Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4700
Revised: September 17, 2024
Accepted: October 16, 2024
Published online: December 15, 2024
Processing time: 148 Days and 22.5 Hours
The 5-year survival rate of patients with colorectal cancer (CRC) in China is only 56.9%, highlighting the need for new therapeutic drugs. Previous studies have shown that matrine exhibits antitumor effects by inducing apoptosis. However, the mechanism by which matrine regulates antiapoptotic proteins in CRC remains unclear.
To identify apoptotic proteins from proteomics and investigate the role of matrine in impeding CRC apoptosis by regulating these proteins.
Tumor and adjacent normal tissues were collected from 52 patients with CRC who underwent surgery between January and December 2021. Data-independent acquisition quantitative proteomic analysis was performed to identify differentially expressed apoptotic proteins. The selected apoptotic proteins were identified through their association with tumor-node-metastasis (TNM) stage and prognosis, then confirmed by immunohistochemical (IHC) staining in validation cohort. In vitro, the role of matrine or apoptotic proteins on cancer cells were analyzed.
Compared to normal tissues, 88 anti-apoptotic proteins from proteomic results were selected. Among them, Shank-associated RH domain interactor (SHARPIN) was identified because of its relationship with TNM stage and overall survival in TCGA database. In the IHC-confirmed cohort, SHARPIN was highly expressed in CRC tissues and localized in the cytoplasm. Higher SHARPIN expression was associated with TNM stage, carbohydrate antigen 153 levels, and gross type compared to low expression. SHARPIN knockdown promoted apoptosis, significantly upregulated the expression of Bcl-2 associated agonist of cell death, Bcl-2 associated X protein, caspase 3, and caspase 8, and downregulated B-cell lymphoma-2 (P < 0.05). Importantly, matrine treatment promoted apoptosis and reversed the proliferation, invasion, and migration of CRC cells by repressing SHARPIN.
SHARPIN was identified as an upregulated anti-apoptotic protein in CRC, and matrine exhibited anticancer effects by downregulating its expression. Thus, matrine appears to be a promising drug for CRC.
Core Tip: Despite advances in therapy for colorectal cancer (CRC), the 5-year survival rate for CRC patients in China remains only 56.9%. This study explored the effects of matrine on CRC by targeting a newly identified anti-apoptotic protein, Shank-associated RH domain interactor (SHARPIN). SHARPIN was discovered through proteomic analysis and its expression was validated in both the TCGA database and our patient cohort using immunohistochemistry. Inhibiting SHARPIN expression led to increased apoptosis and reduced proliferation, invasion, and migration of CRC cells in vitro. Matrine's ability to inhibit SHARPIN and induce apoptosis highlights its potential as a promising therapeutic agent for CRC.
- Citation: Zhou YC, Wang QQ, Zhou GYJ, Yin TF, Zhao DY, Sun XZ, Tan C, Zhou L, Yao SK. Matrine promotes colorectal cancer apoptosis by downregulating shank-associated RH domain interactor expression. World J Gastrointest Oncol 2024; 16(12): 4700-4715
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4700.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4700
Colorectal cancer (CRC) is the third most common cancer type worldwide and ranks second in China[1]. It imposes a significant burden on both individuals and the healthcare system because of its high incidence and mortality rate. Despite notable advancements in systemic therapy, CRC prognosis remains poor. In China, although 5-year survival rate increased from 47.2% to 56.9% between 2003 and 2015, it still lagged that of a developed country[2]. The poor prognosis of CRC can be attributed to factors, such as advanced TNM stage, genetic dysregulation or mutation, and drug resistance. Hence, it is imperative to explore novel targets and corresponding drugs to enhance the treatment efficacy.
Cell death resistance is a crucial hallmark of cancer[3]. The dysregulation of apoptotic proteins promotes CRC pro
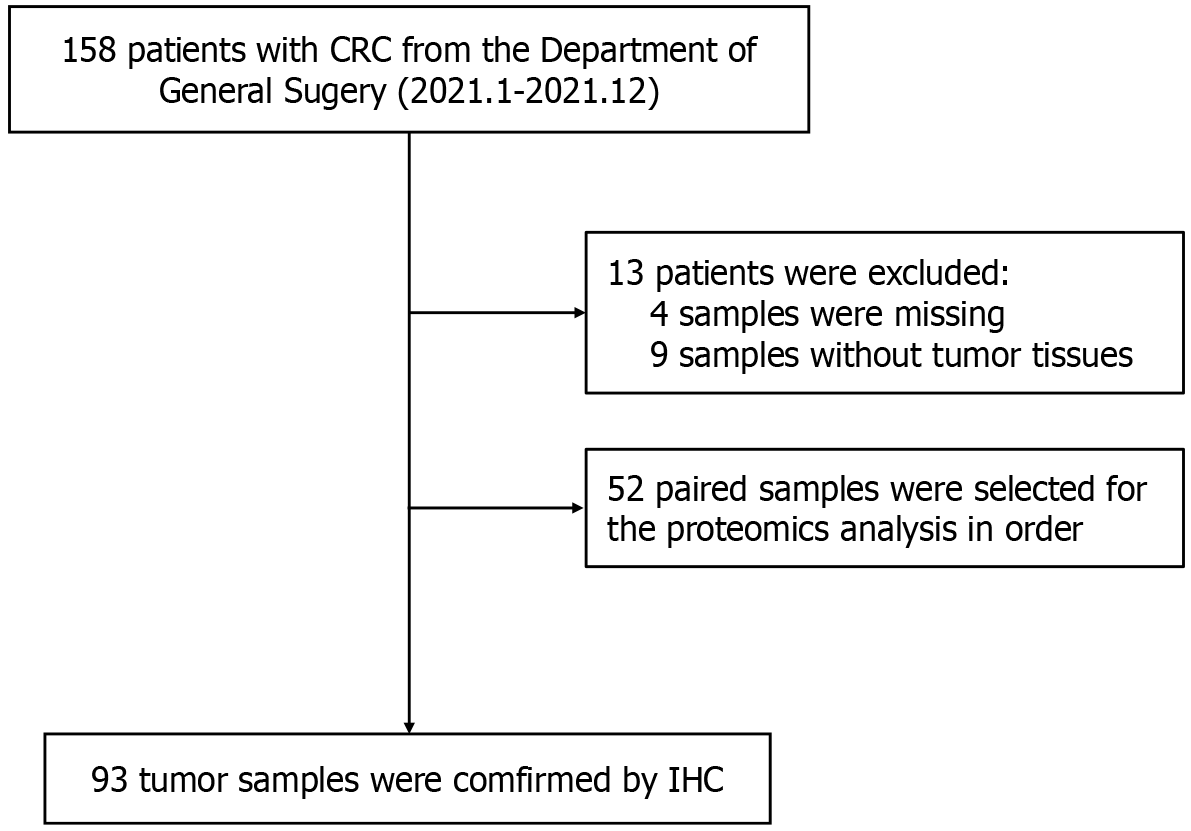
From January 2021 to December 2021, we recruited patients diagnosed with CRC (n = 158) and health control subjects (n = 35) at our hospital. First, 52 paired tumor and adjacent tissues (5 cm away from the tumor) were collected from patients with CRC for proteomic analysis. Four CRC samples were missing, and 9 samples did not contain tumor tissue. Finally, 93 tumor tissues from patients with CRC (Figure 1) and 35 normal tissues from healthy subjects were used as a validation cohort to examine protein expression using immunohistochemistry (IHC). Clean Tissues were transferred by liquid nitrogen and stored in a -80 °C freezer. Other tissues were fixed and processed into sections.
Surgical resection samples diagnosed with CRC were included. Inflammatory bowel disease–associated, hereditary, and neuroendocrine CRC were excluded. Additionally, patients who had received chemoradiotherapy, targeted therapy, immunotherapy, or participated in clinical trials within the past 6 months were also excluded. All enrolled patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of the China-Japan Friendship Hospital (2018-116-K85).
The 52 pairs of CRC tissue samples were analyzed using high-performance liquid chromatography–mass spectrometry/mass spectrometry to identify differentially expressed proteins (DEPs). Samples were prepared through various processes, including protein denaturation, reduction, alkylation, tryptic digestion, and peptide clean-up, using an iST Sample Preparation Kit (PreOmics, Germany). The eluted peptides were lyophilized using SpeedVac. The peptides were then redissolved in solvent A (0.1% formic acid in water) and analyzed using an Orbitrap Fusion Lumos Tribrid coupled to an EASY-nano LC 1200 system (Thermo Fisher Scientific, MA, United States). The mass spectrometer was run in data independent acquisition (DIA) mode with a hybrid data strategy[4].
Raw DIA data were processed and analyzed using Spectronaut 15.0 (Biognosys AG, Switzerland) with default settings. The spectra were configured to search the UniProt-Homo sapiens database (version201907, 20428 entries), assuming trypsin as the digestion enzyme. The Q value false discovery rate (FDR) cutoff was set to 1% at both the precursor and protein levels. Decoy generation was set to mutated, which is similar to scrambled generation but only applies a random number of amino acid position swamps (min = 2, max = length/2). A normalization strategy was set for local normalization. The average of the top three filtered peptides that passed the 1% Q value cutoff was used to calculate the major group quantities. DEPs were selected if their Padj value was < 0.05, and absolute fold change (FC) > 2 after the t-test.
Quality control showed a low coefficient of variation and good reliability of the proteomic results: (1) DEPs were analyzed using R packages such as BiocManager, org.Hs.eg.db, colorspace, stringi, and ggplot2, and pairwise comparisons between two groups. The differential protein screening criteria were a t-test with Benjamini & Hochberg-corrected P value < 0.05 and an absolute FC > 2. Principal component analysis (PCA) was performed to determine the differences between tumor and normal samples. The upregulated and downregulated DEPs are displayed as volcano plots, and hierarchical clustering analysis was performed for expressed proteins and samples simultaneously, with the results displayed as heatmaps; and (2) Functional annotation and enrichment analyses were performed on all identified DEPs, and their functions were marked using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG).
RNA sequencing quantification and relevant clinical and survival data[5] of CRC tissues (n = 647) and normal colorectal tissues (n = 51) were obtained from TCGA database. (https://portal.gdc.cancer.gov). The ggplot2 and survminer packages were used to visualize the analyzed data.
Methanal-Fixed and Paraffin-Embedded tumors were stained for the Shank-associated RH domain interactor (SHARPIN) protein. Antigen retrieval was performed using an automatic antigen repair instrument (LBP-5196-II; Guangzhou LBP Medicine Science & Technology Co., Ltd) for 15 minutes in Tris-EDTA buffer (pH 9.0), followed by treatment with an enhanced endogenous peroxidase-blocking buffer (P0100B, Beyotime, Shanghai, China) for 10 minutes. Specimens were incubated overnight at 4 °C with polyclonal antibodies against human SHARPIN (1:100; 14626-1-AP, Proteintech, Wuhan, China). After rinsing three times with phosphate buffered saline (PBS), the slides were incubated with goat anti-rabbit IgG (1:2000, ab6721; Abcam) for 1 hour at room temperature. A coloring reaction was performed using a 3,3N-Diaminobenzidine Tertrahydrochloride chromogenic kit (ZLI-9017; Zhongshan Golden Bridge, Beijing, China). Nuclei were stained with Harris hematoxylin and mounted with neutral gum. Finally, the slides were photographed using a BX53 upright bright-field microscope (Olympus).
The ImageJ software was used for image quantification. SHARPIN expression was quantified using the H-score method in ImageJ; weak immunostaining was assigned a score of 1, moderate immunoreactivity was assigned a score of 2, and strong immunostaining was assigned a score of 3. The percentage of tumor cells stained at each intensity level was multiplied by the respective numeric score of the staining intensity, and the results were summed to quantify the total H-score.
Demographic data, clinicopathological features, and tumor markers, including age, sex, gross type, differentiation, tumor size, tumor location, histopathological type and manifestation, microsatellite instability, TNM stage, carcinoembryonic antigen, carbohydrate antigen 199 (CA199), CA125, CA153, and CA724 were collected from the medical record system.
Two CRC cells, HCT116 and SW480, obtained from the Procell Life Science & Technology Co., Ltd (Wuhan, China), were cultured in McCoy’s 5A or DMEM supplemented 10% fetal bovine serum (FBS) (Gibco), 1% penicillin/streptomycin solution (C125C5, NCM Biotech) at 37.7 °C in a humidified atmosphere with 5% CO2.
CRC cells were transfected with SHARPIN small interfering RNA (GenePharma) or negative control siRNA (GenePharma). The sequence pairs of the SHARPIN were as followed: Sense, 5′-CCUGAGGCAGAUCUUCCUATT-3’, antisense, 5′-UAGGAAGAUCUGCCUCAGGTT-3’. Lipofectamine 3000 Transfection Reagent (Invitrogen) was used to transfect CRC cells with the above siRNAs, according to the manufacturer’s instructions. After 24 hours, the CRC cells were collected for subsequent experiments.
Matrine (MCE) stock solution was diluted with dimethyl sulfoxide and stored in -20 °C in the dark. CRC cells were treated with matrine at concentration of 2.5 mg/mL for 48 hours, which refer to previously described[6].
Total RNA was extracted from the cells using TRIzol reagent (Vazyme, Nanjing, China). The following primers were used for SHARPIN: Forward 5′- TGTTCTCAGAGCTCGGTTT -3′ and reverse 5′- AAGTTCCCCGTCCATCTT -3′. Fluorescence was detected using a CFX Connect instrument (Bio-Rad). Each sample was run in triplicate and compared using GAPDH as the reference gene. Results were analyzed using the 2-ΔΔCT method for the relative quantification of mRNA expression.
Cell lysis buffer for western blotting, IP (P0013, Beyotime), and a protease and phosphatase inhibitor cocktail (P1045, Beyotime) were used for protein extraction. The BCA Protein Assay Kit (P0012S, Beyotime) was used to measure the amount of protein.
Total proteins were subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis and electrotransferred to PVDF membranes (Merck Millipore, China). Nonspecific binding sites were blocked using 5% nonfat milk for 2 hours and the membranes were incubated at 4 °C overnight with SHARPIN Monoclonal antibody (1:1000, Immunoway, United States), Caspase 3/p17/p19 Polyclonal antibody (1:1000, Proteintech), Caspase 8/p43/p18 Polyclonal antibody (1:1000, Proteintech), B-cell lymphoma-2 (Bcl-2) Polyclonal antibody (1:1000, Proteintech), Bcl-2 associated agonist of cell death (BAD) Polyclonal antibody (1:1000, Proteintech), Bcl-2 associated X protein (BAX) Polyclonal antibody (1:1000, Proteintech), GAPDH antibody (1:4000; Abcam), β-actin (1:1000, Proteintech). The PVDF membranes were then treated for 1 hour with a goat anti-rabbit secondary antibody (1:10000; D21109-35, IRDye® 800CW, LI-COR, United States). Fluorescence imaging was performed using an LI-COR Odyssey CLx imaging system. ImageJ software was used to quantify the chemiluminescent signals of the protein bands, using GAPDH, β-actin or tubulin as an internal control.
An Annexin V-fluorescein isothiocyanate/propidium iodide apoptosis kit (556547, BD, United States) was used to detect the proportion of apoptotic cells after transfection. All procedures were performed according to manufacturer’s instructions. Flow cytometry (FACSCanto II, BD, United States) and FlowJo (V_10.8.1) software were used to determine cell apoptosis rates.
The collected CRC cells were seeded in each well of a 96-well plate at a density of 1 × 104 cells/well. Then, at 0 hour, 24 hours, 48 hours and 72 hours, 10 μL of cell counting kit-8 (CCK-8) reagent (C0037, Beyotome) and 90 μL of medium were added after the previous medium was removed. Then, the cells were incubated for 1 hours at 37 °C with 5% CO2, and the optical density was detected at 450 nm using a microplate reader (Tecan infinite M200, Switzerland).
CRC cells were suspended in 200 μL of serum-free medium and seeded in the upper chambers coated with matrigel (Corning, United States). The bottom 24-well plates were filled with 600 μL of medium containing 10% FBS as a chemoattractant. After incubation for 72 hours, the cells remaining in the upper chamber were gently removed using cotton swabs, and the cells on the lower surface were photographed and counted after fixing with 4% paraformaldehyde and staining with 0.1% crystal violet.
CRC cells were cultured in 6-well plates for 24 hours, and cell transfection was performed as described above. After incubation for 48 hours, a wound was scratched with a 200 µL sterile pipette tip. The cells were then washed with PBS 3 times and incubated in a serum-free medium. Representative images were captured 0 and 24 hours after scratching. The total wound area was measured using the ImageJ software, and the relative migration rate was calculated.
Statistical programs for R software (version 4.0.3) and GraphPad Prism (version 9) were used to perform statistical analyses. Quantitative data with a normal distribution are presented as the mean ± SD and were analyzed using Student’s t-test or one-way analysis of variance, while variables with a non-normal distribution are presented as the median and interquartile range and were compared using a Mann-Whitney U test or Kruskal-Wallis’s test. Categorical variables were analyzed using the χ2 test or a nonparametric test. Receiver operating characteristic curves were constructed to examine the diagnostic efficiency of RNA molecules by assessing the area under the curve. Statistical significance was set at P < 0.05.
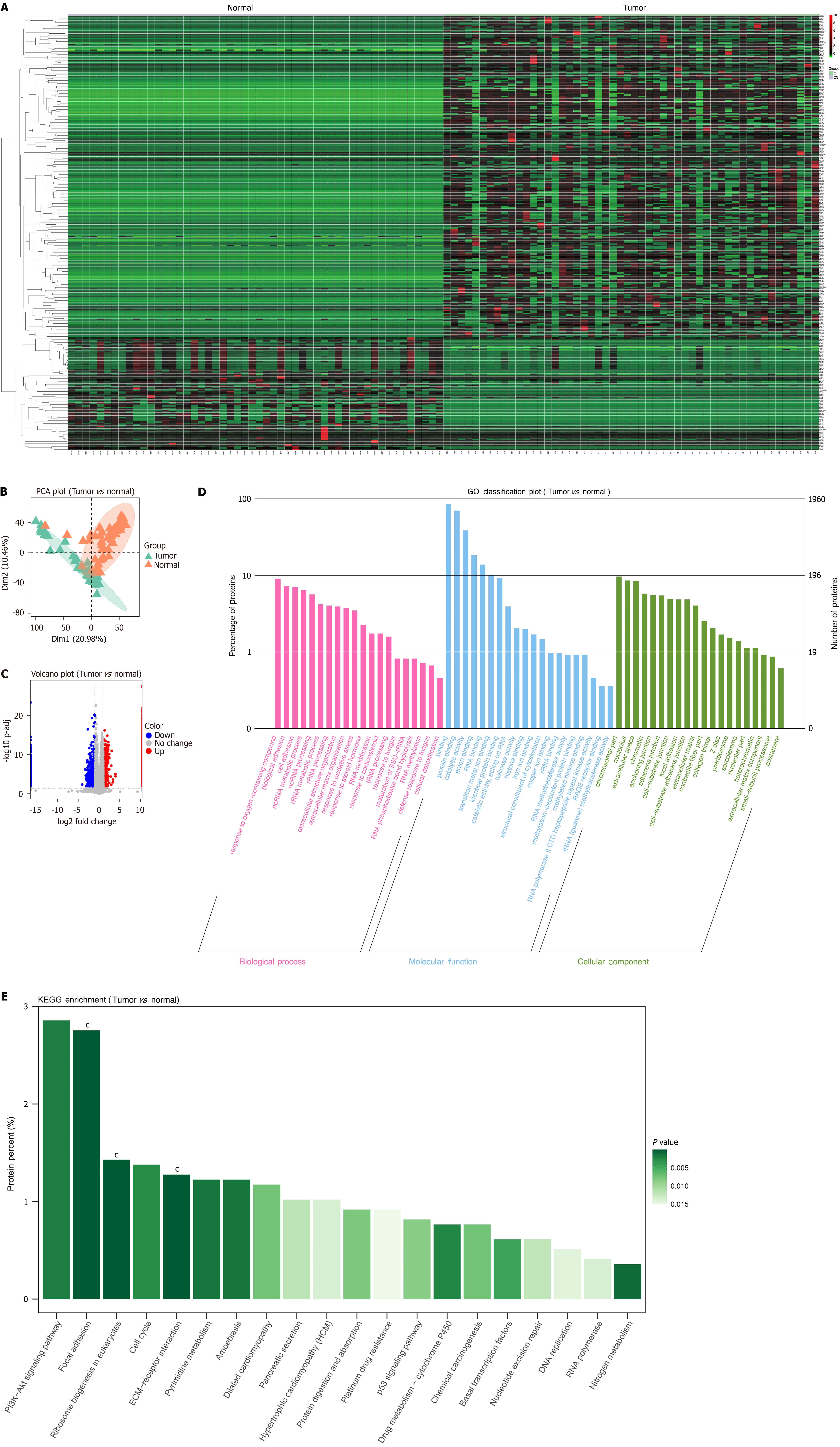
52 pairs of cancerous and matched adjacent normal tissues were collected from patients with CRC after surgical treatment for quantitative proteomic detection. 46.2% CRC patients were male, with a mean age of 64.6 ± 15.1 yeas old (Supplementary Table 1). A total of 7666 proteins were identified, and PCA demonstrated a clear separation between the CRC and normal groups (Figure 2A and B). Among these, 1960 DPEs with an FDR of < 1% were identified between the two groups (Figure 2C).
GO and KEGG enrichment analyses were performed for 1960 DEPs. GO enrichment analysis (Figure 2D) revealed the significant enrichment of various biological processes, molecular functions, and cellular components. In the biological processes, enrichment was observed in response to stimuli (oxygen-containing compounds, oxidative stress, and corticosteroids), RNA metabolic processes (ncRNA metabolic processes, rRNA metabolic processes, etc.), and other processes. In terms of molecular functions, enrichment was noted for binding (proteins, ions, etc.) and catalytic activity (acting on RNA). Among the cellular components, enrichment was found in organelles (chromosomal part, nucleolus, etc.), extracellular regions (extracellular space, extracellular matrix, etc.), and cell junctions (anchoring junction, adheres junction, etc.). KEGG pathway enrichment analysis (Figure 2E) indicated the activation of several pathways, including the PI3K-Akt signaling pathway, focal adhesion, cell cycle, and extracellular matrix-receptor interaction.
The enrichment of responses to stimuli in biological processes is often associated with cell death. Resistance to cell death is a crucial hallmark of cancer and the upregulation of anti-apoptotic proteins is known to promote tumor pro
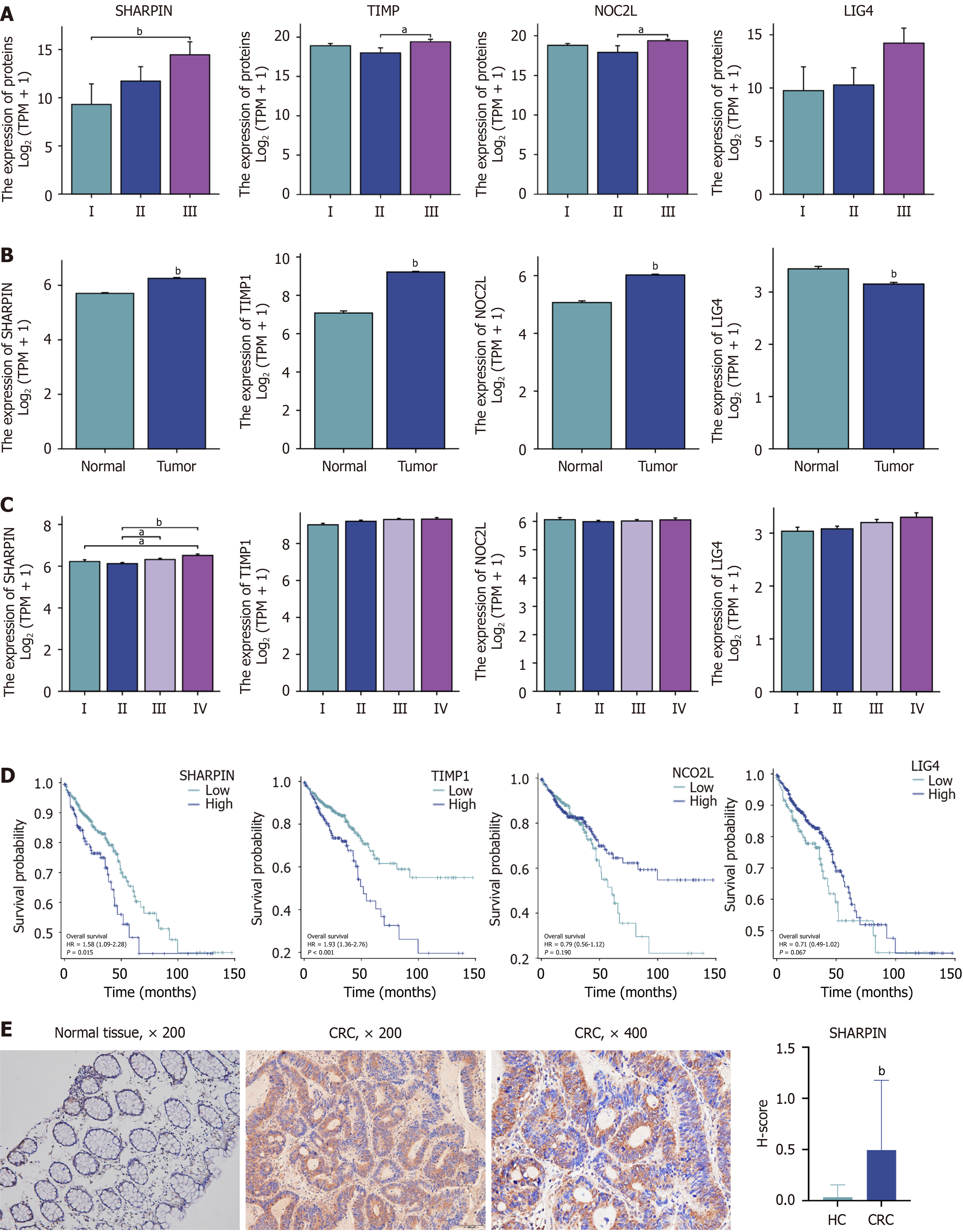
To identify these anti-apoptotic proteins, 88 proteins were selected by negative regulation of apoptotic proteins based on GO enrichment analysis and were upregulated in tumor tissue (Supplementary Table 2). SHARPIN, metalloproteinase inhibitor 1 (TIMP1), nucleolar complex protein 2 homolog (NOC2 L), and DNA ligase 4 (LIG4) were significantly different among the TNM stages (Supplementary Table 3). As shown in Figure 3A, the expression of SHARPIN was significantly higher in stage III than in stage I. TIMP1 and NOC2 L expression levels were significantly higher in stage III than in stage II. With the advancement of TNM staging, the expression of SHARPIN was gradually increased, but not that of the others.
SHARPIN, TIMP1, NOC2 L, and LIG4 were further confirmed using TCGA database. As shown in Figure 3B, expect for LIG4, the expression of SHARPIN, TIMP1, NOC2 L was higher in tumor tissues than in normal tissues. As shown in Figure 3C, only SHARPIN was associated with the TNM stage. In the survival analysis, higher expression of SHARPIN and TIMP1 significantly decreased the overall survival (Figure 3D). Thus, upregulation SHARPIN was associated with the pathological stage and prognosis of CRC.
IHC was conducted to investigate the expression of SHARPIN between CRC tumor tissues (n = 93) and normal tissues (n = 35). Compared with normal tissues (Figure 3E), CRC tissues showed significantly higher SHARPIN expression (P < 0.01). SHARPIN was hardly stained in normal tissues but was highly expressed in the cytoplasm of CRC cells.
93 CRC patients were divided into two groups according to their mean H-score. As indicated in Table 1, the average age was 67.5 ± 10.9 years, and 49 participants (52.7%) were male. SHARPIN expression correlated with serum CA153 Levels, gross type, T stage, N stage, and TNM stage (all P < 0.05; Table 1).
| Variables | Total (n = 93) | Low (n = 50) | High (n = 43) | P value |
| Sex, male | 49 (52.7) | 28 (56) | 21 (48.8) | 0.49 |
| Age, years | 67.5 ± 10.9 | 67.4 ± 10.6 | 67.7 ± 11.4 | 0.89 |
| Location | 0.38 | |||
| Left | 75 (80.6) | 42 (84) | 33 (76.7) | |
| Right | 18 (19.4) | 8 (16) | 10 (23.3) | |
| Gross type | 0.03 | |||
| Fungating | 32 (34.4) | 21 (42) | 11 (25.6) | |
| Ulceroinfiltrative | 54 (58.1) | 23 (46) | 31 (72.1) | |
| Ulcerofungating | 7 (7.5) | 6 (12) | 1 (2.3) | |
| Differentiation | 0.443 | |||
| Poor | 10 (11.1) | 3 (10.3) | 4 (12.5) | |
| Moderate | 70 (77.8) | 21 (72.4) | 26 (81.2) | |
| Well | 10 (11.1) | 5 (17.2) | 2 (6.2) | |
| Tumor size, ≥ 5 cm | 48 (51.6) | 24 (48) | 24 (55.8) | 0.45 |
| Histopathologic type | 0.76 | |||
| AC | 79 (84.9) | 43 (86) | 36 (83.7) | |
| MAC | 14 (15.1) | 7 (14) | 7 (16.3) | |
| Venous invasion | 21 (22.6) | 10 (20) | 11 (25.6) | 0.52 |
| Perineural invasion | 48 (51.6) | 24 (48) | 24 (55.8) | 0.45 |
| MSI | 7 (7.5) | 3 (6) | 4 (9.3) | 0.70 |
| T stage | 0.02 | |||
| 1 | 4 (4.3) | 4 (8) | 0 (0) | |
| 2 | 17 (18.3) | 13 (26) | 4 (9.3) | |
| 3 | 60 (64.5) | 29 (58) | 31 (72.1) | |
| 4 | 12 (12.9) | 4 (8) | 8 (18.6) | |
| N stage | 0.02 | |||
| 0 | 63 (67.7) | 40 (80) | 23 (53.5) | |
| 1 | 18 (19.4) | 7 (14) | 11 (25.6) | |
| 2 | 12 (12.9) | 3 (6) | 9 (20.9) | |
| M stage | 0.09 | |||
| 0 | 87 (93.5) | 49 (98) | 38 (88.4) | |
| 1 | 6 (6.5) | 1 (2) | 5 (11.6) | |
| TNM stage | 0.01 | |||
| Ⅰ | 20 (21.5) | 16 (32) | 4 (9.3) | |
| Ⅱ | 41 (44.1) | 22 (44) | 19 (44.2) | |
| Ⅲ | 26 (28.0) | 11 (22) | 15 (34.9) | |
| Ⅳ | 6 (6.5) | 1 (2) | 5 (11.6) | |
| CA125, ng/mL | 9.0 (7.0, 14.5) | 9.0 (7.0, 14.5) | 9.0 (7.1, 13.7) | 0.96 |
| CA199, U/mL | 14.1 (9.2, 24.0) | 14.5 (8.0, 23.0) | 13.6 (10.0, 26.0) | 0.97 |
| CA724, U/mL | 1.7 (1.5, 4.7) | 1.9 (1.5, 4.9) | 1.6 (1.5, 2.8) | 0.61 |
| CEA, ng/mL | 4.8 (2.5, 11.1) | 5.5 (2.6, 11.5) | 3.5 (2.5, 8.8) | 0.26 |
| CA153, U/mL | 8.1 (6.0, 12.4) | 7.2 (5.3, 10.6) | 9.2 (6.9, 13.3) | 0.04 |
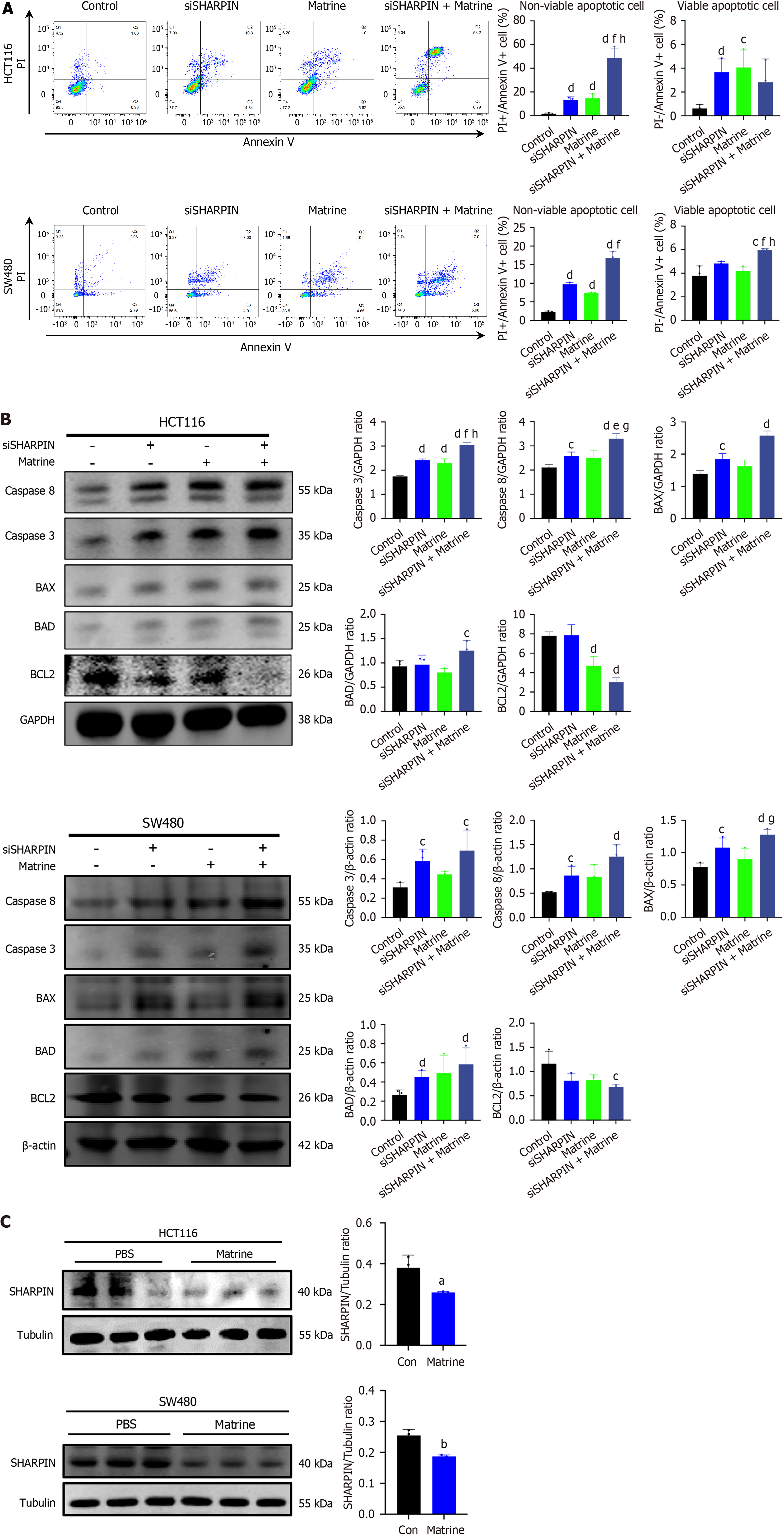
To further investigate the effects of SHARPIN on the apoptosis of two CRC cell lines (HCT116 and SW480), siRNA was used to knockdown the expression of SHARPIN. The efficiency of SHARPIN knockdown was confirmed by Quantitative reverse transcription-PCR and western blot analysis (Supplementary Figure 1). Apoptotic cells were assessed by flow cytometry, and the expression of apoptotic proteins (Caspase 3, caspase 8, BAD, BAX, and Bcl-2) was determined by western blotting. The results showed that SHARPIN knockdown promoted apoptosis in CRC cells, as indicated by the increased proportion of apoptotic cells in the siSHARPIN group compared to that in the control group (Figure 4A; P < 0.05). Additionally, the expression levels of Caspase 3, Caspase 8, BAD, and BAX were significantly upregulated in the siSHARPIN group, whereas the expression level of Bcl-2 was downregulated in siSHARPIN + Matrine group (Figure 4B).
Subsequently, we explored the role of SHARPIN in suppressing matrine-induced apoptosis in CRC cells. We observed the apoptosis-promoting effects of matrine in CRC cells. In CRC cell lines treated with matrine, there was a significant downregulation of SHARPIN expression (Figure 4C) and significant increase in the number of apoptotic cells (Figure 4A). The siSHARPIN + matrine group enhanced this effect. As shown in Figure 4, caspase 3 was significantly upregulated and Bcl-2 was significantly downregulated in the matrine group. Furthermore, in the siSHARPIN + matrine group, the expression of caspase 3, caspase 8, BAD, and BAX was significantly upregulated, whereas that of Bcl-2 was significantly downregulated.
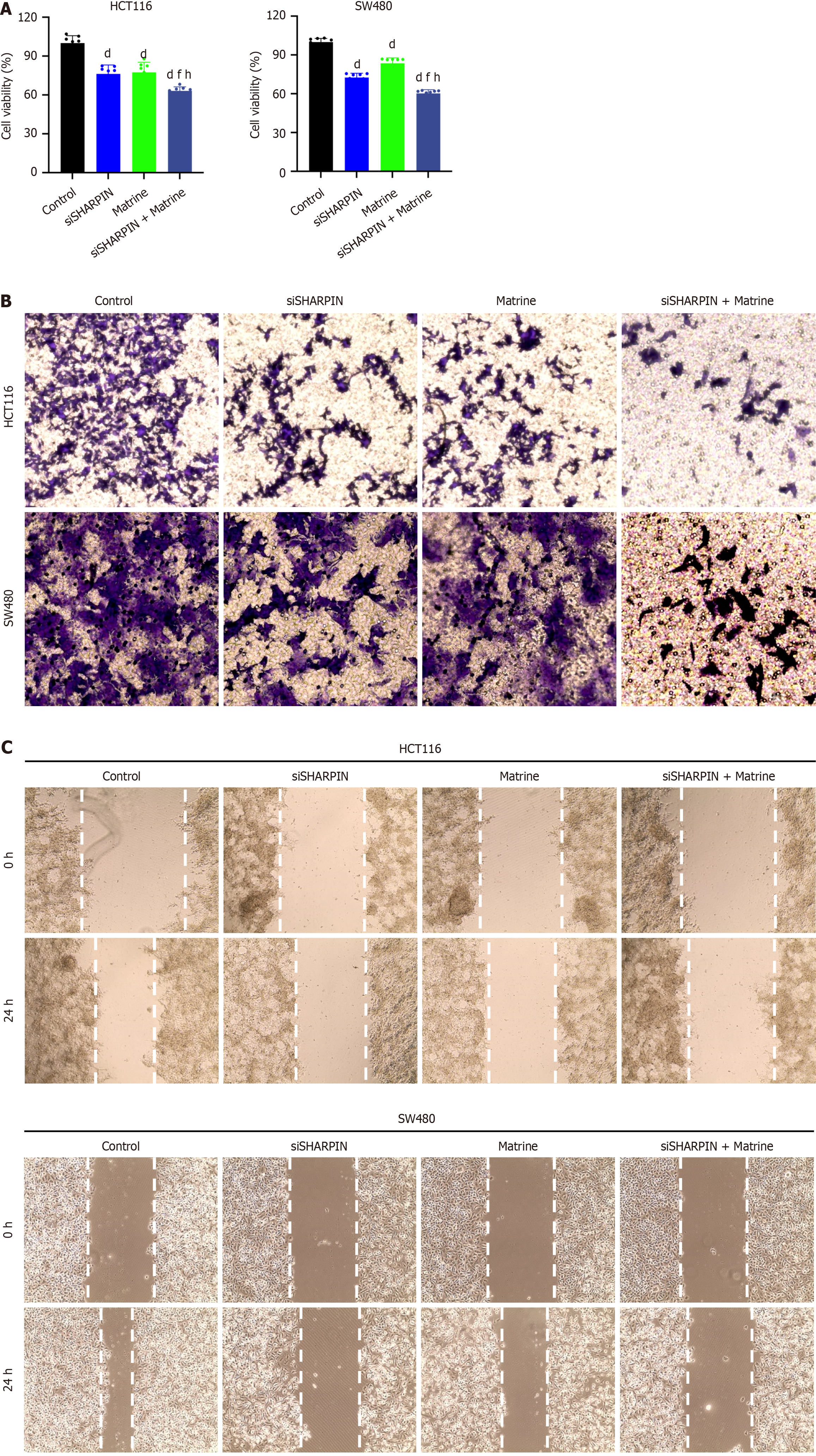
Next, we examined the effects of matrine and SHARPIN knockdown on the proliferation, invasion, and migration of CRC cells. The viability of CRC cells was assessed using the CCK-8 assay, whereas cell invasion and migration were evaluated using transwell invasion and wound-healing assays, respectively.
The viability of CRC cells was significantly inhibited in the siSHARPIN, matrine, and siSHARPIN + matrine groups compared to that in the control group (P < 0.01; Figure 5A). In the transwell invasion assay, the number of invasive cells markedly decreased in the siSHARPIN, matrine, and siSHARPIN + matrine groups (Figure 5B). Furthermore, the wound healing rates of CRC cells were significantly reduced in the siSHARPIN, matrine, and siSHARPIN + matrine groups, indicating a decreased migratory capacity (Figure 5C).
The incidence and mortality rates of CRC are on the rise, and despite advances in treatment strategies, improvements in prognosis have been modest. Resistance to cell death is a critical hallmark of cancer, and there are relatively few drugs specifically designed to induce apoptosis in cancer cells. Apoptotic drugs hold promise for tumor treatment, and further exploration of molecules involved in regulating the apoptotic pathway is essential for advancing CRC therapies. In the present study, SHARPIN was both found to be upregulated in CRC tissues based on our proteomic and TCGA database and validated by IHC staining in our CRC cohort. Additionally, the expression of SHARPIN was associated with different TNM stages and a poor prognosis. In vitro experiments, this study demonstrated that the downregulation of SHARPIN induces apoptosis in CRC cells. Matrine treatment also induced apoptosis and inhibited the proliferation, invasion, and migration of CRC cells by downregulating SHARPIN expression. Therefore, the role of SHARPIN in CRC was initially validated in our study, providing further evidence supporting the potential of matrine in preventing CRC and colorectal adenoma. Our study identifies SHARPIN as a potential anti-apoptotic molecule, shedding light on the mechanisms of matrine’s impact on CRC. Moreover, matrine has a favorable safety profile with minimal side effects. In the future, it is interesting to explore the effect of matrine in preventing tumor progression in high-risk patients.
SHARPIN is enriched at synaptic sites in mature neurons[7]. Located in the cytoplasm, SHARPIN is an approximately 40 kDa conserved protein that is ubiquitously expressed in various human tissues[8]. Furthermore, SHARPIN is amplified in some cancers, such as prostate, breast, and ovarian cancers. Zhang et al[9] demonstrated that upregulated SHARPIN activated the NF-κB pathway and suppressed cell apoptosis, potentially contributing to prostate cancer progression[9]. Overexpression of SHARPIN has been shown to increase the breast cancer risk[10] and promote the breast cancer progression by regulating p53 and ERα[11]. Knockdown of SHARPIN expression leads to apoptosis and inhibits the proliferation, invasion, and migration of ovarian cancer cells[12]. However, the role of SHAPRIN in CRC has not yet been reported. In the present study, SHARPIN expression was significantly higher in tumor tissues than in normal tissues in our proteomic data (n = 52), TCGA database (n = 698), and IHC-confirmed cohort (n = 93). Additionally, higher SHARPIN expression was associated with poor overall survival in CRC patients compared to that in the low expression group.
In a 14-year-old boy, a homozygous deficiency of SHARPIN led to a shift in signaling from pro-survival to cell death in immune cells, contributing to an observed discrepancy in immune function[13]. Because of the effect of SHARPIN deficiency on cell death, knockdown expression of SHARPIN may induce apoptosis in cancer. As the component of the linear ubiquitin chain activation complex, SHARPIN plays an important role in inflammatory response and apoptosis by regulating canonical NF-κB signaling[8,14,15]. For anti-tumor effect, NF-kB pathway was activated by SHARPIN overexpression with upregulated anti-apoptosis proteins in prostate cancer[9,16]. As a p53 mediator, SHARPIN may facilitate its degradation or ubiquitination in a mouse double minute 2 homolog-dependent manner[17], which is an inhibitor of p53 transcriptional activation[18]. SHARPIN is a negative regulator of PTEN in human tumor cell lines and primary cervical cancer cells both in vitro and in vivo. SHARPIN activates the PI3K/AKT pathway and induces tumorigenesis by interacting with PTEN.
Matrine is an alkaloid isolated from the traditional Chinese medicine, Sophora flavescens Aiton. In China, matrine injections are used to treat hepatitis. Owing to its anti-inflammatory effects, matrine also exhibits antitumor properties, especially apoptosis. Previous studies have shown that matrine inducs autophagy and apoptosis by downregulating β-catenin and mediating the JNK-Bcl-2/Bcl-xL-Bax/Bak pathway in hepatocellular carcinoma cells[19,20]. In CRC cells, matrine induces apoptosis by regulating associated proteins. Zhang et al[21] demonstrated matrine reduce the ratio of Bcl-2/Bax and increase caspase-9 in vitro. Gu et al[22] explored effects of matrine on Bcl-2, Bax and caspase-3 in several cell lines. And Chang et al[23] showed that matrine activated apoptosis by regulating BAX,Bcl-2 and Cyto C. However, the precise mechanism underlying the matrine-induced apoptosis in CRC cells remains unclear. In the current study, we found that matrine induced cell apoptosis by inhibiting SHARPIN expression, while also repressing the proliferation, invasion, and migration of CRC cells. Targeted SHARPIN drugs might lead to various adverse effects, while matrine shows potential in multiple therapeutic roles, making it a promising candidate for treatment.
Our study has some limitations. First, the follow-up time of the enrolled 52 patients was not available, and the association between SHARPIN expression and CRC survival needs to be investigated in Chinese population. Second, the therapeutic effects of matrine on CRC need to be validated in vivo and in clinical trials. Matrine injection has been used to improve hepatitis; however, the dosage of matrine that inhibits tumor progression remains to be explored. Third, the mechanism of matrine repressing SHARPIN expression remains to be conducted in the further study.
In conclusion, this study identified overexpressed SHARPIN in CRC tissues. High expression SHARPIN is associated with TNM stage and poor prognosis. Matrine promotes cell apoptosis and inhibits proliferation, invasion, and migration by repressing SHARPIN expression in CRC. Thus, matrine could be considered as a novel therapeutic agent for CRC.
The authors thank Wei-Liang Sun (China-Japan Friendship Hospital, Beijing, PhD) and Tian-Hui Zhou (Graduate School of Beijing University of Chinese Medicine, Beijing University of Chinese Medicine, MD) for their technical assistance.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64104] [Article Influence: 16026.0] [Reference Citation Analysis (174)] |
| 2. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 960] [Article Influence: 137.1] [Reference Citation Analysis (2)] |
| 3. | Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 4873] [Article Influence: 1624.3] [Reference Citation Analysis (0)] |
| 4. | Prakash A, Peterman S, Ahmad S, Sarracino D, Frewen B, Vogelsang M, Byram G, Krastins B, Vadali G, Lopez M. Hybrid data acquisition and processing strategies with increased throughput and selectivity: pSMART analysis for global qualitative and quantitative analysis. J Proteome Res. 2014;13:5415-5430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee AV, Omberg L, Wolf DM, Shriver CD, Thorsson V; Cancer Genome Atlas Research Network, Hu H. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell. 2018;173:400-416.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2534] [Cited by in RCA: 2329] [Article Influence: 332.7] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Xu H, Bi X, Hou G, Liu A, Zhao Y, Wang G, Cao X. Src acts as the target of matrine to inhibit the proliferation of cancer cells by regulating phosphorylation signaling pathways. Cell Death Dis. 2021;12:931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Seymour RE, Hasham MG, Cox GA, Shultz LD, Hogenesch H, Roopenian DC, Sundberg JP. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Wang Z, Potter CS, Sundberg JP, Hogenesch H. SHARPIN is a key regulator of immune and inflammatory responses. J Cell Mol Med. 2012;16:2271-2279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Huang H, Zhou H, Du T, Zeng L, Cao Y, Chen J, Lai Y, Li J, Wang G, Guo Z. Activation of nuclear factor κB pathway and downstream targets survivin and livin by SHARPIN contributes to the progression and metastasis of prostate cancer. Cancer. 2014;120:3208-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | De Melo J, Tang D. Elevation of SIPL1 (SHARPIN) Increases Breast Cancer Risk. PLoS One. 2015;10:e0127546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Tian Z, Tang J, Yang Q, Li X, Zhu J, Wu G. Atypical ubiquitin-binding protein SHARPIN promotes breast cancer progression. Biomed Pharmacother. 2019;119:109414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wang G, Zhuang Z, Cheng J, Yang F, Zhu D, Jiang Z, Du W, Shen S, Huang J, Hua L, Chen Y. Overexpression of SHARPIN promotes tumor progression in ovarian cancer. Exp Mol Pathol. 2022;104806. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Oda H, Manthiram K, Chavan PP, Rieser E, Veli Ö, Kaya Ö, Rauch C, Nakabo S, Kuehn HS, Swart M, Wang Y, Çelik NI, Molitor A, Ziaee V, Movahedi N, Shahrooei M, Parvaneh N, Alipour-Olyei N, Carapito R, Xu Q, Preite S, Beck DB, Chae JJ, Nehrebecky M, Ombrello AK, Hoffmann P, Romeo T, Deuitch NT, Matthíasardóttir B, Mullikin J, Komarow H, Stoddard J, Niemela J, Dobbs K, Sweeney CL, Anderton H, Lawlor KE, Yoshitomi H, Yang D, Boehm M, Davis J, Mudd P, Randazzo D, Tsai WL, Gadina M, Kaplan MJ, Toguchida J, Mayer CT, Rosenzweig SD, Notarangelo LD, Iwai K, Silke J, Schwartzberg PL, Boisson B, Casanova JL, Bahram S, Rao AP, Peltzer N, Walczak H, Lalaoui N, Aksentijevich I, Kastner DL. Biallelic human SHARPIN loss of function induces autoinflammation and immunodeficiency. Nat Immunol. 2024;25:764-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 14. | Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 513] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 15. | Ikeda F, Deribe YL, Skånland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011;471:637-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 609] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 16. | Li J, Lai Y, Cao Y, Du T, Zeng L, Wang G, Chen X, Chen J, Yu Y, Zhang S, Zhang Y, Huang H, Guo Z. SHARPIN overexpression induces tumorigenesis in human prostate cancer LNCaP, DU145 and PC-3 cells via NF-κB/ERK/Akt signaling pathway. Med Oncol. 2015;32:444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Yang H, Yu S, Wang W, Li X, Hou Y, Liu Z, Shi Y, Mu K, Niu G, Xu J, Wang H, Zhu J, Zhuang T. SHARPIN Facilitates p53 Degradation in Breast Cancer Cells. Neoplasia. 2017;19:84-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Lessel D, Wu D, Trujillo C, Ramezani T, Lessel I, Alwasiyah MK, Saha B, Hisama FM, Rading K, Goebel I, Schütz P, Speit G, Högel J, Thiele H, Nürnberg G, Nürnberg P, Hammerschmidt M, Zhu Y, Tong DR, Katz C, Martin GM, Oshima J, Prives C, Kubisch C. Dysfunction of the MDM2/p53 axis is linked to premature aging. J Clin Invest. 2017;127:3598-3608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Xie BS, He XX, Ai ZL, Yao SK. Involvement of β-catenin in matrine-induced autophagy and apoptosis in WB-F344 cells. Mol Med Rep. 2014;9:2547-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Yang J, Yao S. JNK-Bcl-2/Bcl-xL-Bax/Bak Pathway Mediates the Crosstalk between Matrine-Induced Autophagy and Apoptosis via Interplay with Beclin 1. Int J Mol Sci. 2015;16:25744-25758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Zhang S, Cheng B, Li H, Xu W, Zhai B, Pan S, Wang L, Liu M, Sun X. Matrine inhibits proliferation and induces apoptosis of human colon cancer LoVo cells by inactivating Akt pathway. Mol Biol Rep. 2014;41:2101-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Gu YY, Chen MH, May BH, Liao XZ, Liu JH, Tao LT, Man-Yuen Sze D, Zhang AL, Mo SL. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via Bcl-2 and caspase-3. Phytomedicine. 2018;51:214-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Chang C, Liu SP, Fang CH, He RS, Wang Z, Zhu YQ, Jiang SW. Effects of matrine on the proliferation of HT29 human colon cancer cells and its antitumor mechanism. Oncol Lett. 2013;6:699-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |