Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4565
Revised: August 30, 2024
Accepted: September 19, 2024
Published online: December 15, 2024
Processing time: 146 Days and 20.2 Hours
Within the intricate milieu of colorectal cancer (CRC) tissues, cancer-associated fibroblasts (CAFs) act as pivotal orchestrators, wielding considerable influence over tumor progression. This review endeavors to dissect the multifaceted functions of CAFs within the realm of CRC, thereby highlighting their indispensability in fostering CRC malignant microenvironment and indicating the deve
Core Tip: Within the intricate milieu of colorectal cancer (CRC) tissues, cancer-associated fibroblasts (CAFs) act as pivotal orchestrators, wielding considerable influence over tumor progression. This review endeavors to dissect the multifaceted functions of CAFs within the realm of CRC, thereby highlighting their indispensability in fostering CRC malignant microenvironment and indicating the development of CAFs-targeted therapeutic interventions. Through a comprehensive synthesis of current knowledge, this review delineates insights into CAFs-mediated modulation of cancer cell proliferation, invasiveness, immune evasion, and neovascularization, elucidating the intricate web of interactions that sustain the pro-tumor metabolism and secretion of multiple factors. Additionally, recognizing the high level of heterogeneity within CAFs is crucial, as they encompass a range of subtypes, including myofibroblastic CAFs, inflammatory CAFs, antigen-presenting CAFs, and vessel-associated CAFs. Innovatively, the symbiotic relationship between CAFs and the intestinal microbiota is explored, shedding light on a novel dimension of CRC pathogenesis. Despite remarkable progress, the orchestrated dynamic functions of CAFs remain incompletely deciphered, underscoring the need for continued research endeavors for therapeutic advancements in CRC management.
- Citation: Cui JY, Ma J, Gao XX, Sheng ZM, Pan ZX, Shi LH, Zhang BG. Unraveling the role of cancer-associated fibroblasts in colorectal cancer. World J Gastrointest Oncol 2024; 16(12): 4565-4578
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4565.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4565
Malignant tumors have become a significant factor affecting the mortality worldwide, with colorectal cancer (CRC) being among the crucial types of malignancies. CRC is the third most common cancer and the second leading cause of cancer-related death worldwide[1]. It is well known that the development of tumors is not solely due to changes of genes within tumor cells, and the alterations in the surrounding microenvironment also play a significant role in tumor progression.
Over 100 years ago, Paget and others elucidated the importance of the tumor microenvironment (TME) with the “seed and soil” hypothesis which implied a synergistic interaction between tumors and their microenvironment[2]. Numerous studies have confirmed that the stromal components of the TME have crucial impacts on the occurrence and evolution of tumors. As the most abundant non-cancerous stromal cell type, cancer-associated fibroblasts (CAFs) play vital roles in the development of CRC by influencing the proliferation, invasion, and metastasis of cancer cells as well as angiogenesis and immune response through reshaping the extracellular matrix (ECM), secreting soluble factors (chemokines and growth factors), and modulating the microbial community[3,4].
In this review, we aim to delve into the crucial role of CAFs in the progression and treatment of CRC. Additionally, we provide a comprehensive overview of the diverse functions of CAFs, including their impact on cancer cell proliferation, invasiveness, immune evasion, and neovascularization, as well as their role in shaping the TME and influencing tumor growth and metastasis. This exploration highlights the intricate network between CRC and CAFs and clarifies how these interactions affect the disease’s prognosis and treatment efficacy. Moreover, we introduce a novel perspective on the symbiotic relationship between CAFs and the intestinal microbiota, potentially opening new avenues for targeting CAFs.
Normal fibroblasts (NFs) are spindle-shaped mesenchymal cells possessing non-epithelial, non-endothelial, and non-immunological characteristics[5,6] which commonly locate in the connective tissue and help to maintain tissue homeostasis[7]. Functionally, fibroblasts synthesize and secrete laminin, type IV collagen, and other basement membrane-related proteins under normal physiological conditions. Particularly, fibroblasts in intestinal tissue exhibit specialized functions that are crucial for supporting epithelial cells and maintaining barrier integrity[8]. Their roles in ECM production, tissue remodeling, cell communication, immunoregulation, and wound healing collectively contribute to the maintenance of the intestinal epithelial function. In addition, fibroblasts exhibit inhibitory effects on the occurrence, development, invasion, and metastasis of tumor cells through various mechanisms including direct cell-to-cell contact, secretion of soluble factors, and preservation of an intact ECM environment[9]. However, in the context of malignant tumors, changes in the structure and function of the TME lead fibroblasts to transition from their initial anti-tumor characteristics to pro-tumor preference[10].
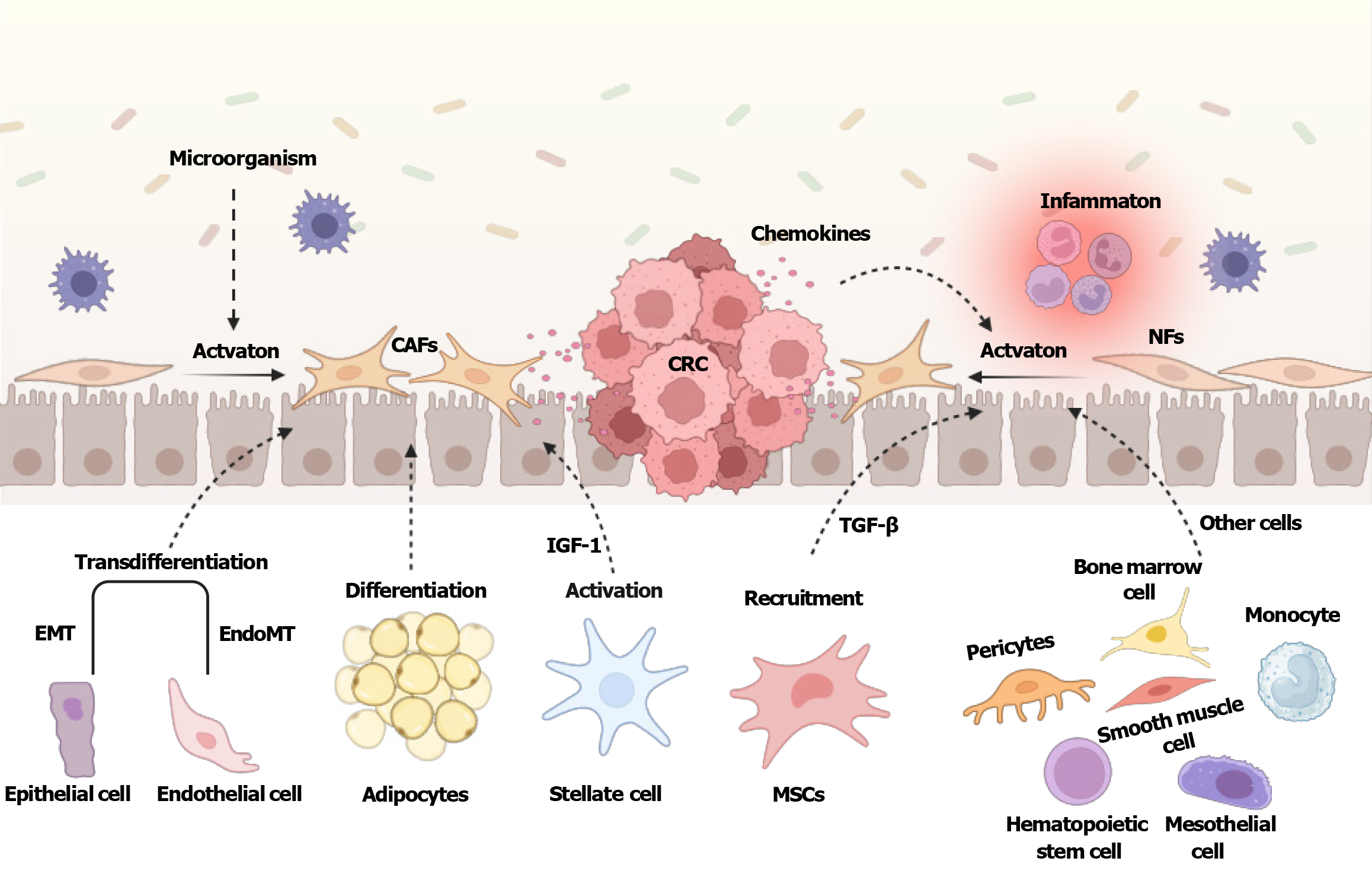
CAFs are generally considered to be all fibroblasts embedded within and around cancerous tissues, representing a crucial component of TME[11]. Progenitor cells of CAFs are recruited from several sources and developed by distinct pathways[12], and most of the available evidence supports that the majority of CAFs may originate from the activation of locally resident fibroblasts[13]. Tissue-resident quiescent fibroblasts can be activated by various factors including inflammatory cytokines, constipation, microorganisms, and chemokines [interleukin (IL)-6], as well as growth factors such as transforming growth factor-β (TGF-β)[14] (Figure 1). Persisted activation tends to cause morphological and functional transformations of NFs into CAFs which are involved in cancer initiation, progression, and chemoresistance[15]. It should be noted that CAFs and NFs are interconvertible, although the underlying mechanism is still poorly understood. Fibrocytes represent a quiescent fibroblast state, which are also considered as one of the sources of CAFs[16,17]. For example, fibrocytes derived from hepatic stellate cells (HSCs) expressing fibroblast growth factor receptor 2 were identified as the origin of CAFs in esophageal squamous cell carcinoma[18].
Mesenchymal stem cells (MSCs) are proposed as potential precursors for CAFs, and Notch and Akt signaling pathways have been reported to be involved in the differentiation of bone marrow MSCs into CAFs[19,20] (Figure 1). In addition, MSCs could acquire CAF-like characteristics while stimulated by TGF-β, tumor necrosis factor-α, and IL-1β[21]. Intriguingly, macrophages can assist MSCs in developing CAF-like features[22]. Stellate cells are also an alternative origin of CAFs in certain tumors[23,24] (Figure 1). Insufficient vitamin A can trigger the transformation of pancreatic stellate cells into CAFs[25], and insulin-like growth factor-1 (IGF-1) signaling is crucial for activating HSCs into CAFs[26]. Adipocytes are recognized as another category of CAFs precursors[17,27] (Figure 1). Adipocytes may play a role in tumorigenesis through a mechanism of adipocyte-fibroblast transition[28]. Tumor cells could induce morphological changes and, meanwhile, decrease lipid content and marker expression of adipocytes. Bochet et al[29] found that adipocytes may dedifferentiate into cancer-associated adipocytes, and cancer-associated adipocytes become less adipocyte-like and phenotypically mirror fibroblasts with increased expression of ECM components. Moreover, epithelial and endothelial cells can differentiate into CAFs through epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition[30-33]. Through the TGF-β-mediated EMT, epithelial cells are able to differentiate into functional CAFs expressing ferroptosis suppressor protein 1 and α-fibroblast activation protein (FAP)[34], whereas during the endothelial-to-mesenchymal transition, endothelial cells acquire a mesenchymal-like phenotype as well as invasive and migratory properties[35]. Cell types, including pericytes[36], monocytes[37], mesothelial cells[38], hematopoietic stem cells[39], circulating bone marrow cells[40], and smooth muscle cells[41], are also identified as potential precursors for CAFs. Understanding the origins of CAFs can provide new targets for cancer stromal remodeling process.
Heterogeneity transcends not only between individuals with the same tumor but also manifests as striking variations within a single tumor[42]. This dynamic variability evolves continuously over time and across spatial dimensions, interacting intricately with the surrounding microenvironment, making it a perpetually shifting and complex process. Distinct subtypes of CAFs are characterized by unique biological markers. In CRC, commonly identified CAF biomarkers include α-smooth muscle actin (α-SMA), FAP, vimentin, fibroblast specific protein 1, podoplanin, and platelet-derived growth factor receptors α/β (PDGFRα/β)[43]. With the advent of sequencing technologies, researchers have begun to analyze CAF populations at the single-cell level and revealed substantial heterogeneity among CAFs[44]. Single-cell sequencing has identified two principal CAF subsets named CAF-A and CAF-B in human CRC: CAF-A expresses high levels of FAP, matrix metalloproteinase-2 (MMP-2), and collagen type I alpha 2 while CAF-B is characterized by the expression of myofibroblastic markers such as α-SMA, transgelin, and PDGFα[45].
Additionally, recent studies have revealed two distinct subpopulations of CAFs in CRC: Myofibroblastic CAFs (myCAFs), characterized by high α-SMA and IL-6 expression, and inflammatory CAFs (iCAFs), which exhibit low α-SMA and high IL-6 expression[23,46-51]. myCAFs are primarily involved in regulating the ECM, collagen deposition, cellular contraction, and adhesion, whereas iCAFs are distinguished by their secretion of cytokines and chemokines and their interactions with immune cells[50,51]. Interestingly, we discovered that iCAFs were particularly concentrated around dilated blood vessels at the colonic luminal margin and demonstrated greater activity than myCAFs in mismatch repair-deficient tumors[46].
As research continues to deepen, the classification of CAF subtypes has become increasingly nuanced. Khaliq et al[51] utilized large-scale single-cell sequencing across diverse ethnic groups to achieve a more detailed categorization of myCAF and iCAF subtypes in CRC tissue, drawing on classification methods previously used in breast cancer[49]. The myCAF group was further subdivided into ecm-myCAFs, associated with ECM proteins; wound-myCAFs, linked to wound healing signaling; and TGFβ-myCAFs, dependent on the TGFβ pathway[51]. Similarly, the iCAF group was refined to include IL-iCAFs, related to IL signaling, and detox-iCAFs, associated with detoxification pathways. In addition to myCAFs and iCAFs, a type of vascular-associated CAFs expressing pericyte markers and hypoxia-inducible factor 2 has also been identified in CRC, participating in angiogenesis and vascular development and potentially contributing to tumor invasion and metastasis[51-53].
CAFs are frequently linked to the promotion of CRC, but recent studies have also uncovered their potential role in inhibiting CRC growth[54,55]. Kobayashi et al[56] discovered that CAFs can polarize into GREM1+ CAFs, which promote tumor progression, and ISLR+ CAFs, which inhibit tumor progression, under the regulation of the TGFβ-FOXL1-GREM1/ISLR axis. Although the specific mediators secreted by ISLR+ CAFs that inhibit CRC progression remain unidentified, it is clear that these mediators contribute to activating bone morphogenetic protein signaling in CRC cells.
The heterogeneity of CAFs is continuously evolving with technological and temporal advancements. Single-cell sequencing and spatial transcriptomics have significantly enhanced our understanding of CAFs’ diversity. However, a thorough classification of the functional diversity among different CAF subtypes in CRC remains incomplete, and more effective approaches for elucidating the identities and states of CAF subtypes are urgently needed[57,58].
In recent years, an increasing awareness has emerged regarding the pre-existing tumorigenic state of fibroblasts even prior to the onset of malignant cell transformation[13]. To delve deeper into the pathogenic intricacies of CRC, inves
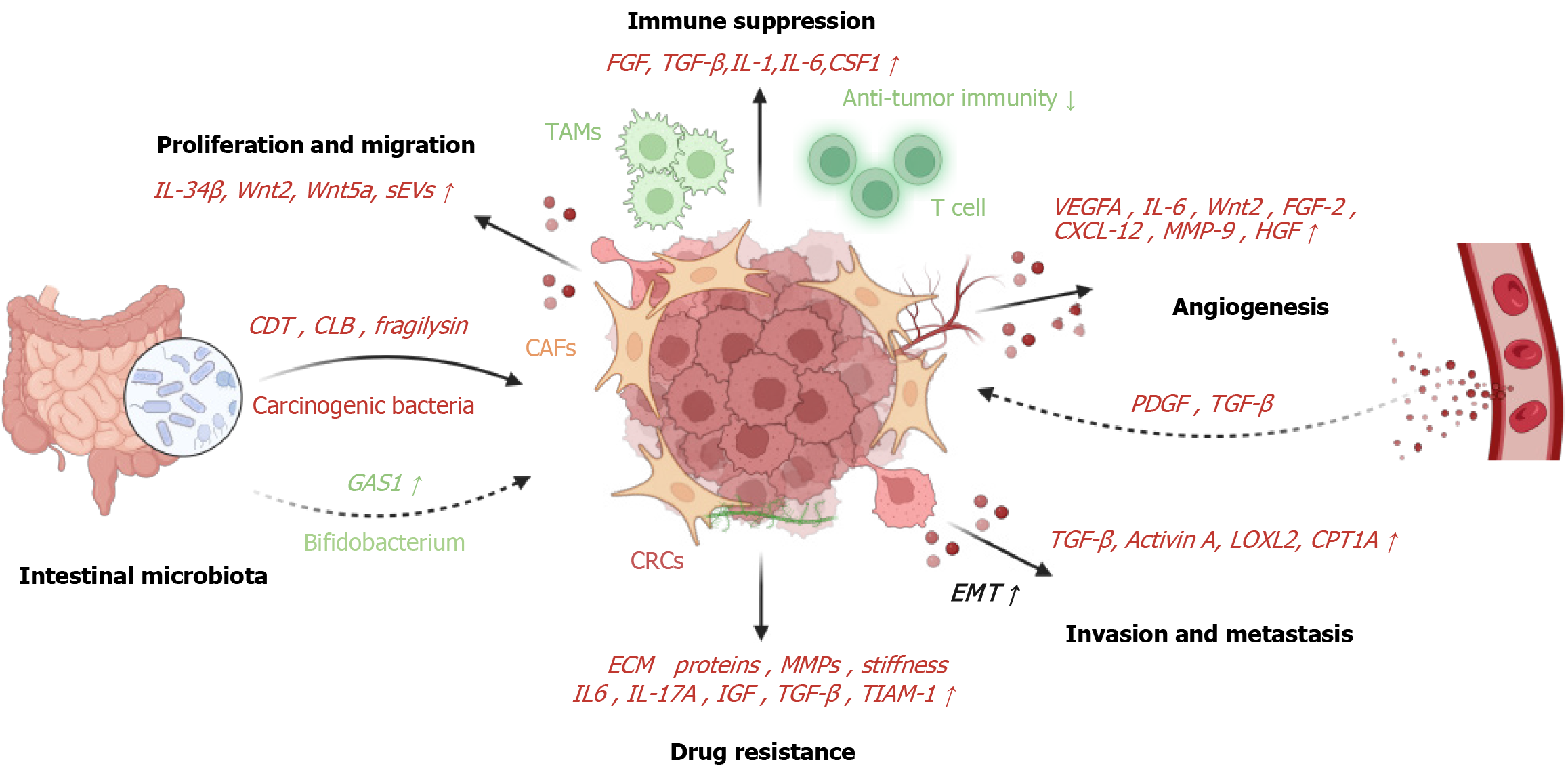
On the other hand, the intricate crosstalks between CAFs and immune cells also lead to tumor immune evasion by mediating immune cell recruitment and functional differentiation within the TME[64] (Figure 2). In the context of CRCs, the MSCs, as precursors to CAFs, exacerbate tumor progression by intensifying the inhibition of immune cells. Studies found that the sialylation profile of stromal cells is an important mechanism by which MSCs/CAFs modulate T cell exhaustion. Targeting stromal cell sialylation may overcome immunosuppression in the CRC TME[65]. The investigations spearheaded by Yang et al[66] illuminated the exacerbation of CAFs’ pro-tumor role with the loss of the BCL9 gene, culminating in the aberrant activation of the Wnt/β-catenin signaling pathway, thereby impeding T-cell-mediated anti-tumor immune responses.
Tumor-associated macrophages contribute to all aspects of tumor progression. Colony-stimulating factor 1 produced by tumor cells caused histone deacetylase 2-mediated downregulation of granulocyte-specific chemokine expression in CAFs. Treatment with colony-stimulating factor 1 receptor inhibitors disrupted this crosstalk and triggered a profound increase in granulocyte recruitment to tumors[67]. Moreover, colony-stimulating factor 1 receptor inhibitors have been utilized as agents targeting tumor-associated macrophages in clinical trials for solid tumors, including intestinal cancers[68]. However, their efficacy has been limited. This limitation has been attributed to CAFs’ capability to counteract the therapeutic effects of these agents by recruiting polymorphonuclear myeloid-derived suppressor cells to tumor sites, thereby shaping an immunosuppressive TME[67]. Concomitantly, the involvement of pentraxin 3, fostering M2-like polarization of macrophages, surfaces in the context of CAF-mediated immune suppression, with elevated pentraxin 3 expression correlating positively with fibroblasts and inflammatory response signals in CRC patients, prognosticating unfavorable survival outcomes[69]. The pervasive influence of CAFs also extends to shaping the cytotoxicity of T lymphocytes, fortifying tumor cells in their evasion of the body’s immune response[70].
A noteworthy revelation surfaces from recent research, underscoring the tumor-suppressive function of α-SMA+ CAFs within a genetically engineered mouse model of metastatic CRC. The α-SMA(+) CAFs in CRC exert tumor-restraining functions via bone morphogenetic protein 4/TGF-β1 paracrine signaling that serves to suppress Lgr5(+) CSCs and promote anti-tumor immunity, ultimately limiting CRC progression[71]. However, the nuanced impact of the depletion strategy, predominantly targeting α-SMA+ CAFs, on other α-SMA+ cells dispersed throughout the body, introduces an element of uncertainty regarding its potential perturbation of the observed outcomes. While there is a growing interest in cancer immunology, our comprehension of how CAFs participate in tumor immune surveillance is still in its infancy. More extensive investigations into CAFs are imperative to pinpoint promising target molecules or subsets of CAFs and to devise innovative therapies that can enhance the clinical efficacy of existing immunotherapies.
Angiogenesis stands out as a pivotal step throughout diverse stages of tumor progression, playing a crucial role in the invasive growth of CRC. The significance lies in its ability to provide abundant blood vessels, furnishing oxygen and nutrients indispensable for the proliferation of tumor tissues. As a hallmark of malignant tumors, angiogenesis is closely associated with tumor growth, metastasis, and patient prognosis and is promoted by the most potent pro-angiogenic factor, vascular endothelial growth factor (VEGF), which stimulates angiogenesis by binding to its corresponding receptor VEGF receptor 2 (VEGFR2) on endothelial cells[72-74].
Neovascularization in cancer is governed not solely by tumor cells but also by stromal cells[75]. In fact, CAFs directly foster tumor angiogenesis by releasing pro-angiogenic factors and indirectly by generating the ECM[5,75]. In the context of CRC, cancer cells exhibit minimal VEGF secretion whereas CAFs represent a significant origin of VEGFA through the abundant release of IL-6[75,76]. The intricate interplay between colon cancer cells and the TME facilitates the transformation of NFs into CAFs, positively modulating IL-6 secretion, thereby amplifying VEGF secretion and fostering tumor angiogenesis[77]. Additionally, Unterleuthner et al[78] discovered that WNT2 secreted by CAFs plays a pivotal role in promoting the formation of tumor blood vessels in CRC. Silencing WNT2 in CAFs significantly diminishes the angiogenic potential of tumor vessels, highlighting that elevated WNT2 expression in CAFs renders tumors more susceptible to invasion and metastasis. Moreover, CAFs promote tumor initiation by secreting pro-tumor factors (Figure 2) that enhance angiogenesis, including VEGF-α, fibroblast growth factor 2, CXC motif chemokine ligand 12, MMP-9, and hepatocyte growth factor (HGF)[79]. In turn, the permeable vasculature within tumors leads to platelet extravasation and the subsequent release of pro-angiogenic factors such as PDGF and TGFβ through degranulation, which further activate fibroblasts[80]. Collectively, these studies offer a compelling rationale for considering CAFs as a viable therapeutic target to modify tumor vasculature.
In the intricate landscape of tumorigenesis, CAFs stand as instrumental architects, actively promoting the insidious progression of tumor invasion and metastasis[81,82]. As a substantial component of the TME, CAFs wield their influence in fostering the malignant behaviors of cancer cells. The multifaceted role of CAFs is underscored by their ability to induce EMT, a pivotal process that endows cancer cells with the traits essential for invasion and metastasis. Research illuminates the mechanistic intricacies, revealing that factors (Figure 2) such as TGF-β activation of colonic stromal fibroblasts and the secretion of activin A contribute to the heightened migratory capacity and EMT in colonic epithelial cells, thereby rendering CRC cells more prone to metastasis[83].
The dynamic interplay extends to specific molecular signaling pathways, as evidenced by studies elucidating the stimulation of EMT in CRC cells through the secretion of lysyl oxidase-like 2 by CAFs (Figure 2), activating the focal adhesion kinase signaling pathway[84]. Inhibition of this process presents a potential avenue for curtailing CRC cell invasion and metastasis. Additionally, the orchestration of EMT by CAFs involves regulatory elements such as myosin light chain 9, which influences the secretion of C-C motif ligand 2 and TGF-β, thereby shaping the TME and impacting CRC invasion and metastasis[85].
Beyond their involvement in EMT, CAFs exert a substantial impact on the metabolic landscape of CRC cells, enhancing their invasion and metastasis. Through the upregulation of key factors like carnitine palmitoyl-transferase 1A (Figure 2)[86], CAFs promote fatty acid oxidation while minimizing glycolysis, ultimately fostering a milieu conducive to tumor growth and invasion. Woven into this intricate narrative is the expression of WNT2 in CAFs, contributing to the invasion of CRC cells and unveiling additional dimensions to the role of CAFs in promoting metastasis[87]. As the exploration of CAFs’ influence on tumor invasion and metastasis unfolds, their capacity to produce signaling molecules, including TGF-β, leukemia inhibitory factor, and HGF, emerges as another crucial facet[88]. These molecules act as potent drivers, propelling cancer cell proliferation and invasion behaviors. TGF-β, in particular, emerges as an effective inducer of EMT through paracrine signaling, endowing premalignant cells with mesenchymal properties that facilitate invasion and metastasis[89]. Furthermore, CAFs play a pivotal role in the colonization of distant organs during metastasis, either by creating a supportive microenvironment for cancer cells or by accompanying them in their journey[90].
Examining factors that foster invasion and metastasis through the lenses of EMT and metabolism is imperative. Concurrently, we need to delve into the repercussions of cancer cell EMT on CAFs subtypes. A crucial aspect to explore involves comprehending the influence of EMT-inducing transcription factors on stromal cells. Existing studies underscore a direct correlation between the expression of EMT transcription factors and the presence of CAFs[91]. Noteworthy is the work of Franci et al[92], who discerned predominant Snail expression in fibroblasts proximate to tumor cells in CRC. Furthermore, CRCs exhibiting mesenchymal gene expression traits display a heightened proclivity for distant metastasis, marked by the accumulation of CAFs in the stromal milieu. And findings indicate that fibroblasts within epithelial tumors exhibit elevated miR-200 expression and reduced levels of actin alpha 2 and fibronectin 1 compared to their mesenchymal counterparts[93]. This discovery unveils a novel mechanism for the heterogeneity of CRC CAFs, elucidating how miRNA transfer via extracellular vesicles contributes to this phenomenon. It also offers insights into why CRCs with augmented metastatic potential boast an abundance of CAFs.
Recent investigations underscore the pivotal role of the TME in shaping tumor cell resistance, elucidating the substantial impact of CAFs in promoting drug resistance across diverse cancers, notably CRC[94]. The intricate interplay involves CAFs’s production of ECM proteins and matrix remodeling MMPs, forming both a physical barrier and elevating matrix stiffness and interstitial pressure (Figure 2). This dual effect impedes the efficient penetration of chemotherapy drugs and targeted therapies, contributing significantly to drug resistance[95]. Moreover, the secretion of key soluble factors (Figure 2) by CAFs, including IL-6, IL-17A, and IGF, intricately mediates chemotherapy resistance and amplifies cytokine secretion post-treatment, consequently intensifying drug resistance[96,97].
Beyond their physical and soluble influences, CAFs play a pivotal role in immune suppression within the TME[13]. The accumulation of aberrant ECM components exacerbates immune suppression, adversely impacting the efficacy of immune checkpoint inhibitors. The involvement of the TGF-β signaling pathway is particularly noteworthy in the context of immune therapy resistance. It is intriguing to observe that the blockade of TGF-β signaling, in conjunction with receptor kinase inhibitors and anti-programmed cell death protein 1/programmed cell death ligand 1 immunotherapy, exhibits a synergistic effect in a murine model of CRC liver metastasis[98,99]. Notably, an elevated compound stromal score, characterized by three stromal components (CAFs, leukocytes, and endothelial cells), has the potential to anticipate resistance to radiotherapy in rectal cancer patients[100].
Regarding molecularly targeted medications, the release of HGF and IGF2 by CAFs plays a role in conferring resistance to tyrosine kinase inhibitors[101]. Notably, the concurrent inhibition of epidermal growth factor receptor (EGFR) and MET, or insulin receptor and IGF1 receptor, which facilitate the IGF2/insulin receptor/IGF1 receptor signaling pathway, has been shown to augment the therapeutic efficacy of an EGFR inhibitor in xenograft models of colon cancer, respectively[102]. In the realm of CRC, CAFs further exhibit their intricate influence by secreting IL-17A, which acts on the IL-17A receptor on CSCs. This action maintains CSPDCs’ stem cell characteristics, upregulates nuclear factor-κB expression, and induces resistance in cancer cells[97]. Additionally, the secretion of exosomes by CAFs is implicated in promoting the stemness and chemotherapy resistance of CRC. The interaction with eukaryotic initiation factor 4A-III and the exosomal miR-625-3p blockade of the CELF2/WWOX pathway underscore the multifaceted mechanisms through which CAFs potentiate drug resistance[103,104]. Furthermore, CAFs contribute to chemotherapy resistance in CRC cells by inducing the overexpression of T-lymphoma invasion and metastasis-inducing protein-1, presenting T-lymphoma invasion and metastasis-inducing protein-1 as a promising therapeutic target[105]. In a co-culture setting, CAF-conditioned medium induces the overexpression of the RBCK1 gene, augmenting the stemness and resistance of CRC cells[106]. This intricate web of interactions emphasizes the significant and varied roles of CAFs in fueling drug resistance in CRC, urging further exploration for targeted therapeutic interventions.
As a crucial component of the intestinal barrier, the intestinal microbiota intricately weaves a biofilm, actively participating in intestinal functions and establishing a conducive survival environment for intestinal cells. Notably, this microbial consortium is implicated in various stages of CRC, ranging from benign precursor lesions (polyps) to in situ growth and metastasis. The concept of “carcinogenic bacteria” introduced in 2018, encompasses species like Escherichia coli, Fusobacterium nucleatum, and enterotoxigenic Bacteroides fragilis, underscoring their pivotal role in CRC pathogenesis. Concurrently, bacterial toxins such as cytolethal distending toxin, colibactin, and fragilysin exert their influence on the TME, fostering tumor development and immune escape. Noteworthy insights illuminate the intricate interplay between Helicobacter pylori, fibroblasts, and cancer cells, leading to the induction of Serpin E1 expression. This induction propels the transformation of NFs into CAFs, contributing to the onset of gastric cancer[107]. The complexity of these interactions emphasizes the multifaceted role of the intestinal microbiota in orchestrating the TME and influencing cancer progression.
Crucially, the nuanced roles of distinct intestinal bacteria must be acknowledged, as some confer beneficial effects on the human body. For instance, the work of Chen et al[108] highlights the positive impact of youth-associated Bifidobacterium, activating CD143+ CAFs, thereby initiating the Wnt signaling pathway and upregulating growth arrest specific 1 expression. This cascade of events manifests in anticancer effects, unveiling a promising therapeutic target for CRC[108]. These findings underscore the dynamic nature of the interactions between the intestinal microbiota, fibroblasts, and cancer cells, offering a profound understanding of the intricate molecular mechanisms shaping the TME and presenting potential avenues for therapeutic interventions.
Alongside surgical resection, the most current clinical approaches to treating CRC involve chemotherapy and radiotherapy, which exert diverse effects on the various cellular constituents of the TME[109]. A comparative study of CRC specimens from patients before and after undergoing cytotoxic treatment revealed a marked rise in CAFs[97]. CAFs exposed to chemotherapy were observed to prolong tumor cell survival and accelerate growth compared to untreated CAFs, indicating that CAFs may shield tumor cells from the growth-inhibitory effects of chemotherapy[109]. In contrast to the genetic instability often observed in malignant tumor cells of CRC, CAFs exhibit genetic stability. This characteristic distinction prompts the exploration of combined therapies targeting both CRC cells and CAFs, envisaged as a novel strategy to augment treatment effectiveness and overcome resistance in CRC. These strategies encompass three primary methods: (1) The elimination of “harmful” CAFs[110]; (2) The reprogramming of CAFs into “normal” fibroblasts or anti-tumor “beneficial” CAF subtypes[111]; and (3) The blockade of signals or ECM components derived from CAFs[112,113]. Successful implementation of these approaches necessitates a precise definition and classification of CAFs, coupled with an enhanced understanding of their diverse functions. Despite extensive research endeavors, several strategies targeting CAFs are yet to yield satisfactory clinical outcomes[14,114,115].
Currently, numerous anticancer drugs targeting CAFs are undergoing preclinical research or clinical trial phases. These drugs predominantly exert direct damage to CAFs by targeting specific surface molecules or impede the secretion of pro-cancer factors and associated signaling pathways in CAFs. The inaugural phase I clinical trial targeting gastrointestinal cancers, employing autologous MSCs genetically modified to express herpes simplex virus-thymidine kinase, has demonstrated acceptable safety and tolerability. These MSCs facilitate the conversion of the pro-drug ganciclovir into its active cytotoxic metabolite. Furthermore, researchers are exploring the combination of TGF-β inhibitors or Hedgehog inhibitors with standard chemotherapies or immunotherapies[116]. This combination strategy aims to obstruct pro-tumorigenic signaling pathways pertinent to CAFs in gastrointestinal cancers[99,117,118]. Moreover, the differentiation of HSCs into CAFs via the C-X-C chemokine receptor type 4/TGF-β1 signaling axis promotes CRC liver metastasis. Consequently, blocking the C-X-C chemokine receptor type 4/TGF-β1 signaling axis emerges as a beneficial strategy for anti-metastasis treatment in CRC[119].
As research on the TME advances, exosomes have emerged as a promising avenue for therapeutic development. Among many miRNA molecules, miRNA-20a was found to inhibit the secretion of CXC motif chemokine ligand 8 from CAFs, thereby inhibiting tumor growth[120]. CAFs are also capable of secreting the lncRNAs CCAL and H19, thereby inducing drug resistance in cancer cells through the activation of β-catenin signaling[121,122]. Furthermore, CAFs release exosomal circSLC7A6 to stimulate the proliferation of CRC cells, an effect that can be inhibited by matrine, a compound derived from Sophora flavescens[123]. Additionally, CAFs secrete exosomal miR-93-5p and miR-21, which are transferred into CRC cells, consequently enhancing tumor resistance to chemotherapy[124-126].
Diverse attempts have been made to directly target specific subsets of CAFs. Some researchers have encapsulated drugs within lipid nanocapsules to directly target CAFs in the TME, discovering that paclitaxel and acetylcholinesterase are particularly promising for inhibiting CAF development[127]. Notably, a DNA vaccine inducing CD8 T cell killing of FAP+ CAFs has demonstrated the depletion of FAP+ CAFs and the inhibition of tumor progression in a CRC mouse model[128]. However, clinical trials evaluating anti-FAP monoclonal antibodies have not yielded the anticipated efficacy in advanced CRC patients[129]. Combining the chemotherapeutic drug oxaliplatin with the inhibitor PT-100 in a CRC mouse model significantly increased the sensitivity of tumor tissue to chemotherapy, concurrently reducing the recruitment of pro-tumor cells and angiogenesis[130]. Additionally, calcium/calmodulin-dependent protein kinase II has emerged as a pivotal mediator of TFG-β1-induced CAFs activation and participation in CRC cell signaling, positioning calcium/calmodulin-dependent protein kinase II as a promising target for future CRC treatment[131]. Furthermore, CAFs enhance the chemotherapy resistance of CRC cells by receiving signals through their receptor C-C motif chemokine receptor 5, suggesting that targeting C-C motif chemokine receptor 5 may offer a potential approach for treating CRC[132]. In the meanwhile, phosphorylated signal transducer and activator of transcription 3 in CAFs can be used as a tool to judge the prognosis of patients with CRC, and the activation of CAFs may be a therapeutic target for CRC[133]. Moreover, inhibitors of HGF activation, such as SRI 31215, offer a novel strategy to disrupt autocrine and paracrine oncogenic HGF/MET signaling, thereby preventing HGF-dependent cancer cell proliferation, EMT, and migration[134]. Simultaneously targeting HGF and EGFR can effectively block both primary and acquired fibroblast-mediated resistance to EGFR inhibitors in colon cancer cells.
In CRC, CAFs predominantly reside within the tumor or adjacent tissues, secreting factors that fuel tumor initiation, progression, invasion, and metastasis. Notably, CAFs play a pivotal role in aiding tumor cells in evading the body’s immune responses, thereby fortifying resistance to radiation and chemotherapy. Consequently, the targeting of CAFs has emerged as an innovative and promising approach in the treatment landscape of CRC.
Anticipating future developments in the realm of CAFs, particularly in CAF subpopulation biology, there has been rapid and significant progress. A growing body of evidence underscores the crucial roles played by specific subgroups of CAFs in tumor initiation, development, metastasis, and resistance to treatment. Nevertheless, the heterogeneity in phenotype and function exhibited by CAFs introduces variability in their roles across different types of tumors and at various stages of the same tumor. Therefore, further exploration is imperative to unravel the nuanced functions of CAFs in different pathological subtypes of CRC and at distinct developmental stages.
Recent reports add an intriguing layer to our understanding, revealing that CAFs not only promote tumor cell development but also exhibit inhibitory effects on tumors. Investigating the underlying mechanisms and pathways through which CAFs themselves exert inhibitory effects represents a promising avenue for future research. The advent of modern scientific technologies, such as single-cell sequencing and spatial transcriptomics, has empowered researchers to delve into the study of CAFs subgroups at unprecedented levels. However, despite the valuable insights gained from single-cell technologies in identifying CAFs subgroups, comprehensive characterization and in-depth functional analyses remain essential to validate data and establish meaningful connections.
In the future trajectory of research, dedicated efforts should be directed towards comprehending the dynamic nature of CAFs, exploring their plasticity, deciphering the factors influencing their transformation, and investigating the potential reversibility of these changes. This concerted endeavor holds the promise of offering more precise and effective strategies for cancer treatment, ultimately paving the way for improved therapeutic outcomes and enhanced well-being for patients.
We sincerely thank our colleagues for their valuable suggestions and technical assistance for this research. We thank BioRender.com for expert assistance in the pattern drawing.
| 1. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1344] [Article Influence: 336.0] [Reference Citation Analysis (5)] |
| 2. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 3. | Wang W, Li Q, Yamada T, Matsumoto K, Matsumoto I, Oda M, Watanabe G, Kayano Y, Nishioka Y, Sone S, Yano S. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630-6638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1207] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 5. | Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2209] [Cited by in RCA: 2941] [Article Influence: 326.8] [Reference Citation Analysis (0)] |
| 6. | Tarin D, Croft CB. Ultrastructural features of wound healing in mouse skin. J Anat. 1969;105:189-190. [PubMed] |
| 7. | Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S, Brenner M, Buckley CD. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21:704-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 342] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 9. | Alkasalias T, Moyano-Galceran L, Arsenian-Henriksson M, Lehti K. Fibroblasts in the Tumor Microenvironment: Shield or Spear? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Adv Drug Deliv Rev. 2016;99:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 688] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 12. | Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev. 2021;101:147-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 767] [Article Influence: 191.8] [Reference Citation Analysis (0)] |
| 13. | Abudukelimu S, de Miranda NFCC, Hawinkels LJAC. Fibroblasts in Orchestrating Colorectal Tumorigenesis and Progression. Cell Mol Gastroenterol Hepatol. 2024;17:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Koliaraki V, Pallangyo CK, Greten FR, Kollias G. Mesenchymal Cells in Colon Cancer. Gastroenterology. 2017;152:964-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Feng B, Wu J, Shen B, Jiang F, Feng J. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell Int. 2022;22:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 16. | Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1940] [Cited by in RCA: 2448] [Article Influence: 489.6] [Reference Citation Analysis (0)] |
| 17. | Xu Y, Li W, Lin S, Liu B, Wu P, Li L. Fibroblast diversity and plasticity in the tumor microenvironment: roles in immunity and relevant therapies. Cell Commun Signal. 2023;21:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 18. | Qiu H, Zhang X, Qi J, Zhang J, Tong Y, Li L, Fu L, Qin YR, Guan X, Zhang L. Identification and characterization of FGFR2(+) hematopoietic stem cell-derived fibrocytes as precursors of cancer-associated fibroblasts induced by esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2022;41:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Friedman G, Levi-Galibov O, David E, Bornstein C, Giladi A, Dadiani M, Mayo A, Halperin C, Pevsner-Fischer M, Lavon H, Mayer S, Nevo R, Stein Y, Balint-Lahat N, Barshack I, Ali HR, Caldas C, Nili-Gal-Yam E, Alon U, Amit I, Scherz-Shouval R. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4(+) and PDPN(+) CAFs to clinical outcome. Nat Cancer. 2020;1:692-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 20. | Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 869] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 21. | Rubinstein-Achiasaf L, Morein D, Ben-Yaakov H, Liubomirski Y, Meshel T, Elbaz E, Dorot O, Pichinuk E, Gershovits M, Weil M, Ben-Baruch A. Persistent Inflammatory Stimulation Drives the Conversion of MSCs to Inflammatory CAFs That Promote Pro-Metastatic Characteristics in Breast Cancer Cells. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Chai S, Wang W, Wan C, Zhang F, Li Y, Wang F. Macrophages activate mesenchymal stem cells to acquire cancer-associated fibroblast-like features resulting in gastric epithelial cell lesions and malignant transformation in vitro. Oncol Lett. 2019;17:747-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1020] [Cited by in RCA: 1756] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
| 24. | Trivedi P, Wang S, Friedman SL. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021;33:242-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 25. | Zhou Y, Zhou J, Sun B, Xu W, Zhong M, Li Y, He C, Chen Y, Wang X, Jones PM, Sun Z. Vitamin A deficiency causes islet dysfunction by inducing islet stellate cell activation via cellular retinol binding protein 1. Int J Biol Sci. 2020;16:947-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Xie Z, Gao Y, Ho C, Li L, Jin C, Wang X, Zou C, Mao Y, Wang X, Li Q, Fu D, Zhang YF. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut. 2022;71:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259-5266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Downer MA, Griffin MF, Morgan AG, Parker JB, Li DJ, Berry CE, Liang NE, Kameni L, Cotterell AC, Akras D, Valencia C, Longaker MT, Wan DC. Understanding the Role of Adipocytes and Fibroblasts in Cancer. Ann Plast Surg. 2023;91:779-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, Couderc B, Escourrou G, Valet P, Muller C. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657-5668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 30. | Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1434] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 31. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1666] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 32. | Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 433] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 33. | Morgan A, Griffin M, Kameni L, Wan DC, Longaker MT, Norton JA. Medical Biology of Cancer-Associated Fibroblasts in Pancreatic Cancer. Biology (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 34. | Chen PY, Wei WF, Wu HZ, Fan LS, Wang W. Cancer-Associated Fibroblast Heterogeneity: A Factor That Cannot Be Ignored in Immune Microenvironment Remodeling. Front Immunol. 2021;12:671595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Adjuto-Saccone M, Soubeyran P, Garcia J, Audebert S, Camoin L, Rubis M, Roques J, Binétruy B, Iovanna JL, Tournaire R. TNF-α induces endothelial-mesenchymal transition promoting stromal development of pancreatic adenocarcinoma. Cell Death Dis. 2021;12:649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 36. | Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 37. | Huang X, He C, Hua X, Kan A, Mao Y, Sun S, Duan F, Wang J, Huang P, Li S. Oxidative stress induces monocyte-to-myofibroblast transdifferentiation through p38 in pancreatic ductal adenocarcinoma. Clin Transl Med. 2020;10:e41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Huang H, Wang Z, Zhang Y, Pradhan RN, Ganguly D, Chandra R, Murimwa G, Wright S, Gu X, Maddipati R, Müller S, Turley SJ, Brekken RA. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell. 2022;40:656-673.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 309] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 39. | McDonald LT, Russell DL, Kelly RR, Xiong Y, Motamarry A, Patel RK, Jones JA, Watson PM, Turner DP, Watson DK, Soloff AC, Findlay VJ, LaRue AC. Hematopoietic stem cell-derived cancer-associated fibroblasts are novel contributors to the pro-tumorigenic microenvironment. Neoplasia. 2015;17:434-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Punt CJ, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol. 2017;14:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 448] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 43. | Kobayashi H, Gieniec KA, Lannagan TRM, Wang T, Asai N, Mizutani Y, Iida T, Ando R, Thomas EM, Sakai A, Suzuki N, Ichinose M, Wright JA, Vrbanac L, Ng JQ, Goyne J, Radford G, Lawrence MJ, Sammour T, Hayakawa Y, Klebe S, Shin AE, Asfaha S, Bettington ML, Rieder F, Arpaia N, Danino T, Butler LM, Burt AD, Leedham SJ, Rustgi AK, Mukherjee S, Takahashi M, Wang TC, Enomoto A, Woods SL, Worthley DL. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology. 2022;162:890-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 44. | Chen Y, Liang Z, Lai M. Targeting the devil: Strategies against cancer-associated fibroblasts in colorectal cancer. Transl Res. 2024;270:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 45. | Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, Wong M, Choi PJ, Wee LJK, Hillmer AM, Tan IB, Robson P, Prabhakar S. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet. 2017;49:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 760] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 46. | Pelka K, Hofree M, Chen JH, Sarkizova S, Pirl JD, Jorgji V, Bejnood A, Dionne D, Ge WH, Xu KH, Chao SX, Zollinger DR, Lieb DJ, Reeves JW, Fuhrman CA, Hoang ML, Delorey T, Nguyen LT, Waldman J, Klapholz M, Wakiro I, Cohen O, Albers J, Smillie CS, Cuoco MS, Wu J, Su MJ, Yeung J, Vijaykumar B, Magnuson AM, Asinovski N, Moll T, Goder-Reiser MN, Applebaum AS, Brais LK, DelloStritto LK, Denning SL, Phillips ST, Hill EK, Meehan JK, Frederick DT, Sharova T, Kanodia A, Todres EZ, Jané-Valbuena J, Biton M, Izar B, Lambden CD, Clancy TE, Bleday R, Melnitchouk N, Irani J, Kunitake H, Berger DL, Srivastava A, Hornick JL, Ogino S, Rotem A, Vigneau S, Johnson BE, Corcoran RB, Sharpe AH, Kuchroo VK, Ng K, Giannakis M, Nieman LT, Boland GM, Aguirre AJ, Anderson AC, Rozenblatt-Rosen O, Regev A, Hacohen N. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021;184:4734-4752.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 431] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 47. | Sebastian A, Hum NR, Martin KA, Gilmore SF, Peran I, Byers SW, Wheeler EK, Coleman MA, Loots GG. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 48. | Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M, Chin L, Filliol A, Wen W, Song X, Decker A, Worley J, Caviglia JM, Yu L, Yin D, Saito Y, Savage T, Wells RG, Mack M, Zender L, Arpaia N, Remotti HE, Rabadan R, Sims P, Leblond AL, Weber A, Riener MO, Stockwell BR, Gaublomme J, Llovet JM, Kalluri R, Michalopoulos GK, Seki E, Sia D, Chen X, Califano A, Schwabe RF. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866-882.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 228] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 49. | Kieffer Y, Hocine HR, Gentric G, Pelon F, Bernard C, Bourachot B, Lameiras S, Albergante L, Bonneau C, Guyard A, Tarte K, Zinovyev A, Baulande S, Zalcman G, Vincent-Salomon A, Mechta-Grigoriou F. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020;10:1330-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 542] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 50. | Peng Z, Ye M, Ding H, Feng Z, Hu K. Spatial transcriptomics atlas reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment components in colorectal cancer. J Transl Med. 2022;20:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 51. | Khaliq AM, Erdogan C, Kurt Z, Turgut SS, Grunvald MW, Rand T, Khare S, Borgia JA, Hayden DM, Pappas SG, Govekar HR, Kam AE, Reiser J, Turaga K, Radovich M, Zang Y, Qiu Y, Liu Y, Fishel ML, Turk A, Gupta V, Al-Sabti R, Subramanian J, Kuzel TM, Sadanandam A, Waldron L, Hussain A, Saleem M, El-Rayes B, Salahudeen AA, Masood A. Correction: Refining colorectal cancer classification and clinical stratification through a single-cell atlas. Genome Biol. 2022;23:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, Madsen CD, Lindgren D, Pekar G, Karlsson G, Ringnér M, Bergh J, Björklund Å, Pietras K. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9:5150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 581] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 53. | Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge C, Liu Y, Hao Y, Zhang D, Shi G, Gong Y, Ni Y, Wang C, Zhang Y, Xi J, Wang S, Shi L, Zhang L, Yue W, Pei X, Liu B, Yan X. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73:1118-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 54. | Miyai Y, Esaki N, Takahashi M, Enomoto A. Cancer-associated fibroblasts that restrain cancer progression: Hypotheses and perspectives. Cancer Sci. 2020;111:1047-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 55. | Wang Z, Yang Q, Tan Y, Tang Y, Ye J, Yuan B, Yu W. Cancer-Associated Fibroblasts Suppress Cancer Development: The Other Side of the Coin. Front Cell Dev Biol. 2021;9:613534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 56. | Kobayashi H, Gieniec KA, Wright JA, Wang T, Asai N, Mizutani Y, Lida T, Ando R, Suzuki N, Lannagan TRM, Ng JQ, Hara A, Shiraki Y, Mii S, Ichinose M, Vrbanac L, Lawrence MJ, Sammour T, Uehara K, Davies G, Lisowski L, Alexander IE, Hayakawa Y, Butler LM, Zannettino ACW, Din MO, Hasty J, Burt AD, Leedham SJ, Rustgi AK, Mukherjee S, Wang TC, Enomoto A, Takahashi M, Worthley DL, Woods SL. The Balance of Stromal BMP Signaling Mediated by GREM1 and ISLR Drives Colorectal Carcinogenesis. Gastroenterology. 2021;160:1224-1239.e30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 57. | Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023;56:2188-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 372] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 58. | Caligiuri G, Tuveson DA. Activated fibroblasts in cancer: Perspectives and challenges. Cancer Cell. 2023;41:434-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 182] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 59. | Fernando-Macías E, Fernández-García MT, García-Pérez E, Porrero Guerrero B, López-Arévalo C, Rodríguez-Uría R, Sanz-Navarro S, Vázquez-Villa JF, Muñíz-Salgueiro MC, Suárez-Fernández L, Galván JA, Barneo-Caragol C, García-Ocaña M, de Los Toyos JR, Barneo-Serra L. A new aggressive xenograft model of human colon cancer using cancer-associated fibroblasts. PeerJ. 2020;8:e9045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Yang K, Zhang F, Luo B, Qu Z. CAFs-derived small extracellular vesicles circN4BP2L2 promotes proliferation and metastasis of colorectal cancer via miR-664b-3p/HMGB3 pathway. Cancer Biol Ther. 2022;23:404-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 61. | Hirashima T, Karasawa H, Aizawa T, Suzuki T, Yamamura A, Suzuki H, Kajiwara T, Musha H, Funayama R, Shirota M, Ohnuma S, Nakayama K, Unno M. Wnt5a in cancer-associated fibroblasts promotes colorectal cancer progression. Biochem Biophys Res Commun. 2021;568:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Aizawa T, Karasawa H, Funayama R, Shirota M, Suzuki T, Maeda S, Suzuki H, Yamamura A, Naitoh T, Nakayama K, Unno M. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019;8:6370-6382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 63. | Stadler M, Pudelko K, Biermeier A, Walterskirchen N, Gaigneaux A, Weindorfer C, Harrer N, Klett H, Hengstschläger M, Schüler J, Sommergruber W, Oehler R, Bergmann M, Letellier E, Dolznig H. Stromal fibroblasts shape the myeloid phenotype in normal colon and colorectal cancer and induce CD163 and CCL2 expression in macrophages. Cancer Lett. 2021;520:184-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 64. | Musa M, Ali A. Cancer-associated fibroblasts of colorectal cancer and their markers: updates, challenges and translational outlook. Future Oncol. 2020;16:2329-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Egan H, Treacy O, Lynch K, Leonard NA, O'Malley G, Reidy E, O'Neill A, Corry SM, De Veirman K, Vanderkerken K, Egan LJ, Ritter T, Hogan AM, Redmond K, Peng L, Che J, Gatlin W, Jayaraman P, Sheehan M, Canney A, Hynes SO, Kerr EM, Dunne PD, O'Dwyer ME, Ryan AE. Targeting stromal cell sialylation reverses T cell-mediated immunosuppression in the tumor microenvironment. Cell Rep. 2023;42:112475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 66. | Yang M, Wei Z, Feng M, Zhu Y, Chen Y, Zhu D. Pharmacological Inhibition and Genetic Knockdown of BCL9 Modulate the Cellular Landscape of Cancer-Associated Fibroblasts in the Tumor-Immune Microenvironment of Colorectal Cancer. Front Oncol. 2021;11:603556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, Mulligan C, Nam B, Hockstein N, Denstman F, Shakamuri S, Speicher DW, Weeraratna AT, Chao T, Vonderheide RH, Languino LR, Ordentlich P, Liu Q, Xu X, Lo A, Puré E, Zhang C, Loboda A, Sepulveda MA, Snyder LA, Gabrilovich DI. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell. 2017;32:654-668.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 494] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 68. | Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 588] [Cited by in RCA: 775] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 69. | Chen FW, Wu YL, Cheng CC, Hsiao YW, Chi JY, Hung LY, Chang CP, Lai MD, Wang JM. Inactivation of pentraxin 3 suppresses M2-like macrophage activity and immunosuppression in colon cancer. J Biomed Sci. 2024;31:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 70. | Monteran L, Erez N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front Immunol. 2019;10:1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 484] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 71. | McAndrews KM, Vázquez-Arreguín K, Kwak C, Sugimoto H, Zheng X, Li B, Kirtley ML, LeBleu VS, Kalluri R. αSMA(+) fibroblasts suppress Lgr5(+) cancer stem cells and restrain colorectal cancer progression. Oncogene. 2021;40:4440-4452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Lu Y, Qin T, Li J, Wang L, Zhang Q, Jiang Z, Mao J. Correction: MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther. 2020;27:838-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 73. | Ding C, Luo J, Fan X, Li L, Li S, Wen K, Feng J, Wu G. Elevated Gab2 induces tumor growth and angiogenesis in colorectal cancer through upregulating VEGF levels. J Exp Clin Cancer Res. 2017;36:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Bagheri A, Kumar P, Kamath A, Rao P. Association of angiogenic cytokines (VEGF-A and VEGF-C) and clinical characteristic in women with unexplained recurrent miscarriage. Bratisl Lek Listy. 2017;118:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 1303] [Article Influence: 162.9] [Reference Citation Analysis (0)] |
| 76. | Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 696] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 77. | Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 78. | Unterleuthner D, Neuhold P, Schwarz K, Janker L, Neuditschko B, Nivarthi H, Crncec I, Kramer N, Unger C, Hengstschläger M, Eferl R, Moriggl R, Sommergruber W, Gerner C, Dolznig H. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 79. | Ben Baruch B, Mantsur E, Franco-Barraza J, Blacher E, Cukierman E, Stein R. CD38 in cancer-associated fibroblasts promotes pro-tumoral activity. Lab Invest. 2020;100:1517-1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Yan M, Jurasz P. The role of platelets in the tumor microenvironment: From solid tumors to leukemia. Biochim Biophys Acta. 2016;1863:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 81. | Drev D, Harpain F, Beer A, Stift A, Gruber ES, Klimpfinger M, Thalhammer S, Reti A, Kenner L, Bergmann M, Marian B. Impact of Fibroblast-Derived SPARC on Invasiveness of Colorectal Cancer Cells. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Tommelein J, Verset L, Boterberg T, Demetter P, Bracke M, De Wever O. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front Oncol. 2015;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 83. | Bauer J, Emon MAB, Staudacher JJ, Thomas AL, Zessner-Spitzenberg J, Mancinelli G, Krett N, Saif MT, Jung B. Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci Rep. 2020;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 84. | Xuefeng X, Hou MX, Yang ZW, Agudamu A, Wang F, Su XL, Li X, Shi L, Terigele T, Bao LL, Wu XL. Epithelial-mesenchymal transition and metastasis of colon cancer cells induced by the FAK pathway in cancer-associated fibroblasts. J Int Med Res. 2020;48:300060520931242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Deng S, Cheng D, Wang J, Gu J, Xue Y, Jiang Z, Qin L, Mao F, Cao Y, Cai K. MYL9 expressed in cancer-associated fibroblasts regulate the immune microenvironment of colorectal cancer and promotes tumor progression in an autocrine manner. J Exp Clin Cancer Res. 2023;42:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 86. | Peng S, Chen D, Cai J, Yuan Z, Huang B, Li Y, Wang H, Luo Q, Kuang Y, Liang W, Liu Z, Wang Q, Cui Y, Wang H, Liu X. Enhancing cancer-associated fibroblast fatty acid catabolism within a metabolically challenging tumor microenvironment drives colon cancer peritoneal metastasis. Mol Oncol. 2021;15:1391-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 87. | Kramer N, Schmöllerl J, Unger C, Nivarthi H, Rudisch A, Unterleuthner D, Scherzer M, Riedl A, Artaker M, Crncec I, Lenhardt D, Schwarz T, Prieler B, Han X, Hengstschläger M, Schüler J, Eferl R, Moriggl R, Sommergruber W, Dolznig H. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene. 2017;36:5460-5472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 88. | Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, Ridinger-Saison M, DelGiorno KE, Antal CE, Liang G, Atkins AR, Erikson G, Sun H, Meisenhelder J, Terenziani E, Woo G, Fang L, Santisakultarm TP, Manor U, Xu R, Becerra CR, Borazanci E, Von Hoff DD, Grandgenett PM, Hollingsworth MA, Leblanc M, Umetsu SE, Collisson EA, Scadeng M, Lowy AM, Donahue TR, Reya T, Downes M, Evans RM, Wahl GM, Pawson T, Tian R, Hunter T. Author Correction: Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2021;600:E18. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 89. | Massagué J, Ganesh K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021;11:971-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 225] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 90. | Gonzalez-Zubeldia I, Dotor J, Redrado M, Bleau AM, Manrique I, de Aberasturi AL, Villalba M, Calvo A. Co-migration of colon cancer cells and CAFs induced by TGFβ₁ enhances liver metastasis. Cell Tissue Res. 2015;359:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 91. | Baulida J. Epithelial-to-mesenchymal transition transcription factors in cancer-associated fibroblasts. Mol Oncol. 2017;11:847-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 92. | Francí C, Takkunen M, Dave N, Alameda F, Gómez S, Rodríguez R, Escrivà M, Montserrat-Sentís B, Baró T, Garrido M, Bonilla F, Virtanen I, García de Herreros A. Expression of Snail protein in tumor-stroma interface. Oncogene. 2006;25:5134-5144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 93. | Bhome R, Emaduddin M, James V, House LM, Thirdborough SM, Mellone M, Tulkens J, Primrose JN, Thomas GJ, De Wever O, Mirnezami AH, Sayan AE. Epithelial to mesenchymal transition influences fibroblast phenotype in colorectal cancer by altering miR-200 levels in extracellular vesicles. J Extracell Vesicles. 2022;11:e12226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 94. | Yan H, Guo BY, Zhang S. Cancer-associated fibroblasts attenuate Cisplatin-induced apoptosis in ovarian cancer cells by promoting STAT3 signaling. Biochem Biophys Res Commun. 2016;470:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 95. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1639] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 96. | Ireland L, Santos A, Ahmed MS, Rainer C, Nielsen SR, Quaranta V, Weyer-Czernilofsky U, Engle DD, Perez-Mancera PA, Coupland SE, Taktak A, Bogenrieder T, Tuveson DA, Campbell F, Schmid MC, Mielgo A. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016;76:6851-6863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 97. | Lotti F, Jarrar AM, Pai RK, Hitomi M, Lathia J, Mace A, Gantt GA Jr, Sukhdeo K, DeVecchio J, Vasanji A, Leahy P, Hjelmeland AB, Kalady MF, Rich JN. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 98. | Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors. 2011;29:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 99. | Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1332] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 100. | Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore A, Mellano A, Senetta R, Cassenti A, Sonetto C, Inghirami G, Trusolino L, Fekete Z, De Ridder M, Cassoni P, Storme G, Bertotti A, Medico E. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015;47:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 486] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 101. | Luraghi P, Reato G, Cipriano E, Sassi F, Orzan F, Bigatto V, De Bacco F, Menietti E, Han M, Rideout WM 3rd, Perera T, Bertotti A, Trusolino L, Comoglio PM, Boccaccio C. MET signaling in colon cancer stem-like cells blunts the therapeutic response to EGFR inhibitors. Cancer Res. 2014;74:1857-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 102. | Vaquero J, Lobe C, Tahraoui S, Clapéron A, Mergey M, Merabtene F, Wendum D, Coulouarn C, Housset C, Desbois-Mouthon C, Praz F, Fouassier L. The IGF2/IR/IGF1R Pathway in Tumor Cells and Myofibroblasts Mediates Resistance to EGFR Inhibition in Cholangiocarcinoma. Clin Cancer Res. 2018;24:4282-4296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 103. | Qu Z, Yang KD, Luo BH, Zhang F. CAFs-secreted exosomal cricN4BP2L2 promoted colorectal cancer stemness and chemoresistance by interacting with EIF4A3. Exp Cell Res. 2022;418:113266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 104. | Zhang Y, Yin C, Wei C, Xia S, Qiao Z, Zhang XW, Yu B, Zhou J, Wang R. Exosomal miR-625-3p secreted by cancer-associated fibroblasts in colorectal cancer promotes EMT and chemotherapeutic resistance by blocking the CELF2/WWOX pathway. Pharmacol Res. 2022;186:106534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 105. | Izumi D, Toden S, Ureta E, Ishimoto T, Baba H, Goel A. TIAM1 promotes chemoresistance and tumor invasiveness in colorectal cancer. Cell Death Dis. 2019;10:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 106. | Liu ML, Zang F, Zhang SJ. RBCK1 contributes to chemoresistance and stemness in colorectal cancer (CRC). Biomed Pharmacother. 2019;118:109250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Chen X, Chen W, Zhao Y, Wang Q, Wang W, Xiang Y, Yuan H, Xie Y, Zhou J. Interplay of Helicobacter pylori, fibroblasts, and cancer cells induces fibroblast activation and serpin E1 expression by cancer cells to promote gastric tumorigenesis. J Transl Med. 2022;20:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 108. | Chen S, Fan L, Lin Y, Qi Y, Xu C, Ge Q, Zhang Y, Wang Q, Jia D, Wang L, Si J, Wang L. Bifidobacterium adolescentis orchestrates CD143(+) cancer-associated fibroblasts to suppress colorectal tumorigenesis by Wnt signaling-regulated GAS1. Cancer Commun (Lond). 2023;43:1027-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 109. | Yan L, Zheng J, Wang Q, Hao H. Role of cancer-associated fibroblasts in colorectal cancer and their potential as therapeutic targets. Biochem Biophys Res Commun. 2023;681:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 110. | Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, O'Brien S, Evans RA, Bajor DJ, Clendenin C, Durham AC, Buza EL, Vonderheide RH, June CH, Albelda SM, Puré E. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75:2800-2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 401] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 111. | Ferrer-Mayorga G, Gómez-López G, Barbáchano A, Fernández-Barral A, Peña C, Pisano DG, Cantero R, Rojo F, Muñoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 112. | Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci. 2013;34:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 113. | Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212-20217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1566] [Cited by in RCA: 1553] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 114. | Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, Hermann RC, Mulcahy MF, Zalupski MM, Mackey HM, Yauch RL, Graham RA, Bray GL, Low JA. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Cancer Res. 2013;19:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 115. | Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol Cancer Ther. 2018;17:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 116. | von Einem JC, Guenther C, Volk HD, Grütz G, Hirsch D, Salat C, Stoetzer O, Nelson PJ, Michl M, Modest DP, Holch JW, Angele M, Bruns C, Niess H, Heinemann V. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. Int J Cancer. 2019;145:1538-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 117. | Vennin C, Murphy KJ, Morton JP, Cox TR, Pajic M, Timpson P. Reshaping the Tumor Stroma for Treatment of Pancreatic Cancer. Gastroenterology. 2018;154:820-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 118. | Jia SN, Han YB, Yang R, Yang ZC. Chemokines in colon cancer progression. Semin Cancer Biol. 2022;86:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 119. | Tan HX, Gong WZ, Zhou K, Xiao ZG, Hou FT, Huang T, Zhang L, Dong HY, Zhang WL, Liu Y, Huang ZC. CXCR4/TGF-β1 mediated hepatic stellate cells differentiation into carcinoma-associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol Ther. 2020;21:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 120. | Signs SA, Fisher RC, Tran U, Chakrabarti S, Sarvestani SK, Xiang S, Liska D, Roche V, Lai W, Gittleman HR, Wessely O, Huang EH. Stromal miR-20a controls paracrine CXCL8 secretion in colitis and colon cancer. Oncotarget. 2018;9:13048-13059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, Wang Y, Wang T, Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932-3948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 558] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 122. | Deng X, Ruan H, Zhang X, Xu X, Zhu Y, Peng H, Zhang X, Kong F, Guan M. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int J Cancer. 2020;146:1700-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 123. | Gu C, Lu H, Qian Z. Matrine reduces the secretion of exosomal circSLC7A6 from cancer-associated fibroblast to inhibit tumorigenesis of colorectal cancer by regulating CXCR5. Biochem Biophys Res Commun. 2020;527:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 124. | Bhome R, Goh RW, Bullock MD, Pillar N, Thirdborough SM, Mellone M, Mirnezami R, Galea D, Veselkov K, Gu Q, Underwood TJ, Primrose JN, De Wever O, Shomron N, Sayan AE, Mirnezami AH. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging (Albany NY). 2017;9:2666-2694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 125. | Bullock MD, Pickard KM, Nielsen BS, Sayan AE, Jenei V, Mellone M, Mitter R, Primrose JN, Thomas GJ, Packham GK, Mirnezami AH. Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. 2013;4:e684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 126. | Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y, Sun Y, Ge H, Liu Y. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J Exp Clin Cancer Res. 2020;39:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 127. | Fourniols T, Bastien E, Canevat A, Feron O, Préat V. Inhibition of colorectal cancer-associated fibroblasts by lipid nanocapsules loaded with acriflavine or paclitaxel. Int J Pharm. 2020;584:119337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 128. | Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 534] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 129. | Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jäger D, Renner C, Tanswell P, Kunz U, Amelsberg A, Kuthan H, Stehle G. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 130. | Li M, Li M, Yin T, Shi H, Wen Y, Zhang B, Chen M, Xu G, Ren K, Wei Y. Targeting of cancerassociated fibroblasts enhances the efficacy of cancer chemotherapy by regulating the tumor microenvironment. Mol Med Rep. 2016;13:2476-2484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 131. | Chen W, Chen Y, Su J, Kang J, Ding Y, Ai W, Zhang J, Luo H, An P. CaMKII Mediates TGFβ1-Induced Fibroblasts Activation and Its Cross Talk with Colon Cancer Cells. Dig Dis Sci. 2022;67:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 132. | Tanabe Y, Sasaki S, Mukaida N, Baba T. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancer-associated fibroblast accumulation. Oncotarget. 2016;7:48335-48345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 133. | Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, Thoma OM, Kramer V, Waldner MJ, Büttner C, Farin HF, Pešić M, Knieling F, Merkel S, Grüneboom A, Gunzer M, Grützmann R, Rose-John S, Koralov SB, Kollias G, Vieth M, Hartmann A, Greten FR, Neurath MF, Neufert C. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69:1269-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 134. | Owusu BY, Bansal N, Venukadasula PK, Ross LJ, Messick TE, Goel S, Galemmo RA, Klampfer L. Inhibition of pro-HGF activation by SRI31215, a novel approach to block oncogenic HGF/MET signaling. Oncotarget. 2016;7:29492-29506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |