Published online Feb 15, 2024. doi: 10.4251/wjgo.v16.i2.527
Peer-review started: November 13, 2023
First decision: December 15, 2023
Revised: December 19, 2023
Accepted: January 5, 2024
Article in press: January 5, 2024
Published online: February 15, 2024
Processing time: 81 Days and 6.8 Hours
An increasing number of studies have focused on the role of cellular metabolism in the development of colorectal cancer (CRC). However, no work is currently available to synthesize the field through bibliometrics.
To analyze the development in the field of “glucose metabolism” (GM), “amino acid metabolism” (AM), “lipid metabolism” (LM), and “nucleotide metabolism” (NM) in CRC by visualization.
Articles within the abovementioned areas of GM, AM, LM and NM in CRC, which were published from January 1, 1991, to December 31, 2022, are retrieved from the Web of Science Core Collection and analyzed by CiteSpace 6.2.R4 and VOSviewer 1.6.19.
The field of LM in CRC presented the largest number of annual publications and the fastest increase in the last decade compared with the other three fields. Meanwhile, China and the United States were two of the most prominent contri
Research in “cellular metabolism in CRC” is all the rage at the moment, and researchers are particularly interested in exploring the mechanism to explain the metabolic alterations in CRC. Targeting metabolic vulnerability appears to be a promising direction in CRC therapy.
Core Tip: This paper performs a bibliometric analysis of the articles in the field of cellular metabolism in colorectal cancer (CRC). In detail, we first analyze the development and current status of this area, and finally explore the future development trend. In addition, we also summarize some hot topics on cellular metabolism in CRC, which will help researchers identify potential future research directions.
- Citation: Liu YC, Gong ZC, Li CQ, Teng P, Chen YY, Huang ZH. Global research trends and prospects of cellular metabolism in colorectal cancer. World J Gastrointest Oncol 2024; 16(2): 527-542
- URL: https://www.wjgnet.com/1948-5204/full/v16/i2/527.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i2.527
Colorectal cancer (CRC) is the 3rd most frequently diagnosed malignant tumor worldwide, while simultaneously having the 2nd highest mortality rate, imposing a significant disease burden worldwide[1]. Although significant progress has been made in treatment strategies for CRC, the prognosis of patients with CRC is still far from satisfactory[2].
Energy metabolism reprogramming is one of the most prominent hallmarks of cancer[3]. Research on cancer meta
Numerous studies have revealed the important role of amino acids in cancer metabolism. In addition to being important fuels that support cancer development, amino acids also function in redox balance and homeostatic maintenance[8]. For example, glutamine supports the TCA cycle as a fuel by relinquishing the two amine groups[9]. In addition to glutamine, some branched-chain amino acids, such as valine, leucine, and isoleucine, can also provide fuel for the TCA cycle[10]. Additionally, glutamate, glycine and cysteine in cells mediate the redox balance by synthesizing glutathione, thus preventing cancer cell death[11-13]. Although NADPH from glycolysis and the PPP is usually considered to be a major contributor to redox homeostasis, NADPH produced by serine-derived single-carbon units driven by NADPH also makes a very important contribution[14].
Researchers have discovered early on the association of lipids with the etiology of cancer. In the 1960s, researchers revealed, through the detection of radiolabeled substrates, that the lipid metabolism (LM) of cancer cells was altered, as evidenced by the active synthesis and uptake of lipids[15]. In the mid-1990s, the connection between LM and cancer was prompted by the discovery of OA-519, an oncogenic antigen highly expressed in human breast cancer that encodes fatty acid synthase (FASN), a key enzyme involved in LM[16]. In addition, many studies have identified LM as one of the primary influences on tumorigenesis. Enhanced de novo synthesis of lipids mediated by upregulating the needed enzymes is considered to be a universal hallmark of tumorigenesis[17].
In the progression of tumors, the increased nitrogen demand of cells is considered one of the most important metabolic features, where nucleotides synthesized by proliferating cells are considered important molecules for maintaining proliferation in cancer[3], and nucleotide metabolism (NM) is considered to be the most critical aspect of tumorigenesis and cancer cell replication. First, the tumor microenvironment cannot provide sufficient amounts or nucleotide ratios unless proliferating cells upregulate the integrative metabolism of nonessential amino acids, ribose, and single carbon donors to synthesize these complex molecules[18]. Second, cancer cells can utilize dysregulated NM to enhance proliferation and progression[19]. In addition, many oncogenic drivers have been shown to upregulate nucleotide biosynthesis, which is critical for cancer initiation and oncogene activation[20].
Bibliometrics is a branch of scientometrics in informatics. It functions as a quantitative analysis of patterns in the scientific literature to understand emerging trends and the structure of knowledge in a study field[21,22], which provides a quick reference guide for interdisciplinary researchers on how scientific experts evaluate the field[23]. Here, we visualized the trends and research hotspots in the field of “cellular metabolism in CRC” through bibliometric analysis so that researchers can obtain a more detailed understanding of the work in this area.
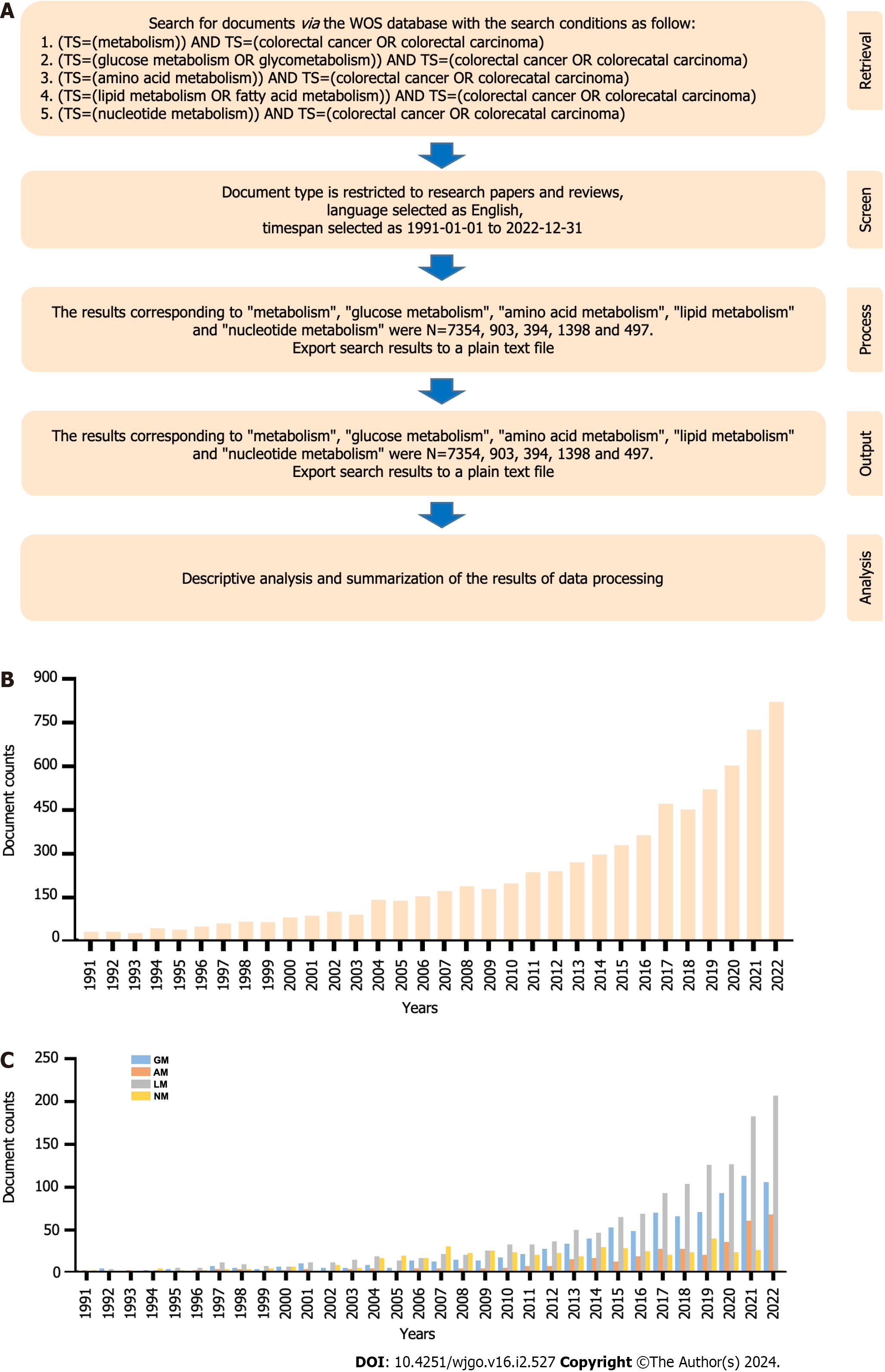
The topics “cellular metabolism in CRC”, GM, AM, LM, and NM in CRC were searched on the Web of Science Core Collection (WOSCC). The search criteria were (TS=(metabolism)) AND TS=(colorectal cancer OR colorectal carcinoma), (TS=(glucose metabolism OR glycometabolism)) AND TS=(colorectal cancer OR colorectal carcinoma), (TS=(amino acid metabolism)) AND TS=(colorectal cancer OR colorectal carcinoma), (TS=(lipid metabolism OR fatty acid metabolism)) AND TS=(colorectal cancer OR colorectal carcinoma), (TS=(nucleotide metabolism)) AND TS=(colorectal cancer OR colorectal carcinoma), the time set to January 1, 1991 to December 31, 2022, the language set to English, and the article type set to research paper and review. The retrieved documents were saved in plain text format.
All of our analysis methods can be summarized as performance analysis and scientometric analysis. For performance analysis, we performed the analysis of annual publication data in CiteSpace 6.2.R4, and the analysis of country contribution was conducted in VOSviewer 1.6.19, while the cooperation between countries was performed on https://bibliometric.com/app. The scientometric analyses, including citation analysis, cocitation analysis, coauthorship analysis, bibliographic coupling analysis, and co-occurrence analysis, were all performed on VOSviewer 1.6.19, while the burst word analysis was performed on CiteSpace 6.2.R4. In addition, the analysis of keywords was performed after data cleansing.
To analyze the trend of research concerning “cellular metabolism in CRC”, we conducted a bibliometric analysis of the relevant documents published between 1991 and 2022, and the whole workflow is illustrated below (Figure 1A). From January 1, 1991, to December 31, 2022, a total of 7354 articles were published in the field of “metabolism in CRC”, 903 articles in GM, 394 articles in amino acid metabolism (AM), 1398 articles in LM, and 497 articles in NM. Generally, articles concerning “cellular metabolism in CRC” showed a rising trend during this period, especially in the last decade (2012-2022), with a rapid outburst of articles, which exceeded more than three times the number of articles published a decade earlier (Figure 1B). Furthermore, documents on GM, AM, and LM in CRC presented a gradually increasing trend, especially on LM, which had the largest volume and fastest growth rate among these studies (Supplementary Table 1). However, the development of studies on NM was very limited, exhibiting a declining trend over the past four years (Figure 1C).
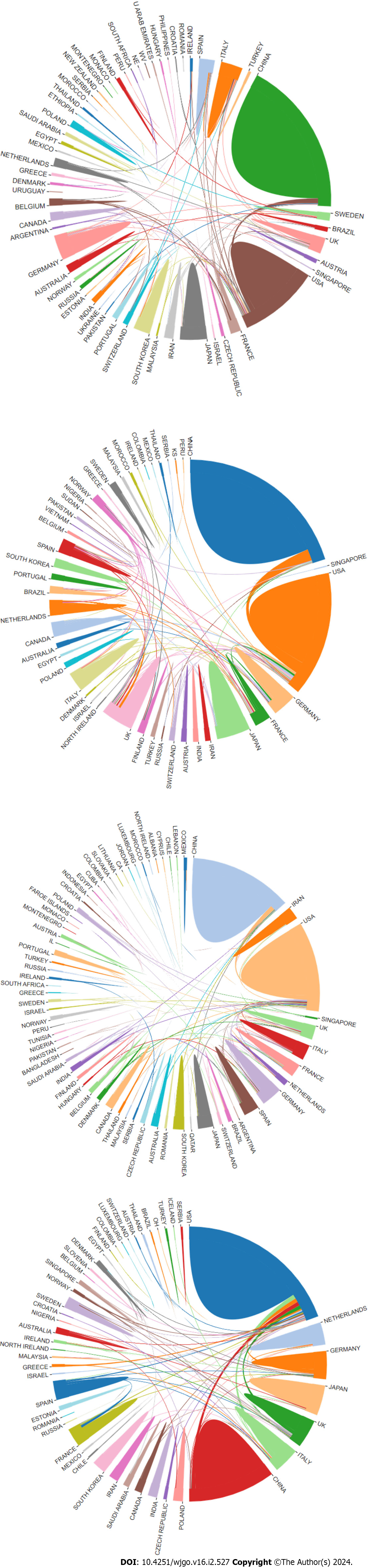
We conducted a performance analysis to evaluate the contribution of different countries and organizations in the field of “cellular metabolism in CRC”. The results of citation analyses suggested that 1347 institutions from 57 country regions contributed to the area of GM, 718 institutions from 50 countries contributed to the area of AM, 1884 organizations from 70 countries contributed to the area of LM, and 49 countries from 897 contributed to the area of NM. Notably, we found that China and the United States took the top two places in terms of publications in all fields (Figure 2), in which China ranked first in GM (318 papers), AM (129 papers), and LM (389 papers), while the United States ranked first in NM (167 papers) (Table 1). Meanwhile, in terms of the number of citations, the United States ranked first in all four areas, with 15929 citations in GM, 6754 citations in AM, 23819 citations in LM, and 7464 citations in NM (Table 1). For the average number of citations, Japan ranked first in AM (68 citations/paper) and NM (48 citations/paper) in CRC, while the United States ranked first in GM (83 citations/paper) and LM (63 citations/paper) (Table 1).
| Theme | Rank | Country | Publications/citations | Rank | Organizations | Publications/citations |
| Glucose metaboslim | 1 | China | 318/7804 | 1 | Shanghai Jiao Tong University (China) | 36/1518 |
| 2 | United States | 191/15929 | 2 | Fudan University (China) | 31/1052 | |
| 3 | Germany | 57/2864 | 3 | China Medical University (China) | 16/169 | |
| 4 | Japan | 56/2305 | 4 | Sun Yat Sen University (China) | 15/448 | |
| 5 | Italy | 54/3218 | 5 | Chinese Academy of Science (China) | 13/656 | |
| Amino acid metabolism | 1 | China | 129/2431 | 1 | Shanghai Jiao Tong University (China) | 14/510 |
| 2 | United States | 106/6754 | 2 | Zhejiang University (China) | 13/265 | |
| 3 | Japan | 38/2575 | 3 | National Cancer Institute (United States) | 13/818 | |
| 4 | Germany | 31/2053 | 4 | Chinese Academy of Science (China) | 10/170 | |
| 5 | England | 28/1024 | 5 | Sun Yat Sen University (China) | 10/348 | |
| Lipid metabolism | 1 | China | 389/11486 | 1 | Shanghai Jiao Tong University (China) | 27/1863 |
| 2 | United States | 376/23819 | 2 | Sun Yat Sen University (China) | 21/644 | |
| 3 | Germany | 93/3743 | 3 | National Cancer Institute (United States) | 20/1115 | |
| 4 | England | 87/4166 | 4 | Fudan University (China) | 19/1026 | |
| 5 | Italy | 80/4145 | 5 | Central South University (China) | 18/368 | |
| Nucleotide metabolism | 1 | United States | 167/7464 | 1 | Fred Hutchinson Cancer Center (United States) | 15/689 |
| 2 | China | 84/2166 | 2 | German Cancer Research Center (Germany) | 15/481 | |
| 3 | Italy | 51/1554 | 3 | National Cancer Institute (United States) | 15/572 | |
| 4 | Germany | 42/1358 | 4 | Harvard University (United States) | 10/486 | |
| 5 | Japan | 34/1647 | 5 | Brigham and Women’s Hospital (United States) | 9/438 |
Concerning research institutions, all of them, apart from the German Cancer Research Center (German), were from China and the United States. Among them, Shanghai Jiao Tong University (China) ranked first in the number of publications in the fields of GM (36 papers), AM (14 papers), and LM (27 papers), while Fred Hutchinson Cancer Center (United States), German Cancer Research Center (German), and National Cancer Institute (United States) tied for first place in the field of NM (15 papers). Regarding citations, Shanghai Jiao Tong University (China) ranked first in GM (1518 citations) and LM (1863 citations), National Cancer Institute (United States) ranked first in AM (818 citations), and Fred Hutchinson Cancer Center (United States) ranked first in NM (689 citations) (Table 1).
According to the coauthorship analysis, we found that China and the United States had the closest cooperation in the areas of GM, AM, and LM, while in the areas of NM, China, Germany, and Italy all had strong cooperation with the United States (Supplementary Figure 1). Overall, research on “cellular metabolism in CRC” spanned a wide range of work globally, and good partnerships between most countries facilitated advances in the field.
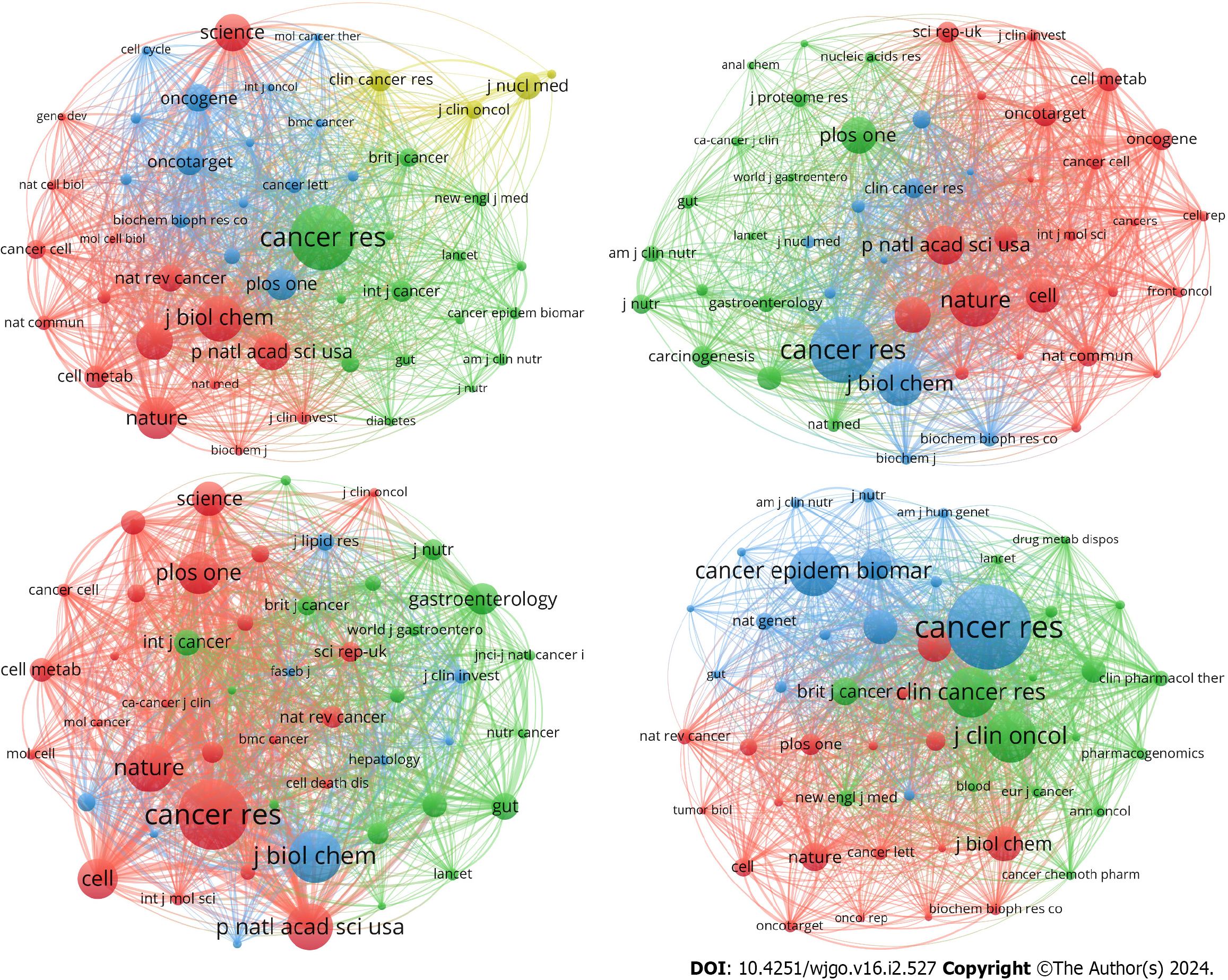
Another important indicator to track the development of a discipline is major publications and cocited journals. A total of 442 journals reported articles on GM, 233 journals reported articles on AM, 555 journals reported articles on LM, and 264 journals reported articles on NM. Meanwhile, 4538, 3158, 6480, and 3053 journals were cocited within these four areas respectively.
Then, we listed the top 10 journals in the field of “metabolism in CRC” by number of publications and cocitations. Oncotarget published the highest number of publications on GM (29 articles), while the Journal of Nuclear Medicine showed a much higher average number of citations than the other journals (112 citations/paper). Cancers ranked first in the number of publications on AM (11 papers) and LM (31 papers); additionally, Cancer Research (134 citations/paper) and Scientific Reports (52 citations/paper) ranked first in the average cited number within the above two areas. Cancer Epidemiology Biomarkers & Prevention issued the most publications on NM (21 papers), while Cancer Research had the highest average number of citations (142 citations/paper) (Supplementary Table 2).
Notably, Cancer Research had the highest number of cocitations in all four areas. We found that 87.5% of the top 10 journals in terms of publications were in Journal Citation Reports (JCR) Quartile in Category 2 (Q2) and above. In addition, 95% of the top 10 journals in terms of cocitations were in JCR Q2 and above (Supplementary Table 2). All of the above data illustrated the productive and high-quality results in the field of “metabolism in CRC”, and the cocitation analysis also indicated that journals in different fields were relatively well connected, without any isolated journals (Figure 3).
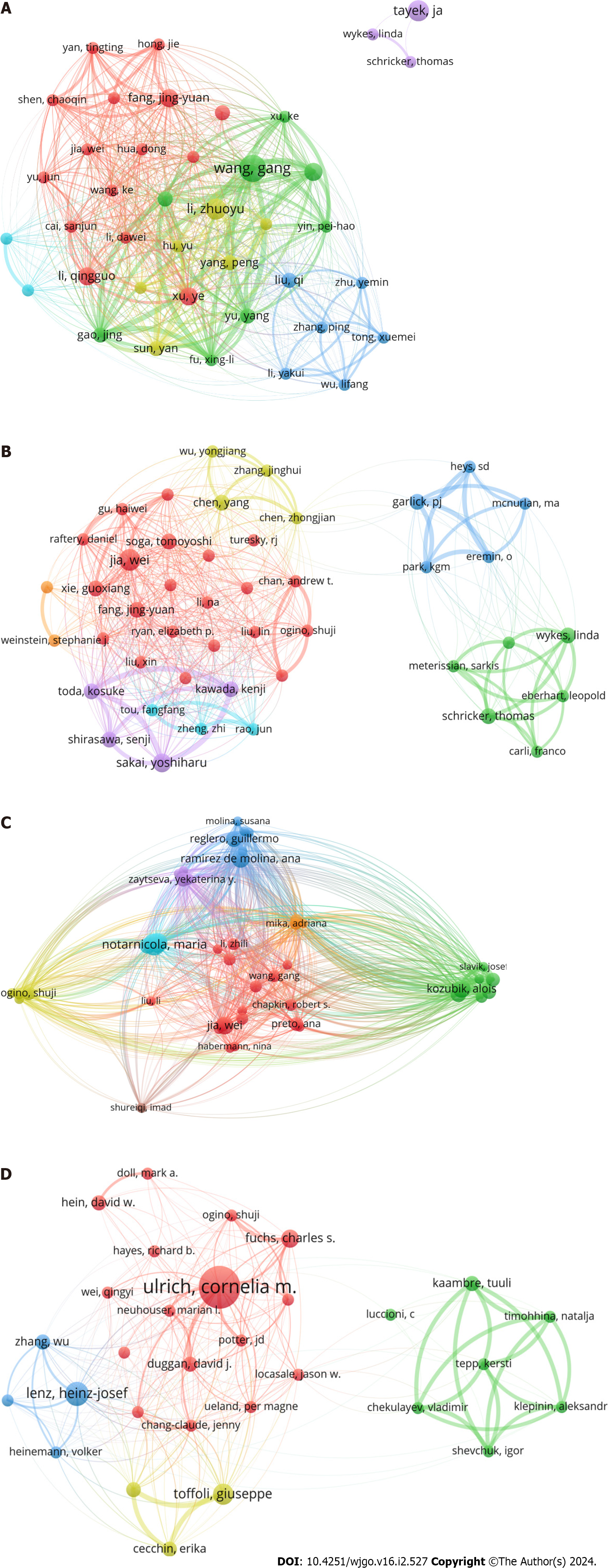
A total of 5826, 2599, 8436, and 3290 authors had outputs of articles in GM, AM, LM, and NM, respectively. Gang Wang had the highest number of publications in GM (9 papers), followed by Zhuo-Yu Li (7 papers) and John Tayek (7 papers), and Jing-Yuan Fang had the highest average number of citations (80 citations/paper) in this field. In the field of AM, Wei Jia produced the most publications (6 papers), followed by Yoshiharu Sakai (5 papers) and Kenji Kawada (4 papers), while Senji Shirasawa had a much higher average cited number (119 citations/papers) than the other authors. In the field of LM, Maria Notarnicola ranked first in the number of publications (11 papers), followed by Valeria Tutino (10 papers) and Alois Kozubík (9 papers), while Wei Jia ranked first in terms of the average number of citations (147 citations/paper). Cornelia Ulrich had the most publications (14 papers) in NM, followed by Heinz-Josef Lenz (8 papers) and Giuseppe Toffoli (7 papers), while Charles Fuchs was cited the most times per article (61 citations/paper) (Table 2).
| Theme | Rank | Author | Publications/citations | Rank | Cocited author | Publications/citations |
| Glucose metaboslim | 1 | Gang Wang | 9/180 | 1 | Otto Warburg | 241/2009 |
| 2 | Zhuo-Yu Li | 7/290 | 2 | Matthew Vander Heiden | 153/1409 | |
| 3 | John Tayek | 7/316 | 3 | Douglas Hanahan | 139/1106 | |
| 4 | Yu-Zhu Wang | 6/143 | 4 | Ralph DeBerardinis | 115/1291 | |
| 5 | Jing-Yuan Fang | 6/477 | 5 | Gregg Semenza | 107/979 | |
| Amino acid metabolism | 1 | Wei Jia | 6/137 | 1 | Ralph DeBerardinis | 67/328 |
| 2 | Yoshiharu Sakai | 5/208 | 2 | Otto Warburg | 61/243 | |
| 3 | Kenji Kawada | 4/196 | 3 | Matthew Vander Heiden | 42/202 | |
| 4 | Kosuke Toda | 4/196 | 4 | Douglas Hanahan | 39/127 | |
| 5 | Senji Shirasawa | 4/477 | 5 | David R.Wise | 37/169 | |
| Lipid metabolism | 1 | Maria Notarnicola | 11/220 | 1 | Douglas Hanahan | 163/2712 |
| 2 | Valeria Tutino | 10/200 | 2 | Javier A. Menendez | 151/2322 | |
| 3 | Alois Kozubík | 9/155 | 3 | Bandaru S.Reddy | 145/1992 | |
| 4 | Ana Ramírez de Molina | 9/377 | 4 | Otto Warburg | 143/2259 | |
| 5 | Wei Jia | 9/1325 | 5 | Rebecca Siegel | 124/1651 | |
| Nucleotide metabolism | 1 | Cornelia Ulrich | 14/712 | 1 | Cornelia Ulrich | 136/1970 |
| 2 | Heinz-Josef Lenz | 8/216 | 2 | André van Kuilenburg | 123/2054 | |
| 3 | Giuseppe Toffoli | 7/245 | 3 | Jia Chen | 96/1151 | |
| 4 | Charles Fuchs | 6/368 | 4 | Young-In Kim | 87/972 | |
| 5 | Erika Cecchin | 5/117 | 5 | Koichi Kawakami | 85/1611 |
Through the cocitation analysis, we found that the most cocited author in the field of GM was Otto Warburg (2009 times), followed by Matthew Vander Heiden (1409 times), Douglas Hanahan (1106 times) and others. Ralph DeBerardinis was the most cocited author in the field of AM (328 times), followed by Otto Warburg (243 times) and Matthew Vander Heiden (202 times). In the field of LM, Douglas Hanahan ranked first (2712 times), followed by Javier A. Menendez (2322 times), and Bandaru S. Reddy (1992 times). In the field of NM, Cornelia Ulrich was cited 1970 times, followed by André van Kuilenburg (2054 times), and Jia Chen (1151 times) (Table 2). Bibliographic coupling analysis showed that significant research directions were formed within all fields, and the links between authors were relatively tight, except John Tayek et al in GM, whose research directions had little connection to others (Figure 4). In contrast, the coauthorship analysis showed that collaborative relationships between authors were generally relatively decentralized (Supple
The key literature was the milestone in the development of a discipline. We showed the top ten cited articles in the four fields, where in GM, “Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice” was the most cited document with 1214 citations. “Serine, glycine and one-carbon units: cancer metabolism in a full circle”, which was cited 983 times, was the most cited article simultaneously in AM and NM. The most cited document in the field of LM was “The gut microbiota, bacterial metabolites and colorectal cancer”, which was cited 1571 times (Supplementary Table 3).
Cocited references are defined as documents that are cited by two or more documents simultaneously. The most cocited reference, “Understanding the Warburg effect: the metabolic requirements of cell proliferation” was the most cocited document in the field of GM (147 times) and AM (39 times). “Hallmarks of cancer: the next generation” was the most cocited document in LM (133 times). “A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase” was the most cited document in NM (44 times) (Supplementary Table 4).
Analyses of occurring keywords and burst words assist us in identifying the research hotspots and trends in the development of a discipline. From the results of the most occurring words, we found that terms concerning aerobic glycolysis occupied the most position of the analyses in GM. In contrast, the keywords in the area of AM were more thematically dispersed; nonetheless, we found that glutamine metabolism was the most mentioned among all the kinds of AM. In LM, we concluded that terms including fatty acids were referred to most of the time. In addition, the lexicon on 5-FU chemotherapy-related processes in CRC was of greatest interest in NM (Supplementary Table 5).
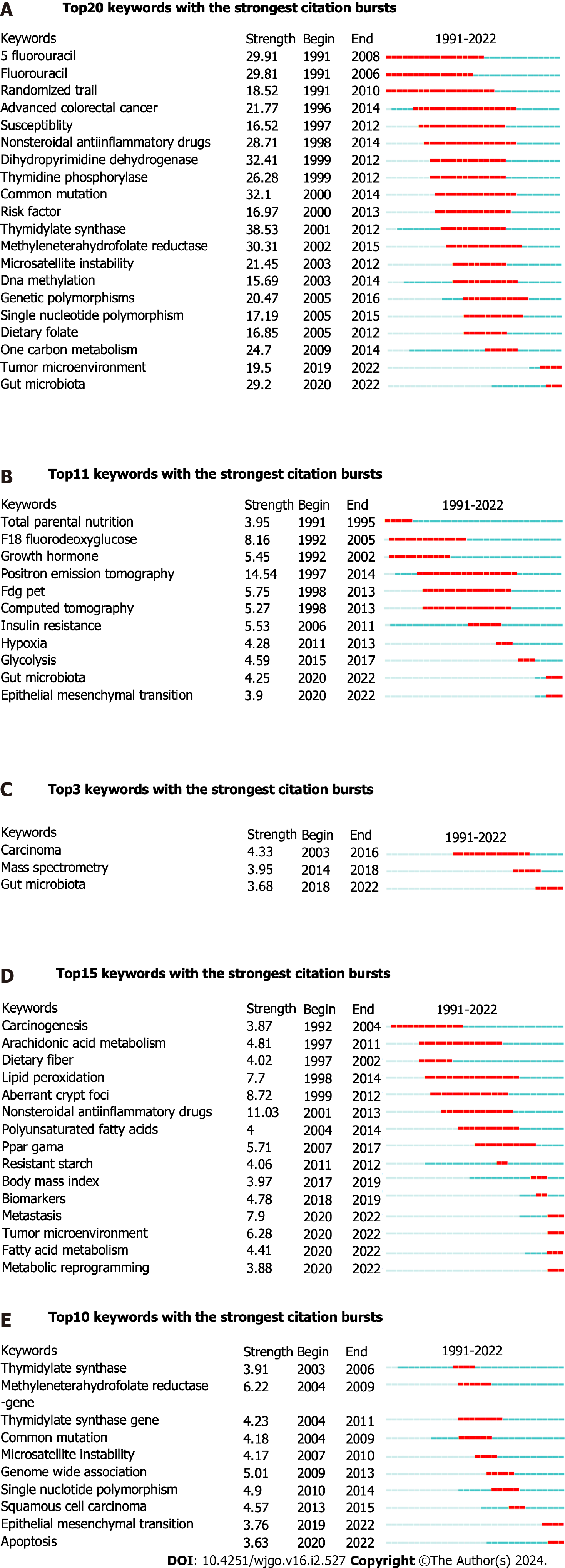
We finally showed the burst words in different fields from 1991 to 2022. As was shown in the field of “metabolism in CRC”, the burst words at the forefront were “tumor microenvironment” and “gut microbiota”. In the field of GM, the present burst words were “gut microbiota” and “epithelial mesenchymal transition”. The latest burst word was “gut microbiota” in the field of AM. In the field of LM, the up-to-date burst words were “metastasis”, “tumor microenvironment”, “fatty acid metabolism”, and “metabolic reprogramming”. In the field of NM, the most advanced burst words were “epithelial mesenchymal transition” and “apoptosis” (Figure 5).
Keeping track of research hotspots and staying abreast of the latest findings are essential for researchers. Therefore, scholars are required to be equipped with bibliographic retrieval and knowledge management. Bibliometrics is a cross science with the advantage of summarizing developments in specialized research areas and analyzing research hotspots[24]. We first summarize papers concerning “cellular metabolism in CRC” by means of bibliometrics, revealing the development in this discipline and predicting research hotspots in the future.
Generally, the development of research on "cellular metabolism in CRC" showed an upward trend. In particular, the most attention and investment of researchers was attracted in "LM in CRC". We assume that such a boom in the study of lipid metabolism in CRC is due to the development of effective tools for lipid measurements, including mass spectrometry-based lipidomics, which is making lipids a central role in cancer biology[17]. In addition, China and the United States not only had the largest outputs, but also cooperated tightly with each other and promoted most of the inter-country cooperation in the four areas.
In detail, scientific mapping can help us quantify the structure of the research. As we can see in the analyses of the keywords (Supplementary Table 5), “Aerobic glycolysis”, “Glycolysis”, and “Warburg effect” are all contained in the frequently co-occurring words, which suggests that the glycolytic process is one of the most important directions in GM research. Numerous studies have reported that HK2, which catalyzes the first irreversible step in glycolysis, is upregulated in CRC and contributes to CRC progression. For instance, Yan et al[25] found that lncSLCC1 activated the expression of HK2 promoting tumor growth. Similarly, Shen et al[26] also showed that methyltransferase-like 3 stabilized HK2 and activated the glycolytic pathway, which in turn promoted tumor progression. It is noteworthy that all the articles cited above have the participation of Dr. Jing-Yuan Fang, which is consistent with the results of high-output authors in GM that we analyzed.
Our analyses of outbreak terms suggest that research on “gut microbiota” has recently become popular in GM. Enolase 1 (ENO 1) promotes the formation of phosphoenolpyruvate through the catalysis of 2-phosphoglycerate and the production of ATP during glycolysis[27]. Fusobacterium (F.) nucleatum promoted the expression of ENO 1 by upregulating the transcription of the lncRNA enolase1-intronic transcript 1 (ENO1-IT1), which induced glycolysis and oncogenesis in CRC cells[28]. Many studies have reported the role of glycolytic enzymes in the development of CRC, suggesting that in-depth research on glucose metabolism may provide new ideas for the treatment of CRC.
AM also plays an important role in the development of CRC[29]. For instance, serine-glycine-one-carbon metabolism, glutamine metabolism, etc., have all been reported to participate in the bioprocesses of CRC[30,31]. Phosphoglycerate dehydrogenase (PHGDH) is a key enzyme that catalyzes serine biosynthesis by shifting the flux of glycolysis through the catalytic production of serine. Our previous study proved that PHGDH expression in CRC cells could be restored by hsa_circ_0062682, which ultimately promoted serine metabolism and tumor growth in CRC[32]. Likewise, Pan et al[33] recently revealed that PHGDH can serve as a novel substrate for GSK3β/FBXW7β, and that eIF3f can enhance the serine one-carbon metabolic pathway to promote CRC progression by deubiquitinating PHGDH. In contrast, increased alcohol dehydrogenase 1C was found to reduce serine levels by simultaneously inhibiting the expression of PHGDH, thereby inhibiting the growth, migration, and invasion of CRC cells[34].
As we mentioned before, with advances in technology, LM has become more concentrated as a process that converts nutrients into metabolic intermediates for energy storage and generation of the signaling molecules in CRC. FAs, which are the principal constituents of lipids[35], appeared in the most frequently occurring and outbreak words simultaneously. It should be mentioned that FASN, which emerged as the latest buzzword, utilizes acetyl-CoA and malonyl-CoA to resynthesize FAs. Wei et al[36] reported that COP9 signalosome subunit 6 (CSN6) and the tumor suppressor F-box and WD repeat domain-containing 7 (FBXW7β) prevented the degradation of FASN; as a result, the CSN6-FASN axis eventually promoted lipogenesis and tumor growth. Likewise, FASN could be restored by the deubiquitinase ubiquitin-specific protease 22, resulting in accelerated lipid synthase and tumorigenesis[37]. In addition, the upregulation of FASN also induces metastasis and oxaliplatin resistance in CRC[38,39]. Instead, inhibition of FASN diminished CD44 expression, which overwhelmingly restricted hepatic metastasis in CRC[39]. The antitumor activity of FASN attaches great significance in its prospect of being a promising therapeutic strategy for CRC. Intriguingly, Ye et al[40] recently found that fatty acid-binding protein 5 degrades FASN; however, this process ultimately promotes lipid accumulation and inhibits mTOR signaling, which facilitates cell autophagy. Taken together, lipid metabolism, especially fatty acid metabolism, plays a fundamental role in CRC growth, metastasis and chemoresistance. Particularly, the study of FASN has recently drawn great interest from many researchers. Therefore, it is a meaningful exploration to target enzymes in CRC lipid metabolism for therapy.
Research in the area of NM has been quite sluggish in recent years, while the co-cited publications on “NM in CRC” were relatively independent of the other three fields. Specifically, the cocited literature mainly showed commonalities in studying the metabolic function of thymidylate synthase (TYMS) in CRC. Meanwhile, “5-fluorouracil”, “thymidylate synthase” ranked the second and third respectively in the co-occurring keywords. To the best of our knowledge, TYMS, a folate-dependent essential enzyme that produces the sole intracellular source of dTMP needed for DNA synthesis and repair, is widely recognized as a target for the action of 5-FU[41].
Our study uses the WOSCC database for analyses of four principal areas in “cellular metabolism in CRC”, while the setup of the parameters may not be completely comprehensive. For instance, researchers worldwide are going to deepen the study of cancer metabolism, while more branches of research are now being studied. Overall, we provide a comprehensive overview of the knowledge structure and outlook of the area of “cellular metabolism in CRC “.
This study presents the process of development in “cellular metabolism in CRC” between 1991 and 2022 as well as the current status through bibliometric analysis. The results showed that LM has the largest annual number of publications and growth rate, while NM has experienced a decline in recent years. China and the United States are the two largest contributors to all four fields, while the United States leads in the number of citations in all four areas. Correspondingly, research on “cellular metabolism in CRC” has very good cooperation among all countries. In terms of journals, 87.5% of the top 10 publications reach JCR Q2 and above, and 95% of the top 10 cocitation journals reach JCR Q2 and above. Moreover, the citation relationship among all journals is very tight. Although the citation relationship between articles is strong, it is not that close in author collaboration. In regard to key documents, many well-known reviews serve as references for these fields, especially in GM, AM, and LM, while the key references in the field of NM are relatively independent of the other three fields. Last, we notice that the foci of research in recent years have been more focused on the mechanism. In conclusion, this study suggests that research on “cellular metabolism in CRC” is all the rage currently, and researchers are interested in exploring the mechanism to explain the metabolic alterations in CRC. Targeting metabolic vulnerability appears to be a very promising direction for CRC therapy.
An increasing number of studies have focused on the role of cellular metabolism in the development of colorectal cancer (CRC).
No work is currently available to synthesize the field through bibliometrics.
To analyze the development in the field of “glucose metabolism” (GM), “amino acid metabolism” (AM), “lipid metabolism” (LM), and “nucleotide metabolism” (NM) in CRC by visualization.
Articles within the abovementioned areas of GM, AM, LM and NM in CRC, which were published from January 1, 1991, to December 31, 2022, are retrieved from the Web of Science Core Collection and analyzed by Citespace 6.2.R4 and VOSviewer 1.6.19.
The field of LM in CRC presented the largest number of annual publications and the fastest increase in the last decade compared with the other three fields. Meanwhile, cooperation within the countries and authors is also growing. Besides, the research on tumor metabolism in CRC is also gradually deepening the mechanism.
Research in “cellular metabolism in CRC” is all the rage at the moment, and researchers are particularly interested in exploring the mechanism to explain the metabolic alterations in CRC. Targeting metabolic vulnerability appears to be a promising direction in CRC therapy.
This study tells researchers that metabolic alteration in CRC is one of the most important hallmarks. Future research will not only expand in the four directions listed in the paper, but also produce many other branches and more refined metabolic types. At present, the research on lipid metabolism in CRC is very hot, which provides a future research direction for researchers to study the pathogenesis and development of CRC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M, United States S-Editor: Gong ZM L-Editor: A P-Editor: Zhang XD
| 1. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1586] [Article Influence: 264.3] [Reference Citation Analysis (2)] |
| 2. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3029] [Article Influence: 504.8] [Reference Citation Analysis (3)] |
| 3. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47162] [Article Influence: 3368.7] [Reference Citation Analysis (5)] |
| 4. | WARBURG O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9935] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 5. | Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1757] [Cited by in RCA: 2264] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 6. | Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 825] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 7. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11817] [Article Influence: 738.6] [Reference Citation Analysis (0)] |
| 8. | Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52:15-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 519] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 9. | Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 948] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 10. | Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP, Metallo CM. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12:15-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 339] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 11. | Lo M, Ling V, Wang YZ, Gout PW. The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer. 2008;99:464-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25:7101-7110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 14. | Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 933] [Cited by in RCA: 864] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 15. | Spector AA, Steinberg D. Relationship between fatty acid and glucose utilization in Ehrlich ascites tumor cells. J Lipid Res. 1966;7:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Jensen V, Ladekarl M, Holm-Nielsen P, Melsen F, Soerensen FB. The prognostic value of oncogenic antigen 519 (OA-519) expression and proliferative activity detected by antibody MIB-1 in node-negative breast cancer. J Pathol. 1995;176:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Butler LM, Perone Y, Dehairs J, Lupien LE, de Laat V, Talebi A, Loda M, Kinlaw WB, Swinnen JV. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv Drug Deliv Rev. 2020;159:245-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 18. | Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017;168:657-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1574] [Article Influence: 196.8] [Reference Citation Analysis (0)] |
| 19. | Aird KM, Zhang R. Nucleotide metabolism, oncogene-induced senescence and cancer. Cancer Lett. 2015;356:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Mullen NJ, Singh PK. Nucleotide metabolism: a pan-cancer metabolic dependency. Nat Rev Cancer. 2023;23:275-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 192] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 21. | Liu X, Zhao S, Tan L, Tan Y, Wang Y, Ye Z, Hou C, Xu Y, Liu S, Wang G. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens Bioelectron. 2022;201:113932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 22. | Miao YD, Quan W, Dong X, Gan J, Ji CF, Wang JT, Zhang F. A bibliometric analysis of ferroptosis, necroptosis, pyroptosis, and cuproptosis in cancer from 2012 to 2022. Cell Death Discov. 2023;9:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Zyoud SH, Smale S, Waring WS, Sweileh W, Al-Jabi SW. Global research trends in the microbiome related to irritable bowel syndrome: A bibliometric and visualized study. World J Gastroenterol. 2021;27:1341-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Shen Z, Hu J, Wu H, Chen Z, Wu W, Lin J, Xu Z, Kong J, Lin T. Global research trends and foci of artificial intelligence-based tumor pathology: a scientometric study. J Transl Med. 2022;20:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Yan T, Shen C, Jiang P, Yu C, Guo F, Tian X, Zhu X, Lu S, Han B, Zhong M, Chen J, Liu Q, Chen Y, Zhang J, Hong J, Chen H, Fang JY. Risk SNP-induced lncRNA-SLCC1 drives colorectal cancer through activating glycolysis signaling. Signal Transduct Target Ther. 2021;6:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X, Zhang X, Cao Y, Ma D, Zhu X, Zhang Y, Fang JY, Chen H, Hong J. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 27. | Ji H, Wang J, Guo J, Li Y, Lian S, Guo W, Yang H, Kong F, Zhen L, Guo L, Liu Y. Progress in the biological function of alpha-enolase. Anim Nutr. 2016;2:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Hong J, Guo F, Lu SY, Shen C, Ma D, Zhang X, Xie Y, Yan T, Yu T, Sun T, Qian Y, Zhong M, Chen J, Peng Y, Wang C, Zhou X, Liu J, Liu Q, Ma X, Chen YX, Chen H, Fang JY. F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut. 2021;70:2123-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 29. | Peng X, Zheng T, Guo Y, Zhu Y. Amino acid metabolism genes associated with immunotherapy responses and clinical prognosis of colorectal cancer. Front Mol Biosci. 2022;9:955705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Sun H, Zhang C, Zheng Y, Liu C, Wang X, Cong X. Glutamine deficiency promotes recurrence and metastasis in colorectal cancer through enhancing epithelial-mesenchymal transition. J Transl Med. 2022;20:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 31. | Li K, Wu JL, Qin B, Fan Z, Tang Q, Lu W, Zhang H, Xing F, Meng M, Zou S, Wei W, Chen H, Cai J, Wang H, Fang L, Bian X, Chen C, Lan P, Ghesquière B, Lee MH. ILF3 is a substrate of SPOP for regulating serine biosynthesis in colorectal cancer. Cell Res. 2020;30:163-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 32. | Sun S, Li C, Cui K, Liu B, Zhou M, Cao Y, Zhang J, Bian Z, Fei B, Huang Z. Hsa_circ_0062682 Promotes Serine Metabolism and Tumor Growth in Colorectal Cancer by Regulating the miR-940/PHGDH Axis. Front Cell Dev Biol. 2021;9:770006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Pan Q, Yu F, Jin H, Zhang P, Huang X, Peng J, Xie X, Li X, Ma N, Wei Y, Wen W, Zhang J, Zhang B, Yu H, Xiao Y, Liu RY, Liu Q, Meng X, Lee MH. eIF3f Mediates SGOC Pathway Reprogramming by Enhancing Deubiquitinating Activity in Colorectal Cancer. Adv Sci (Weinh). 2023;10:e2300759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Li S, Yang H, Li W, Liu JY, Ren LW, Yang YH, Ge BB, Zhang YZ, Fu WQ, Zheng XJ, Du GH, Wang JH. ADH1C inhibits progression of colorectal cancer through the ADH1C/PHGDH /PSAT1/serine metabolic pathway. Acta Pharmacol Sin. 2022;43:2709-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 551] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 36. | Wei W, Qin B, Wen W, Zhang B, Luo H, Wang Y, Xu H, Xie X, Liu S, Jiang X, Wang M, Tang Q, Zhang J, Yang R, Fan Z, Lyu H, Lin J, Li K, Lee MH. FBXW7β loss-of-function enhances FASN-mediated lipogenesis and promotes colorectal cancer growth. Signal Transduct Target Ther. 2023;8:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 37. | Han Z, Liu M, Xie Y, Zeng K, Zhan Z, Chen Y, Wang L, Chen X, Luo Y, Zeng Y, Zhan H, Lin Y, Zhang K, Zhu X, Liu S, Luo X, Zhou A. Derepression of the USP22-FASN axis by p53 Loss under oxidative stress drives lipogenesis and tumorigenesis. Cell Death Discov. 2022;8:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 38. | Han L, Dai W, Luo W, Ye L, Fang H, Mo S, Li Q, Xu Y, Wang R, Cai G. Enhanced De Novo Lipid Synthesis Mediated by FASN Induces Chemoresistance in Colorectal Cancer. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O'Connor K, Morris AJ, Sunkara M, Weiss HL, Lee EY, Evers BM. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012;72:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 40. | Ye M, Hu C, Chen T, Yu P, Chen J, Lu F, Xu L, Zhong Y, Yan L, Kan J, Bai J, Li X, Tian Y, Tang Q. FABP5 suppresses colorectal cancer progression via mTOR-mediated autophagy by decreasing FASN expression. Int J Biol Sci. 2023;19:3115-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 41. | Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11:282-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |