Published online Feb 15, 2024. doi: 10.4251/wjgo.v16.i2.331
Peer-review started: October 17, 2023
First decision: November 16, 2023
Revised: December 5, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: February 15, 2024
Processing time: 107 Days and 17.3 Hours
Colorectal cancer is the third most prevalent malignancy globally and ranks second in cancer-related mortality, with the liver being the primary organ of metastasis. Preoperative chemotherapy is widely recommended for initially or potentially resectable colorectal liver metastases (CRLMs). Tumour pathological response serves as the most important and intuitive indicator for assessing the efficacy of chemotherapy. However, the postoperative pathological results reveal that a considerable number of patients exhibit a poor response to preoperative chemotherapy. Body mass index (BMI) is one of the factors affecting the tumorigenesis and progression of colorectal cancer as well as prognosis after various antitumour therapies. Several studies have indicated that overweight and obese patients with metastatic colorectal cancer experience worse prognoses than those with normal weight, particularly when receiving first-line chemotherapy regimens in combination with bevacizumab.
To explore the predictive value of BMI regarding the pathologic response following preoperative chemotherapy for CRLMs.
A retrospective analysis was performed in 126 consecutive patients with CRLM who underwent hepatectomy following preoperative chemotherapy at four different hospitals from October 2019 to July 2023. Univariate and multivariate logistic regression models were applied to analyse potential predictors of tumour pathological response. The Kaplan-Meier method with log rank test was used to compare progression-free survival (PFS) between patients with high and low BMI. BMI < 24.0 kg/m2 was defined as low BMI, and tumour regression grade 1-2 was defined as complete tumour response.
Low BMI was observed in 74 (58.7%) patients and complete tumour response was found in 27 (21.4%) patients. The rate of complete tumour response was significantly higher in patients with low BMI (29.7% vs 9.6%, P = 0.007). Multivariate analysis revealed that low BMI [odds ratio (OR) = 4.56, 95% confidence interval (CI): 1.42-14.63, P = 0.011], targeted therapy with bevacizumab (OR = 3.02, 95%CI: 1.10-8.33, P = 0.033), preoperative carcinoembryonic antigen level < 10 ng/mL (OR = 3.84, 95%CI: 1.19-12.44, P = 0.025) and severe sinusoidal dilatation (OR = 0.17, 95%CI: 0.03-0.90, P = 0.037) were independent predictive factors for complete tumour response. The low BMI group exhibited a significantly longer median PFS than the high BMI group (10.7 mo vs 4.7 mo, P = 0.011).
In CRLM patients receiving preoperative chemotherapy, a low BMI may be associated with better tumour response and longer PFS.
Core Tip: Increasing evidence suggests that body mass index (BMI) is a significantly underestimated predictor in the diagnosis and treatment of colorectal cancer. However, few literatures have evaluated the relationship between BMI and tumour response to chemotherapy in colorectal liver metastases (CRLMs). In this study, we found that a low BMI was associated with an improved tumour response to preoperative chemotherapy. This finding may provide a novel perspective for the individualized treatment of CRLMs.
- Citation: Song HC, Zhou HC, Gu P, Bao B, Sun Q, Mei TM, Cui W, Yao K, Yao HZ, Zhang SY, Wang YS, Song RP, Wang JZ. Tumour response following preoperative chemotherapy is affected by body mass index in patients with colorectal liver metastases. World J Gastrointest Oncol 2024; 16(2): 331-342
- URL: https://www.wjgnet.com/1948-5204/full/v16/i2/331.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i2.331
Colorectal cancer is the third most prevalent malignancy globally and ranks second in cancer-related mortality, with the liver being the primary organ of metastasis[1]. Colorectal cancer liver metastasis (CRLM) is the primary cause of mortality in patients with colorectal cancer. At present, radical surgery is the most efficacious therapeutic approach for curing CRLMs, with a median overall survival of 5.2 years following hepatectomy[2]. Preoperative chemotherapy, including chemotherapy regimens based on oxaliplatin and irinotecan with or without targeted therapy, is widely recommended for initially or potentially resectable CRLM patients due to its potential to reduce tumour load, increase resection rate, and consolidate surgical results[3].
Tumour pathological response serves as the most important and intuitive indicator for assessing the efficacy of chemotherapy, while also playing a crucial role as a long-term prognostic factor in patients with CRLM receiving chemo
Obesity, an important risk factor threatening human health, is strongly associated with the occurrence and progression of numerous diseases[9]. Epidemiological evidence suggests that overweight and obesity are associated with an increased risk of colorectal carcinogenesis and poorer tumour outcomes. Several studies have indicated that overweight and obese patients with metastatic colorectal cancer experience worse prognoses than those with normal weight, particularly when receiving first-line chemotherapy regimens in combination with bevacizumab[10-12]. Furthermore, research has demonstrated that obesity and an increase in adipocytes are associated with therapeutic resistance in patients with colorectal cancer. In this study, we mainly investigated the correlation between body mass index (BMI) and pathological responses in CRLM patients receiving preoperative chemotherapy.
We retrospectively collected and analysed the clinical and pathological data of 126 patients with CRLM who underwent radical hepatectomy after receiving preoperative chemotherapy at the First Affiliated Hospital of University of Science and Technology of China, Suzhou Hospital Affiliated to Anhui Medical University, Tongcheng People’s Hospital and Xuancheng People’s Hospital from October 2019 to July 2023. The inclusion criteria were as follows: (1) Patients received first-line chemotherapy preoperatively, including neoadjuvant and conversion therapies; and (2) The postoperative pathology findings are consistent with CRLM. The exclusion criteria were as follows: (1) Patients who had received radiotherapy, transarterial chemoembolization, or other local treatments for CRLM before hepatectomy; and (2) Combined with other malignant tumours. The data collection plan received approval from the Institutional Ethics Review Committee of the hospital.
The personalized treatment decisions of patients were deliberated by a multidisciplinary team (MDT) consisting of experts in hepatobiliary surgery, oncology, gastroenterology, imaging, pathology and ultrasound. All patients underwent preoperative chemotherapy with oxaliplatin and/or irinotecan, and this was followed by individualized decision-making regarding the addition of targeted therapy based on their specific tumour characteristics. The targeted therapies included cetuximab and bevacizumab. Subsequently, radical hepatectomy was performed for all patients after preoperative chemotherapy.
Considering variations in adipose tissue distribution across diverse ethnic populations, a BMI < 24.0 kg/m2 was defined as low BMI within this study[13]. A weight change exceeding 5% was recorded as either weight gain or weight loss during the period between the initiation of neoadjuvant chemotherapy and the time of surgery. Both weight and height data were measured on the day of patient admission before surgery. Postoperative complication grading was based on the Clavien-Dindo classification system[14]. The occurrence of complications at Clavien-Dindo grade II and higher was recorded. More definitions of variables are summarized in Supplementary Table 1.
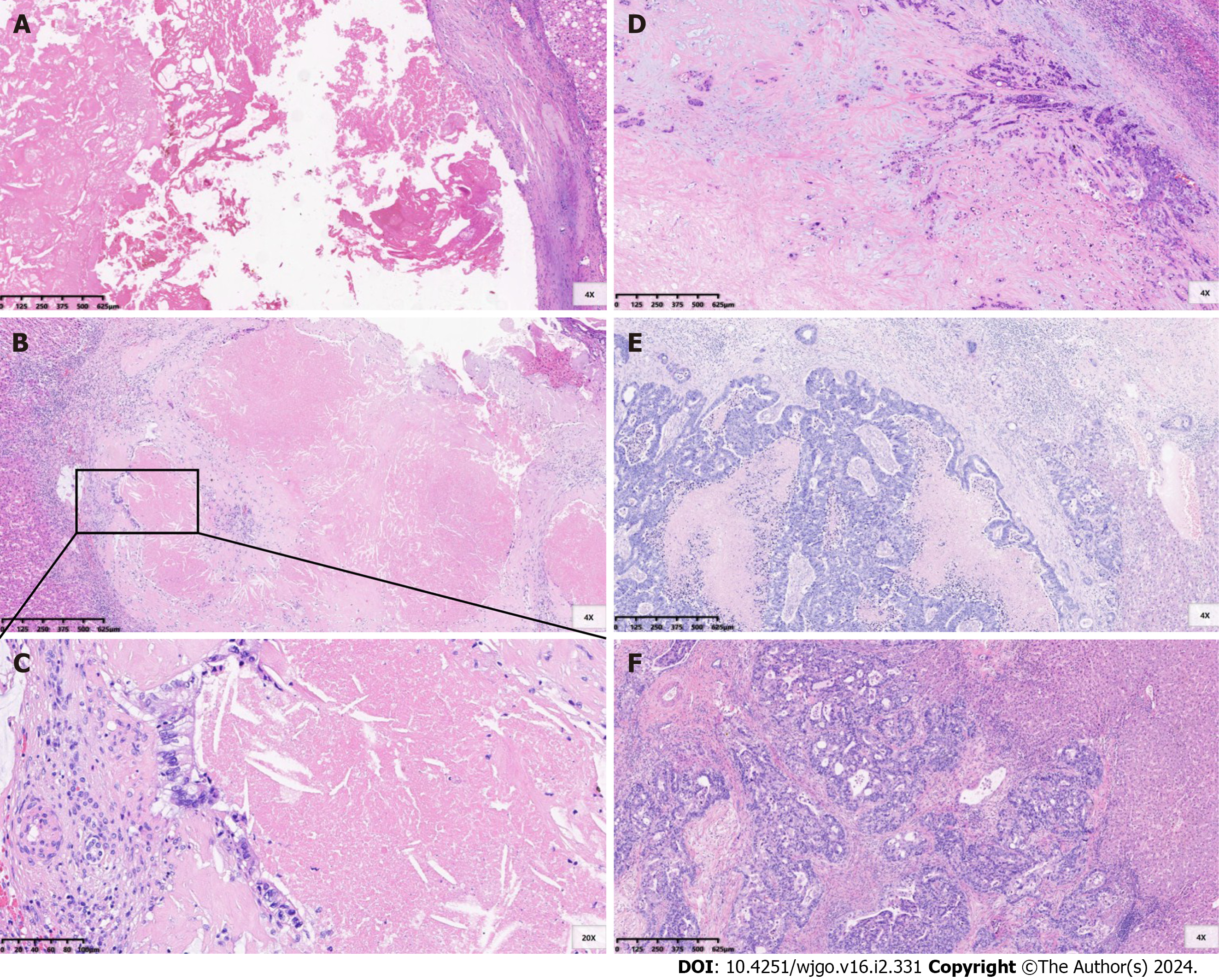
The metastatic specimens obtained after liver resection were evaluated by professional pathologists. The assessment of pathological responses to chemotherapy was based on the tumour regression grade (TRG) system proposed by Mandard et al[15] (Figure 1). Grade 1 indicates the absence of residual cancer and extensive fibrosis. Grade 2 signifies rare scattered residual cancer cells within fibrosis. Grade 3 demonstrates a higher presence of residual tumour cells, although fibrosis remains predominant. Grade 4 is characterized by a predominance of residual tumour cells. Grade 5 denotes the absence of signs indicating regression. TRG grade 1-2 was defined as a complete tumour response, while TRG grade 3-5 was defined as a poor tumour response[4,5].
Considering that steatosis and sinusoidal dilatation are the two most common liver injuries associated with oxaliplatin, irinotecan, and 5-fluorouracil chemotherapy for CRLM, we conducted a comprehensive assessment of the extent of these chemotherapy-associated liver injuries in the liver area surrounding metastases. The percentage of steatosis in involved hepatocytes was assessed and graded into the following categories: Absent (0%), grade 1 (1%-30%), grade 2 (31%-60%) and grade 3 (> 60%)[4]. Sinusoidal dilatation was scored according to the pathological grade system published by Rubbia-Brandt et al[16]: Absent, grade 1, grade 2 and grade 3. Grade 2-3 steatosis and sinusoidal dilatation were defined as severe liver injuries[5].
Lung, abdominal and pelvic contrast-enhanced computed tomography scans (liver contrast-enhanced magnetic resonance imaging examinations if deemed necessary) were performed at 3-mo intervals during the initial 2-year period postsurgery, followed by biannual assessments thereafter.
The data were analysed using the statistical software packages R version 4.2.0 (The R Foundation; http://www.R-project.org) and EmpowerStats (www.empowerstats.com; X&Y Solutions, Boston, MA, United States). T tests and the Kruskal-Wallis rank sum test were employed to test normally and nonnormally distributed continuous variables, respectively. χ2 tests were used to compare categorical variables. The relevant factors associated with tumour histopathological response were examined using univariate and multivariate logistic regression models. Predictors with a significance level of P < 0.10 in the univariate analysis were included in the multivariate models. The Kaplan-Meier method with log rank test was used to compare progression-free survival (PFS) between patients with high and low BMI. Statistical significance was considered at P < 0.05.
A total of 126 patients were enrolled in this study, including 85 males and 41 females. The median age at the time of hepatectomy was 58 years [interquartile range (IQR) 51.0-67.0]. The median BMI was 23.3 (IQR 20.8-25.9) kg/m2, with low BMI observed in 74 (58.7%) patients. Hypertension was present in 50 (39.7%) patients, and diabetes was diagnosed in 20 (15.9%) patients. Among the patients in the cohort, the rectum was the primary tumour site in 64 (50.8%) patients. Synchronous liver metastases were identified in 83 (65.9%) patients, with multiple liver metastases observed in 61 (48.4%) patients. The median diameter of the largest metastatic lesion was 2.7 cm (IQR 1.7-4.2). A preoperative carcinoembryonic antigen (CEA) level ≥ 10 ng/mL was observed in 52 (41.3%) patients.
Ninety-five (75.4%) patients received an oxaliplatin-based preoperative chemotherapy regimen. Targeted therapy was administered to 77 (61.1%) patients, including bevacizumab in 45 (35.7%) patients and cetuximab in 32 (25.4%) patients. The median number of chemotherapy cycles was 5 (IQR 3.0-7.0), with more than 6 cycles being administered in 32 (25.4%) patients preoperatively. Laparoscopic procedures were performed in 101 (80.2%) patients, while simultaneous surgery was conducted in 35 (27.8%) patients. The Pringle maneuver was utilized in the surgical approach for a total of 89 (70.6%) patients. Complications of grade II or higher were observed in 29 (23%) patients. The median duration of postoperative hospital stay was recorded as approximately 8 (IQR 6.0-10.8) d. Different degrees of steatosis and sinusoidal dilatation were observed in 56 (44.4%) and 103 (82.4%) patients, respectively. A complete tumour response was found in 27 (21.4%) patients (Table 1).
| Item | All patients (n= 126) | Low BMI (n= 74) | High BMI (n= 52) | P value |
| General characteristics | ||||
| Male | 85 (67.5) | 48 (64.9) | 37 (71.2) | 0.458 |
| Age ≥ 60 yr | 54 (42.9) | 37 (50.0) | 17 (32.7) | 0.053 |
| BMI, kg/m2 | 23.6 ± 3.5 | 21.2 ± 1.8 | 27.0 ± 2.3 | < 0.001 |
| Weight gain ≥ 5% | 34 (27.0) | 18 (24.3) | 16 (30.8) | 0.254 |
| Weight loss ≥ 5% | 17 (13.5) | 13 (17.6) | 4 (7.7) | 0.254 |
| Hypertension | 50 (39.7) | 23 (31.1) | 27 (51.9) | 0.019 |
| Diabetes | 20 (15.9) | 10 (13.5) | 10 (19.2) | 0.387 |
| Primary colorectal tumour | ||||
| Primary site, colon | 62 (49.2) | 37 (50.0) | 25 (48.1) | 0.832 |
| Lymph nodal metastasis1 | 77 (68.8) | 49 (73.1) | 28 (62.2) | 0.222 |
| Liver metastases | ||||
| Synchronous | 83 (65.9) | 52 (70.3) | 31 (59.6) | 0.214 |
| Bilobar distribution | 47 (37.3) | 26 (35.1) | 21 (40.4) | 0.549 |
| Diameter of largest metastases ≥ 3.0 cm | 46 (36.5) | 24 (32.4) | 22 (42.3) | 0.257 |
| Multiple metastases | 61 (48.4) | 36 (48.6) | 25 (48.1) | 0.950 |
| Preoperative chemotherapy details | ||||
| Oxaliplatin-based regimen | 95 (75.4) | 60 (81.1) | 35 (67.3) | 0.077 |
| Bevacizumab | 45 (35.7) | 26 (35.1) | 19 (36.5) | 0.871 |
| Cycles > 6 | 32 (25.4) | 18 (24.3) | 14 (26.9) | 0.741 |
| Therapy delayed or dose reduced | 15 (11.9) | 9 (12.2) | 6 (11.5) | 0.915 |
| Surgical details | ||||
| Preoperative CEA ≥ 10 ng/mL | 52 (41.3) | 27 (36.5) | 25 (48.1) | 0.193 |
| Laparoscopic | 101 (80.2) | 59 (79.7) | 42 (80.8) | 0.885 |
| Simultaneous | 35 (27.8) | 16 (21.6) | 19 (36.5) | 0.066 |
| Pringle manoeuver | 89 (70.6) | 51 (68.9) | 38 (73.1) | 0.614 |
| Postoperative details | ||||
| Morbidity (Dindo-Clavien II-V) | 29 (23.0) | 17 (23.0) | 12 (23.1) | 0.989 |
| Length of hospital stay (median, Q1-Q3) | 8.0 (6.0-10.8) | 8.0 (6-10) | 8.0 (6-11.0) | 0.539 |
| Pathology details | ||||
| Complete tumour response | 27 (21.4) | 22 (29.7) | 5 (9.6) | 0.007 |
| Steatosis | 56 (44.4) | 30 (40.5) | 26 (50.0) | 0.293 |
| Sinusoidal dilatation | 103 (82.4) | 62 (84.9) | 41 (78.8) | 0.379 |
| Severe steatosis (grade 2-3) | 17 (13.5) | 6 (8.1) | 11 (21.2) | 0.035 |
| Severe sinusoidal dilatation (grade 2-3) | 30 (23.8) | 19 (25.7) | 11 (21.2) | 0.557 |
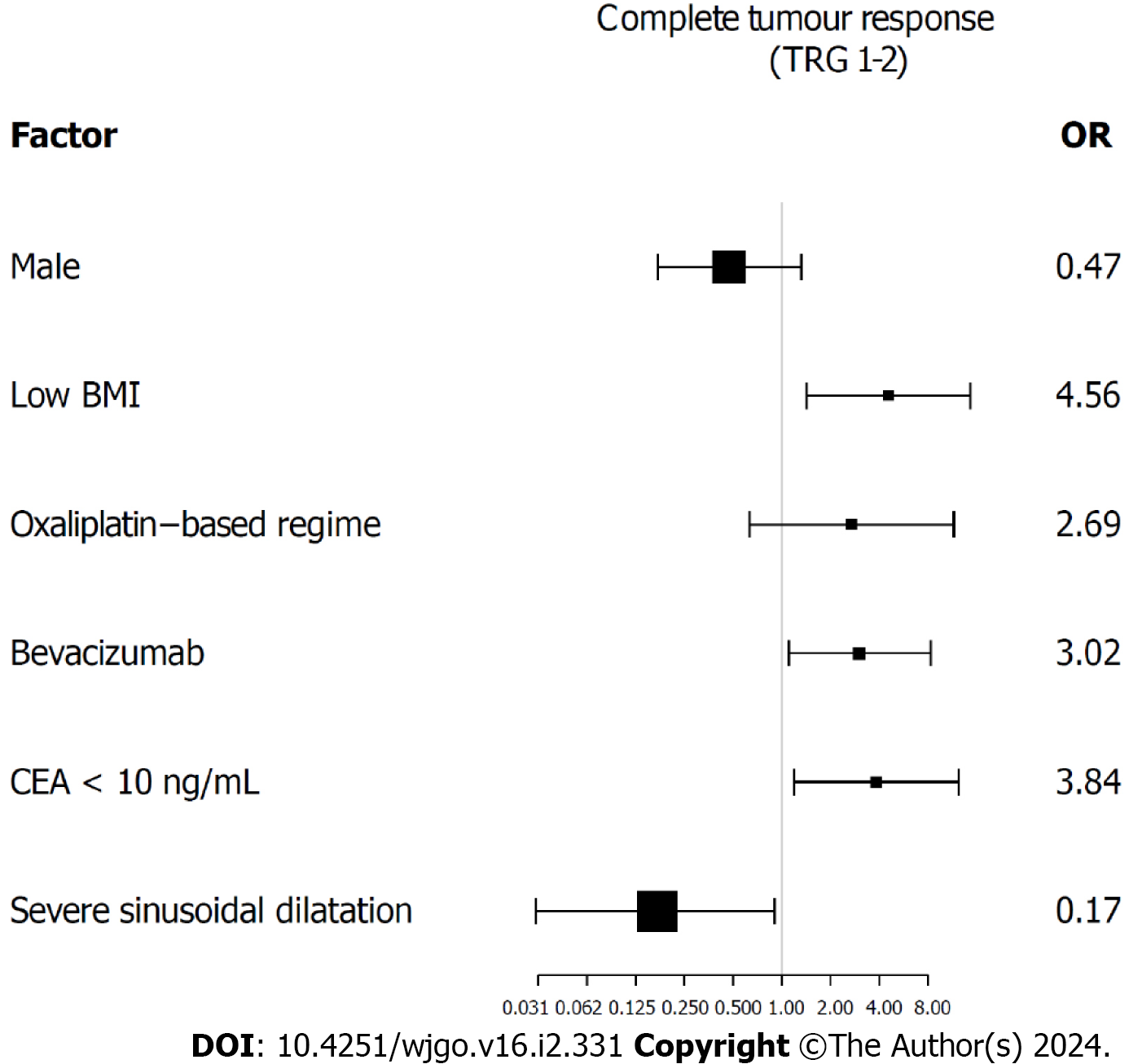
The baseline clinicopathologic and treatment characteristics based on BMI grouping are summarized in Table 1. The characteristics of the two groups were basically similar, with the high BMI group demonstrating a higher incidence of hypertension (51.9% vs 31.1%, P = 0.019) and severe steatosis (21.2% vs 8.1%, P = 0.035) than the low BMI group. The rate of complete tumour response was significantly higher in patients with low BMI (29.7% vs 9.6%, P = 0.007). Univariate analysis identified that low BMI [odds ratio (OR) = 3.98, 95% confidence interval (CI): 1.39-11.34, P = 0.010], targeted therapy with bevacizumab (OR = 2.88, 95%CI: 1.20-6.87, P = 0.018), preoperative CEA level < 10 ng/mL (OR = 3.98, 95%CI: 1.39-11.34, P = 0.010) and severe sinusoidal dilatation (OR = 0.20, 95%CI: 0.05-0.91, P = 0.038) were significant factors associated with complete tumour response (Table 2). Multivariate analysis revealed that low BMI (OR = 4.56, 95%CI: 1.42-14.63, P = 0.011), targeted therapy with bevacizumab (OR = 3.02, 95%CI: 1.10-8.33, P = 0.033), preoperative CEA level < 10 ng/mL (OR = 3.84, 95%CI: 1.19-12.44, P = 0.025) and severe sinusoidal dilatation (OR = 0.17, 95%CI: 0.03-0.90, P = 0.037) were independent predictive factors for complete tumour response (Figure 2 and Supplementary Table 2). The effect of BMI on tumour histological response was consistent across subgroups (Supplementary Table 3).
| Factor | n (%) | Complete tumour response (TRG1-2) | |
| OR (95%CI) | P value | ||
| Male | 85 (67.5) | 0.42 (0.18-1.02) | 0.054 |
| Age ≥ 60 yr | 54 (42.9) | 0.90 (0.38-2.13) | 0.802 |
| Low BMI | 74 (58.7) | 3.98 (1.39-11.34) | 0.010 |
| Weight gain ≥ 5% | 34 (27.0) | 0.68 (0.24-1.90) | 0.460 |
| Weight loss ≥ 5% | 17 (13.5) | 0.68 (0.18-2.63) | 0.575 |
| Hypertension | 50 (39.7) | 0.57 (0.23-1.43) | 0.232 |
| Diabetes | 20 (15.9) | 0.60 (0.16-2.23) | 0.449 |
| Primary site, colon | 62 (49.2) | 0.78 (0.33-1.84) | 0.577 |
| Lymph nodal metastasis1 | 77 (68.8) | 0.88 (0.35-2.22) | 0.789 |
| Synchronous | 83 (65.9) | 2.09 (0.77-5.65) | 0.147 |
| Bilobar distribution | 47 (37.3) | 1.78 (0.75-4.20) | 0.192 |
| Diameter ≥ 3.0 cm | 46 (36.5) | 1.53 (0.64-3.63) | 0.336 |
| Multiple metastases | 61 (48.4) | 1.19 (0.51-2.79) | 0.687 |
| Oxaliplatin-based regimen | 95 (75.4) | 3.15 (0.88-11.32) | 0.078 |
| Bevacizumab | 45 (35.7) | 2.88 (1.20-6.87) | 0.018 |
| Cycles > 6 | 32 (25.4) | 0.80 (0.29-2.21) | 0.669 |
| Preoperative CEA < 10 ng/mL | 74 (58.7) | 3.98 (1.39-11.34) | 0.010 |
| Steatosis | 73 (57.9) | 1.07 (0.45-2.55) | 0.875 |
| Sinusoidal dilatation | 103 (82.4) | 0.64 (0.22-1.85) | 0.412 |
| Severe steatosis (grade 2-3) | 17 (13.5) | 1.65 (0.53-5.17) | 0.392 |
| Severe sinusoidal dilatation (grade 2-3) | 30 (23.8) | 0.20 (0.05-0.91) | 0.038 |
The incidence of severe steatosis in patients with high BMI (21.2% vs 8.1% P = 0.035) was significantly higher than that in patients with low BMI, with no statistically significant difference in sinusoidal dilatation. No statistically significant difference was observed in the incidence of postoperative complications or the length of hospital stay postoperatively (Table 1).
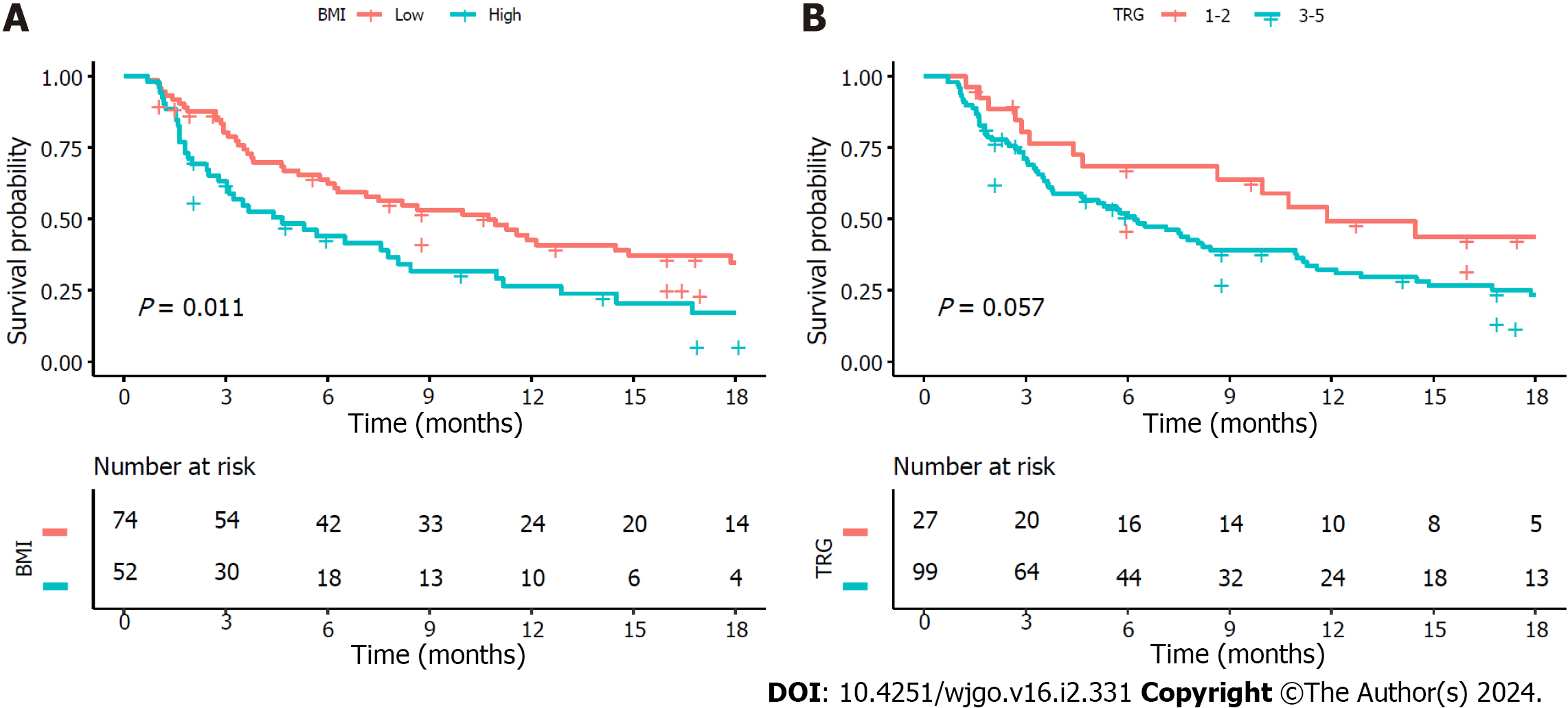
The median follow-up for PFS was 5.7 (IQR 2.4-12.8) mo. At the time of analysis, disease progression was observed in 87 (69.0%) patients. The low BMI group exhibited a significantly longer median PFS than the high BMI group, with median PFS of 10.7 (95%CI: 6.3-17.9) mo and 4.7 (95%CI: 3.0-8.4) mo, respectively (P = 0.011) (Figure 3A). The median PFS was preferable in patients with complete tumour response compared with poor response, although this difference did not reach statistical significance (10.7 mo vs 6.0 mo, P = 0.057) (Figure 3B).
Obesity (or high BMI) is associated with an increased risk of colorectal carcinogenesis and unfavourable prognosis[9]. However, the impact of BMI on the pathological response of CRLMs to preoperative chemotherapy has been reported in a limited number of studies. In this study, we observed that overweight and obese patients with CRLM exhibited a poorer response to chemotherapy and appeared to have shorter PFS than patients with low BMI. The World Health Organization standard defines adult overweight as having a BMI ≥ 25.0 kg/m2, while obesity is defined as having a BMI ≥ 30.0 kg/m2. Nevertheless, considering variations in average BMI among different ethnic groups, the Chinese standards employed a threshold of BMI ≥ 24.0 kg/m2 to define overweight and ≥ 28.0 kg/m2 for obesity[13]. Therefore, a BMI < 24.0 kg/m2 was defined as low BMI within the study.
Similar findings have been reported in some studies of primary and metastatic colorectal cancer. Previous studies have shown that obesity is associated with a lower rate of pathological complete response and a higher risk for local recurrence after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer[17,18]. A retrospective study showed that high BMI, visceral fat area and subcutaneous fat area were significantly associated with nonresponse (stable disease or progressive disease) in metastatic colorectal cancer patients treated with a bevacizumab-based chemotherapeutic regimen. Patients with high BMI had a significantly shorter mean time to progression than patients with normal BMI (9 vs 12 mo, P = 0.01). In the multivariate analysis, a high visceral fat area independently predicted nonresponse [hazard ratio (HR) = 7.18, 95%CI: 1.69-30.6], a shorter time to progression (HR = 2.80, 95%CI: 1.35-5.79) and shorter survival (HR = 2.88, 95%CI: 1.13-7.32)[12]. An analysis of 563 patients with metastatic colorectal cancer who had received first-line chemotherapy in combination with bevacizumab showed that the nonobese group exhibited a longer median PFS (10.8 vs 9 mo, P = 0.030) and a higher 2-year survival rate (34% vs 23%, P = 0.036) than the obese group[11]. A similar result of low BMI being associated with longer median PFS (24 vs 17.9 mo, P = 0.04) in CRLM patients was also found in the work by Hopirtean et al[19].
At the initiation of chemotherapy, the dosage of first-line chemotherapy agents commonly used for CRLM, such as oxaliplatin, irinotecan, 5-fluorouracil and capecitabine, is determined based on body surface area, while bevacizumab is dosed according to weight. However, in our study, there was no statistically significant difference in the chemotherapy cycle delays or dose reductions (11.5% in the low BMI group and 7% in the high BMI group) due to factors between the two patient cohorts (Table 1). Therefore, we propose that these findings are not attributable to relatively inadequate medication dosages or substantially lower systemic drug concentrations among patients with higher BMI compared to those with lower BMI. The inferior pathological response and prognosis observed in patients with high BMI may arise from more aggressive tumour biology or increased susceptibility to chemotherapy resistance of CRLMs in obese patients involving insulin resistance and adipose tissue-derived factors[12].
Previous studies have demonstrated that adipose tissue secretes various growth factors and cytokines, including leptin, adiponectin, vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), and angiopoietins (Angs)[20]. The systemic or local internal environment is then altered to facilitate tumour growth, metastasis and angiogenesis as well as confer resistance to chemotherapy and targeted therapies[20-22]. Angiogenic factors such as leptin, VEGF, IGF-1 and Angs exert a direct effect on promoting cancer angiogenesis, which may be the main reason for the resistance of obese patients to anti-VEGF therapy such as bevacizumab and tumour progression[12,20]. Chen et al[23] discovered that obesity-associated leptin promotes chemoresistance towards the chemotherapy drug 5-fluorouracil in colorectal cancer. Elevated IGF-1 levels elicit various biological responses, including enhanced tumour proliferation and aggressiveness, resistance to apoptosis, promotion of an inflammatory tumour microenvironment, and obesity-related resistance to chemotherapy[22]. Dallas et al[24] found that the levels of activated IGF-1 receptors were elevated in chemoresistant (5-fluorouracil and oxaliplatin) colorectal cancer cell lines compared with parent cell lines. Additionally, a cross-resistance phenomenon was observed between 5-fluorouracil and oxaliplatin[24]. Moreover, heightened blood insulin levels in obese patients induce resistance to oxaliplatin colon cancer cells by activating the PI3K/Akt pathway. Simultaneously, there is an augmented proliferative response of chemoresistant cancer cells to insulin that is accompanied by decreased insulin metabolism[25].
The poor outcomes associated with obesity may be attributed to insufficient engagement in appropriate physical activities and an unhealthy dietary regimen among patients with CRLM. Increased levels of physical activity and a lower BMI have been found to correlate with extended disease-free survival in individuals diagnosed with colorectal cancer, apparently enhancing their long-term survival outcomes[26,27]. In metastatic colorectal cancer, high levels of physical activity were associated with longer PFS and lower treatment-related adverse events[28]. Physical activity may ameliorate insulin resistance and mitigate the effects of obesity-related growth factors, including insulin, IGF-1, and adiponectin, thereby enhancing tumour outcomes[29-36]. Western dietary patterns, high glycaemic load, and total carbohydrate intake exhibit a positive correlation with an elevated risk of colorectal cancer recurrence and mortality[37-39]. Therefore, physical activity and a controlled diet are recommended for patients diagnosed with colorectal cancer to improve prognosis[40]. Furthermore, research findings have demonstrated that bariatric surgery in obese adults can significantly reduce both obesity-related cancers and cancer-related mortality[41]. The incidence of colorectal cancer in obese individuals exhibits a significant reduction after bariatric surgery[42-44]. Similar results have been found in other tumours, and diverse modalities of weight loss can enhance outcomes in breast cancer patients[45-47]. Additionally, bariatric surgery has the potential to improve the response to immune checkpoint blockade in breast cancer patients[48].
First-line chemotherapy combined with the anti-VEGF agent bevacizumab in the treatment of CRLM can improve the resection rates, objective response rates and overall survival rates[5,49,50]. Consistent with these findings, our study revealed a superior pathological response when preoperative chemotherapy was combined with bevacizumab for CRLMs. Given the comparable pathological response between patients treated with cetuximab and those without targeted therapy (complete response rates of 15.6% and 14.3%, respectively), both groups exhibited inferior outcomes compared to the bevacizumab-treated group (complete response rate of 33.3%). Consequently, we categorized patients receiving cetuximab or no targeted therapy as the nonbevacizumab group. Although previous studies have shown that the effectiveness of first-line bevacizumab-based treatment for CRLMs is more likely to be affected by BMI in metastatic colorectal cancer[10-12], our findings indicated that, whether receiving bevacizumab or not, patients with high BMI exhibited poorer pathological responses.
The present study found that a preoperative CEA level < 10 ng/mL was associated with favourable tumour response rates as mentioned in a previous study[50]. However, further research is needed to explore the underlying mechanism responsible for this association. Consistent with previous studies, this study revealed that severe sinusoidal dilatation (grade 2-3) was also significantly associated with poor pathological response rates[4,5]. This involves various alterations, including the loss of fenestrae in sinusoidal endothelial cells, weakened hepatic microcirculation, limited transportation of drugs, and hypoxia associated with sinusoidal dilatation. Collectively, these factors contribute to inferior tumour response rates.
There were several limitations in this study. First, genetic factors such as RAS and mismatch repair/microsatellite instability status that have been shown to be important prognostic factors in colorectal cancer were not included in the analysis because the recorded data were partially missing. Second, the study needed a review of tumour pathological sections after hepatectomy to assess the TRG of liver metastases, resulting in a study population that included only resectable CRLM patients, inevitably resulting in selection bias. Third, due to the small sample size, we did not conduct a subgroup analysis of underweight patients. Nevertheless, we believe that this study provides useful information for clinical practice.
This study provides evidence that tumour response following preoperative chemotherapy is affected by BMI in patients with CRLM, and this finding may serve as a reference for developing personalized treatment strategies. For example, neoadjuvant chemotherapy is not recommended for initially resectable CRLM patients with high BMI. Next, it is worth further exploring whether deliberately increasing physical activity and weight loss can improve the response to chemotherapy in obese CRLM patients.
From this study of CRLM patients receiving preoperative chemotherapy, low BMI appears to be associated with better tumour response and longer PFS. The MDT should consider this factor when designing personalized treatment strategies for the optimal management of CRLM patients.
Radical surgery is the most efficacious therapeutic approach for curing colorectal liver metastases (CRLMs). Preoperative chemotherapy is widely recommended for patients with CRLM. However, a considerable number of patients exhibit poor response to preoperative chemotherapy.
Epidemiological evidence suggests that body mass index (BMI) is associated with colorectal carcinogenesis and outcomes. Exploring the relationship between BMI and pathological response to preoperative chemotherapy may help us better manage CRLM patients.
This study was designed to explore the predictive value of BMI regarding the pathologic response following preoperative chemotherapy for CRLMs.
A retrospective analysis was conducted on the clinical and pathological data of 126 CRLM patients who underwent hepatectomy after preoperative chemotherapy. We analysed the potential predictive factors affecting tumour regression grade (TRG). TRG 1-2 was defined as a complete pathological response.
The rate of complete tumour response was significantly higher in patients with low BMI. Multivariate analysis revealed that low BMI independently predicted complete tumour response to preoperative chemotherapy. The low BMI group exhibited a longer median progression-free survival (PFS) than the high BMI group.
In CRLM patients receiving preoperative chemotherapy, a low BMI may be associated with better tumour response and longer PFS.
Whether deliberately increasing physical activity and weight loss may improve the response to chemotherapy in obese CRLM patients is an interesting topic worthy of further exploration.
Thanks to all authors for their efforts in this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martinou E, United Kingdom; Pavlidis TE, Greece S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64342] [Article Influence: 16085.5] [Reference Citation Analysis (175)] |
| 2. | Ecker BL, Lee J, Saadat LV, Aparicio T, Buisman FE, Balachandran VP, Drebin JA, Hasegawa K, Jarnagin WR, Kemeny NE, Kingham TP, Groot Koerkamp B, Kokudo N, Matsuyama Y, Portier G, Saltz LB, Soares KC, Wei AC, Gonen M, D'Angelica MI. Recurrence-free survival versus overall survival as a primary endpoint for studies of resected colorectal liver metastasis: a retrospective study and meta-analysis. Lancet Oncol. 2022;23:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 805] [Article Influence: 402.5] [Reference Citation Analysis (34)] |
| 4. | Viganò L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258:731-40; discussion 741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Zhao J, Sawo P, Rensen SS, Rouflart MMJ, Winstanley A, Vreuls CPH, Verheij J, van Mierlo KMC, Lodewick TM, van Woerden V, van Tiel FH, van Dam RM, Dejong CHC, Olde Damink SWM. Impact of chemotherapy-associated liver injury on tumour regression grade and survival in patients with colorectal liver metastases. HPB (Oxford). 2018;20:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Anaya DA, Dogra P, Wang Z, Haider M, Ehab J, Jeong DK, Ghayouri M, Lauwers GY, Thomas K, Kim R, Butner JD, Nizzero S, Ramírez JR, Plodinec M, Sidman RL, Cavenee WK, Pasqualini R, Arap W, Fleming JB, Cristini V. A Mathematical Model to Estimate Chemotherapy Concentration at the Tumor-Site and Predict Therapy Response in Colorectal Cancer Patients with Liver Metastases. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Serayssol C, Maulat C, Breibach F, Mokrane FZ, Selves J, Guimbaud R, Otal P, Suc B, Berard E, Muscari F. Predictive factors of histological response of colorectal liver metastases after neoadjuvant chemotherapy. World J Gastrointest Oncol. 2019;11:295-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Baldin P, Carrasco J, Beniuga G, Jouret-Mourin A, Demolin G, Roland S, D'Hondt L, Vergauwe P, Van Daele D, Mailleux M, Sinapi I, De Cuyper A, Blétard N, Massart B, Delos M, Castella ML, van Maanen A, Van den Eynde M. Randomized Phase 2 Study Comparing Pathological Responses of Resected Colorectal Cancer Metastases after Bevacizumab with mFOLFOX6 or FOLFIRI (BEV-ONCO Trial). Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 587] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 10. | Patel GS, Ullah S, Beeke C, Hakendorf P, Padbury R, Price TJ, Karapetis CS. Association of BMI with overall survival in patients with mCRC who received chemotherapy versus EGFR and VEGF-targeted therapies. Cancer Med. 2015;4:1461-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Artaç M, Korkmaz L, Coşkun HŞ, Dane F, Karabulut B, Karaağaç M, Çabuk D, Karabulut S, Aykan NF, Doruk H, Avcı N, Turhal NS. Bevacuzimab May Be Less Effective in Obese Metastatic Colorectal Cancer Patients. J Gastrointest Cancer. 2019;50:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, Krausé D, Hillon P, Borg C, Chauffert B, Ghiringhelli F. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 876] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 14. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24754] [Article Influence: 1178.8] [Reference Citation Analysis (0)] |
| 15. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 16. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, Mentha G, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 763] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 17. | Park IJ, You YN, Skibber JM, Rodriguez-Bigas MA, Das P, Eng C, Kopetz S, Wolff RA, Crane CH, Krishnan S, Minsky B, Hu CY, Nguyen S, Chang GJ. Oncologic and Functional Hazards of Obesity Among Patients With Locally Advanced Rectal Cancer Following Neoadjuvant Chemoradiation Therapy. Am J Clin Oncol. 2017;40:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Sun Y, Xu Z, Lin H, Lu X, Huang Y, Huang S, Wang X, Chi P. Impact of body mass index on treatment outcome of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur J Surg Oncol. 2017;43:1828-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Hopirtean C, Ciuleanu T, Cainap C, Todor N, Nagy V. body mass index as a prognostic factor for disease progression in patients with metastatic colorectal cancer treated with bevacizumab based systemic therapy. Acta Endocrinol (Buchar). 2017;13:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 520] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 21. | Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond). 2005;29:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Lashinger LM, Rossi EL, Hursting SD. Obesity and resistance to cancer chemotherapy: interacting roles of inflammation and metabolic dysregulation. Clin Pharmacol Ther. 2014;96:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Chen YC, Chien CY, Hsu CC, Lee CH, Chou YT, Shiah SG, Liu SY, Yen CY, Hsieh AC, Wabitsch M, Shieh YS. Obesity-associated leptin promotes chemoresistance in colorectal cancer through YAP-dependent AXL upregulation. Am J Cancer Res. 2021;11:4220-4240. [PubMed] |
| 24. | Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G 2nd, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 452] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 25. | Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi Y, Seke Etet PF. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer. 2013;20:R1-R17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 26. | Himbert C, Ose J, Gigic B, Viskochil R, Santuci K, Lin T, Ashworth A, Cohan JN, Scaife CL, Jedrzkiewicz J, Damerell V, Atkins KM, Gong J, Mutch MG, Bernadt C, Felder S, Sanchez J, Cohen SA, Krane MK, Hinkle N, Wood E, Peoples AR, Figueiredo JC, Toriola AT, Siegel EM, Li CI, Shibata D, Boucher K, Round JL, Ulrich AB, Schneider M, Huang LC, Hardikar S, Ulrich CM. Associations of combined physical activity and body mass index groups with colorectal cancer survival outcomes. BMC Cancer. 2023;23:300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527-3534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 584] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 28. | Guercio BJ, Zhang S, Ou FS, Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, O'Neil BH, Shaw JE, Polite BN, Hochster HS, Atkins JN, Goldberg RM, Sato K, Ng K, Van Blarigan E, Mayer RJ, Blanke CD, O'Reilly EM, Fuchs CS, Meyerhardt JA. Associations of Physical Activity With Survival and Progression in Metastatic Colorectal Cancer: Results From Cancer and Leukemia Group B (Alliance)/SWOG 80405. J Clin Oncol. 2019;37:2620-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, Pollak MN, Giovannucci EL, Fuchs CS. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 31. | Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, Adloff K, Keshaviah A, Winer EP. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Chu SH, Park JH, Lee MK, Jekal Y, Ahn KY, Chung JY, Lee DH, Kim ES, Naruse M, Im JA, Kong ID, Chung CH, Lee JW, Chung KM, Kim YB, Jeon JY. The association between pentraxin 3 and insulin resistance in obese children at baseline and after physical activity intervention. Clin Chim Acta. 2012;413:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Kim ES, Im JA, Kim KC, Park JH, Suh SH, Kang ES, Kim SH, Jekal Y, Lee CW, Yoon YJ, Lee HC, Jeon JY. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity (Silver Spring). 2007;15:3023-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, Irwin ML, Rudolph RE, Rajan KB, Stanczyk F, Bowen D, Weigle DS, Potter JD, McTiernan A. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 883] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 36. | Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 37. | Lund EK, Belshaw NJ, Elliott GO, Johnson IT. Recent advances in understanding the role of diet and obesity in the development of colorectal cancer. Proc Nutr Soc. 2011;70:194-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 39. | Meyerhardt JA, Sato K, Niedzwiecki D, Ye C, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, Wigler DS, Venook A, Fuchs CS. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst. 2012;104:1702-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 40. | Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015;33:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Aminian A, Wilson R, Al-Kurd A, Tu C, Milinovich A, Kroh M, Rosenthal RJ, Brethauer SA, Schauer PR, Kattan MW, Brown JC, Berger NA, Abraham J, Nissen SE. Association of Bariatric Surgery With Cancer Risk and Mortality in Adults With Obesity. JAMA. 2022;327:2423-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 201] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 42. | Pararas N, Pikouli A, Dellaportas D, Nastos C, Charalampopoulos A, Muqresh MA, Bagias G, Pikoulis E, Papaconstantinou D. The Protective Effect of Bariatric Surgery on the Development of Colorectal Cancer: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Khalid SI, Maasarani S, Wiegmann J, Wiegmann AL, Becerra AZ, Omotosho P, Torquati A. Association of Bariatric Surgery and Risk of Cancer in Patients With Morbid Obesity. Ann Surg. 2022;275:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Almazeedi S, El-Abd R, Al-Khamis A, Albatineh AN, Al-Sabah S. Role of bariatric surgery in reducing the risk of colorectal cancer: a meta-analysis. Br J Surg. 2020;107:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 45. | Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 558] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 46. | Sundaram S, Le TL, Essaid L, Freemerman AJ, Huang MJ, Galanko JA, McNaughton KK, Bendt KM, Darr DB, Troester MA, Makowski L. Weight Loss Reversed Obesity-Induced HGF/c-Met Pathway and Basal-Like Breast Cancer Progression. Front Oncol. 2014;4:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Patterson RE, Marinac CR, Sears DD, Kerr J, Hartman SJ, Cadmus-Bertram L, Villaseñor A, Flatt SW, Godbole S, Li H, Laughlin GA, Oratowski-Coleman J, Parker BA, Natarajan L. The Effects of Metformin and Weight Loss on Biomarkers Associated With Breast Cancer Outcomes. J Natl Cancer Inst. 2018;110:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Sipe LM, Chaib M, Korba EB, Jo H, Lovely MC, Counts BR, Tanveer U, Holt JR, Clements JC, John NA, Daria D, Marion TN, Bohm MS, Sekhri R, Pingili AK, Teng B, Carson JA, Hayes DN, Davis MJ, Cook KL, Pierre JF, Makowski L. Response to immune checkpoint blockade improved in pre-clinical model of breast cancer after bariatric surgery. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Tang W, Ren L, Liu T, Ye Q, Wei Y, He G, Lin Q, Wang X, Wang M, Liang F, Cui Y, Xu J. Bevacizumab Plus mFOLFOX6 Versus mFOLFOX6 Alone as First-Line Treatment for RAS Mutant Unresectable Colorectal Liver-Limited Metastases: The BECOME Randomized Controlled Trial. J Clin Oncol. 2020;38:3175-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 50. | Chen Q, Wu C, Zhao H, Wu J, Zhao J, Bi X, Li Z, Huang Z, Zhang Y, Zhou J, Cai J. Neo-adjuvant Chemotherapy-Induced Neutropenia Is Associated with Histological Responses and Outcomes after the Resection of Colorectal Liver Metastases. J Gastrointest Surg. 2020;24:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |