Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.1046
Peer-review started: December 21, 2023
First decision: January 13, 2024
Revised: January 14, 2024
Accepted: February 4, 2024
Article in press: February 4, 2024
Published online: March 15, 2024
Processing time: 82 Days and 7 Hours
Gastric cancer (GC) is the fifth most commonly diagnosed malignancy worldwide, with over 1 million new cases per year, and the third leading cause of cancer-related death.
To determine the optimal perioperative treatment regimen for patients with locally resectable GC.
A comprehensive literature search was conducted, focusing on phase II/III randomized controlled trials (RCTs) assessing perioperative chemotherapy and chemoradiotherapy in treating locally resectable GC. The R0 resection rate, overall survival (OS), disease-free survival (DFS), and incidence of grade 3 or higher nonsurgical severe adverse events (SAEs) associated with various perioperative regimens were analyzed. A Bayesian network meta-analysis was performed to compare treatment regimens and rank their efficacy.
Thirty RCTs involving 8346 patients were included in this study. Neoadjuvant XELOX plus neoadjuvant radiotherapy and neoadjuvant CF were found to significantly improve the R0 resection rate compared with surgery alone, and the former had the highest probability of being the most effective option in this context. Neoadjuvant plus adjuvant FLOT was associated with the highest probability of being the best regimen for improving OS. Owing to limited data, no definitive ranking could be determined for DFS. Considering nonsurgical SAEs, FLO has emerged as the safest treatment regimen.
This study provides valuable insights for clinicians when selecting perioperative treatment regimens for patients with locally resectable GC. Further studies are required to validate these findings.
Core Tip: This study provides an update of the literature on perioperative therapy for locally resectable gastric cancer (GC) as of April 21, 2023. This study aimed to provide a multidimensional approach to perioperative treatment regimens for resectable GC using Bayesian network meta-analysis.
- Citation: Kuang ZY, Sun QH, Cao LC, Ma XY, Wang JX, Liu KX, Li J. Efficacy and safety of perioperative therapy for locally resectable gastric cancer: A network meta-analysis of randomized clinical trials. World J Gastrointest Oncol 2024; 16(3): 1046-1058
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/1046.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.1046
Gastric cancer (GC) is the fifth most commonly diagnosed malignancy worldwide, with over 1 million new cases per year, and is the third leading cause of cancer-related deaths[1]. It is highly prevalent in Asia, South America, Southern Africa, and Eastern Europe[2]. The incidence of GC is associated with various factors, with Helicobacter pylori infection being the most significant[3]. Other factors include dietary habits, smoking, heavy alcohol consumption, age, and genetic predisposition[4,5]. Gastroesophageal reflux disease is also linked to gastric-esophageal junction cancers[6]. Although the global incidence of GC has declined due to improved living conditions and early screening[2], the number of new cases and deaths remains significant, likely due to population growth and aging[7].
Surgical or endoscopic resection remains the only curative treatment for GC[8], especially in patients with resectable GC without distant metastases[9]. However, even after radical resection, the prognosis for node-positive patients remains poor, with a five-year survival rate of < 50%[8]. Consequently, the management of GC has shifted from a singular surgical approach to a multidisciplinary approach. Several clinical trials such as MAGIC[10], FNCLCC and FFCD[11], and FLOT[12] have established the therapeutic value of perioperative chemotherapy for locally resectable GC. Perioperative chemotherapy improves the survival of patients with GC of stage IB or higher[13]. However, guidelines such as the National Comprehensive Cancer Network[1], European Society for Medical Oncology (ESMO)[14], and Chinese Society of Clinical Oncology[14] offer varying recommendations regarding the choice of perioperative chemotherapy regimens for GC, leading to confusion among clinicians. Although perioperative radiotherapy has been shown to improve overall survival (OS) in patients with GC[15,16], its role in the treatment of resectable GC remains controversial[17].
Network meta-analysis (NMA) is an extension of traditional meta-analysis[18] that overcomes some of the limitations of pairwise meta-analysis by enabling indirect comparisons of multiple interventions and the sequencing of individual interventions[19]. Accordingly, it facilitates clinicians' decision-making regarding chemotherapy regimens[20]. This study aimed to conduct a systematic search for randomized controlled trials (RCTs) involving resectable GC treated with perioperative chemotherapy and/or radiotherapy and rank them based on R0 resection rate, OS, disease-free survival (DFS), and safety using Bayesian NMA. The ultimate goal of this study was to identify an optimal treatment regimen and provide valuable clinical guidance.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement[21] (Supplementary Table 1) and was registered in the International Prospective Register of Systematic Reviews (CRD420
PubMed, Embase, and the Cochrane Library were searched from their inception to April 21, 2023, without language restrictions, using the terms Stomach, Gastric, Cancer, Tumor, Neoplasm, Carcinoma, Neoadjuvant, Preoperative, Perioperative, Adjuvant, Chemoradiotherapy, Radiotherapy, Chemotherapy, and Random. The search was conducted by Kuang ZY, Sun QH, and Cao LC, and any disagreements were resolved through discussions with three other authors (Ma XY, Wang JX, and Liu KX). All articles were screened using Endnote 20, and the search details are provided in Supplementary material.
Studies meeting the following criteria were included: (1) Type: Phase II or III RCTs, with or without blinding; (2) Participants: Participants with locally resectable GC and gastroesophageal junctions according to the eighth edition of the tumor–node–metastasis (TNM) classification issued by the International Union against Cancer were included if they met the criteria of stage IB-III or cT2-4NanyM0 and had not received treatment before joining the clinical trial. Pathologically, the tumor was an adenocarcinoma. No sex-related limitations were observed in this study; (3) Interventions: Neoadjuvant chemotherapy and/or radiotherapy combined with postoperative adjuvant chemotherapy and/or radiotherapy, neoadjuvant chemotherapy and/or radiotherapy, and adjuvant chemotherapy and/or radiotherapy. There were no restrictions on specific regimens, and the surgical approach involved D2 Lymph node dissection based on the patient’s condition; and (4) Outcomes: At least one of the following clinical outcomes should be reported: R0 resection rate, OS, DFS, incidence of non-surgical grade 3 or higher nonsurgical severe adverse events (SAEs).
Studies meeting the following criteria were excluded: (1) Multiple cancer; (2) Studies involving targeted immunotherapy and alternative therapies; (3) Studies lacking detailed information on treatment regimens; and (4) Studies that were reported repeatedly, lacked full-text availability, or had unavailable data.
We documented literature information, including the first author, year of publication, demographic data, and interventions. Data extraction for outcomes, such as the R0 excision rate, OS, DFS, and nonsurgical SAEs, was performed independently by two authors (Wang JX and Liu KX), and Kuang ZY was involved in cases of disagreement. For articles lacking survival data but providing survival curves, we used Engauge Digitizer software to extract the hazard ratio (HR) value and 95% confidence interval (95%CI) from the survival curve, as described by Tierney et al[22].
We assessed the risk of bias using Review Manager (5.4.1) following the guidelines provided in the Cochrane Handbook[23]. In the case of disputes, the assessment was carried out independently by two authors (Wang JX and Liu KX) and a third author (Kuang ZY).
The primary outcome of this review was OS, whereas the secondary outcomes were R0 resection rate, DFS, and non-surgical SAEs. The study was divided into two phases. For the R0 resection rate, we compared studies related to neoadjuvant treatment regimens, while the outcome measures, OS, DFS, and non-surgical SAEs, were analyzed in studies involving neoadjuvant therapy, surgery, and postoperative adjuvant treatment regimens simultaneously. We assessed the risk ratio (RR) and 95%CI for dichotomous outcomes (R0 excision rate and non-surgical SAE) and converted the HR and 95%CI to lnHR and selnHR for outcomes such as OS and DFS.
We assessed the heterogeneity between studies using the Q-test and I2 statistics. Unless I2 exceeded 50% and the P value was less than 0.05, a fixed-effects model was employed. Intervention network diagrams were generated using Stata 15.0, and the mapping of the dichotomous variable surface under the cumulative ranking (SUCRA) was conducted under a Bayesian framework using the "GeMTC" software package in R 4.3.0. A model convergence diagnosis, heterogeneity testing, and consistency testing were performed. For outcomes for which NMA was not feasible, pairwise direct comparisons were performed using the Review Manager software. Publication bias was assessed by plotting funnels and Egger's test.
There are three ways to assess convergence in an NMA. The trajectory graph depicts the fluctuation of the Markov Monte Carlo chain during iterative calculations. If the chains demonstrated stable fusion and substantial overlap, the convergence was considered satisfactory. The density map compares the distribution patterns of the posterior values with a preset distribution; a smaller bandwidth value indicates a closer match. The Brooks-Gelman-Rubin diagnosis plot combines graphical evaluation and quantitative analysis using the potential scale reduction factor (PSRF), with a value closer to 1 indicating satisfactory convergence.
SUCRA is an indicator of the cumulative ranking probability. A SUCRA value of 1 signifies absolute effectiveness, whereas a value of 0 indicates complete ineffectiveness. Interventions can be ranked according to their effectiveness based on SUCRA values.
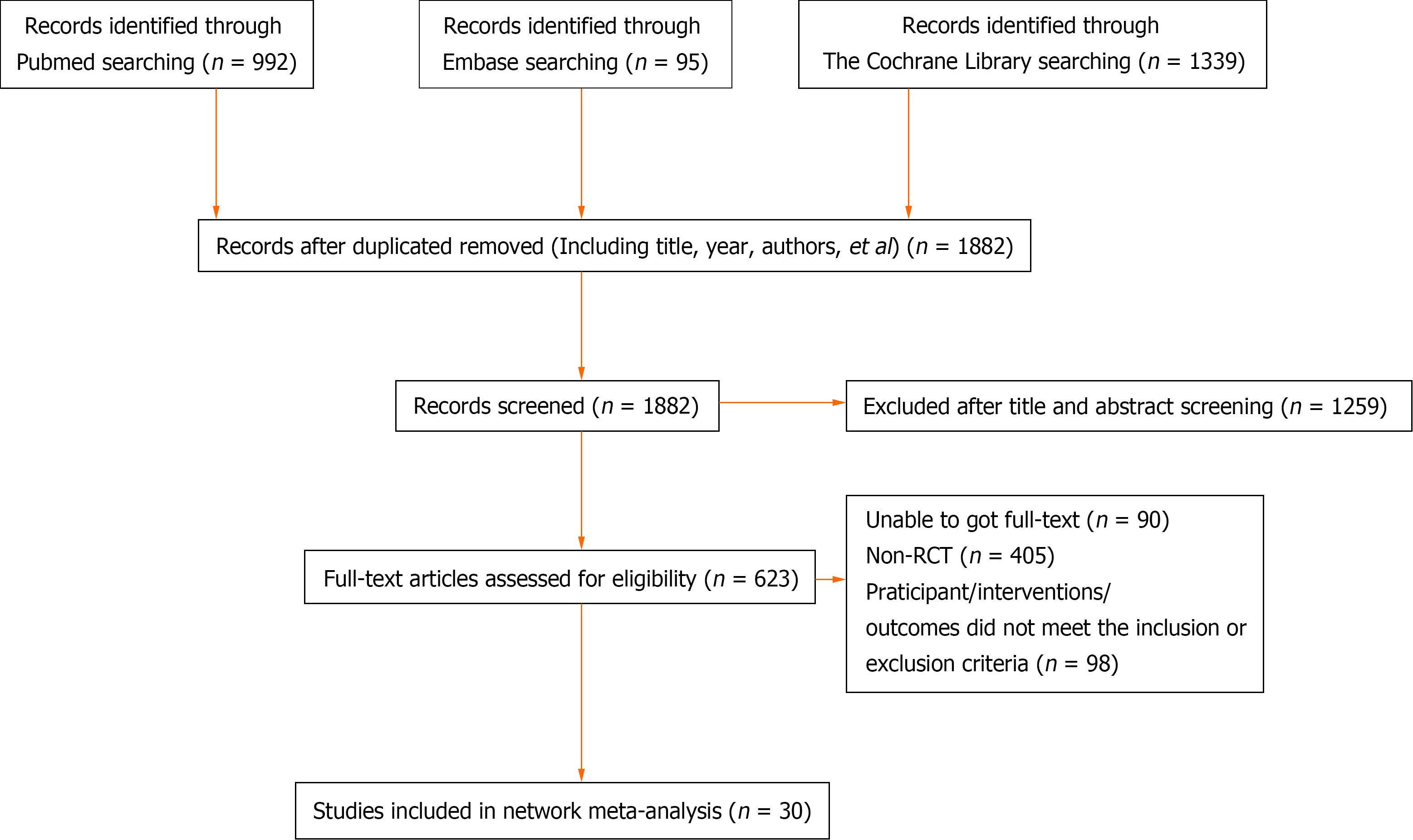
A total of 2426 articles were initially retrieved. Among them, 544 duplicate articles were identified and manually removed. Additionally, 1259 non-clinical studies, including reviews, systematic reviews, and protocols, and 593 articles that did not meet the inclusion criteria were excluded. As a result, a total of 30 RCTs were included in the analysis[10-12,24-50] (Figure 1 and Supplementary Table 2).
The characteristics of the 30 RCTs are summarized in Supplementary Table 3. The bias risk assessment of these studies is presented in Supplementary Figures 1 and 2.
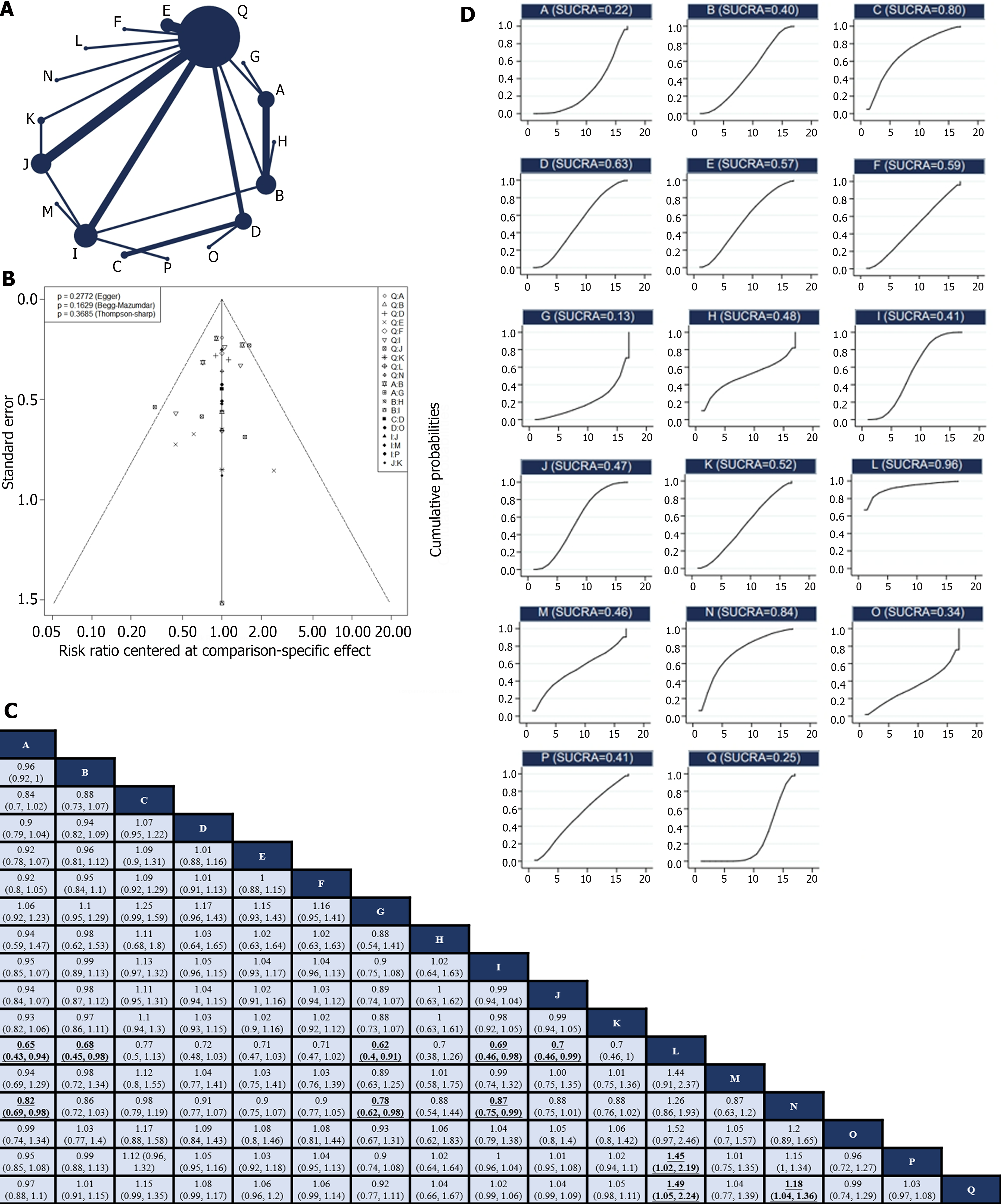
Of the 30 RCTs, 28[10-12,24-29,31-44,46-50] reported the R0 resection rate. Among them, there were 17 direct or indirect comparisons between the preoperative neoadjuvant regimens (Figure 2A). Some control groups where surgery was performed directly without neoadjuvant therapy were considered as the “surgery alone” group. Global inconsistency detection yielded an I2 value of 34%. Accordingly, a fixed-effects model was used for effect size pooling. The trace plot, density plot, and Brooks-Gelman-Rubin diagnosis plot showed good convergence (Supplementary Figures 3 and 4), and the PSRF was 1, further indicating good convergence. Local inconsistencies were found between neoadjuvant SOX vs neoadjuvant FLOT, and neoadjuvant SOX vs surgery alone (Supplementary Figure 5). The Funnel plot indicated no evidence of publication bias (P = 0.2772; Figure 2B).
Pairwise comparisons between treatments showed that neoadjuvant XELOX plus neoadjuvant radiotherapy (RR: 1.49; 95%CI: 1.05–2.24) and neoadjuvant CF (RR: 1.18; 95%CI: 1.04–1.36) significantly improved the R0 resection rate compared with surgery alone. However, the remaining neoadjuvant regimens failed to improve the R0 resection rates. In addition, neoadjuvant ECF (RR: 0.65; 95%CI: 0.43–0.94), neoadjuvant FLOT (RR: 0.68; 95%CI: 0.45–0.98), neoadjuvant ECF plus neoadjuvant radiotherapy (RR: 0.62; 95%CI: 0.4–0.91), neoadjuvant SOX (RR: 0.69; 95%CI: 0.46–0.98), and neoadjuvant XELOX (RR: 0.7; 95%CI: 0.46–0.99) exhibited lower R0 resection rates compared to neoadjuvant XELOX plus neoadjuvant radiotherapy. Neoadjuvant ECF (RR: 0.82; 95%CI: 0.69–0.98), neoadjuvant ECF plus neoadjuvant radiotherapy (RR: 0.78; 95%CI: 0.62–0.98), and neoadjuvant SOX (RR: 0.87; 95%CI: 0.75–0.99) had inferior R0 resection rates compared to neoadjuvant CF. Notably, the R0 excision rate of neoadjuvant XELOX plus neoadjuvant radiotherapy was higher than that of neoadjuvant FOLFOX (RR: 1,45; 95%CI: 1.02–2.19; Figure 2C). Neoadjuvant XELOX combined with neoadjuvant radiotherapy resulted in the highest SUCRA value (0.96; Figure 2D). Taken together, neoadjuvant XELOX plus neoadjuvant radiotherapy appear to be the most effective neoadjuvant regimen.
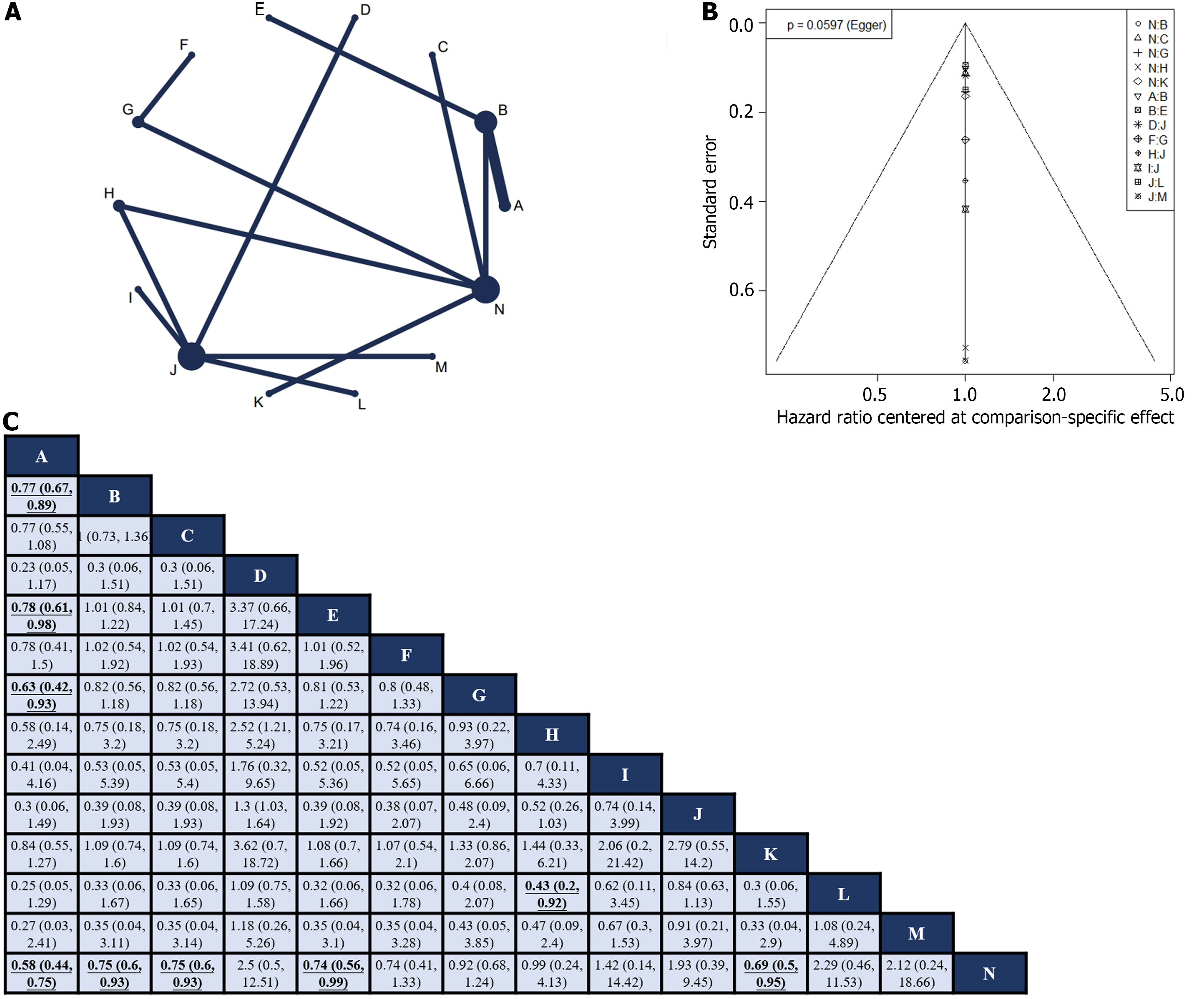
Fourteen RCTs[10-12,24-28,31,41,42,45,47,50] reported HR values for OS with corresponding 95%CIs for 14 interventions (Figure 3A). Global inconsistency detection yielded an I2 value of 0%. Accordingly, the effect size was pooled using a fixed effects model. Convergence was confirmed by the trace plot, density plot, and Brooks-Gelman-Rubin diagnosis plot (Supplementary Figures 6 and 7), with a PSRF of 1, indicating good convergence. No local inconsistencies were detected in any study (Supplementary Figure 8). The Funnel plot showed no evidence of a publication bias (Figure 3B).
Pairwise comparisons of treatments revealed that neoadjuvant plus adjuvant FLOT (HR: 0.58; 95%CI: 0.44–0.75), neoadjuvant plus adjuvant ECF (HR: 0.75; 95%CI: 0.6–0.93), neoadjuvant plus adjuvant DCF (HR: 0.75; 95%CI: 0.6–0.93), neoadjuvant ECF plus adjuvant ECF and radiotherapy (HR: 0.74; 95%CI: 0.56–0.99), and neoadjuvant plus adjuvant CF (HR: 0.69; 95%CI: 0.5–0.95) significantly improved OS compared to surgery alone. In addition, neoadjuvant plus adjuvant FLOT outperformed neoadjuvant plus adjuvant ECF (HR: 0.77; 95%CI: 0.67–0.89), neoadjuvant ECF plus adjuvant ECF and radiotherapy (HR: 0.78; 95%CI: 0.61–0.98), and neoadjuvant CS plus adjuvant S-1 (HR: 0.63; 95%CI: 0.42–0.93) in terms of OS. Furthermore, neoadjuvant plus adjuvant XELOX showed superior OS compared with neoadjuvant plus adjuvant FOLFOX (HR: 0.43; 95%CI: 0.2–0.92). No statistically significant differences were observed in other intervention comparisons (Figure 3C). The neoadjuvant plus adjuvant FLOT group had the highest SUCRA value (0.91). Therefore, neoadjuvant plus adjuvant FLOT is likely to offer the best OS outcome (Table 1).
| Rank | Intervention | SUCRA value |
| 1 | Neoadjuvant plus adjuvant FLOT | 0.91 |
| 2 | Neoadjuvant plus adjuvant CF | 0.74 |
| 3 | Neoadjuvant DCS plus adjuvant S-1 | 0.67 |
| 4 | Neoadjuvant ECF plus adjuvant ECF and radiotherapy | 0.67 |
| 5 | Neoadjuvant plus adjuvant DCF | 0.66 |
| 6 | Neoadjuvant plus adjuvant ECF | 0.65 |
| 7 | Neoadjuvant plus adjuvant XELOX | 0.57 |
| 8 | Neoadjuvant SOX and radiotherapy plus adjuvant SOX | 0.46 |
| 9 | Neoadjuvant CS plus adjuvant S-1 | 0.46 |
| 10 | Surgery alone | 0.37 |
| 11 | Neoadjuvant plus adjuvant SOX | 0.28 |
| 12 | Adjuvant SOX | 0.27 |
| 13 | Neoadjuvant plus adjuvant FOLFOX | 0.17 |
| 14 | Adjuvant XELOX | 0.11 |
Six RCTs[11,12,25-27,50] reported the HR values and 95%CIs for DFS. Due to the limited number of included studies, only direct comparisons were conducted (Table 2). Neoadjuvant plus adjuvant FLOT demonstrated superior DFS compared to neoadjuvant plus adjuvant ECF (HR: 0.75; 95%CI: 0.65–0.86). Neoadjuvant plus adjuvant CF outperformed surgery alone (HR: 0.69; 95%CI: 0.50–0.95). However, there was no statistically significant difference between Neoadjuvant plus adjuvant XELOX and surgery alone (HR: 0.96; 95%CI: 0.25–3.66). In addition, no significant difference was observed between the neoadjuvant plus adjuvant SOX and adjuvant SOX alone groups (HR: 1.28; 95%CI: 0.33–4.93). Neoadjuvant plus adjuvant SOX outperformed adjuvant XELOX (HR: 0.77; 95%CI: 0.61–0.97).
| Intervention 1 | Intervention 2 | Study number | I2 | P value | HR/95%CI |
| Neoadjuvant plus adjuvant FLOT | Neoadjuvant plus adjuvant ECF | 2 | 0 | 1.00 | 0.75 (0.65, 0.86) |
| Neoadjuvant plus adjuvant CF | Surgery alone | 1 | - | - | 0.69 (0.50, 0.95) |
| Neoadjuvant plus adjuvant XELOX | Surgery alone | 1 | - | - | 0.96 (0.25, 3.66) |
| Neoadjuvant plus Adjuvant SOX | Adjuvant SOX | 1 | - | - | 1.28 (0.33, 4.93) |
| Neoadjuvant plus adjuvant SOX | Adjuvant XELOX | 1 | - | - | 0.77 (0.61, 0.97) |
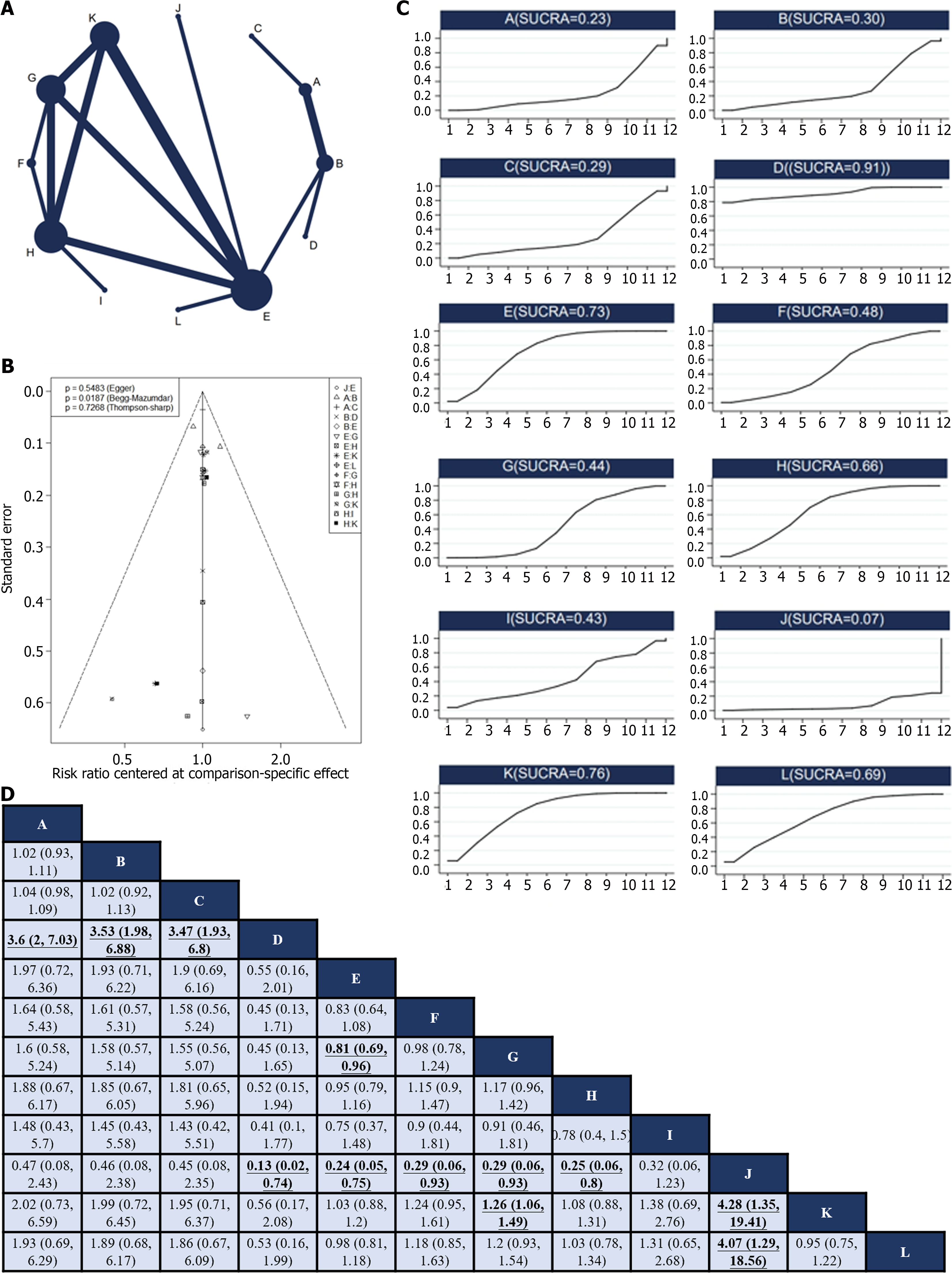
Twelve RCTs[12,24,27,28,30-33,37,38,45,49] reported 12 treatments for nonsurgical SAEs (Figure 4A). Global inconsistency detection yielded an I2 value of 6%. Accordingly, the effect size was pooled using a fixed effects model. Convergence was confirmed by the trace plot, density plot, and Brooks-Gelman-Rubin diagnosis plot (Supplementary Figures 9 and 10), with a PSRF of 1, suggesting good convergence, and no local inconsistencies were detected (Supplementary Figure 11). The Funnel plot indicated no evidence of a publication bias (P = 0.5483; Figure 4B).
Pairwise comparisons of interventions showed that neoadjuvant chemotherapy plus adjuvant ECF (RR: 3.6; 95%CI: 2–7.03), neoadjuvant chemotherapy plus adjuvant FLOT (RR: 3.53; 95%CI: 1.98–6.88), and neoadjuvant chemotherapy plus adjuvant ECF and radiotherapy (RR: 3.47; 95%CI: 1.93–6.8) were associated with a higher occurrence of non-surgical SAEs than neoadjuvant chemotherapy plus adjuvant FLO. Conversely, neoadjuvant plus adjuvant FLO (RR: 0.13; 95%CI: 0.02–0.74), neoadjuvant plus adjuvant SOX (RR: 0.24; 95%CI: 0.05–0.75), neoadjuvant DOX plus adjuvant SOX (RR: 0.29; 95%CI: 0.06–0.93), neoadjuvant plus adjuvant XELOX (RR: 0.29; 95%CI: 0.06–0.93), and adjuvant XELOX (RR: 0.25; 95%CI: 0.06–0.8) were associated with fewer non-surgical SAEs during treatment compared to neoadjuvant SOX and radiotherapy plus adjuvant SOX. Neoadjuvant plus adjuvant SOX had fewer non-surgical SAEs compared to neoadjuvant plus adjuvant XELOX (RR: 0.81; 95%CI: 0.69–0.96). Neoadjuvant plus adjuvant XELOX had more non-surgical SAEs compared to adjuvant SOX (RR: 1.26; 95%CI: 1.06–1.49). Neoadjuvant SOX and radiotherapy plus adjuvant SOX had a higher occurrence of non-surgical SAEs compared to adjuvant SOX (RR: 4.28; 95%CI: 1.35–19.41) and neoadjuvant plus adjuvant FOLFOX (RR: 4.07; 95%CI: 1.29–18.56; Figure 4C). The SUCRA value of the neoadjuvant plus adjuvant FLO regimen was the highest (0.91), indicating that this regimen had the lowest probability of nonsurgical SAEs. Conversely, the neoadjuvant SOX and radiotherapy plus adjuvant SOX regimens (SUCRA, 0.06) were associated with the highest probability of nonsurgical SAEs (Figure 4D).
We evaluated the R0 resection rate, OS, DFS, and nonsurgical SAEs using the GRADE assessment tool, and the results indicated that all four outcomes were assessed as low-quality evidence (Supplementary Table 4).
Advancements in biological science have deepened our understanding of GC characteristics[51,52]. Numerous biomar
These results indicate that only the neoadjuvant XELOX plus neoadjuvant radiotherapy and neoadjuvant CF regimens effectively improved the R0 resection rate. However, this result was inconsistent with those of some of the included studies. For example, Zhao et al[25] reported that neoadjuvant XELOX increased the R0 resection rate (P = 0.04) compared to surgery alone, but indirect comparisons in NMA showed no significant difference. Similarly, Al-Batran et al[12] found that preoperative FLOT chemotherapy was superior to preoperative ECF in terms of R0 resection rate (P = 0.0162), whereas indirect comparisons showed no significant difference. Based on the SUCRA values, we inferred that neoadjuvant XELOX plus neoadjuvant radiotherapy might be the most effective regimen for improving the R0 resection rate, supporting its short-term efficacy. However, there is insufficient data available to determine the long-term survival benefits. Moreover, recommendations for preoperative chemotherapy combined with radiotherapy for locally resectable GC remain unclear among various guidelines. Therefore, caution should be exercised when interpreting these results.
Neoadjuvant FLOT plus adjuvant FLOT showed the highest probability of being the most effective regimen for OS, which is consistent with the ESMO guidelines. FLOT is currently the mainstream three-drug perioperative chemotherapy regimen used in Europe and has been shown to effectively prolong OS and DFS[12,58]. However, its impact on the R0 resection rate appears to be minimal and requires further investigation. Interestingly, neoadjuvant therapy plus adjuvant SOX did not show a survival benefit compared to surgery alone. The SOX regimen is widely used as a perioperative chemotherapy regimen for GC in Asia, and several phase III clinical trials conducted in Asia have established its role in locally resectable GC[27,59]. However, the results of this study suggest that perioperative SOX regimens may not confer a survival benefit compared to surgery alone. This discrepancy could be attributed to the limited number of available studies and the uncertainties associated with indirect comparisons. Further clinical studies involving direct comparisons are required to validate these findings.
Unfortunately, we could not rank the regimens based on DFS because of insufficient data. Only direct head-to-head comparisons were made between the regimens, and further clinical studies are required to gain a better understanding. Therefore, the safety of this regimen is crucial, particularly in the context of radical GC resection. This study suggests that FLO may be the safest perioperative treatment option, whereas neoadjuvant SOX and radiotherapy plus adjuvant SOX may be associated with a higher risk of adverse effects, presumably owing to the increased toxicity of this combination.
This study has several limitations. First, most of the included studies were open-label studies, which may have introduced some degree of bias into the conclusions. Second, there is ongoing controversy regarding the classification of malignant tumors[60]. Although classified as a distinct type of malignant tumor, gastroesophageal junction tumors are often combined with gastric or esophageal cancers in clinical studies. However, their unique pathological characteristics require caution when combined with general oncological principles[61]. Another limitation of this study was the limited number of direct comparisons between interventions, with most comparisons being indirect. Then, SUCRA values have limitations and do not necessarily imply statistical differences, so caution is needed when interpreting intervention rankings based on SUCRA values. Finally, caution must be exercised when applying findings from Eastern countries to Western countries and vice versa, as the biology of patients with GC may vary from country to country.
In this study, perioperative chemoradiotherapy regimens for locally resectable GC were analyzed and ranked using a Bayesian NMA. Our findings may guide clinicians in selecting appropriate treatment regimens. However, it is important to consider the limitations of this study and exercise caution when interpreting its conclusions. Future RCTs with rigorous designs and large sample sizes are needed to validate these findings. Given the advancements in targeted therapy and immunotherapy, it would be valuable to further explore the potential survival benefits of combining basic chemotherapy with targeted therapies and immunotherapy for locally resectable GC in future research.
Gastric cancer (GC) is the fifth most commonly diagnosed malignancy worldwide, with over 1 million new cases per year, and the third leading cause of cancer-related death.
To conduct a systematic search for randomized controlled trials (RCTs) involving resectable GC with perioperative chemotherapy and/or radiotherapy and rank them based on R0 resection rate, overall survival (OS), disease-free survival (DFS), and safety using Bayesian NMA. The ultimate goal was to identify the optimal treatment regimen and provide valuable clinical guidance.
To determine the optimal perioperative treatment regimen for locally resectable GC.
A comprehensive literature search was conducted focusing on phase II/III RCTs assessing perioperative chemotherapy and chemoradiotherapy in locally resectable GC. The R0 resection rate, OS, DFS, and incidence of grade 3 or non-surgical grade 3 or higher nonsurgical severe adverse events (SAEs) associated with various perioperative regimens were analyzed. Bayesian network meta-analysis was performed to compare the treatment regimens and rank their efficacy.
A total of 30 RCTs involving 8346 patients were included in this study. Neoadjuvant XELOX plus neoadjuvant radiotherapy and neoadjuvant CF were found to significantly improve the R0 resection rate compared to surgery alone, and the former had the highest probability of being the most effective option in this context. Neoadjuvant plus adjuvant FLOT was associated with the highest probability of being the best regimen for OS. Due to limited data, no definitive ranking could be determined for DFS. Considering non-surgical SAEs, FLO emerged as the safest regimen.
A total of 30 RCTs involving 8346 patients were included in this study. Neoadjuvant XELOX plus neoadjuvant radiotherapy and neoadjuvant CF were found to significantly improve the R0 resection rate compared to surgery alone, and the former had the highest probability of being the most effective option in this context. Neoadjuvant plus adjuvant FLOT was associated with the highest probability of being the best regimen for OS. Due to limited data, no definitive ranking could be determined for DFS. Considering non-surgical SAEs, FLO emerged as the safest regimen.
Our findings may provide some guidance to clinicians in selecting the appropriate treatment regimens. However, it is important to consider the limitations of this study and exercise caution when interpreting its conclusions. Future RCTs with rigorous designs and large sample sizes are needed to validate the findings. Given the advancements in targeted therapy and immunotherapy, it would be valuable to further explore the potential survival benefits of combining basic chemotherapy with targeted therapies and immunotherapy for locally resectable GC in future research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vorobjova T, Estonia S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 941] [Article Influence: 313.7] [Reference Citation Analysis (0)] |
| 2. | GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 431] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 3. | Yang L, Kartsonaki C, Millwood IY, Chen Z. Helicobacter pylori infection and risk of gastric cancer - Authors' reply. Lancet Public Health. 2022;7:e303. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac J Cancer Prev. 2018;19:591-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 161] [Reference Citation Analysis (0)] |
| 5. | Hatakeyama M. Malignant Helicobacter pylori-Associated Diseases: Gastric Cancer and MALT Lymphoma. Advances in Experimental Medicine and Biology. 2019;1149:135-149. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Velanovich V, Hollingsworth J, Suresh P, Ben-Menachem T. Relationship of gastroesophageal reflux disease with adenocarcinoma of the distal esophagus and cardia. Dig Surg. 2002;19:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Hayashi M, Abe M, Fujita T, Matsushita H. Prognostic effect of categorized tumor deposits in gastric cancer: A single-center retrospective study. Surgery. 2024;175:373-379. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14 Suppl 2:ii31-ii36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Xiong HQ, Gunderson LL, Yao J, Ajani JA. Chemoradiation for resectable gastric cancer. Lancet Oncol. 2003;4:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4587] [Article Influence: 241.4] [Reference Citation Analysis (0)] |
| 11. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1494] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 12. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1625] [Article Influence: 270.8] [Reference Citation Analysis (0)] |
| 13. | Bouvier AM, Créhange G, Azélie C, Cheynel N, Jouve JL, Bedenne L, Faivre J, Lepage C, Maingon P. Adjuvant treatments for gastric cancer: from practice guidelines to clinical practice. Dig Liver Dis. 2014;46:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 456] [Article Influence: 114.0] [Reference Citation Analysis (1)] |
| 15. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM, Stemmermann GN, Blanke CD, Macdonald JS. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 626] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 16. | Zhou ML, Yang W, Wang YQ, Mo M, Hu R, Wang Y, Yang JN, Li GC, Wang YN, Zhang Z. Adjuvant chemoradiotherapy versus adjuvant chemotherapy for patients with N3 gastric cancer after D2/R0 resection: a retrospective study based on propensity score analyses. Cancer Manag Res. 2019;11:4855-4870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK; ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. 2021;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 18. | Lee A. The development of network meta-analysis. J R Soc Med. 2022;115:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Tian J, Gao Y, Zhang J, Yang Z, Dong S, Zhang T, Sun F, Wu S, Wu J, Wang J, Yao L, Ge L, Li L, Shi C, Wang Q, Li J, Zhao Y, Xiao Y, Yang F, Fan J, Bao S, Song F. Progress and challenges of network meta-analysis. J Evid Based Med. 2021;14:218-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Antoniou SA, Koelemay M, Antoniou GA, Mavridis D. A Practical Guide for Application of Network Meta-Analysis in Evidence Synthesis. Eur J Vasc Endovasc Surg. 2019;58:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22118] [Cited by in RCA: 18202] [Article Influence: 2600.3] [Reference Citation Analysis (1)] |
| 22. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4941] [Article Influence: 274.5] [Reference Citation Analysis (0)] |
| 23. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2871] [Article Influence: 478.5] [Reference Citation Analysis (0)] |
| 24. | Zhao Q, Lian C, Huo Z, Li M, Liu Y, Fan L, Tan B, Zhao X, Zhang Z, Wang D, Guo H, Yang P, Tian Y, Li Y. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial. Cancer Med. 2020;9:5731-5745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Zhao Q, Li Y, Tan BB, Tian Y, Jiao ZK, Zhao XF, Zhang ZD, Wang D, Yang PG. [Effects of XELOX regimen as neoadjuvant chemotherapy on radical resection rate and prognosis in patients with advanced gastric cancer]. Zhonghua Zhong Liu Za Zhi. 2013;35:773-777. [PubMed] |
| 26. | Zhao Q, Li Y, Huang J, Fan L, Tan B, Tian Y, Yang P, Jiao Z, Zhao X, Zhang Z, Wang D, Liu Y. Short-term curative effect of S-1 plus oxaliplatin as perioperative chemotherapy for locally advanced gastric cancer: a prospective comparison study. Pharmazie. 2017;72:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, Wang X, Wu A, Li G, Su X, Xiao G, Cui M, Wu D, Wu X, Zhou Y, Zhang L, Dang C, He Y, Zhang Z, Sun Y, Li Y, Chen H, Bai Y, Qi C, Yu P, Zhu G, Suo J, Jia B, Li L, Huang C, Li F, Ye Y, Xu H, Yuan Y, E JY, Ying X, Yao C, Shen L, Ji J; RESOLVE study group. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 255] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 28. | Yu J, Gao Y, Chen L, Wu D, Shen Q, Zhao Z, Liu W, Yang H, Zhang Q, Wang X, Hu P, Zheng Z, Liu H, Xu Z, Yan Z, Wu Y, Jin M, Liu X, Zhu K, Shou C. Effect of S-1 Plus Oxaliplatin Compared With Fluorouracil, Leucovorin Plus Oxaliplatin as Perioperative Chemotherapy for Locally Advanced, Resectable Gastric Cancer: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e220426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, Hirabayashi N, Mikata S, Iwahashi M, Fukushima R, Takiguchi N, Miyashiro I, Morita S, Miyashita Y, Tsuburaya A, Sakamoto J. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS trial. Ann Surg Oncol. 2014;21:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Xue K, Ying X, Bu Z, Wu A, Li Z, Tang L, Zhang L, Zhang Y, Ji J. Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II-III randomized trial. Chin J Cancer Res. 2018;30:516-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Wang X, Zhao DB, Yang L, Chi Y, Zhao H, Jiang LM, Jiang J, Tang Y, Li N, Liu WY, Dou LZ, Zou SM, Xue LY, Ren JS, Tian YT, Che X, Guo CG, Bai XF, Sun YM, Wang SL, Song YW, Liu YP, Fang H, Li YX, Jin J. Preoperative Concurrent Chemoradiotherapy Versus Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: Phase II Randomized Study. Front Oncol. 2022;12:870741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Wang F, Qu A, Sun Y, Zhang J, Wei B, Cui Y, Liu X, Tian W, Li Y. Neoadjuvant chemoradiotherapy plus postoperative adjuvant XELOX chemotherapy versus postoperative adjuvant chemotherapy with XELOX regimen for local advanced gastric cancer-A randomized, controlled study. Br J Radiol. 2021;94:20201088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Tian Y, Zhao Q, Li Y, Fan L, Zhang Z, Zhao X, Tan B, Wang D, Yang P. Efficacy of Neoadjuvant Chemotherapy DOX and XELOX Regimens for Patients with Resectable Gastric or Gastroesophageal Junction Adenocarcinoma. Gastroenterol Res Pract. 2021;2021:5590626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito Y, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Boku N, Sano T, Sasako M; Stomach Cancer Study Group, Japan Clinical Oncology Group. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. 2019;22:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Sun XC, Lin J, Ju AH. Treatment of Borrmann type IV gastric cancer with a neoadjuvant chemotherapy combination of docetaxel, cisplatin and 5-fluorouracil/leucovorin. J Int Med Res. 2011;39:2096-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Sun G, Wang S, Liu G. Preoperative neoadjuvant chemotherapy on surgical condition and oncogene expression in advanced gastric cancer. Pak J Med Sci. 2020;36:485-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Sah BK, Zhang B, Zhang H, Li J, Yuan F, Ma T, Shi M, Xu W, Zhu Z, Liu W, Yan C, Li C, Liu B, Yan M. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun. 2020;11:6093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 38. | Lorenzen S, Pauligk C, Homann N, Schmalenberg H, Jäger E, Al-Batran SE. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br J Cancer. 2013;108:519-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Leong T, Smithers BM, Haustermans K, Michael M, Gebski V, Miller D, Zalcberg J, Boussioutas A, Findlay M, O'Connell RL, Verghis J, Willis D, Kron T, Crain M, Murray WK, Lordick F, Swallow C, Darling G, Simes J, Wong R. TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017;24:2252-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 40. | Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, Ryu MH, Rha SY, Chung IJ, Kim IH, Oh SC, Park YS, Son T, Jung MR, Heo MH, Kim HK, Park C, Yoo CH, Choi JH, Zang DY, Jang YJ, Sul JY, Kim JG, Kim BS, Beom SH, Cho SH, Ryu SW, Kook MC, Ryoo BY, Yoo MW, Lee NS, Lee SH, Kim G, Lee Y, Lee JH, Noh SH. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol. 2021;39:2903-2913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 41. | Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. 2021;24:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 42. | Hayashi T, Yoshikawa T, Sakamaki K, Nishikawa K, Fujitani K, Tanabe K, Misawa K, Matsui T, Miki A, Nemoto H, Fukunaga T, Kimura Y, Hihara J. Primary results of a randomized two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of cisplatin/S-1 and docetaxel/cisplatin/S-1 as neoadjuvant chemotherapy for advanced gastric cancer. Ann Gastroenterol Surg. 2020;4:540-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Hashemzadeh S, Pourzand A, Somi MH, Zarrintan S, Javad-Rashid R, Esfahani A. The effects of neoadjuvant chemotherapy on resectability of locally-advanced gastric adenocarcinoma: a clinical trial. Int J Surg. 2014;12:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Fazio N, Biffi R, Maibach R, Hayoz S, Thierstein S, Brauchli P, Bernhard J, Stupp R, Andreoni B, Renne G, Crosta C, Morant R, Chiappa A, Luca F, Zampino MG, Huber O, Goldhirsch A, de Braud F, Roth AD; Swiss Group for Clinical Cancer Research SAKK; European Institute of Oncology, Milan, Italy. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann Oncol. 2016;27:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, Meershoek-Klein Kranenbarg E, Boot H, Trip AK, Swellengrebel HAM, van Laarhoven HWM, Putter H, van Sandick JW, van Berge Henegouwen MI, Hartgrink HH, van Tinteren H, van de Velde CJH, Verheij M; CRITICS investigators. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 46. | Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F, Bonomo G, Crosta C, Huber O. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol. 2010;16:868-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 47. | Basi A, Sohrabkhani S, Zamani F, Baghai-Wadji M, Rabiei N, Razavi SM, Ajdarkosh H. Comparing Efficacy of Preoperative neo-Adjuvant Chemotherapy and Surgery versus Surgery Alone in Patients with Resectable Gastroesophageal Cancer. Int J Hematol Oncol Stem Cell Res. 2013;7:24-28. [PubMed] |
| 48. | Aoyama T, Nishikawa K, Fujitani K, Tanabe K, Ito S, Matsui T, Miki A, Nemoto H, Sakamaki K, Fukunaga T, Kimura Y, Hirabayashi N, Yoshikawa T. Early results of a randomized two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of cisplatin/S-1 and docetaxel/cisplatin/S-1 as neoadjuvant chemotherapy for locally advanced gastric cancer. Ann Oncol. 2017;28:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, Probst S, Koenigsmann M, Egger M, Prasnikar N, Caca K, Trojan J, Martens UM, Block A, Fischbach W, Mahlberg R, Clemens M, Illerhaus G, Zirlik K, Behringer DM, Schmiegel W, Pohl M, Heike M, Ronellenfitsch U, Schuler M, Bechstein WO, Königsrainer A, Gaiser T, Schirmacher P, Hozaeel W, Reichart A, Goetze TO, Sievert M, Jäger E, Mönig S, Tannapfel A. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 507] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 50. | Adenis A, Samalin E, Mazard T, Portales F, Mourregot A, Ychou M. [Does the FLOT regimen a new standard of perioperative chemotherapy for localized gastric cancer?]. Bull Cancer. 2020;107:54-60. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Blum A, Wang P, Zenklusen JC. SnapShot: TCGA-Analyzed Tumors. Cell. 2018;173:530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 448] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 52. | Zeng D, Wu J, Luo H, Li Y, Xiao J, Peng J, Ye Z, Zhou R, Yu Y, Wang G, Huang N, Rong X, Sun L, Sun H, Qiu W, Xue Y, Bin J, Liao Y, Li N, Shi M, Kim KM, Liao W. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 53. | Huynh J, Patel K, Gong J, Cho M, Malla M, Parikh A, Klempner S. Immunotherapy in Gastroesophageal Cancers: Current Evidence and Ongoing Trials. Curr Treat Options Oncol. 2021;22:100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1070] [Article Influence: 267.5] [Reference Citation Analysis (0)] |
| 55. | Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 56. | Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 697] [Article Influence: 139.4] [Reference Citation Analysis (0)] |
| 57. | Wang Y, Zhang L, Yang Y, Lu S, Chen H. Progress of Gastric Cancer Surgery in the era of Precision Medicine. Int J Biol Sci. 2021;17:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 58. | Dos Santos M, Lequesne J, Leconte A, Corbinais S, Parzy A, Guilloit JM, Varatharajah S, Brachet PE, Dorbeau M, Vaur D, Weiswald LB, Poulain L, Le Gallic C, Castera-Tellier M, Galais MP, Clarisse B. Perioperative treatment in resectable gastric cancer with spartalizumab in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT): a phase II study (GASPAR). BMC Cancer. 2022;22:537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 59. | Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 397] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 60. | Chen L, Liu FL. [Dilemmas in definition and classification of adenocarcinoma of esophagogastric junction: from history to current status]. Zhonghua Wai Ke Za Zhi. 2022;60:813-818. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Chevallay M, Bollschweiler E, Chandramohan SM, Schmidt T, Koch O, Demanzoni G, Mönig S, Allum W. Cancer of the gastroesophageal junction: a diagnosis, classification, and management review. Ann N Y Acad Sci. 2018;1434:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |