Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4746
Revised: September 29, 2024
Accepted: October 18, 2024
Published online: December 15, 2024
Processing time: 99 Days and 3.9 Hours
Signet-ring cell carcinoma (SRCC) is a rare subtype of colorectal cancer. The incidence of primary colonic SRCC is relatively rare in pediatric patients, with a limited number of reported cases currently available. The prognosis for this specific tumor type is unfavorable, and the preoperative diagnosis presents challenges, potentially leading to misdiagnosis. This case report describes the diagnosis of primary SRCC in the colon of a 10-year-old girl.
The patient was admitted to the hospital due to abdominal pain and vomiting. A computed tomography scan revealed an irregular mass with soft tissue density in her transverse colon, showing uneven density and multiple calcifications. The patient underwent surgical resection of the affected bowel and lymph node dissection, which was confirmed by pathological examination to be SRCC infiltrating both nerves and the entire intestinal wall. Additionally, tumor thrombus formation was observed in blood vessels and lymphatic vessels, multiple can
Primary colonic SRCC is a rare malignant tumor with atypical clinical symptoms, and timely identification and intervention are crucial for improving the prognosis.
Core Tip: We present a case of primary signet-ring cell carcinoma of the colon in a 10-year-old girl. Pediatric signet-ring cell carcinoma is an exceptionally rare condition with atypical clinical symptoms, making early diagnosis challenging. The absence of specific clinical manifestations and the disease’s concealed location often result in oversight by both clinicians and parents. Therefore, when a child presents with persistent abdominal pain, unexplained intestinal obstruction, or refractory ascites, clinicians should strongly consider the possibility of a malignant tumor. Prompt abdominal computed tomography and contrast-enhanced computed tomography scans, along with colonoscopy if indicated, are essential for early detection and timely intervention.
- Citation: Lv L, Song YH, Gao Y, Pu SQ, A ZX, Wu HF, Zhou J, Xie YC. Signet-ring cell carcinoma of the transverse colon in a 10-year-old girl: A case report. World J Gastrointest Oncol 2024; 16(12): 4746-4752
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4746.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4746
The incidence of colorectal cancer (CRC) increases steadily with age, surging significantly after the age of 50 and peaking among individuals aged 71-80[1]. In contrast, the occurrence of CRC in individuals 20 years and younger is exceptionally rare, with an incidence rate of only 1-2 cases per million[2]. Among patients under 15 years old with fatal malignancies, CRC accounts for less than 0.4% of cases[3,4], and only 12%-20% of these patients are below 10 years of age[5]. Adenocarcinoma is the most common subtype of CRC, while mucinous adenocarcinoma (MAC) represents approximately 10%-15% of cases, and signet-ring cell carcinoma (SRCC) accounts for only about 1%[6]. In 2023, a 10-year-old girl was admitted to our hospital with primary SRCC located in the transverse colon. The details of this case are presented below.
The patient presented with a history of persistent symptoms lasting over 20 days, including abdominal pain, distension, and episodes of emesis.
In April 2023, we treated a 10-year-old female patient with unexplained abdominal pain that started 20 days ago. The primary symptom was periumbilical pain accompanied by more than ten episodes of vomiting without any associated diarrhea. Additionally, there was a slight decrease in body weight.
The patient’s parents reported no prior history of abdominal pain or vomiting.
The patient’s parents and relatives have no history of cancer or gastrointestinal polyps.
The patient’s body temperature was 36.5°C. She appeared mildly anemic but showed no signs of jaundice in the skin or sclera. The abdomen was slightly distended, with no gastrointestinal or peristaltic waves visible and no abdominal varices. Tenderness was observed over the umbilicus without rebound pain. The liver and spleen were not palpable beneath the costal margin, and there were no signs of shifting dullness. Bowel sounds were active.
The routine blood test results were as follows: White blood cells 5.60 × 109/L; neutrophil granulocyte percentage 38.7%; red blood cells 3.5 × 1012/L; hemoglobin 92.0 g/L; platelets 336 × 109/L; and C-reactive protein 0.87 mg/L. Liver function and myocardial enzymes were as follows: Alanine transaminase 5.0 U/L; aspartate transaminase 23.0 U/L; alkaline phosphatase 66.0 U/L; γ-Gamma glutamyl transferase 7.0 U/L; total bilirubin 8.7 μmol/L; albumin 37.5 g/L; lactate dehydrogenase 219.0 U/L; creatine kinase 213.0 U/L; creatinine kinase-myocardial band 13.0 U/L; and α-hydroxybutyrate dehydrogenase 147.0 U/L. Tumor marker levels were: Alpha-fetoprotein 1.03 ng/mL; carcinoembryonic antigen 3.39 ng/mL; and neuron-specific enolase 22.34 ng/mL.
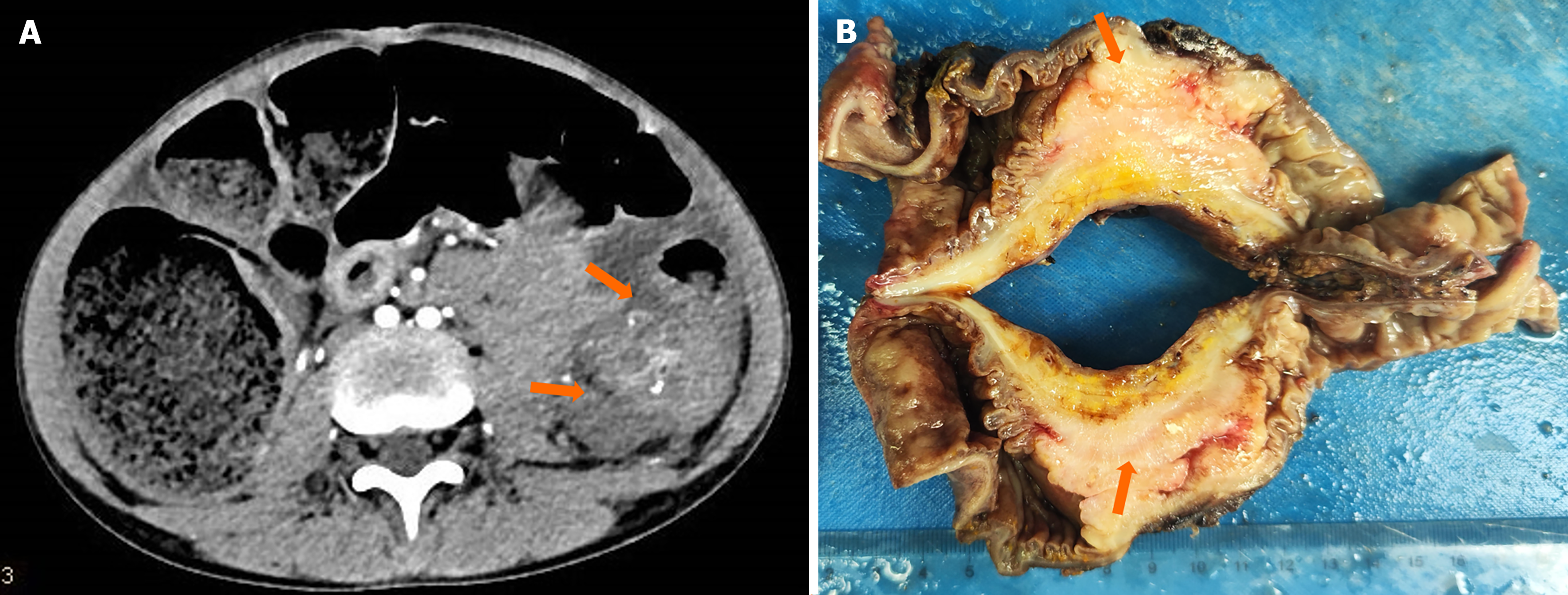
A computed tomography (CT) scan revealed irregular masses of soft tissue density in the transverse colon, exhibiting heterogeneous density and multiple calcifications. The largest cross-sectional dimension measured 4.6 cm × 3.8 cm. Contrast enhancement revealed uneven enhancement patterns and indistinct margins. The lesion exerted pressure on the adjacent descending colon, resulting in obstruction of the proximal transverse colon (Figure 1A).
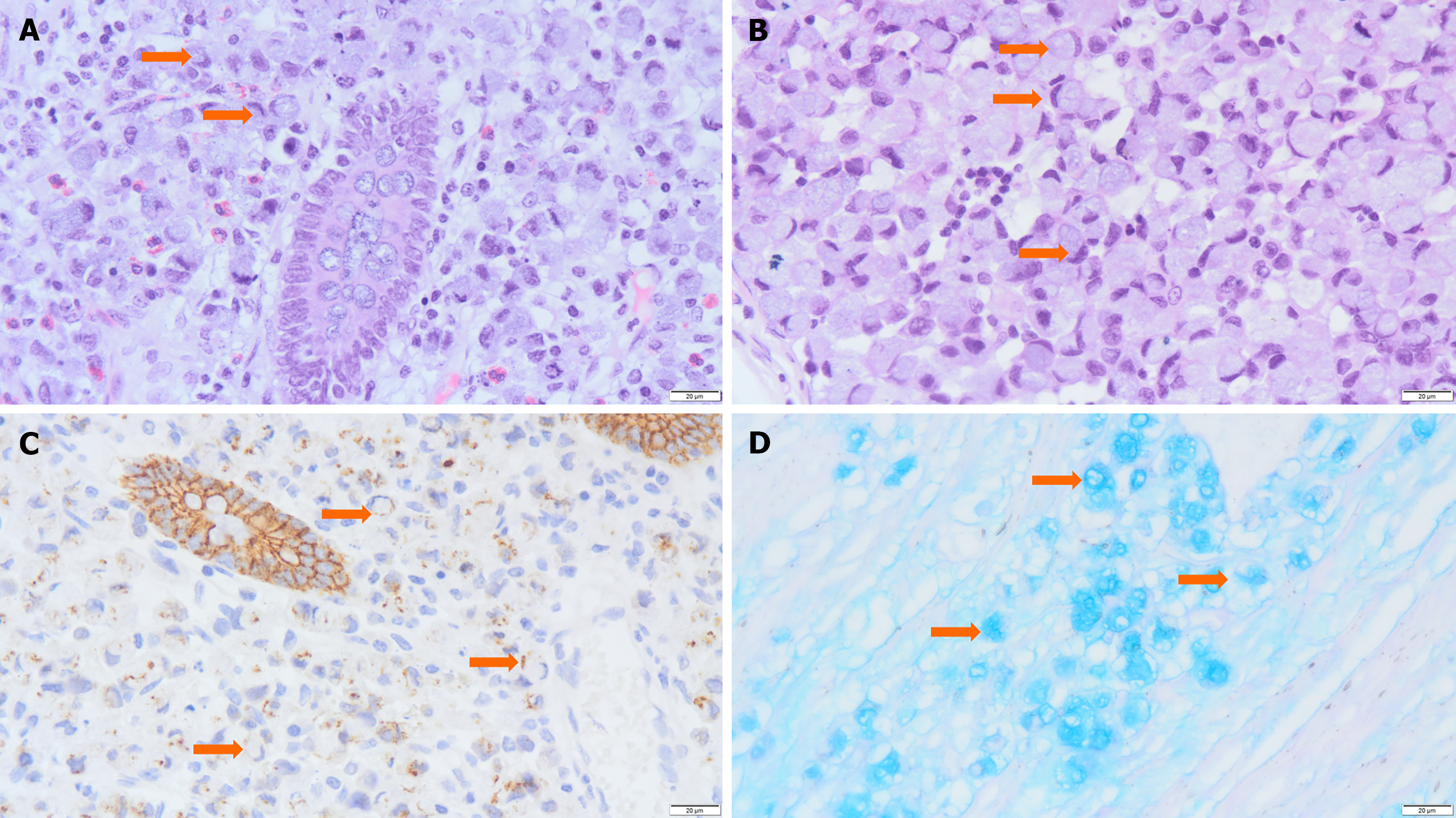
Exploratory surgery and biopsy revealed a transverse colonic mass as well as 26 mesenteric lymph nodes that may contain metastatic tumors. A gross pathological examination revealed a 5 cm × 4 cm × 3 cm mass in the intestinal wall with hardness and deformity, encircling the intestinal lumen. The mass had a mucosal surface and appeared gray-white, solid tissue with a tough texture. Tumor invasion into the serosa and surrounding adipose tissue was evident (Figure 1B). Histological examination revealed that tumor cells were distributed diffusely around the normal mucosal glands (Figure 2A), occurring either individually, in clusters, or small nests (approximately 80%). These cells exhibited weak adhesion and contained mucus. Most cells exhibited a signet-ring appearance due to cytoplasmic mucus crimping their nuclei (Figure 2B). Tumor cells had invaded the mucosal, submucosal, muscular, and serosal layers, with some areas forming mucus lakes (approximately 20%), where cancer cells floated. Nerve invasion was observed, as well as tumor thrombi in blood and lymphatic vessels. Multiple cancerous nodules were present in the omentum, and 18 of 26 mesenteric lymph nodes showed metastasis.
Immunohistochemistry revealed the following results: Cytokeratin pan (Pan-CK) (+) , Carcinoembryonic antigen (CEA) (+), Cytokeratin 7 (CK7) (-), Cytokeratin 20 (CK20) (+), Protein 53 (P53) (+), Ki67 protein (20%+), Cluster of Differentiation 34 (CD34) (-), D2-40 monoclonal antibody (D2-40) (-), S-100 (-), Mutl homolog 1(MLH1) (+), Muts homolog 2 (MSH2) (+), Muts homolog 6 (MSH6) (+), PMS1 homolog 2 (PMS2) (+), Epithelial-cadherin (E-Cadherin) (+, showing E-Cadherin was weaker in tumor cells) (Figure 2C), Caudal type homeobox transcription factor 2 (CDX-2) (+), Epithelial membrane antigen (EMA) (weakly positive), β-Catenin (+), Mucin 1 (MUC1) (+), Mucin 2 (MUC2) (+), Mucin 5AC (MUC5AC) (-), and Mucin 6 (MUC6) (-); Alcian Blue/Phosphoric Acid Schiff staining revealed blue-stained mucus distributed both inside and outside the cells (Figure 2D). The patient underwent genetic testing at other hospitals, and no mutations were observed in the KRAS, NRAS, BRAF, or PIK3CA genes.
The diagnosis of a malignant tumor in the transverse colon [pT4N2M1 IVc, microsatellite stability (MSS), SRCC] (American Joint Committee on Cancer tumor stage 9th edition) was confirmed based on the patient’s medical history and diagnostic findings.
The patient underwent surgery to remove a section of the transverse colon, leaving 4 cm of normal bowel on both ends of the lesion, and mesenteric lymph nodes were removed. After the surgical procedure, the patient underwent a course of 8 cycles of bevacizumab in combination with oxaliplatin, leucovorin, and fluorouracil chemotherapy. The specific treatment options were: Bevacizumab was injected at 100 mg once daily. The first infusion rate was maintained for 90 minutes, and the subsequent infusions were maintained for 60 minutes without any special circumstances. Oxaliplatin was given at a dose of 60 mg/m² after pretreatment with dexamethasone and promethazine to prevent allergic reactions. Additionally, leucovorin at a dose of 0.25 g/m2 was given as rescue therapy during oxaliplatin infusion to avoid cold stimulation and maintain warmth. Fluorouracil was administered initially at a dose of 0.25 g/m² followed by a continuous infusion of 0.75 g/m² over 48 hours. The patient tolerated the treatment well, with no adverse reactions such as vomiting or hair loss.
By May 2024, the patient had completed eight cycles of standard chemotherapy. A 12-month follow-up, including CT reexamination, showed no signs of tumor progression. Blood tests, liver and kidney function assessments, and tumor marker evaluations were also within normal ranges.
SRCC is a rare histological subtype of CRC, which was initially proposed by Saphir and Laufman[7] in 1951, accounting for less than 1% of all histological subtypes. Although SRCC primarily occurs in the stomach, rare cases have been documented in the gallbladder, pancreas, colon, rectum, bladder, and breast[8,9]. The incidence of CRC is much lower in children and adolescents than in adults. There are limited data available on pediatric SRCC, primarily consisting of case reports. Among the retrieved case reports, the youngest patient was 6 years old. SRCC patients often have advanced tumor stages[10]. In the present case report, the patient presented with a tumor in the left transverse colon.
Both MAC and SRCC are rare subtypes of CRC. According to the World Health Organization definition, tumors are classified as SRCC when the proportion of signet ring cell component in the intracellular mucus exceeds 50%. MAC with a signet ring cell component is diagnosed when it contains a notable amount of extracellular mucus forming a mucus pool, with less than 50% signet ring cells[11]. In this particular case, a small amount of mucus pooling was identified along with approximately 80% presence of signet ring cells, confirming the SRCC diagnosis. SRCC is an infrequent and aggressive malignant tumor originating from glandular epithelium in the digestive tract. The tumor cells exhibit a distinctive appearance reminiscent of signet rings, primarily caused by the excessive accumulation of mucin, leading to displacement of the nucleus towards the cell periphery[12]. Signet ring cells typically exist as single or loosely aggregated forms infiltrating diffusely into the mucosa and extending deep into intestinal layers, potentially reaching serosal surfaces and surrounding tissues. However, failure to breach the mucosal layer in some patients may lead to concealed disease presentation and atypical clinical manifestations associated with abnormal bowel movements.
The E-cadherin-catenin complex plays a crucial role in maintaining epithelial cell polarity, as evidenced by the positive expression levels of β-catenin (cell membrane) and E-cadherin (cell membrane and cytoplasm). SRCC is characterized by poor adherence due to dysfunction of the E-cadherin catenin complex, leading to loss of epithelial differentiation and structure or acquisition of a motile and invasive phenotype. In this case, there was a slight reduction in membranous localization of β-catenin protein accompanied by nuclear expression and downregulation of E-cadherin expression level. These alterations enable tumor cells to evade the surrounding microenvironment and exhibit enhanced metastatic potential, diminishing cell adhesion in mucus-rich regions and promoting tumor dissemination[13]. This trait leads to significant intramural infiltration of the tumor, resulting in diffuse thickening of the intestinal wall, luminal constriction, intestinal obstruction, and even inflammatory diseases[14,15]. Due to nonspecific clinical manifestations and inconspicuous localization of the disease, it is susceptible to being overlooked by clinicians and pediatric patients’ parents.
SRCC is a rare subtype of pediatric CRC with a dismal prognosis, necessitating differentiation from the following tumors: (1) Lynch syndrome (LS) is an autosomal dominant tumor syndrome caused by mutations in mismatch repair genes (MMR) or deletions in the EPCAM gene, accounting for 3%-5% of CRC[16]. The National Comprehensive Cancer Network guidelines recommend that all patients with newly diagnosed CRC should undergo microsatellite instability or MMR gene deletion testing[17] to screen for LS. Since there was no history of the disease in our patient’s family, immunohistochemistry was performed to detect MMR proteins MLH1, MSH2, MSH6, and PMS2. The results showed MSS, ruling out LS; and (2) Metastatic gastric SRCC: SRCC typically influences the stomach. Its microscopic morphology resembles that of intestinal SRCC, while its immunohistochemical expression differs. Primary gastric SRCC typically expresses MUC5AC and MUC6, while it lacks expression levels of MUC1 and MUC2. Conversely, primary SRCC of the large intestine expresses MUC1, MUC2, and MUC5A rather than MUC6[18]. EMA is frequently expressed in primary gastric SRCC rather than CDX-2. On the other hand, primary colorectal SRCC mainly exhibits CDX-2 expression without EMA expression; thus, downregulation of EMA may be associated with the carcinogenesis of colorectal SRCC[19]. Therefore, evaluating the apolipoprotein-MUC expression pattern along with EMA and CDX-2 can assist in distinguishing between metastatic sites and primary gastric or colorectal SRCC.
The incidence of pediatric CRC (PCRC) in China is relatively low compared to adults, with a rate of 0.18%, according to a single-center study. Most lesions are found in the transverse colon, and SRCC often shows deep invasion. In the early stages, there are no specific clinical symptoms, which can be similar to inflammatory bowel disease, constipation or pneumatosis intestinalis, and other functional bowel diseases. Due to limited experience with such cases, pediatricians may easily miss or misdiagnose PCRC. Abdominal pain, hematochezia, and intestinal obstruction are the main symptoms observed in later stages of the disease. Therefore, PCRC is often diagnosed at an advanced clinical stage compared to adult cases that commonly involve the rectum and exhibit changes in defecation habits and stool characteristics; they are diagnosed based on elevated blood CEA levels. Adenocarcinoma is the most common type of CRC among both adults and children, but MAC and SRCC predominate among children, leading to poor prognosis[20,21].
The treatment of SRCC in children follows the treatment guidelines for adult CRC, and personalized treatment should be considered. The optimal treatment options primarily consist of surgical intervention and adjuvant chemotherapy. Evidence has demonstrated that removing an adequate number of lymph nodes (≥ 4 regions) during colorectal SRCC surgery could significantly improve the patient’s prognosis. Among patients with stage III colorectal SRCC, those who received adjuvant chemotherapy showed a better prognosis compared with those who did not receive chemotherapy. However, the role of radiotherapy in colorectal SRCC remains elusive, and the evaluation of SRCC tissue alone or in combination with chemotherapy is lacking. Nonetheless, neoadjuvant chemoradiotherapy can yield favorable therapeutic outcomes in the rectal SRCC population.
This case report highlights the rarity of primary SRCC of the colon in children, a condition that often goes unnoticed by clinicians and parents due to the absence of specific clinical manifestations and its concealed location. The lack of distinctive laboratory and imaging findings frequently results in preoperative misdiagnosis. Early diagnosis and timely treatment are crucial for improving survival rates. Clinicians should be vigilant, conduct comprehensive examinations, promptly use endoscopy and imaging for early detection, ensure appropriate surgical intervention, and administer standardized chemotherapy postoperatively to improve prognosis. The incidence of SRCC in children is low and varies among individuals, so this case summary has limitations. More case summaries and further studies on the biology of pediatric SRCC are still needed to accurately understand its molecular mechanism and develop new treatment methods.
| 1. | Young JL Jr, Percy CL, Asire AJ, Berg JW, Cusano MM, Gloeckler LA, Horm JW, Lourie WI Jr, Pollack ES, Shambaugh EM. Cancer incidence and mortality in the United States, 1973-77. Natl Cancer Inst Monogr. 1981;1-187. [PubMed] |
| 2. | Karnak I, Ciftci AO, Senocak ME, Büyükpamukçu N. Colorectal carcinoma in children. J Pediatr Surg. 1999;34:1499-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Angelini C, Crippa S, Uggeri F, Bonardi C, Sartori P, Uggeri F. Colorectal cancer with neuroendocrine differentiation in a child. Pediatr Surg Int. 2005;21:839-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Yamamoto K, Tanaka T, Kuno K, Amoh Y, Takahashi Y, Murakami H. Carcinoma of the colon in children: case report and review of the Japanese literature. J Gastroenterol. 1994;29:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Jain AK, Motil KJ, Olutoye OO, Cope-Yokoyama S, Egler RA, Tatevian N. Colon cancer in a 16-year-old girl: signet-ring cell carcinoma without microsatellite instability--an unusual suspect. J Pediatr Gastroenterol Nutr. 2009;48:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | An Y, Zhou J, Lin G, Wu H, Cong L, Li Y, Qiu X, Shi W. Clinicopathological and Molecular Characteristics of Colorectal Signet Ring Cell Carcinoma: A Review. Pathol Oncol Res. 2021;27:1609859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch Surg. 1951;62:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Kang SH, Chung WS, Hyun CL, Moon HS, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY. A rare case of a signet ring cell carcinoma of the colon mimicking a juvenile polyp. Gut Liver. 2012;6:129-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol. 1996;91:2195-2199. [PubMed] |
| 10. | Wu P, Deng W, Yan L, Wang C, Lou Y, Wang C. Clinicopathologic and prognostic factors for colorectal cancer in children and adolescents: a population-based study. Int J Colorectal Dis. 2023;38:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Tan Y, Fu J, Li X, Yang J, Jiang M, Ding K, Xu J, Li J, Yuan Y. A minor (<50%) signet-ring cell component associated with poor prognosis in colorectal cancer patients: a 26-year retrospective study in China. PLoS One. 2015;10:e0121944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Benedix F, Kuester D, Meyer F, Lippert H. [Influence of mucinous and signet-ring cell differentiation on epidemiological, histological, molecular biological features, and outcome in patients with colorectal carcinoma]. Zentralbl Chir. 2013;138:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Börger ME, Gosens MJ, Jeuken JW, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Signet ring cell differentiation in mucinous colorectal carcinoma. J Pathol. 2007;212:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Zhou JL, Zhao XY, Lin GL, Qiu HZ, Xiao Y, Wu B, Lu JY, Niu BZ, Sun XY, Zhong GX. [Clinicopathological characteristics, diagnosis, and treatment of 29 cases of signet ring cell carcinoma of the rectum and sigmoid colon]. Zhonghua Zhong Liu Za Zhi. 2020;42:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Papp JP Jr, Levine EJ, Thomas FB. Primary linitis plastica carcinoma of the colon and rectum. Am J Gastroenterol. 1995;90:141-145. [PubMed] |
| 16. | Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA, Panescu J, Fix D, Lockman J, LaJeunesse J, Comeras I, de la Chapelle A. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 655] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 17. | Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 567] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 18. | Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:613-621. [PubMed] |
| 19. | Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: III. Expressions of EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:630-638. [PubMed] |
| 20. | Yang LL, Wang M, He P. Clinicopathological characteristics and survival in colorectal signet ring cell carcinoma: a population-based study. Sci Rep. 2020;10:10460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Jayanand SB, Seshadri RA, Tapkire R. Signet ring cell histology and non-circumferential tumors predict pathological complete response following neoadjuvant chemoradiation in rectal cancers. Int J Colorectal Dis. 2011;26:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |