms: A retrospective study based on the population of Hubei Province, China. Front Endocrinol (Lausanne). 2022;13:885895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Lawlor RT, Daprà V, Girolami I, Pea A, Pilati C, Nottegar A, Piccoli P, Parolini C, Sperandio N, Capelli P, Scarpa A, Luchini C. CD200 expression is a feature of solid pseudopapillary neoplasms of the pancreas. Virchows Arch. 2019;474:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Jahangir S, Loya A, Siddiqui MT, Akhter N, Yusuf MA. Accuracy of diagnosis of solid pseudopapillary tumor of the pancreas on fine needle aspiration: A multi-institution experience of ten cases. Cytojournal. 2015;12:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Shen Y, Wang Z, Zhu J, Chen Y, Gu W, Liu Q. α-Methylacyl-CoA racemase (P504S) is a useful marker for the differential diagnosis of solid pseudopapillary neoplasm of the pancreas. Ann Diagn Pathol. 2014;18:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Jiang Y, Xie J, Wang B, Mu Y, Liu P. TFE3 is a diagnostic marker for solid pseudopapillary neoplasms of the pancreas. Hum Pathol. 2018;81:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Guo Y, Yuan F, Deng H, Wang HF, Jin XL, Xiao JC. Paranuclear dot-like immunostaining for CD99: a unique staining pattern for diagnosing solid-pseudopapillary neoplasm of the pancreas. Am J Surg Pathol. 2011;35:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | El-Bahrawy MA, Rowan A, Horncastle D, Tomlinson I, Theis BA, Russell RC, Stamp G. E-cadherin/catenin complex status in solid pseudopapillary tumor of the pancreas. Am J Surg Pathol. 2008;32:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | La Rosa S, Franzi F, Marchet S, Finzi G, Clerici M, Vigetti D, Chiaravalli AM, Sessa F, Capella C. The monoclonal anti-BCL10 antibody (clone 331.1) is a sensitive and specific marker of pancreatic acinar cell carcinoma and pancreatic metaplasia. Virchows Arch. 2009;454:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Zou C, Yang F, Wu W, Fu D. Ki-67 and malignancy in solid pseudopapillary tumor of the pancreas: A systematic review and meta-analysis. Pancreatology. 2020;20:683-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Hao EIU, Hwang HK, Yoon DS, Lee WJ, Kang CM. Aggressiveness of solid pseudopapillary neoplasm of the pancreas: A literature review and meta-analysis. Medicine (Baltimore). 2018;97:e13147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 82. | Kim MJ, Choi DW, Choi SH, Heo JS, Sung JY. Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br J Surg. 2014;101:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 83. | Bosman FTCF, Hruban RH. WHO classification of tumours of the digestive system, 4th ed. Lyon: International Agency for Research on Cancer, 2010. |
| 84. | Lokuhetty DWV, Watanabe R, Cree I. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer, 2018. |
| 85. | Fleming AM, Hendrick LE, Yakoub D, Abdelhafeez H, Deneve JL, Langham MR Jr, Glazer ES, Davidoff AM, Merchant NB, Dickson PV, Murphy AJ. Malignant Solid Pseudopapillary Neoplasm of the Pancreas: An Orthogonal Analysis. Ann Surg Oncol. 2024;31:475-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 86. | Yang F, Jin C, Fu D. Evolution of liver metastasis from solid pseudopapillary tumor of the pancreas. Surgery. 2017;161:1739-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Yang F, Jin C, Long J, Yu XJ, Xu J, Di Y, Li J, Fu de L, Ni QX. Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg. 2009;198:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Zou C, Yang F, Fu D. Meta-analysis of Ki-67 expression for recurrence in patients with solid pseudopapillary tumor of the pancreas. HPB (Oxford). 2020;22:631-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M, Segersvärd R; European Study Group on Cystic Tumours of the Pancreas. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 90. | Standring O, Benitez Sanchez S, Pasha S, Demyan L, Lad N, Ruff SM, Anantha S, Karpeh M, Newman E, Nealon W, Talamini M, Coppa G, Deutsch G, Weiss M, DePeralta DK. Potential Role for Observation in Small Solid Pseudopapillary Neoplasm (SPN). Ann Surg Oncol. 2023;30:5105-5112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 91. | Coelho JCU, da Costa MAR, Ramos EJB, Torres AR, Savio MC, Claus CMP. Surgical Management of Solid Pseudopapillary Tumor of the Pancreas. JSLS. 2018;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Khoury T, Sbeit W, Napoléon B. Endoscopic ultrasound guided radiofrequency ablation for pancreatic tumors: A critical review focusing on safety, efficacy and controversies. World J Gastroenterol. 2023;29:157-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 93. | Choi JH, Seo DW, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 94. | Coupier A, Khoury T, Gincul R, Fumex F, Lisotti A, Leblanc S, Napoléon B. Endoscopic ultrasound-guided radiofrequency ablation for solid pseudopapillary neoplasm of the pancreas. Endoscopy. 2023;55:E951-E952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 95. | Jutric Z, Rozenfeld Y, Grendar J, Hammill CW, Cassera MA, Newell PH, Hansen PD, Wolf RF. Analysis of 340 Patients with Solid Pseudopapillary Tumors of the Pancreas: A Closer Look at Patients with Metastatic Disease. Ann Surg Oncol. 2017;24:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Shyr BS, Wang SE, Chen SC, Shyr YM, Shyr BU. Pancreatic head sparing surgery for solid pseudopapillary tumor in patients with agenesis of the dorsal pancreas. J Chin Med Assoc. 2022;85:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Huang TT, Zhu J, Zhou H, Zhao AM. Solid pseudopapillary neoplasm of pancreas in pregnancy treated with tumor enucleation: Case report and review of the literature. Niger J Clin Pract. 2018;21:1234-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 98. | Hao CY, Lu AP, Xing BC, Huang XF, Gao F, Ji JF. Solid pseudopapillary tumor of the pancreas: report of 8 cases in a single institution and review of the Chinese literature. Pancreatology. 2006;6:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Gao Y, Guo F, Lu Z, Xi C, Wei J, Jiang K, Miao Y, Wu J, Chen J. Perioperative safety and prognosis following parenchyma-preserving surgery for solid pseudopapillary neoplasm of the pancreas. World J Surg Oncol. 2023;21:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 100. | Li YQ, Pan SB, Yan SS, Jin ZD, Huang HJ, Sun LQ. Impact of parenchyma-preserving surgical methods on treating patients with solid pseudopapillary neoplasms: A retrospective study with a large sample size. World J Gastrointest Surg. 2022;14:174-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Tjaden C, Hassenpflug M, Hinz U, Klaiber U, Klauss M, Büchler MW, Hackert T. Outcome and prognosis after pancreatectomy in patients with solid pseudopapillary neoplasms. Pancreatology. 2019;19:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 102. | Cho YJ, Namgoong JM, Kim DY, Kim SC, Kwon HH. Suggested Indications for Enucleation of Solid Pseudopapillary Neoplasms in Pediatric Patients. Front Pediatr. 2019;7:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Wang X, Chen YH, Tan CL, Zhang H, Xiong JJ, Chen HY, Ke NW, Liu XB. Enucleation of pancreatic solid pseudopapillary neoplasm: Short-term and long-term outcomes from a 7-year large single-center experience. Eur J Surg Oncol. 2018;44:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Chen XM, Zhang Y, Sun DL. Laparoscopic central pancreatectomy for solid pseudopapillary tumors of the pancreas: our experience with ten cases. World J Surg Oncol. 2014;12:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Zhang W, Qiu J, Bian W, Sun D, Shi Y, Qin L, Xue X. Clinical characteristics, surgical strategies, and outcome of solid pseudopapillary tumor of the pancreas: retrospective analysis in a single center. Wideochir Inne Tech Maloinwazyjne. 2022;17:163-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 106. | Jin JB, Qin K, Yang Y, Shi YS, Wu ZC, Deng XX, Chen H, Cheng DF, Shen BY, Peng CH. Robotic pancreatectomy for solid pseudopapillary tumors in the pancreatic head: A propensity score-matched comparison and analysis from a single center. Asian J Surg. 2020;43:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Dokmak S, Aussilhou B, Paci M, Ftériche FS, Cros J, Maire F, Soubrane O, Sauvanet A. Extended Laparoscopic Central Pancreatectomy with Clamping of the Mesentericoportal Vein and Resection of the Splenic Vessels for a Large Solid Pseudopapillary Tumor. Ann Surg Oncol. 2019;26:3709-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 108. | Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male vs female patients. Surgery. 2008;143:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 109. | Kodama R, Koh Y, Midorikawa H, Yokota Y, Saegusa H, Ushimaru H. A case of recurrence of a solid pseudopapillary neoplasm of the pancreas effectively treated with proton beam radiotherapy. Clin J Gastroenterol. 2021;14:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Wang WB, Zhang TP, Sun MQ, Peng Z, Chen G, Zhao YP. Solid pseudopapillary tumor of the pancreas with liver metastasis: Clinical features and management. Eur J Surg Oncol. 2014;40:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Dixon M, Cannon J, Kagedan D, Rowsell C, Milot L, Ko YJ, Coburn N. Management of Metastatic Solid Pseudopapillary Cancer of the Pancreas: A Case Report. World J Oncol. 2013;4:201-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 112. | Yoon HJ, Lim JH. Solid pseudopapillary tumor of the pancreas with hepatic metastasis: spontaneous regression over 10-year follow-up period. Korean J Radiol. 2012;13:648-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Li JX, Wu H, Huang JW, Prasoon P, Zeng Y. Synchronous intraoperative radiofrequency ablation for multiple liver metastasis and resection of giant solid pseudopapillary tumors of the pancreas. Chin Med J (Engl). 2012;125:1661-1663. [PubMed] |
| 114. | Sumida W, Kaneko K, Tainaka T, Ono Y, Kiuchi T, Ando H. Liver transplantation for multiple liver metastases from solid pseudopapillary tumor of the pancreas. J Pediatr Surg. 2007;42:e27-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Hassan I, Celik I, Nies C, Zielke A, Gerdes B, Moll R, Ramaswamy A, Wagner HJ, Bartsch DK. Successful treatment of solid-pseudopapillary tumor of the pancreas with multiple liver metastases. Pancreatology. 2005;5:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Hofmann H, von Haken R, Werner J, Kortes N, Bergmann F, Schemmer P, Jäger D, Radeleff B, Schulze-Bergkamen H. Unresectable isolated hepatic metastases from solid pseudopapillary neoplasm of the pancreas: a case report of chemosaturation with high-dose melphalan. Pancreatology. 2014;14:546-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Maffuz A, Bustamante Fde T, Silva JA, Torres-Vargas S. Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas. Lancet Oncol. 2005;6:185-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 118. | Honore C, Goere D, Dartigues P, Burtin P, Dumont F, Elias D. Peritoneal carcinomatosis from solid pseudopapillary neoplasm (Frantz's tumour) of the pancreas treated with HIPEC. Anticancer Res. 2012;32:1069-1073. [PubMed] |
| 119. | Wang X, Zhu D, Bao W, Li M, Wang S, Shen R. Case Report: Targeted Therapy for Metastatic Solid Pseudopapillary Neoplasm of the Pancreas With CTNNB1 and PTEN Mutations. Front Oncol. 2021;11:729151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Jorgensen MS, Velez-Velez LM, Asbun H, Colon-Otero G. Everolimus Is Effective Against Metastatic Solid Pseudopapillary Neoplasm of the Pancreas: A Case Report and Literature Review. JCO Precis Oncol. 2019;3:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 121. | Cuglievan B, Subbiah V, Wang H, Morani A, Meric-Bernstam F, Holla V, Herzog CE. Response to Mammalian Target of Rapamycin-Based Therapy and Incidental Finding of Lynch Syndrome in a Patient With Solid Pseudopapillary Neoplasm of the Pancreas With AKT1_E17K Mutation. JCO Precis Oncol. 2018;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 122. | Kornietskaya A, Evdokimova S, Kachmazov A, Fedenko A, Bolotina L, Sidorov D, Volchenko N, Goeva N, Govaleshko A, Kaprin A. Endocrine therapy for metastatic solid pseudopapillary neoplasm of the pancreas: A case report. Front Oncol. 2022;12:970142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 123. | Mazzarella G, Muttillo EM, Coletta D, Picardi B, Rossi S, Rossi Del Monte S, Gomes V, Muttillo IA. Solid pseudopapillary tumor of the pancreas: A systematic review of clinical, surgical and oncological characteristics of 1384 patients underwent pancreatic surgery. Hepatobiliary Pancreat Dis Int. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 124. | Song H, Dong M, Zhou J, Sheng W, Zhong B, Gao W. Solid Pseudopapillary Neoplasm of the Pancreas: Clinicopathologic Feature, Risk Factors of Malignancy, and Survival Analysis of 53 Cases from a Single Center. Biomed Res Int. 2017;2017:5465261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 125. | Irtan S, Galmiche-Rolland L, Elie C, Orbach D, Sauvanet A, Elias D, Guérin F, Coze C, Faure-Conter C, Becmeur F, Demarche M, Galifer RB, Galloy MA, Podevin G, Aubert D, Piolat C, De Lagausie P, Sarnacki S. Recurrence of Solid Pseudopapillary Neoplasms of the Pancreas: Results of a Nationwide Study of Risk Factors and Treatment Modalities. Pediatr Blood Cancer. 2016;63:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 126. | Butte JM, Brennan MF, Gönen M, Tang LH, D'Angelica MI, Fong Y, Dematteo RP, Jarnagin WR, Allen PJ. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011;15:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 127. | Lee SE, Jang JY, Hwang DW, Park KW, Kim SW. Clinical features and outcome of solid pseudopapillary neoplasm: differences between adults and children. Arch Surg. 2008;143:1218-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 128. | Yang F, Bao Y, Zhou Z, Jin C, Fu D. Preoperative neutrophil-to-lymphocyte ratio predicts malignancy and recurrence-free survival of solid pseudopapillary tumor of the pancreas. J Surg Oncol. 2019;120:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Yang F, Fu D. ASO Author Reflections: A Novel Classification System to Predict Outcome in Solid Pseudopapillary Tumor of the Pancreas. Ann Surg Oncol. 2020;27:757-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |