Published online Feb 15, 2024. doi: 10.4251/wjgo.v16.i2.563
Peer-review started: November 7, 2023
First decision: December 4, 2023
Revised: December 9, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: February 15, 2024
Processing time: 86 Days and 19.9 Hours
Early adenocarcinoma mixed with a neuroendocrine carcinoma (NEC) component arising in the gastroesophageal junctional (GEJ) region is rare and even rarer in young patients. Here, we report such a case in a 29-year-old Chinese man.
This patient presented to our hospital with a 3-mo history of dysphagia and regurgitation. Upper endoscopy revealed an elevated nodule in the distal esophagus 1.6 cm above the GEJ line, without Barrett’s esophagus or involvement of the gastric cardia. The nodule was completely resected by endoscopic submu
Early adenocarcinoma with an NEC component arising in the distal esophageal side of the GEJ region showed evidence of gastric origin.
Core Tip: We report a 29-year-old man with a 1.5-cm intramucosal adenocarcinoma with a neuroendocrine carcinoma component, which arose in the columnar-lined esophagus within 1.6 cm above the gastroesophageal junction (GEJ), without Barrett’s esophagus. Next-generation sequencing revealed a novel germline mutation of the ERCC3 gene in the DNA repair pathway of gastric cancer and a germline mutation of the RNF43 gene as a tumor suppressor in gastric cancer. Our findings suggest this early-onset GEJ carcinoma originated from gastric cardiac mucosa with genetic abnormalities involved in the DNA repair pathway.
- Citation: Cheng YQ, Wang GF, Zhou XL, Lin M, Zhang XW, Huang Q. Early adenocarcinoma mixed with a neuroendocrine carcinoma component arising in the gastroesophageal junction: A case report. World J Gastrointest Oncol 2024; 16(2): 563-570
- URL: https://www.wjgnet.com/1948-5204/full/v16/i2/563.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i2.563
Esophageal carcinoma is the eighth most frequently occurring cancer and the sixth leading cause of cancer-related deaths worldwide[1]. In China, esophageal carcinoma remains a major health risk, ranking the fifth in cancer incidence and the fourth in cancer mortality[1]. However, the histological type of esophageal carcinoma varies geographically. For instance, esophageal adenocarcinoma (EAC) is predominant in North America and Europe, where the incidence increases steadily[2-4]. In China, however, esophageal squamous cell carcinoma remains the most prevalent[1], whereas EAC accounts for only 1%-4% of esophageal carcinomas[5-7], among which, < 1% occur in patients aged < 40 years[8]. In Chinese patients, most gastroesophageal junctional (GEJ) carcinomas represent the proximal extension of gastric cardiac cancers, unlike those in the patients from western countries[9], where GEJ adenocarcinoma is largely classified as part of EAC. In this case report, we analyzed the histopathological and genetic characteristics of an adenocarcinoma mixed with a neuroendocrine carcinoma (NEC) component arising on the distal esophageal side of the GEJ in a 29-year-old man.
A previously healthy 29-year-old man complained of dysphagia and gastroesophageal reflux symptoms for 3 mo.
A previously healthy 29-year-old man complained of dysphagia and gastroesophageal reflux symptoms for 3 mo.
The patient denied any specific personal past medical history. He denied alcohol or tobacco abuse. He had no history of Barrett’s esophagus.
The patient denied a family history of cancer.
All vital signs were stable and physical examination revealed no notable abnormalities. His body mass index was 20.22 kg/m2.
Preoperative serum tumor markers, such as a-fetoprotein, neuron-specific enolase, carcinoembryonic antigen, carbo
Endoscopic ultrasonography demonstrated a hypoechoic nodule without the sign of submucosal invasion. Neither distant metastasis nor lymph node involvement was seen on the thoracoabdominal enhanced computed tomography.
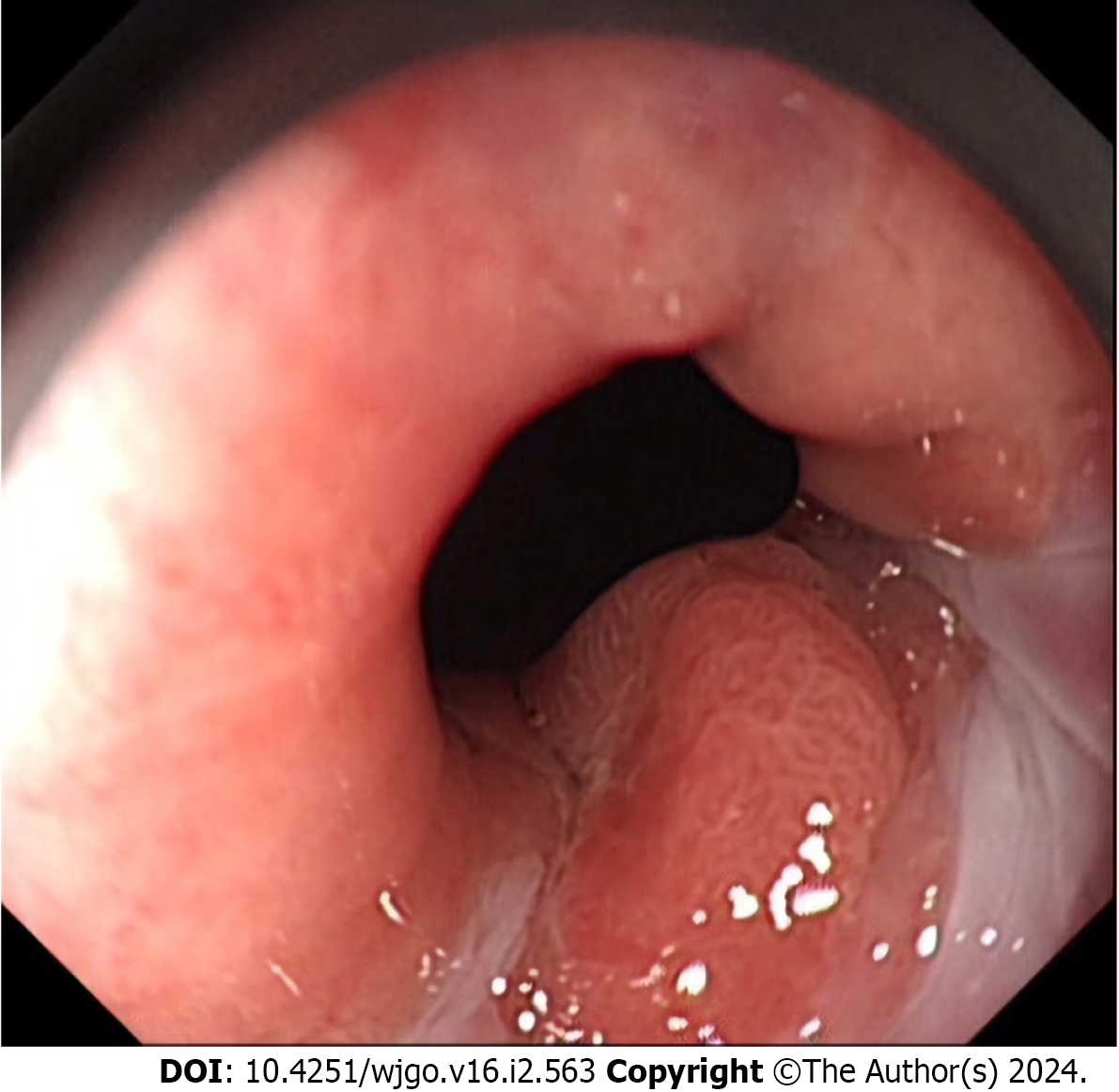
Esophagogastroduodenoscopy in our hospital revealed an elevated small nodular lesion on the esophageal side of the GEJ line without involvement of the proximal gastric cardia under white-light endoscopy (Figure 1). The lesion was surrounded by a short stretch of salmon-red columnar metaplastic esophageal mucosa at a maximum distance of 1.6 cm above the GEJ line. The rest of the esophageal mucosa was unremarkable. No evidence of Barrett’s esophagus in the distal esophagus was noted. Preoperative narrow-band imaging endoscopy showed dilatation and irregular meandering of blood vessels in the lesion along with a demarcation line separating the neoplastic and non-neoplastic mucosae.
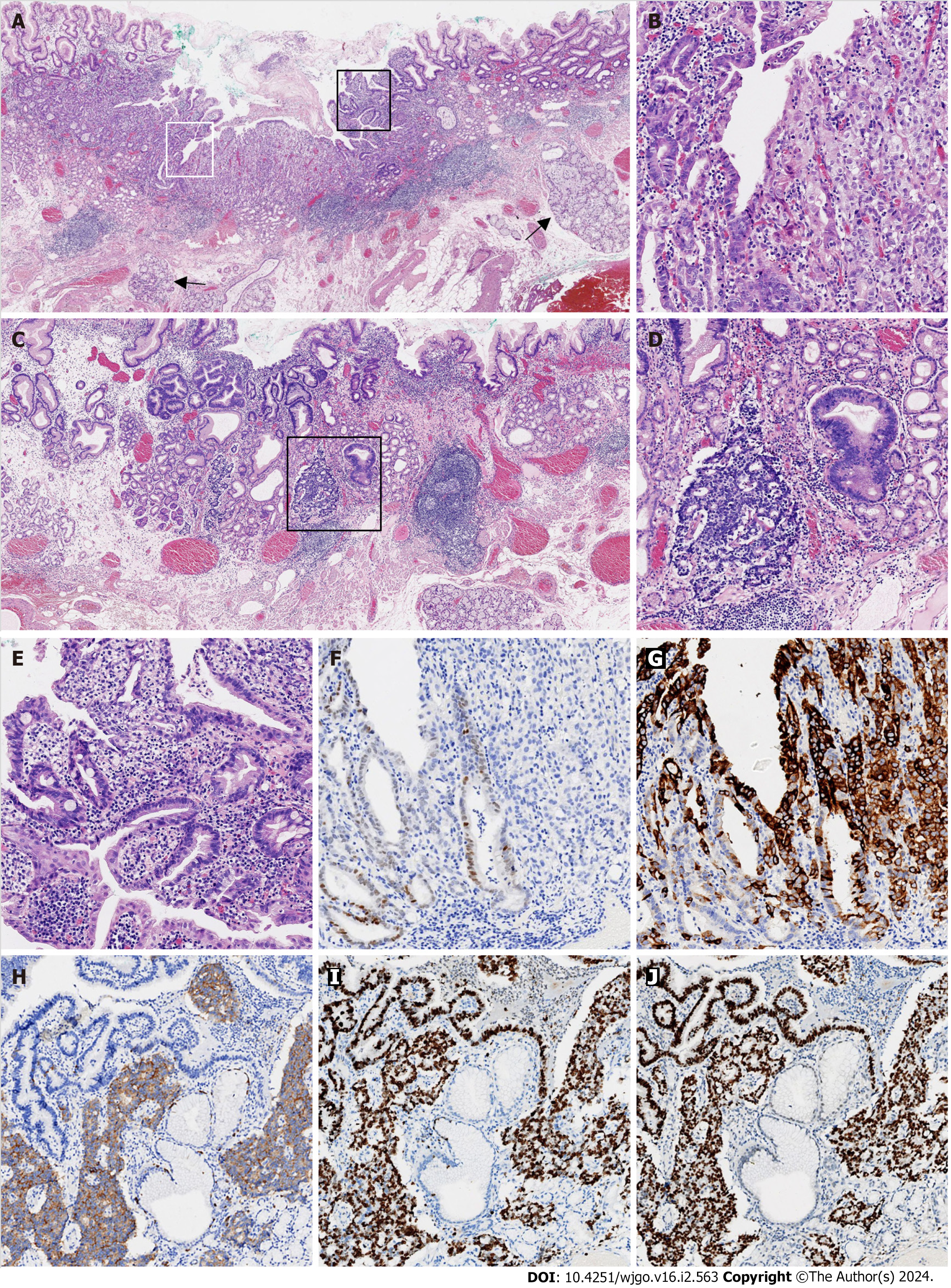
A biopsy of the lesion was performed and diagnosed histopathologically as high-grade glandular dysplasia. After preoperative routine workups, this lesion was completely resected by endoscopic submucosal dissection (ESD). In the ESD resected specimen, a type 0-IIa + IIc lesion measured 11 mm 6 mm grossly. Microscopically, the entire tumor arose in the columnar-lined esophagus (15 mm maximum length), which was confirmed by the presence of submucosal esophageal glands (Figure 2A). The tumor was poorly differentiated in most parts, with only a minor component of moderately differentiated adenocarcinoma (Figure 2B). A small portion of the tumor < 0.1 cm exhibited nuclear features of neuroendocrine differentiation (Figure 2C), including sheets and nests of smaller cells with hyperchromatic nuclei, powdery chromatin patterns, and scant cytoplasm (Figure 2D). No necrosis was noted. The mitotic rate was 4/high-power field. A few dysplastic glands with goblet cells were identified in the adjacent benign metaplastic mucosa (Figure 2E). In the distal part of the specimen, benign gastric cardiac mucosa with a maximum length of 4 mm showed mild chronic inflammation, but no evidence of atrophy or intestinal metaplasia. By routine immunohistochemistry, caudal type homeobox 2 was weakly positive for moderately differentiated adenocarcinoma, but negative for the poorly differentiated adenocarcinoma component (Figure 2F). In contrast, mucin 5AC was diffusely positive for poorly differentiated adenocarcinoma, but only focally positive for moderately differentiated adenocarcinoma (Figure 2G). Chromogranin and synaptophysin were diffusely positive for the cells with neuroendocrine features, but negative for adenocarcinoma (Figure 2H). The Ki-67 proliferative index was approximately 90% for both adenocarcinoma and NEC components (Figure 2I). p53 immunostaining was strongly positive in both components (Figure 2J). The tumor retained nuclear expression of MLH1, PMS2, MSH2, and MSH6. The carcinoma was HER2-negative with a score of 1+. The tumor invaded only muscularis mucosae without the evidence of submucosal, gastric cardiac, lymphovascular, and perineural invasion. Both the lateral and vertical margins of resection were free of dysplasia and carcinoma.
Tumor genetic analysis by next-generation sequencing (NGS) was performed using a 196-gene panel (Genenseeq Technology, 196-cancer-relevant-gene, Nanjing, China) on the Illumina MiSeq platform. NGS data analysis identified 12 genetic mutations; two of which were germline mutations. The novel germline mutation at ERCC3 c.2333A>G was identified, which is in the DNA repair pathway of gastric cancer. The scores of the SIFT and PolyPhen-2 analyses were 0.426 and 0.034, respectively, and predicted the impact of the amino acid substitution of lysine as tolerated and benign. Another germline mutation at RNF43 c.434G>A was also identified. In somatic mutations, pathogenic mutations in TP53 and CHEK2 genes were discovered with allele frequencies of 10.43% and 1.38%, respectively. Other somatic mutations in FANCD2, PALB2, ROS1, PIK3CA, RAD51B, PTEN and BRCA2 genes were found with allele frequencies < 3% (Table 1).
| Gene name | Mutation type | Reference allele | Observed allele | cDNA changed | Amino acid change | Variant type | Exon | Clinical significance | Allele frequency (%) |
| ERCC3 | Germline | T | C | c.2333A>G | p.K778R | Missense | 15 | Uncertain significance | 50.56 |
| RNF43 | Germline | C | T | c.434G>A | p.R145Q | Missense | 4 | Likely benign | 44.79 |
| TP53 | Somatic | C | T | c.853G>A | p.E285K | Missense | 8 | Pathogenic/likely pathogenic | 10.43 |
| ERCC3 | Somatic | G | A | c.377C>T | p.A126V | Missense | 3 | Uncertain significance | 2.24 |
| FANCD2 | Somatic | G | A | c.4297G>A | p.E1433K | Missense | 44 | Uncertain significance | 1.42 |
| CHEK2 | Somatic | G | A | c.538C>T | p.R180* | Stop gained | 4 | Pathogenic | 1.38 |
| PALB2 | Somatic | C | T | c.2986G>A | p.E996K | Missense | 9 | Uncertain significance | 1.33 |
| ROS1 | Somatic | C | T | c.4252G>A | p.V1418I | Missense | 26 | Uncertain significance | 1.30 |
| PIK3CA | Somatic | G | A | c.436G>A | p.V146I | Missense | 2 | Uncertain significance | 1.24 |
| RAD51B | Somatic | G | A | c.140G>A | p.R47Q | Missense | 3 | Uncertain significance | 1.16 |
| PTEN | Somatic | G | A | c.698G>A | p.R233Q | Missense | 7 | Uncertain significance | 1.12 |
| BRCA2 | Somatic | C | A | c.2557C>A | p.Q853K | Missense | 11 | Likely benign | 1.06 |
The patient was diagnosed with intramucosal poorly differentiated tubular adenocarcinoma mixed with a minor (< 1%) NEC component.
En bloc resection of the lesion along with the GEJ was achieved with ESD.
The postoperative course was uneventful. Follow-up esophagogastroduodenoscopy was conducted at 6, 12, 24 and 35 mo after ESD with negative results. A repeated 13C urea breath test at 33 mo after ESD remained negative. Routine computed topography was repeated at 6, 12 and 24 mo after ESD with unremarkable findings. The patient was alive without evidence of the disease 36 mo after ESD resection.
GEJ carcinoma in China is also known as gastric cardiac carcinoma, and not associated with EAC, which is rare in China[9]. A previous study of 5401 esophageal cancers in an endemic region of China identified only 217 (4%) cases of EAC, with the youngest patient aged 44 years[6]. Another study identified EAC in only 2% of 204 resection cases of distal esophageal carcinomas in China[5]. In general, EAC rarely occurs in patients < 40 years, even in western countries. According to two studies in American patients[10,11], young patients with EAC under the age of 40 years accounted for 5%, among which only two of 1146 patients were in their second decade of life. The pathogenesis mechanisms of this cancer in young patients remain unknown.
In Chinese patients, the epicenters of carcinomas that involve the GEJ are most frequently located in the proximal stomach, rather than in the distal esophagus[9]. Moreover, long segment columnar-lined esophagus is rare in Chinese patients, unlike in western patients[6,9,12]. The vast majority of GEJ tumor resection cases with intestinal metaplasia in Chinese patients are the extension of intestinal metaplasia from the adjacent gastric cardia because of the continuity of cardiac mucosa from the gastric cardia to the distal esophagus up to 1.6 cm above the GEJ line[5,13]. In the present case, the tumor was entirely located on the distal esophageal side of the GEJ line without invading the gastric cardia. The adjacent benign mucosa was cardiac mucosa with intestinal metaplasia and focal dysplasia, suggesting the origin of this mixed carcinoma in cardiac mucosa, rather than a proximal extension of H. pylori gastric carditis, which are commonly seen in Chinese patients.
Similar to histological variants in proximal gastric carcinoma (gastric cardiac carcinoma), GEJ carcinomas in Chinese patients also vary widely in histology, including NEC[5,9]. The mixed NEC component in the present case was small in size, with a high Ki-67 index and strong p53 immunoreactivity, showing TP53 gene missense mutation revealed by NGS analysis, which supports the diagnosis of NEC, but not grade 3 neuroendocrine tumor. In our young patient, the tumor arose in cardiac glands, as described previously[14-17], which is unusual, since esophageal NEC is more commonly mixed with squamous cell carcinoma in Chinese patients[18], rather than EAC in American patients[19]. Although there were reports of esophageal carcinoma with mixed adenocarcinoma and NEC components, without the evidence of background columnar-lined esophagus, it might be due to the possibility of destruction of columnar-lined esophagus by advanced large tumors[20,21].
To the best of our knowledge, the present case is the first report of an early-onset early GEJ adenocarcinoma mixed with a small NEC component, with NGS analysis in 196 genes. Somatic mutations of RNF43, which encodes an E3 ubiquitin ligase that negatively regulates Wnt signaling, are frequently revealed in gastric carcinomas, but not in esophageal carcinomas[22,23]. EAC also shows lower APC gene mutation rates than gastric carcinomas, suggesting a less prominent role of Wnt/β-catenin in EAC tumorigenesis[24]. The germline mutation in the RNF43 gene in our case supports the origin of gastric cardiac mucosa. The association between ERCC3 gene, which encodes an ATP-dependent DNA helicase that functions in nucleotide excision repair, and drug resistance in human gastric cancer cells has been reported[25]. Similar ERCC3 gene mutation has also been reported to be associated with ovarian cancer[26]. One of the two pathogenic somatic mutated genes in our case was P53, a well-known driver gene in gastric cancer and EAC[22]. It is of note that the other pathogenic somatic mutation was identified in the CHEK2 gene; an important gene involved in the DNA homologous recombination repair pathway. CHEK2 was reported as a driver mutation in both EAC and gastric cancer[27]. NGS analysis in our case also exhibited somatic mutations in FANCD2, PALB2 and RAD51B genes, which are associated with the DNA homologous recombination repair pathway[28]. The molecular profile of the current case indicates that the genetic abnormalities involved in the DNA repair pathway may play a significant role in tumorigenesis of early-onset GEJ carcinoma of gastric origin.
The incidence of EAC and GEJ in young patients is increasing[29]. These young patients were reported to have a poor prognosis because of advanced stages at initial diagnosis and difficulty in early diagnosis[11,29]. The results from our case illustrate the importance of early diagnosis in young patients with clinical complaints of dysphagia and gastroesophageal reflux symptoms, which should be taken seriously for the possibility of malignancy, and upper endoscopy may help make an appropriate diagnosis. Once the biopsy diagnosis of early carcinoma without nodal and distant metastases is confirmed after routine work-up, ESD appears to be the subsequent step of choice for both diagnosis with staging and treatment with curative intent.
We reported a rare case of GEJ adenocarcinoma mixed with an NEC component with gastric origin in a young adult. Further studies in more cases are required to elucidate the underlying molecular tumorigenesis mechanisms in early-onset GEJ carcinoma.
The authors wish to thank Mr. Yuan Zhou and Mr. Xiaoping Liu of Changzhou Beagle Medical laboratory for their assistance in Next Generation Sequencing data interpretation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ullah K, Pakistan S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | International Agency for Research on Cancer. Cancer Today. [cited 5 November 2022]. Available from: https://gco.iarc.fr/today. |
| 2. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [PubMed] |
| 3. | Wang S, Zheng R, Arnold M, Abnet C, Zeng H, Zhang S, Chen R, Sun K, Li L, An L, Bray F, Wei W, He J. Global and national trends in the age-specific sex ratio of esophageal cancer and gastric cancer by subtype. Int J Cancer. 2022;151:1447-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Dubecz A, Solymosi N, Stadlhuber RJ, Schweigert M, Stein HJ, Peters JH. Does the Incidence of Adenocarcinoma of the Esophagus and Gastric Cardia Continue to Rise in the Twenty-First Century?-a SEER Database Analysis. J Gastrointest Surg. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Huang Q, Shi J, Sun Q, Fan X, Feng A, Wu H, Zhou Q, Yu C, Mashimo H, Lauwers GY. Distal esophageal carcinomas in Chinese patients vary widely in histopathology, but adenocarcinomas remain rare. Hum Pathol. 2012;43:2138-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Liu S, Dai JY, Yao L, Li X, Reid B, Self S, Ma J, Chang Y, Feng S, Tapsoba Jde D, Sun X. Esophageal Adenocarcinoma and Its Rare Association with Barrett's Esophagus in Henan, China. PLoS One. 2014;9:e110348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Huang J, Koulaouzidis A, Marlicz W, Lok V, Chu C, Ngai CH, Zhang L, Chen P, Wang S, Yuan J, Lao XQ, Tse SLA, Xu W, Zheng ZJ, Xie SH, Wong MCS. Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 8. | Lam AK, Kumarasinghe MP. Digestive system tumours. In: WHO Classification of Tumours. Lyon: International Agency for Research on Cancer, 2019: 104-109. |
| 9. | Huang Q, Fan X, Agoston AT, Feng A, Yu H, Lauwers G, Zhang L, Odze RD. Comparison of gastro-oesophageal junction carcinomas in Chinese versus American patients. Histopathology. 2011;59:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Oezcelik A, Ayazi S, DeMeester SR, Zehetner J, Abate E, Dunn J, Grant KS, Lipham JC, Hagen JA, DeMeester TR. Adenocarcinoma of the esophagus in the young. J Gastrointest Surg. 2013;17:1032-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Boys JA, Oh DS, Lewis JS, DeMeester SR, Hagen JA. Esophageal Adenocarcinoma in Patients Younger than 40 Years: A Two-Decade Experience at a Public and Private Hospital. Am Surg. 2015;81:974-978. [PubMed] |
| 12. | Chen X, Zhu LR, Hou XH. The characteristics of Barrett's esophagus: an analysis of 4120 cases in China. Dis Esophagus. 2009;22:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Sun Q, Huang Q, Feng AN, Fan XS, Wu HY, Mashimo H, Zhou Q, Chen J, Lauwers GY. Columnar-lined esophagus in Chinese patients with proximal gastric carcinomas. J Dig Dis. 2013;14:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kaneko Y, Saito S, Takahashi K, Kanamaru R, Hosoya Y, Yamaguchi H, Kitayama J, Niki T, Lefor AK, Sata N. Neuroendocrine carcinoma of the esophagus with an adenocarcinoma component. Clin J Gastroenterol. 2019;12:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Doi S, Matsumoto S, Wakatsuki K, Migita K, Ito M, Kunishige T, Nakade H, Hatakeyama K, Ohbayashi C, Sho M. A neuroendocrine carcinoma with a well-differentiated adenocarcinoma component arising in Barrett's esophagus: a case report and literature review. Surg Case Rep. 2018;4:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Miyaguchi K, Kawasaki T, Tashima T, Ryozawa S. Mixed neuroendocrine-non-neuroendocrine neoplasm arising from long-segment Barrett's esophagus showing exceptionally aggressive clinical behavior. Cancer Rep (Hoboken). 2022;5:e1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Kawazoe T, Saeki H, Edahiro K, Korehisa S, Taniguchi D, Kudou K, Nakanishi R, Kubo N, Ando K, Nakashima Y, Oki E, Fujiwara M, Oda Y, Maehara Y. A case of mixed adenoneuroendocrine carcinoma (MANEC) arising in Barrett's esophagus: literature and review. Surg Case Rep. 2018;4:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, Sun Q, Yang J, Huang L, Ye Q. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37:467-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Maru DM, Khurana H, Rashid A, Correa AM, Anandasabapathy S, Krishnan S, Komaki R, Ajani JA, Swisher SG, Hofstetter WL. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol. 2008;32:1404-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Mendoza-Moreno F, Díez-Gago MR, Mínguez-García J, Tallón-Iglesias B, Zarzosa-Hernández G, Fernández S, Solana-Maoño M, Argüello-De-Andrés JM. Mixed Adenoneuroendocrine Carcinoma of the Esophagus: A Case Report and Review of the Literature. Niger J Surg. 2018;24:131-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Juanmartiñena JF, Fernández-Urién I, Córdoba A, Miranda C, Borda A. Mixed adenoneuroendocrine carcinoma (MANEC) of the gastroesophageal junction: a case report and review of the literature. Rev Esp Enferm Dig. 2017;109:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4859] [Article Influence: 441.7] [Reference Citation Analysis (2)] |
| 23. | Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, Bandla S, Imamura Y, Schumacher SE, Shefler E, McKenna A, Carter SL, Cibulskis K, Sivachenko A, Saksena G, Voet D, Ramos AH, Auclair D, Thompson K, Sougnez C, Onofrio RC, Guiducci C, Beroukhim R, Zhou Z, Lin L, Lin J, Reddy R, Chang A, Landrenau R, Pennathur A, Ogino S, Luketich JD, Golub TR, Gabriel SB, Lander ES, Beer DG, Godfrey TE, Getz G, Bass AJ. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 604] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 24. | Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency; Brigham and Women’s Hospital; Broad Institute; Brown University; Case Western Reserve University; Dana-Farber Cancer Institute; Duke University; Greater Poland Cancer Centre; Harvard Medical School; Institute for Systems Biology; KU Leuven; Mayo Clinic; Memorial Sloan Kettering Cancer Center; National Cancer Institute; Nationwide Children’s Hospital; Stanford University; University of Alabama; University of Michigan; University of North Carolina; University of Pittsburgh; University of Rochester; University of Southern California; University of Texas MD Anderson Cancer Center; University of Washington; Van Andel Research Institute; Vanderbilt University; Washington University; Genome Sequencing Center: Broad Institute; Washington University in St. Louis; Genome Characterization Centers: BC Cancer Agency; Broad Institute; Harvard Medical School; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University; University of North Carolina; University of Southern California Epigenome Center; University of Texas MD Anderson Cancer Center; Van Andel Research Institute; Genome Data Analysis Centers: Broad Institute; Brown University:; Harvard Medical School; Institute for Systems Biology; Memorial Sloan Kettering Cancer Center; University of California Santa Cruz; University of Texas MD Anderson Cancer Center; Biospecimen Core Resource: International Genomics Consortium; Research Institute at Nationwide Children’s Hospital; Tissue Source Sites: Analytic Biologic Services; Asan Medical Center; Asterand Bioscience; Barretos Cancer Hospital; BioreclamationIVT; Botkin Municipal Clinic; Chonnam National University Medical School; Christiana Care Health System; Cureline; Duke University; Emory University; Erasmus University; Indiana University School of Medicine; Institute of Oncology of Moldova; International Genomics Consortium; Invidumed; Israelitisches Krankenhaus Hamburg; Keimyung University School of Medicine; Memorial Sloan Kettering Cancer Center; National Cancer Center Goyang; Ontario Tumour Bank; Peter MacCallum Cancer Centre; Pusan National University Medical School; Ribeirão Preto Medical School; St. Joseph’s Hospital &Medical Center; St. Petersburg Academic University; Tayside Tissue Bank; University of Dundee; University of Kansas Medical Center; University of Michigan; University of North Carolina at Chapel Hill; University of Pittsburgh School of Medicine; University of Texas MD Anderson Cancer Center; Disease Working Group: Duke University; Memorial Sloan Kettering Cancer Center; National Cancer Institute; University of Texas MD Anderson Cancer Center; Yonsei University College of Medicine; Data Coordination Center: CSRA Inc; Project Team: National Institutes of Health. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1458] [Cited by in RCA: 1347] [Article Influence: 168.4] [Reference Citation Analysis (0)] |
| 25. | Li M, Gao M, Xie X, Zhang Y, Ning J, Liu P, Gu K. MicroRNA-200c reverses drug resistance of human gastric cancer cells by targeting regulation of the NER-ERCC3/4 pathway. Oncol Lett. 2019;18:145-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Stradella A, Del Valle J, Rofes P, Vargas-Parra G, Salinas M, González S, Montes E, López-Doriga A, Gómez C, de Cid R, Darder E, Teulé A, Solanes A, Munté E, Capellà G, Pineda M, Feliubadaló L, Brunet J, Lázaro C. ERCC3, a new ovarian cancer susceptibility gene? Eur J Cancer. 2020;141:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Hu N, Kadota M, Liu H, Abnet CC, Su H, Wu H, Freedman ND, Yang HH, Wang C, Yan C, Wang L, Gere S, Hutchinson A, Song G, Wang Y, Ding T, Qiao YL, Koshiol J, Dawsey SM, Giffen C, Goldstein AM, Taylor PR, Lee MP. Genomic Landscape of Somatic Alterations in Esophageal Squamous Cell Carcinoma and Gastric Cancer. Cancer Res. 2016;76:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Ngoi NYL, Tan DSP. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: do we need it? ESMO Open. 2021;6:100144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 29. | Codipilly DC, Sawas T, Dhaliwal L, Johnson ML, Lansing R, Wang KK, Leggett CL, Katzka DA, Iyer PG. Epidemiology and Outcomes of Young-Onset Esophageal Adenocarcinoma: An Analysis from a Population-Based Database. Cancer Epidemiol Biomarkers Prev. 2021;30:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |