Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4625
Revised: August 28, 2024
Accepted: October 9, 2024
Published online: December 15, 2024
Processing time: 187 Days and 8.6 Hours
Cirrhosis is a significant risk factor for the development of hepatocellular carcinoma (HCC). Variability in HCC risk among patients with cirrhosis is notable, particularly when considering the diverse etiologies of cirrhosis.
To identify specific risk factors contributing to HCC development in patients with cirrhosis.
This retrospective study analyzed data from cirrhotic patients at Beijing Youan Hospital from January 1, 2012 to September 30, 2022 with at least 6 mo of follow-up. Patient demographics, medical histories, etiologies, and clinical characteristics were examined. Cox regression analysis was used to analyze correlations of the above parameters with hepatocarcinogenesis, while competing risk regression was used to estimate their adjusted hazard ratios accounting for death. The cumulative incidence was plotted over time.
Overall, 5417 patients with cirrhosis (median age: 54 years; 65.8% males) were analyzed. Hepatitis B virus (HBV) was the most common etiology (23.3%), with 25% (n = 1352) developing HCC over a 2.9-year follow-up period. Patients with multiple etiologies had the HCC highest incidence (30.3%), followed by those with HBV-related cirrhosis (29.5%). Significant risk factors included male sex, advanced age, hepatitis C virus (HCV) infection, elevated blood ammonia, and low platelet count. Men had a higher 5-year HCC risk than women (37.0% vs 31.5%). HBV, HCV, and HBV/HCV co-infected patients had 5-year risks of HCC of 45.8%, 42.9%, and 48.1%, respectively, compared to 29.5% in nonviral hepatitis cases, highlighting the significant HCC risk from viral hepatitis, especially HBV, and underscores the importance of monitoring these high-risk groups.
In conclusion, HBV-related cirrhosis strongly correlates with HCC, with male sex, older age, viral hepatitis, elevated blood ammonia, and lower albumin and platelet levels increasing the risk of HCC.
Core Tip: Our retrospective analysis spanned a decade (January 1, 2012 to September 30, 2022) and included 5417 patients from Beijing Youan Hospital, Capital Medical University. Using Cox regression and competing risk regression models, we identified several key factors that significantly contributed to the risk of hepatocellular carcinoma in patients with cirrhosis. Specifically, this study underscores the increased association of hepatitis B cirrhosis with hepatocellular carcinoma incidence and highlights other significant risk factors, including male sex, advanced age, viral hepatitis-related cirrhosis, elevated blood ammonia, and lower albumin and platelet levels.
- Citation: Zhou DQ, Liu JY, Zhao F, Zhang J, Liu LL, Jia JR, Cao ZH. Risk factors for hepatocellular carcinoma in cirrhosis: A comprehensive analysis from a decade-long study. World J Gastrointest Oncol 2024; 16(12): 4625-4635
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4625.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4625
Cirrhosis is a major cause of morbidity and mortality in patients with chronic liver disease[1]. In 2019, 2.4% of the global deaths were related to cirrhosis. It is among the top 20 causes of death worldwide[2]. Abnormalities in liver function of patients with cirrhosis include varices, gastrointestinal bleeding, ascites, and hepatic encephalopathy. Hepatocellular carcinoma (HCC) is a serious complication. The prognosis of HCC is usually poor[3]. According to global burden statistics, there were approximately 534000 liver cancer cases and 485000 deaths in 2019[4]. With over 290000 people diagnosed with liver cancer and approximately 188000 deaths from liver cancer in 2019[5], China has the highest burden of liver cancer worldwide.
Major causes of cirrhosis include chronic viral hepatitis, such as hepatitis B virus (HBV) and hepatitis C virus (HCV), chronic alcohol abuse, metabolic disorders, and autoimmune liver diseases. Specifically, HBV infection is the leading cause of liver cancer-related mortality and the third leading cause of cirrhosis[6]. While antiviral therapies for chronic hepatitis B have shown efficacy in reducing the risk of cirrhosis, decompensation, and HCC, their ability to significantly affect HBV-related mortality trends remains limited[7]. As of 2019, only 10.3% of chronic HBV infections have been diagnosed, of which only 22.7% are eligible for antiviral treatment[8]. Despite a notable decline in HBV and HCV incidences, China continues to bear the highest global disease burden for HBV (74 million) and HCV (9.48 million) infections, attributed to its vast patient population[9]. Concurrently, the rise in alcohol-associated cirrhosis (AC) and metabolic dysfunction-associated liver disease (MASLD) parallels lifestyle shifts and economic progression, increasing the annual prevalence of advanced liver diseases and the overall disease burden[10].
Cirrhosis is a major risk factor for HCC, with autopsy studies showing cirrhosis in 80%-90% of cases worldwide. In areas with high HBV prevalence, cirrhotic individuals have triple the risk of developing HCC compared to non-cirrhotic individuals and 16 times that of inactive HBV carriers[11]. Beyond progression to HCC, patients with cirrhosis also face an increased risk of liver complications and mortality, particularly as the disease progresses from the compensated to the decompensated stages[12]. The survival rates at 1 and 5 years for patients with compensated cirrhosis were 87% and 67%, in contrast to 75% and 45%, respectively, for patients with decompensated cirrhosis. Compared to the general population, mortality increases five-fold in patients with compensated cirrhosis and ten-fold in decompensated cirrhosis[13]. Notably, chronic comorbidities such as chronic kidney and cardiovascular diseases are often associated with death due to compensatory cirrhosis[14].
To gain a deeper understanding of the prognosis of cirrhosis with different etiologies and liver function states, this retrospective observational study retrieved data from patients with cirrhosis hospitalized at Beijing Youan Hospital over the past 10 years. This study aimed to better understand and guide the clinical management of cirrhosis, as well as to improve the prognosis and personalized treatment planning for patients with cirrhosis.
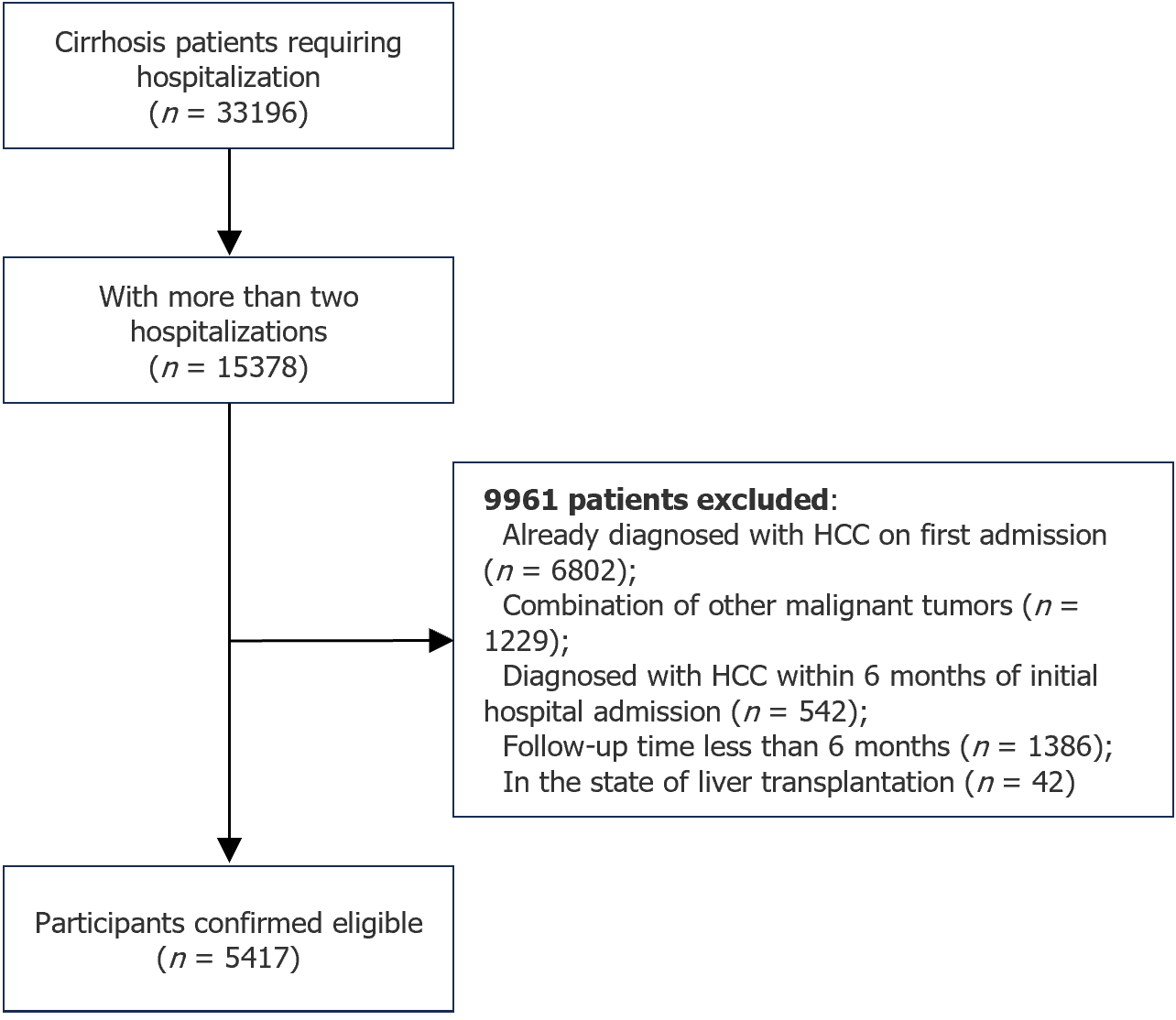
Patients with cirrhosis who were admitted to our hospital from January 1, 2012 to September 30, 2022 were identified from our inpatient system based on the diagnosis code K74 (cirrhosis). The main inclusion criteria were: (1) Diagnosis of cirrhosis based on histology (liver biopsy), imaging (evidence of esophagogastric fundal varices, portal hypertension, splenomegaly, and ascites), or noninvasive examination (FibroScan); (2) Two or more hospitalizations; (3) No diagnosis of HCC at the initial hospitalization and within 6 mo of initial admission; and (4) A minimum of 6 mo of follow-up. Patients with other combined malignancies or those who underwent liver transplantation were excluded.
The time of first admission was defined as the baseline, and clinical characteristics at baseline were collected, including demographics (sex, age, and place of origin), comorbidities (diabetes mellitus, hypertension, and coronary artery disease), liver disease-related complications (esophagogastric fundal varices, gastrointestinal bleeding, ascites, abdominal infections, hepatic encephalopathy, and hepatorenal syndrome), etiology [HBV, HCV, AC, MASLD, primary biliary cirrhosis (PBC), drug-induced cirrhosis, cryptogenic cirrhosis, and mixed cirrhosis], laboratory findings, and liver function scores (model for end-stage liver disease score and Child-Pugh classification).
The primary endpoint was HCC. Observational endpoint was the time to first document HCC. For patients who did not develop HCC, the observational endpoint was death or time to the last follow-up. Death was a competing risk event for hepatocarcinogenesis because patients with cirrhosis who died were no longer at risk of developing HCC.
Pearson χ2 test and Wilcoxon signed-rank test were used to evaluate correlations of categorical and continuous variables with HCC occurrence for clinical characteristics and laboratory values, respectively. The results are expressed as percentages and interquartile ranges. Only patients with cirrhosis for at least 6 mo from baseline to endpoint events were included in the analysis of all HCC-associated risk factors.
Multiple imputations were used to adjust for missing values of all variables. Cox proportional hazards regression analysis was performed on the imputed data set. Clinical and demographic variables that differed significantly between the groups (P < 0.05) were included in the first statistical step of the model by univariate analysis using both forward and backward stepwise Cox regressions. The same set of seven significant risk factors were selected: Age, sex, history of HBV/HCV infection, albumin level, globulin level, platelet count, and blood ammonia level.
The decompensation rate in patients with cirrhosis was 76.6%. Death was considered a competing risk event when assessing the incidence of HCC in patients with decompensated cirrhosis to obtain a reliable risk estimate because the potential risk of death is high[15]. Cox regression analyses were performed for selected multivariate risk factors from the Cox analysis. We then calculated adjusted hazard ratios (aHRs). A fine and gray regression model was used to estimate the cumulative incidence function (CIF) for HCC development in cirrhotic patients of different sexes, ages, and etiologies.
All statistical analyses in this study were performed using R version 4.2.0 and IBM Statistical Product and Service Solutions 27.0.
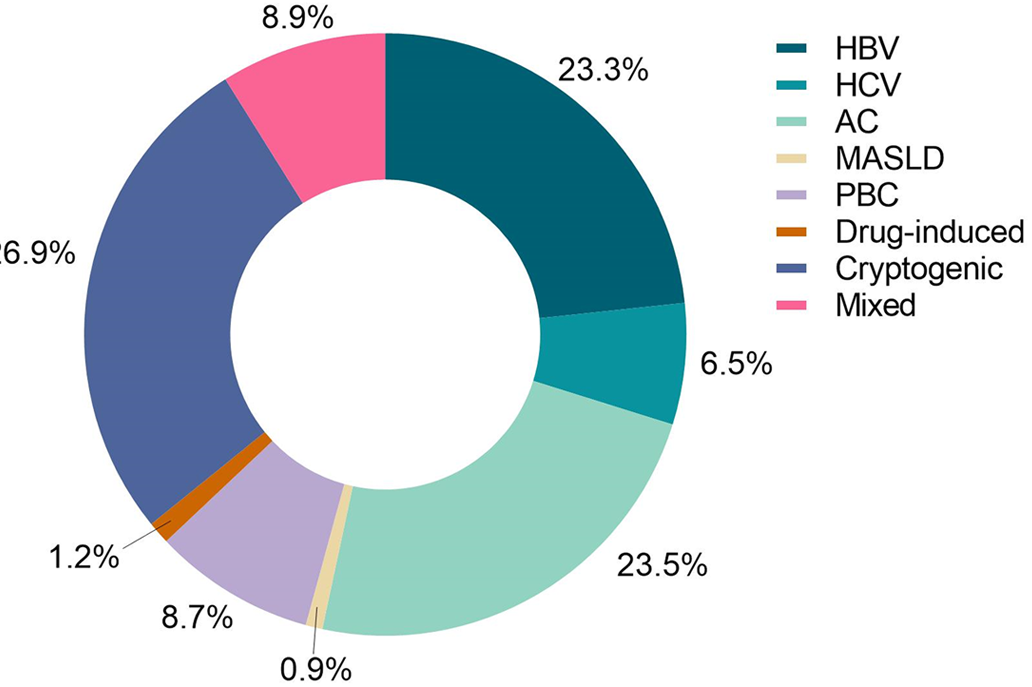
Overall, 5417 patients were included (Figure 1). The median age was 54 years, 3567 (65.8%) patients were male, and 4149 (76.6%) had decompensated cirrhosis. Etiological distribution of cirrhosis is shown in Figure 2: 1261 cases (23.3%) of HBV; 354 cases (6.5%) of HCV; 1274 cases (23.5%) of AC; 51 cases (0.9%) of MASLD; 470 cases (8.7%) of PBC; 66 cases (1.2%) of drug-induced cirrhosis; 1459 cases (26.9%) of cryptogenic cirrhosis; and 482 cases (8.9%) of mixed cirrhosis (two or more etiologies).
HCC occurred in 1352 patients (25%) and 4065 patients did not develop HCC during the in-hospital follow-up period. There were 262 deaths (4.84%) due to complications or other comorbidities among the patients who did not develop HCC.
Based on the occurrence of HCC during follow-up, the observational cohort was divided into HCC and non-HCC groups. The demographic characteristics of the cohort at baseline are presented in Table 1. Patients in the HCC group were more likely to be male (71.7% vs 63.9%, P < 0.001). The median age of the patients was slightly higher (56 vs 54 years, P < 0.001). Decompensated cirrhosis was more common in patients with HCC than in those without (77.5% vs 73.9%), and a greater proportion of patients with HCC had Child-Pugh grades A and B (32.6% vs 29.5% and 49.1% vs 48.0%, respectively, P = 0.003). Gastrointestinal bleeding was more common in patients without HCC (24.1% vs 18.0%).
| Parameter | HCC cases (n = 1352) | Non-HCC controls (n = 4065) | P value |
| Sex (Male), n (%) | 969 (71.7) | 2598 (63.9) | < 0.001 |
| Age | 56 (50, 62) | 54 (46, 62) | < 0.001 |
| Hypertension, n (%) | 255 (18.9) | 727 (17.9) | 0.419 |
| Diabetes, n (%) | 321 (23.7) | 860 (21.2) | 0.046 |
| Coronary disease, n (%) | 35 (2.6) | 154 (3.8) | 0.037 |
| Gastrointestinal bleeding, n (%) | 243 (18.0) | 978 (24.1) | < 0.001 |
| Hepatic encephalopathy, n (%) | 219 (16.2) | 633 (15.6) | 0.584 |
| Decompensated cirrhosis, n (%) | 999 (73.9) | 3150 (77.5) | 0.007 |
| Child-Pugh grade A, n (%) | 430 (32.6) | 1162 (29.5) | 0.003 |
| Child-Pugh grade B, n (%) | 648 (49.1) | 1893 (48.0) | |
| Child-Push grade C, n (%) | 241 (18.3) | 890 (22.6) | |
| HBV, n (%) | 372 (27.5) | 889 (21.9) | < 0.001 |
| HCV, n (%) | 93 (6.9) | 261 (6.4) | |
| AC, n (%) | 261 (19.3) | 1013 (24.9) | |
| MASLD, n (%) | 9 (0.7) | 42 (1.0) | |
| PBC, n (%) | 72 (5.3) | 398 (9.8) | |
| Drug-induced cirrhosis, n (%) | 12 (0.9) | 54 (1.3) | |
| Cryptogenic cirrhosis, n (%) | 387 (28.6) | 1072 (26.4) | |
| Mixed cirrhosis, n (%) | 146 (10.8) | 336 (8.3) | |
| ALT (U/L) | 31.1 (21.2, 53.1) | 27.0 (18.2, 47.5) | < 0.001 |
| AST (U/L) | 47.7 (31.7, 77.4) | 44 (29.1, 79.9) | < 0.001 |
| Albumin (g/L) | 31.2 (27.4, 34.6) | 30.7 (26.8, 34.8) | 0.106 |
| Globin (g/L) | 31.6 (26.5, 37.1) | 31.3 (25.7, 38.0) | 0.268 |
| INR | 1.27 (1.12, 1.45) | 1.32 (1.16, 1.54) | 0.002 |
| Prothrombin time (S) | 14.2 (12.6, 16.3) | 14.8 (13.0, 17.4) | 0.017 |
| Direct bilirubin (μmol/L) | 11.0 (6.3, 19.2) | 12.7 (6.6, 33.6) | 0.001 |
| Triglycerides (mmol/L) | 0.81 (0.62, 1.11) | 0.89 (0.67, 1.26) | < 0.001 |
| Platelets (× 109/L) | 78 (54, 109) | 80 (54, 118) | 0.096 |
| γ-GT (U/L) | 50.1 (26.0, 119.8) | 53.7 (25.4, 143.8) | 0.157 |
| AFP (ng/mL) | 4.64 (2.60, 10.35) | 3.36 (2.02, 6.26) | < 0.001 |
| CA19-9 (U/mL) | 26.72 (13.76, 48.40) | 25.00 (11.93, 52.82) | 0.070 |
| Neutrophils (× 109/L) | 2.30 (1.59, 3.44) | 2.43 (1.59, 3.76) | 0.024 |
| Serum ammonia (μg/dL) | 73 (51, 95) | 67 (46, 95) | < 0.001 |
| MELD | 8 (4, 12) | 10 (5, 15) | < 0.001 |
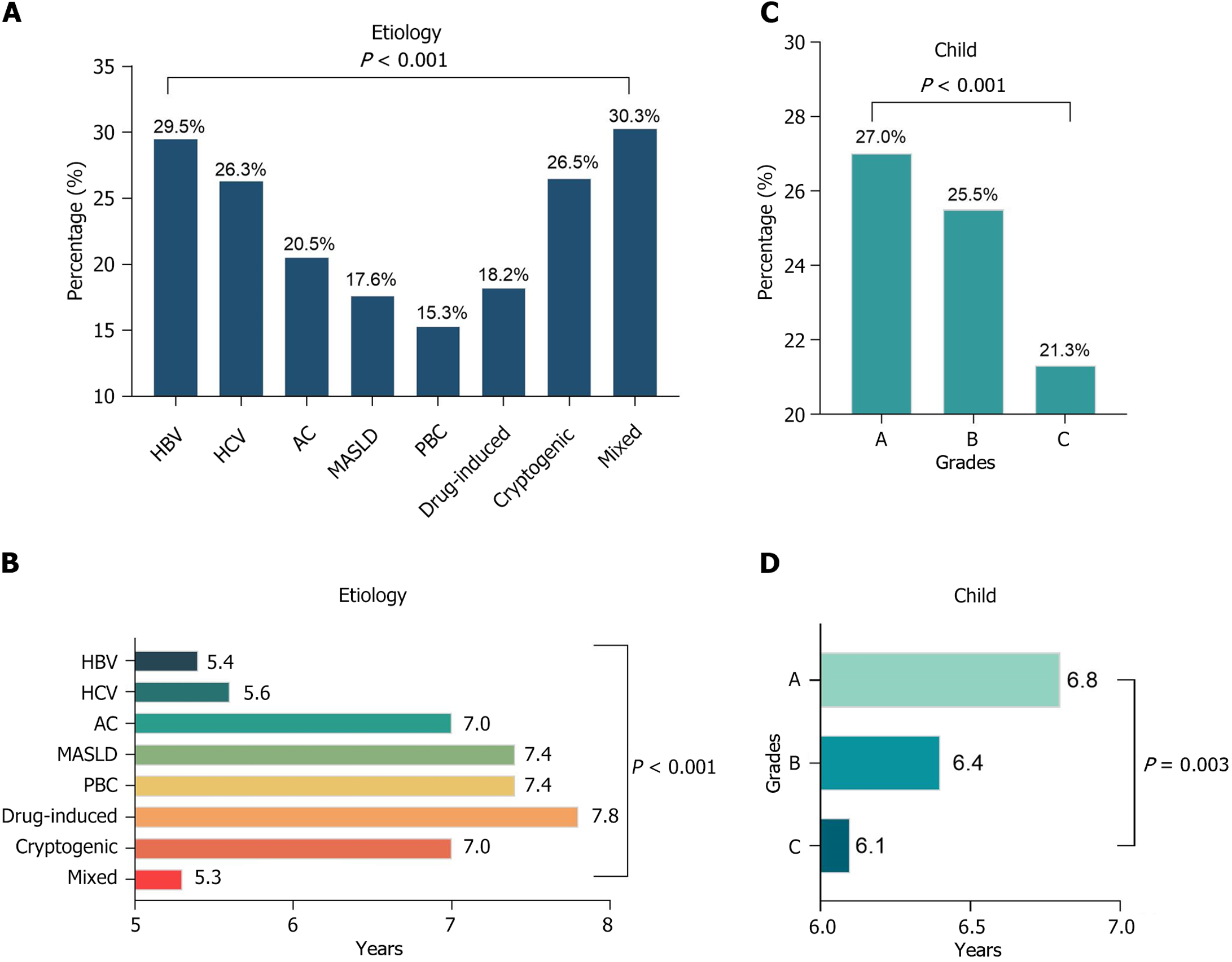
There was a significant difference in the risk of HCC among patients with cirrhosis of different etiologies (P < 0.001). Cirrhotic patients with two or more etiologies had the highest incidence of HCC among all patients with cirrhosis during the entire follow-up period, at 30.3% (Figure 3A), and their mean time to the development of HCC was also the shortest at 5.3 years [95% confidence interval (CI): 4.9-5.7] (Figure 3B). In contrast, patients with HBV cirrhosis had the highest incidence of HCC among single etiologies, at 29.5%, with a median time to HCC was 5.4 years (95%CI: 5.1-5.6 years). Among cirrhotic patients in this study cohort, higher Child-Pugh grades were associated with a lower risk of HCC and shorter survival: The incidence of HCC was 21.3% in patients with Child-Pugh grade C (Figure 3C), and the mean survival was 6.1 years (95%CI: 5.8-6.5 years) (Figure 3D). Compared to compensated cirrhosis, decompensated cirrhosis had a shorter mean time to HCC [6.4 years (95%CI: 6.3-6.6 years) vs 6.8 years (95%CI: 6.5-7.1 years)].
To investigate the risk factors associated with the development of HCC, one-way Cox regression analysis of possible influencing factors was performed, and the following 15 clinical characteristics and laboratory markers were associated with HCC (P < 0.05): Age, sex, HBV/HCV history, compensation status, diabetes history, albumin, globulin, international normalized ratio, prothrombin time, triglycerides, platelets, serum ammonia, and child classification. From these candidate risk factors, the same set of seven significant risk factors (age, sex, HBV/HCV history, albumin, globulin, platelet count, and serum ammonia) was selected using forward and backward stepwise Cox regression, as shown in Table 2.
| Parameter | Univariate Cox regression analysis | Multivariable Cox regression analysis | Multivariable competitive risk regression analysis | |||
| aHR (95%CI) | P value | aHR (95%CI) | P value | aHR (95%CI) | P value | |
| Age | 1.023 (1.018-1.027) | < 0.001 | 1.029 (1.024-1.034) | < 0.001 | 1.025 (1.020-1.030) | < 0.001 |
| Sex (male) | 1.321 (1.174-1.487) | < 0.001 | 1.537 (1.359-1.738) | < 0.001 | 1.482 (1.313-1.673) | < 0.001 |
| Diabetes | 1.155 (1.019-1.309) | 0.025 | ||||
| Hypertension | 1.128 (0.984-1.292) | 0.084 | ||||
| Coronary disease | 0.951 (0.680-1.330) | 0.769 | ||||
| HBV | 1.801 (1.602-2.025) | < 0.001 | 1.963 (1.742-2.211) | < 0.001 | 1.950 (1.734-2.193) | < 0.001 |
| HCV | 1.837 (1.352-1.981) | < 0.001 | 1.567 (1.293-1.900) | < 0.001 | 1.533 (1.274-1.846) | < 0.001 |
| HBV and HCV | 2.062 (1.068-3.980) | 0.031 | 2.456 (1.270-4.747) | 0.008 | 2.074 (1.038-4.143) | 0.039 |
| Decompensation | 1.146 (1.015-1.295) | 0.028 | ||||
| ALT | 1.000 (1.000-1.000) | 0.974 | ||||
| AST | 1.000 (1.000-1.000) | 0.815 | ||||
| Albumin | 0.976 (0.967-0.985) | < 0.001 | 0.986 (0.977-0.995) | 0.002 | 0.995 (0.987-1.005) | 0.381 |
| Globulin | 1.017 (1.010-1.023) | < 0.001 | 1.016 (1.009-1.023) | < 0.001 | 1.012 (1.005-1.019) | < 0.001 |
| INR | 1.271 (1.084-1.490) | 0.003 | ||||
| Prothrombin time | 1.016 (1.002-1.031) | 0.024 | ||||
| Direct bilirubin | 1.000 (0.999-1.001) | 0.943 | ||||
| Triglycerides | 0.854 (0.776-0.939) | 0.001 | ||||
| Hemoglobin | 1.004 (1.002-1.006) | < 0.001 | ||||
| γ-GT | 1.000 (0.999-1.000) | 0.075 | ||||
| AFP | 1.000 (1.000-1.001) | 0.543 | ||||
| CA19-9 | 1.000 (0.999-1.001) | 0.713 | ||||
| Platelets | 0.999 (0.998-1.000) | 0.017 | 0.999 (0.998-1.000) | 0.015 | 0.998 (0.998-1.000) | 0.011 |
| Neutrophils | 0.998 (0.976-1.020) | 0.829 | ||||
| Serum ammonia | 1.002 (1.001-1.003) | 0.004 | 1.002 (1.001-1.003) | 0.003 | 1.002 (1.001-1.003) | < 0.001 |
| MELD | 0.999 (0.990-1.008) | 0.843 | ||||
Considering death as a competing risk event for hepatocarcinogenesis, competing risk regression analysis was performed on the risk factors examined in the Cox regression analysis, and the aHRs of these risk factors were estimated. The results of this analysis are presented in Table 2. In the competing risk regression analysis, a higher risk of liver cancer was associated with male sex (aHR = 1.482; 95%CI: 1.313-1.673), advanced age (aHR = 1.025; 95%CI: 1.020-1.030), HBV infection (aHR = 1.950; 95%CI: 1.734-2.193), HCV infection (aHR = 1.533; 95%CI: 1.274-1.846), and co-infection of HBV and HCV (aHR = 2.074; 95%CI: 1.038-4.143), and higher blood ammonia levels (aHR = 1.002; 95%CI: 1.001-1.003), globulin levels (aHR = 1.012; 95%CI: 1.005-1.019), and lower platelet count (aHR = 0.998; 95%CI: 0.998-1.000) at baseline also showed a significant association with liver cancer risk.
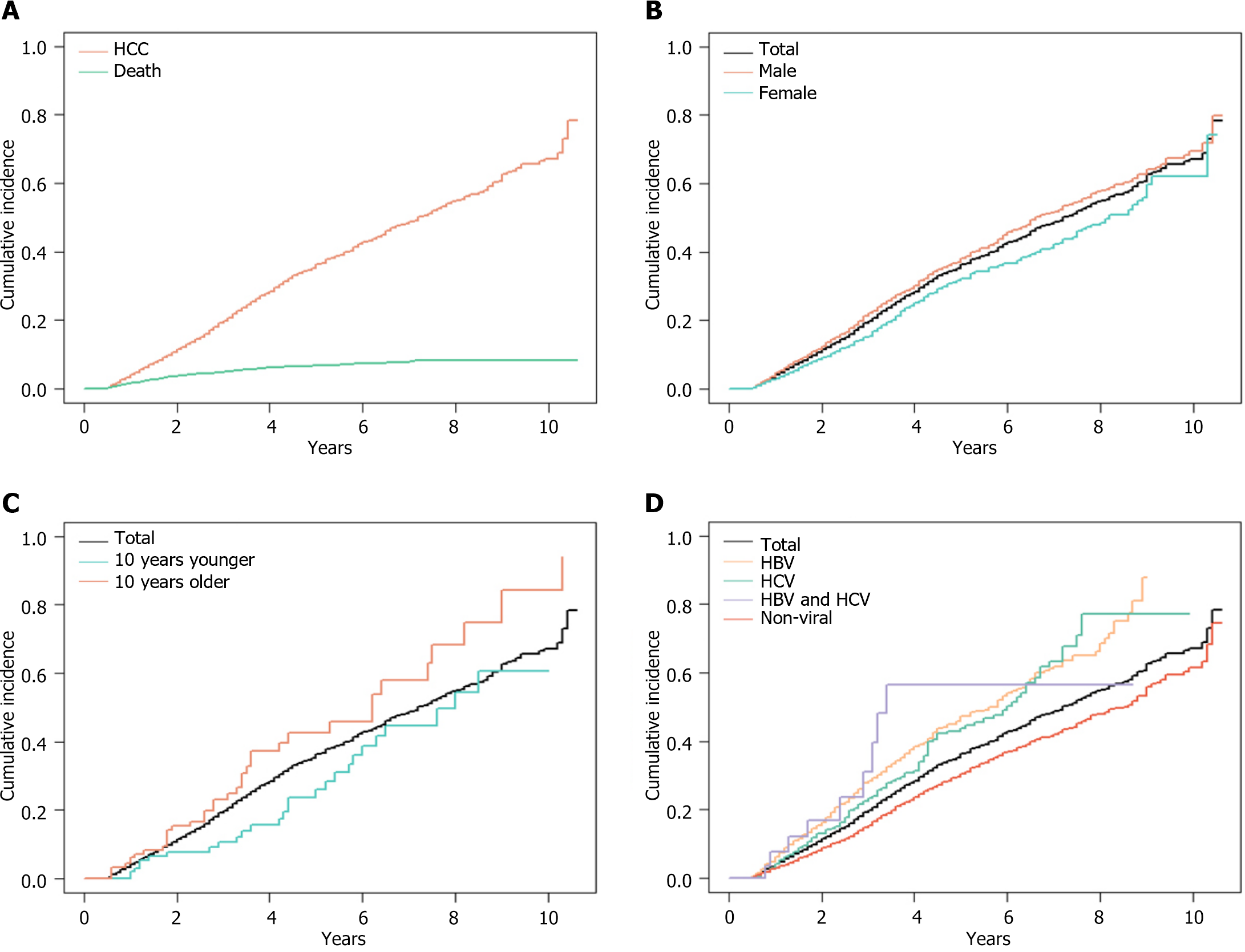
Based on the CIF curves from the competing risk regression analyses (Figure 4A), the risk of HCC in these hospitalized cirrhotic patients was 19.2% (95%CI: 17.9%-20.5%) at 3 years, and 35.2% (95%CI: 33.4%-37.0%) at 5 years. In these patients with cirrhosis, the risk of total mortality was 1.49% (95%CI: 1.15%-1.83%) at 1 year, 4.83% (95%CI: 4.17%-5.50%) at 3 years, and 6.66% (95%CI: 5.81%-7.51%) at 5 years. Men had a higher risk of HCC than women, with a 5-year risk of 37.0% (95%CI: 34.8%-39.3%) vs 31.5% (95%CI: 28.4%-34.7%) (Figure 4B). The risk of HCC increased with age, and the 5-year risk of HCC for decreasing age by 10 years (44) vs increasing age by 10 years (64), based on a median age of 54 years, was 23.6% (95%CI: 12.9%-34.3%) vs 42.4% (95%CI: 29.2%-55.7%) (Figure 4C). The 5-year risk of HCC in cirrhotic patients with HBV, HCV, and HBV/HCV coinfection was 45.8% (95%CI: 42.1%-49.4%), 42.9% (95%CI: 35.9%-49.9%), and 48.1% (95%CI: 20.2%-75.9%), respectively, whereas the 5-year risk of HCC in cirrhotic patients with nonviral hepatitis was only 29.5% (95%CI: 27.3%-31.7%) (Figure 4D).
In this study, we found that patients with hepatitis B cirrhosis had the highest incidence of HCC and the shortest median survival among all causative factors in this retrospective cohort study of hospitalized patients with cirrhosis. The risk of HCC at 5 years was 45.8% (95%CI: 42.0%-49.4%). Among patients with cirrhosis, male sex, older age, and a history of chronic HBV and HCV infections were risk factors for the development of HCC after adjusting for death as a competing risk. Compared with non-viral cirrhosis, viral cirrhosis had a higher cumulative risk of developing HCC within 5 years: 45.3% (95%CI: 42.0%-48.5%) vs 29.5% (95%CI: 27.3%-31.7%).
The Western Pacific Region, including China, has the highest prevalence of HBV infection among patients with cirrhosis (59%), according to the World Health Organization regional classification[16]. These statistics were based on the leading causes of cirrhosis reported between 1993 and 2021. The burden of alcohol-related liver disease, cirrhosis, and HCC is expected to increase with an increase in global alcohol consumption per capita[17]. In our study, 1261 cases (23.3%) of HBV, 1274 cases (23.5%) of AC, and 1459 cases (26.9%) of cryptogenic cirrhosis were the three main etiologies. The proportion of AC almost reached the level of HBV-associated cirrhosis, and even exceeded that of cirrhotic patients with chronic hepatitis B. Screening for alcohol use disorders is urgent. In addition, the prevalence of MASLD by imaging has been estimated to be 25.24% (95%CI: 22.10%-28.65%) worldwide and 27.37% (95%CI: 23.29%-31.88%) in Asia[10]. This study found a significant number of patients with cryptogenic cirrhosis. We speculate that most patients with cryptogenic cirrhosis would develop MASLD. The likely reason is that most hospitalized patients with cirrhosis have a long history of cirrhosis and the initial steatosis has progressed to significant liver fibrosis, making the specific etiology difficult to trace.
Among patients with compensated viral cirrhosis, the cumulative risk of HCC varies according to etiology. The cumulative incidences of HCC in hepatitis B surface antigen (HBsAg), HCV, and HBsAg/HCV positive patients were 10%, 21%, and 23%, respectively, at 5 years, and 16%, 28%, and 45%, respectively, at 10 years[18]. Without HBV and HCV infection, patients with compensated alcoholic cirrhosis have a lower incidence of HCC, with cumulative 1 and 2-year incidence rates of 1% and 5%, respectively[19,20], but a significantly higher risk of death[21]. A prospective study reported that the estimated risk of developing HCC was lower than the observed incidence (2.4% at 1 year and 7.3% at 3 years) in the majority (77.6%) of patients with decompensated cirrhosis. However, only 11.6% of the patients have a 3-year risk, ranging from 10% to 47%[22]. Our study yielded similar results, showing that HBV and HCV infections were associated with a higher risk of HCC after considering death as a competing risk factor for hepatocarcinogenesis. Among cirrhotic patients with HBV, HCV, and HBV/HCV coinfection, the 5-year risk of HCC was 45.8%, 42.9%, and 48.1%, respectively, whereas the 5-year risk of HCC was only 29.5% in patients with cirrhosis who had never had hepatitis.
Male sex and advanced age are risk factors for developing HCC in patients with cirrhosis[5,23], and our study reported similar results. Furthermore, predictors of HCC in patients with cirrhosis include baseline objective and conventional risk indicators, including alpha-fetoprotein, albumin, alanine aminotransferase, and platelet levels[24]. In patients with AC, baseline bilirubin and prothrombin levels were associated with the risk of HCC in a prospective analysis[19]. Our study showed that in addition to sex, age, and hepatitis virus infection, baseline albumin and globulin levels, as well as platelet and blood ammonia levels, were equally significant risk factors for HCC. The differences in the roles of these conventional risk indicators in different studies may be related to the varying etiologic distributions and hepatic function compensation statuses in the corresponding study populations.
Over 10 years of follow-up, large sample size, and detailed objective baseline biochemical data are the strengths of our study. However, there are several limitations to this study: (1) There is no data on the effect of therapeutic interventions in reducing HCC risk; and (2) Our data were collected retrospectively and may be subject to biases associated with retrospective studies. Additionally, there may have been unmeasured confounders, such as family history of cirrhosis, which were not included in the multivariate analysis.
In conclusion, our study showed that viral hepatitis cirrhosis increased the risk of HCC. Male sex and older age are consistently associated with a higher risk of developing HCC in patients with cirrhosis. Furthermore, high blood ammonia levels, and low albumin and platelet levels may predict a higher risk of HCC. These findings emphasize the need to strengthen the management of objective biochemical parameters in patients with cirrhosis.
Of the 5417 patients included, 1352 developed HCC and 262 died. The risk of developing HCC was 19.2% (95%CI: 17.9%-20.5%) at 3 years and 35.2% (95%CI: 33.4%-37.0%) at 5 years. Male sex, older age, and viral hepatitis and cirrhosis were associated with a higher risk of HCC. Increased risk of HCC may also be associated with lower albumin and platelet levels, higher globulin levels, and higher baseline blood ammonia levels.
The authors highly appreciate all the patients who were involved in the present study and our team from Beijing Youan Hospital.
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 839] [Article Influence: 209.8] [Reference Citation Analysis (1)] |
| 2. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2274] [Article Influence: 379.0] [Reference Citation Analysis (0)] |
| 3. | Brar G, Greten TF, Graubard BI, McNeel TS, Petrick JL, McGlynn KA, Altekruse SF. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatol Commun. 2020;4:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 4. | Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, Xu R, Ababneh E, Abbasi-Kangevari M, Abbastabar H, Abd-Elsalam SM, Abdoli A, Abedi A, Abidi H, Abolhassani H, Adedeji IA, Adnani QES, Advani SM, Afzal MS, Aghaali M, Ahinkorah BO, Ahmad S, Ahmad T, Ahmadi A, Ahmadi S, Ahmed Rashid T, Ahmed Salih Y, Akalu GT, Aklilu A, Akram T, Akunna CJ, Al Hamad H, Alahdab F, Al-Aly Z, Ali S, Alimohamadi Y, Alipour V, Aljunid SM, Alkhayyat M, Almasi-Hashiani A, Almasri NA, Al-Maweri SAA, Almustanyir S, Alonso N, Alvis-Guzman N, Amu H, Anbesu EW, Ancuceanu R, Ansari F, Ansari-Moghaddam A, Antwi MH, Anvari D, Anyasodor AE, Aqeel M, Arabloo J, Arab-Zozani M, Aremu O, Ariffin H, Aripov T, Arshad M, Artaman A, Arulappan J, Asemi Z, Asghari Jafarabadi M, Ashraf T, Atorkey P, Aujayeb A, Ausloos M, Awedew AF, Ayala Quintanilla BP, Ayenew T, Azab MA, Azadnajafabad S, Azari Jafari A, Azarian G, Azzam AY, Badiye AD, Bahadory S, Baig AA, Baker JL, Balakrishnan S, Banach M, Bärnighausen TW, Barone-Adesi F, Barra F, Barrow A, Behzadifar M, Belgaumi UI, Bezabhe WMM, Bezabih YM, Bhagat DS, Bhagavathula AS, Bhardwaj N, Bhardwaj P, Bhaskar S, Bhattacharyya K, Bhojaraja VS, Bibi S, Bijani A, Biondi A, Bisignano C, Bjørge T, Bleyer A, Blyuss O, Bolarinwa OA, Bolla SR, Braithwaite D, Brar A, Brenner H, Bustamante-Teixeira MT, Butt NS, Butt ZA, Caetano Dos Santos FL, Cao Y, Carreras G, Catalá-López F, Cembranel F, Cerin E, Cernigliaro A, Chakinala RC, Chattu SK, Chattu VK, Chaturvedi P, Chimed-Ochir O, Cho DY, Christopher DJ, Chu DT, Chung MT, Conde J, Cortés S, Cortesi PA, Costa VM, Cunha AR, Dadras O, Dagnew AB, Dahlawi SMA, Dai X, Dandona L, Dandona R, Darwesh AM, das Neves J, De la Hoz FP, Demis AB, Denova-Gutiérrez E, Dhamnetiya D, Dhimal ML, Dhimal M, Dianatinasab M, Diaz D, Djalalinia S, Do HP, Doaei S, Dorostkar F, Dos Santos Figueiredo FW, Driscoll TR, Ebrahimi H, Eftekharzadeh S, El Tantawi M, El-Abid H, Elbarazi I, Elhabashy HR, Elhadi M, El-Jaafary SI, Eshrati B, Eskandarieh S, Esmaeilzadeh F, Etemadi A, Ezzikouri S, Faisaluddin M, Faraon EJA, Fares J, Farzadfar F, Feroze AH, Ferrero S, Ferro Desideri L, Filip I, Fischer F, Fisher JL, Foroutan M, Fukumoto T, Gaal PA, Gad MM, Gadanya MA, Gallus S, Gaspar Fonseca M, Getachew Obsa A, Ghafourifard M, Ghashghaee A, Ghith N, Gholamalizadeh M, Gilani SA, Ginindza TG, Gizaw ATT, Glasbey JC, Golechha M, Goleij P, Gomez RS, Gopalani SV, Gorini G, Goudarzi H, Grosso G, Gubari MIM, Guerra MR, Guha A, Gunasekera DS, Gupta B, Gupta VB, Gupta VK, Gutiérrez RA, Hafezi-Nejad N, Haider MR, Haj-Mirzaian A, Halwani R, Hamadeh RR, Hameed S, Hamidi S, Hanif A, Haque S, Harlianto NI, Haro JM, Hasaballah AI, Hassanipour S, Hay RJ, Hay SI, Hayat K, Heidari G, Heidari M, Herrera-Serna BY, Herteliu C, Hezam K, Holla R, Hossain MM, Hossain MBH, Hosseini MS, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hsairi M, Huang J, Hugo FN, Hussain R, Hussein NR, Hwang BF, Iavicoli I, Ibitoye SE, Ida F, Ikuta KS, Ilesanmi OS, Ilic IM, Ilic MD, Irham LM, Islam JY, Islam RM, Islam SMS, Ismail NE, Isola G, Iwagami M, Jacob L, Jain V, Jakovljevic MB, Javaheri T, Jayaram S, Jazayeri SB, Jha RP, Jonas JB, Joo T, Joseph N, Joukar F, Jürisson M, Kabir A, Kahrizi D, Kalankesh LR, Kalhor R, Kaliyadan F, Kalkonde Y, Kamath A, Kameran Al-Salihi N, Kandel H, Kapoor N, Karch A, Kasa AS, Katikireddi SV, Kauppila JH, Kavetskyy T, Kebede SA, Keshavarz P, Keykhaei M, Khader YS, Khalilov R, Khan G, Khan M, Khan MN, Khan MAB, Khang YH, Khater AM, Khayamzadeh M, Kim GR, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Kopec JA, Koteeswaran R, Koul PA, Koulmane Laxminarayana SL, Koyanagi A, Kucuk Bicer B, Kugbey N, Kumar GA, Kumar N, Kumar N, Kurmi OP, Kutluk T, La Vecchia C, Lami FH, Landires I, Lauriola P, Lee SW, Lee SWH, Lee WC, Lee YH, Leigh J, Leong E, Li J, Li MC, Liu X, Loureiro JA, Lunevicius R, Magdy Abd El Razek M, Majeed A, Makki A, Male S, Malik AA, Mansournia MA, Martini S, Masoumi SZ, Mathur P, McKee M, Mehrotra R, Mendoza W, Menezes RG, Mengesha EW, Mesregah MK, Mestrovic T, Miao Jonasson J, Miazgowski B, Miazgowski T, Michalek IM, Miller TR, Mirzaei H, Mirzaei HR, Misra S, Mithra P, Moghadaszadeh M, Mohammad KA, Mohammad Y, Mohammadi M, Mohammadi SM, Mohammadian-Hafshejani A, Mohammed S, Moka N, Mokdad AH, Molokhia M, Monasta L, Moni MA, Moosavi MA, Moradi Y, Moraga P, Morgado-da-Costa J, Morrison SD, Mosapour A, Mubarik S, Mwanri L, Nagarajan AJ, Nagaraju SP, Nagata C, Naimzada MD, Nangia V, Naqvi AA, Narasimha Swamy S, Ndejjo R, Nduaguba SO, Negoi I, Negru SM, Neupane Kandel S, Nguyen CT, Nguyen HLT, Niazi RK, Nnaji CA, Noor NM, Nuñez-Samudio V, Nzoputam CI, Oancea B, Ochir C, Odukoya OO, Ogbo FA, Olagunju AT, Olakunde BO, Omar E, Omar Bali A, Omonisi AEE, Ong S, Onwujekwe OE, Orru H, Ortega-Altamirano DV, Otstavnov N, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakshir K, Pana A, Panagiotakos D, Panda-Jonas S, Pardhan S, Park EC, Park EK, Pashazadeh Kan F, Patel HK, Patel JR, Pati S, Pattanshetty SM, Paudel U, Pereira DM, Pereira RB, Perianayagam A, Pillay JD, Pirouzpanah S, Pishgar F, Podder I, Postma MJ, Pourjafar H, Prashant A, Preotescu L, Rabiee M, Rabiee N, Radfar A, Radhakrishnan RA, Radhakrishnan V, Rafiee A, Rahim F, Rahimzadeh S, Rahman M, Rahman MA, Rahmani AM, Rajai N, Rajesh A, Rakovac I, Ram P, Ramezanzadeh K, Ranabhat K, Ranasinghe P, Rao CR, Rao SJ, Rawassizadeh R, Razeghinia MS, Renzaho AMN, Rezaei N, Rezaei N, Rezapour A, Roberts TJ, Rodriguez JAB, Rohloff P, Romoli M, Ronfani L, Roshandel G, Rwegerera GM, S M, Sabour S, Saddik B, Saeed U, Sahebkar A, Sahoo H, Salehi S, Salem MR, Salimzadeh H, Samaei M, Samy AM, Sanabria J, Sankararaman S, Santric-Milicevic MM, Sardiwalla Y, Sarveazad A, Sathian B, Sawhney M, Saylan M, Schneider IJC, Sekerija M, Seylani A, Shafaat O, Shaghaghi Z, Shaikh MA, Shamsoddin E, Shannawaz M, Sharma R, Sheikh A, Sheikhbahaei S, Shetty A, Shetty JK, Shetty PH, Shibuya K, Shirkoohi R, Shivakumar KM, Shivarov V, Siabani S, Siddappa Malleshappa SK, Silva DAS, Singh JA, Sintayehu Y, Skryabin VY, Skryabina AA, Soeberg MJ, Sofi-Mahmudi A, Sotoudeh H, Steiropoulos P, Straif K, Subedi R, Sufiyan MB, Sultan I, Sultana S, Sur D, Szerencsés V, Szócska M, Tabarés-Seisdedos R, Tabuchi T, Tadbiri H, Taherkhani A, Takahashi K, Talaat IM, Tan KK, Tat VY, Tedla BAA, Tefera YG, Tehrani-Banihashemi A, Temsah MH, Tesfay FH, Tessema GA, Thapar R, Thavamani A, Thoguluva Chandrasekar V, Thomas N, Tohidinik HR, Touvier M, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tran MTN, Tripathy JP, Tusa BS, Ullah I, Ullah S, Umapathi KK, Unnikrishnan B, Upadhyay E, Vacante M, Vaezi M, Valadan Tahbaz S, Velazquez DZ, Veroux M, Violante FS, Vlassov V, Vo B, Volovici V, Vu GT, Waheed Y, Wamai RG, Ward P, Wen YF, Westerman R, Winkler AS, Yadav L, Yahyazadeh Jabbari SH, Yang L, Yaya S, Yazie TSY, Yeshaw Y, Yonemoto N, Younis MZ, Yousefi Z, Yu C, Yuce D, Yunusa I, Zadnik V, Zare F, Zastrozhin MS, Zastrozhina A, Zhang J, Zhong C, Zhou L, Zhu C, Ziapour A, Zimmermann IR, Fitzmaurice C, Murray CJL, Force LM. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1180] [Cited by in RCA: 1112] [Article Influence: 370.7] [Reference Citation Analysis (0)] |
| 5. | Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 255] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 6. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 404] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 7. | Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, Singh S, Mohamed EA, Abu Dabrh AM, Prokop LJ, Wang Z, Murad MH, Mohammed K. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 423] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 8. | Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, Séguy N, Rewari BB, Chan PL, Le LV, Doherty M, Luhmann N, Easterbrook P, Dirac M, de Martel C, Nayagam S, Hallett TB, Vickerman P, Razavi H, Lesi O, Low-Beer D. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 156] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 9. | Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 348] [Article Influence: 116.0] [Reference Citation Analysis (1)] |
| 10. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7497] [Article Influence: 833.0] [Reference Citation Analysis (0)] |
| 11. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1787] [Article Influence: 85.1] [Reference Citation Analysis (2)] |
| 12. | Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. 2012;32:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Asrani SK, Hall L, Reddy V, Ogola G, Izzy M. Comorbid Chronic Diseases and Survival in Compensated and Decompensated Cirrhosis: A Population-Based Study. Am J Gastroenterol. 2022;117:2009-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 364] [Article Influence: 52.0] [Reference Citation Analysis (1)] |
| 16. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 352] [Article Influence: 176.0] [Reference Citation Analysis (0)] |
| 17. | Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 516] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 18. | Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132-2137. [PubMed] |
| 19. | Ganne-Carrié N, Chaffaut C, Bourcier V, Archambeaud I, Perarnau JM, Oberti F, Roulot D, Moreno C, Louvet A, Dao T, Moirand R, Goria O, Nguyen-Khac E, Carbonell N, Antonini T, Pol S, de Ledinghen V, Ozenne V, Henrion J, Péron JM, Tran A, Perlemuter G, Amiot X, Zarski JP, Beaugrand M, Chevret S; for CIRRAL Group. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Huang DQ, Tan DJH, Ng CH, Amangurbanova M, Sutter N, Lin Tay PW, Lim WH, Yong JN, Tang A, Syn N, Muthiah MD, Tan EXX, Dave S, Tay B, Majzoub AM, Gerberi D, Kim BK, Loomba R. Hepatocellular Carcinoma Incidence in Alcohol-Associated Cirrhosis: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Jepsen P, Kraglund F, West J, Villadsen GE, Sørensen HT, Vilstrup H. Risk of hepatocellular carcinoma in Danish outpatients with alcohol-related cirrhosis. J Hepatol. 2020;73:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Reddy KR, McLerran D, Marsh T, Parikh N, Roberts LR, Schwartz M, Nguyen MH, Befeler A, Page-Lester S, Tang R, Srivastava S, Rinaudo JA, Feng Z, Marrero JA. Incidence and Risk Factors for Hepatocellular Carcinoma in Cirrhosis: The Multicenter Hepatocellular Carcinoma Early Detection Strategy (HEDS) Study. Gastroenterology. 2023;165:1053-1063.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Rong G, Wang H, Bowlus CL, Wang C, Lu Y, Zeng Z, Qu J, Lou M, Chen Y, An L, Yang Y, Gershwin ME. Incidence and risk factors for hepatocellular carcinoma in primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Kanwal F, Khaderi S, Singal AG, Marrero JA, Asrani SK, Amos CI, Thrift AP, Kramer JR, Yu X, Cao Y, Luster M, Al-Sarraj A, Ning J, El-Serag HB. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023;21:3296-3304.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |