Published online Oct 15, 2024. doi: 10.4251/wjgo.v16.i10.4281
Revised: August 18, 2024
Accepted: August 27, 2024
Published online: October 15, 2024
Processing time: 108 Days and 7.6 Hours
Helicobacter pylori (H. pylori) eradication treatment for primary gastric mucosa-associated lymphoid tissue (MALT) lymphoma has already been established. However, t (11;18) (q21;q21)/API2-MALT1 translocation-positive lesions are a type of primary gastric MALT lymphoma in which a response to eradication trea
A 66-year-old man was diagnosed with MALT lymphoma in the ascending colon by colonoscopy and biopsy. Two years later, esophagogastroduodenoscopy reve
Because the patient had a MALT1 translocation with trisomy 18q21, it was thought that this gastric MALT lymphoma developed independently of H. pylori infection and progressed.
Core Tip: Helicobacter pylori (H. pylori) eradication treatment is expected to be effective for treating mucosa-associated lymphoid tissue (MALT) lymphoma originating not only from the stomach but also from the colon. However, t (11;18) (q21;q21)/API2-MALT1 translocation is difficult to achieve a response to H. pylori eradication treatment for primary gastric MALT lymphoma. In this study, H. pylori eradication treatment performed for chronic atrophic gastritis was not only ineffective against initial colonic MALT lymphoma but also failed to prevent the subsequent development of gastric MALT lymphoma and further transformed diffuse large B-cell lymphoma. Because this lesion had a MALT1 translocation with trisomy 18q21, it was thought that the disease developed independently of H. pylori infection and progressed.
- Citation: Saito M, Tanei ZI, Tsuda M, Suzuki T, Yokoyama E, Kanaya M, Izumiyama K, Mori A, Morioka M, Kondo T. Transformed gastric mucosa-associated lymphoid tissue lymphoma originating in the colon and developing metachronously after Helicobacter pylori eradication: A case report. World J Gastrointest Oncol 2024; 16(10): 4281-4288
- URL: https://www.wjgnet.com/1948-5204/full/v16/i10/4281.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i10.4281
Mucosa-associated lymphoid tissue (MALT) lymphoma, proposed by Isaacson and Wright[1] in 1984, is an indolent lymphoma that develops due to chronic inflammation in various extranodal organs[1]. The stomach is the most common site of occurrence, and MALT lymphoma accounts for approximately half of all primary gastric lymphomas and is dichotomous with diffuse large B-cell lymphoma (DLBCL)[2]. In addition, Helicobacter pylori (H. pylori) is often involved in the development of primary gastric MALT lymphoma, and eradication therapy has long been established as a treatment for this disease[3,4]. Gastric MALT lymphoma independent of H. pylori infection, which is difficult to eradicate, is characterized by: (1) H. pylori negativity; (2) t (11;18) (q21;q21)/API2-MALT1 translocation; (3) Deep gastric wall invasion; and (4) An advanced disease stage[3,5,6]. In addition, trisomy 18 may be associated with DLBCL transformation of gastric MALT lymphoma[7].
On the other hand, primary colorectal lymphoma accounts for < 1% of all colorectal malignancies[8], and colorectal MALT lymphoma is an even rarer disease[9,10]. A study of a small number of patients reported that one-third of those individuals with colonic MALT lymphoma also had gastric MALT lymphoma[11]. In addition, a standard treatment for primary colonic MALT lymphoma has not yet been established, and there are reports that suggest the effectiveness of H. pylori eradication therapy[12,13].
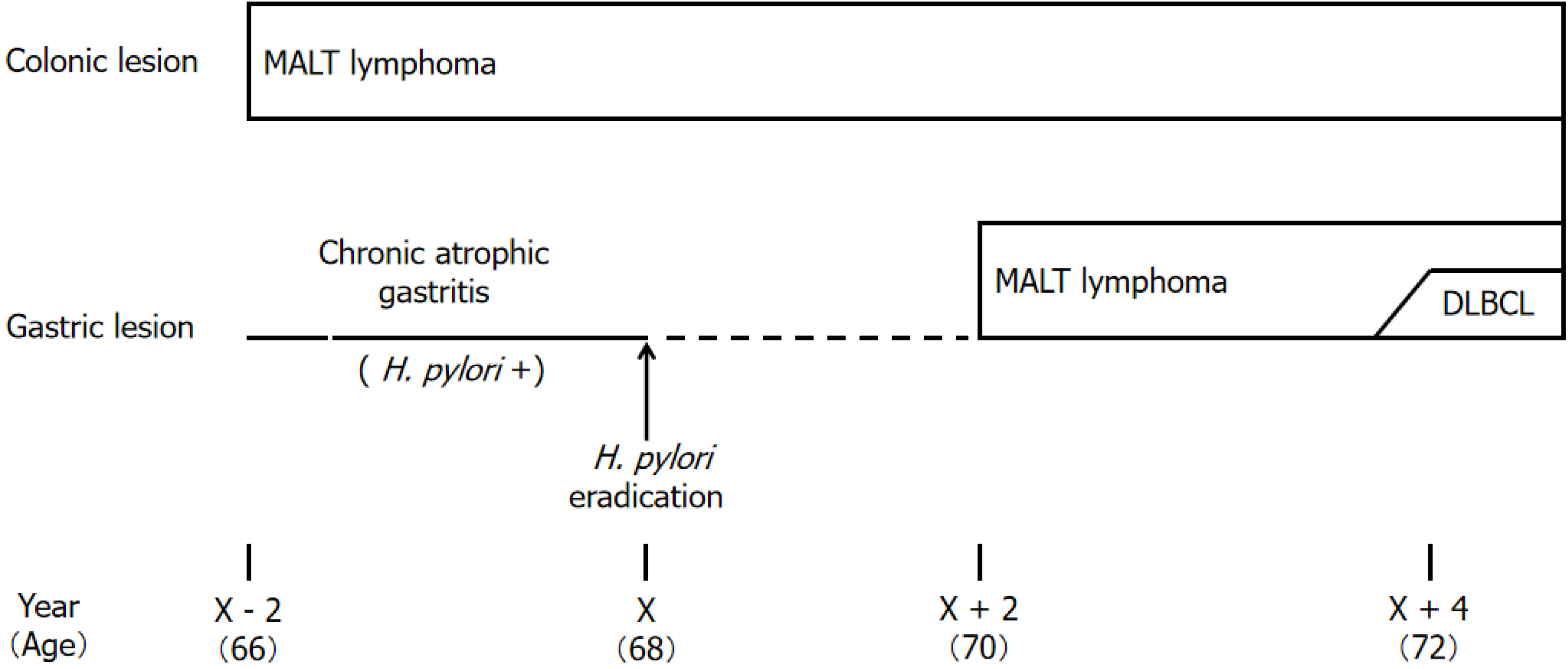
In this study, we reported that MALT lymphoma initially developed in the colon. After H. pylori was eradicated for chronic atrophic gastritis, the colonic lesions remained unchanged, and MALT lymphoma developed metachronously in the stomach, in which multiple lesions were observed, one of which transformed into DLBCL. This case was considered highly suggestive of the mechanism by which gastric MALT lymphoma progresses from its onset.
A 72-year-old man, who was asymptomatic, was transferred to our department for detailed examination and treatment for gastrointestinal lymphomas, as described below.
The biopsy suggested DLBCL, and the patient was referred and admitted to our department.
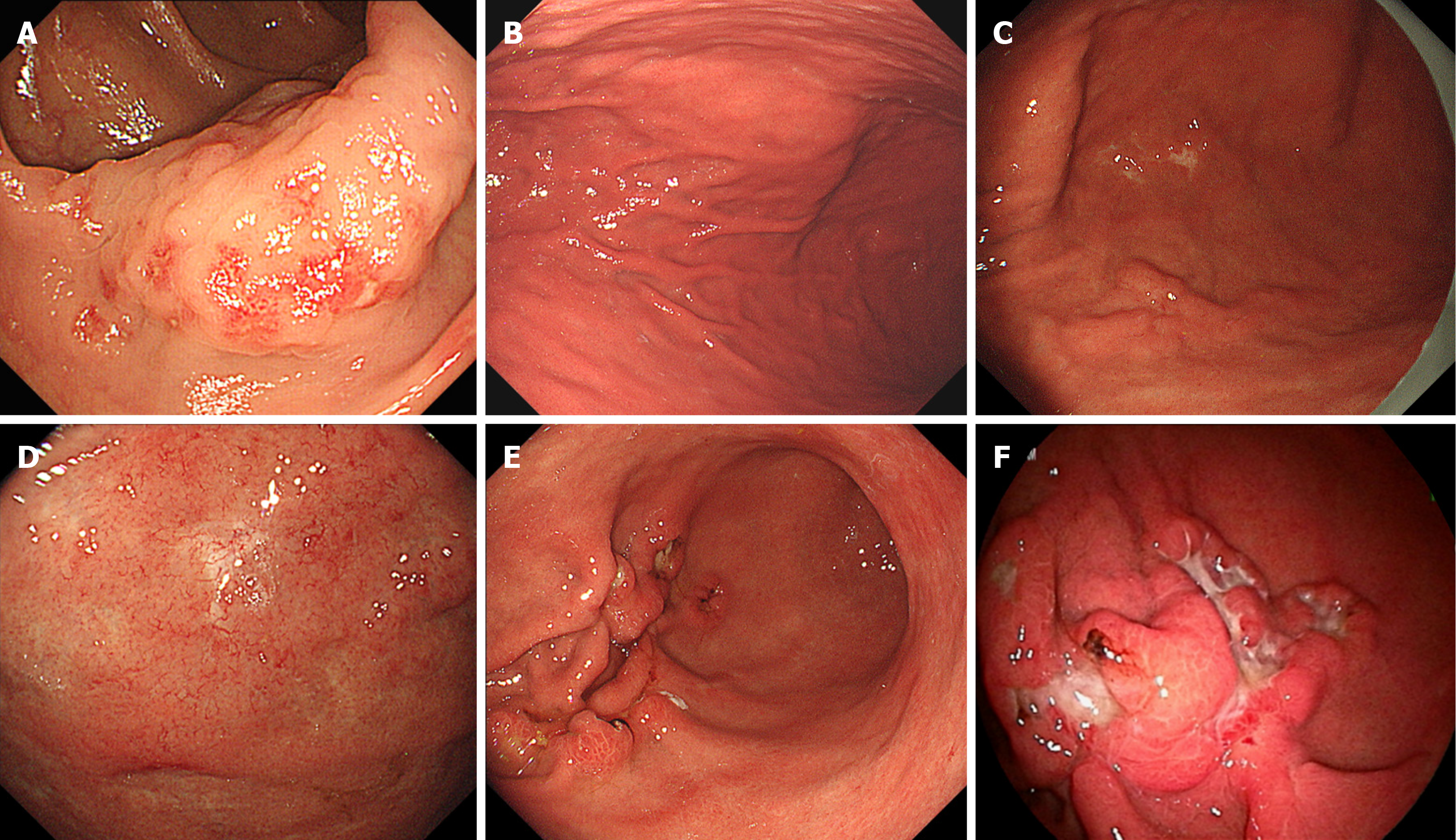
Figure 1 shows the clinical course chart. At the age of 66, owing to a positive fecal occult blood test, the patient underwent a colonoscopy at the hospital in charge of his treatment at that time; a tumor lesion was found in the ascending colon (Figure 2A), and a biopsy suggested MALT lymphoma. Two years later (at the age of 68), esophagogastroduodenoscopy revealed chronic atrophic gastritis that was positive for H. pylori (Figure 2B). Oral administration of vonoprazan fumarate, amoxicillin hydrate and clarithromycin (20 mg, 750 mg and 200 mg, respectively, twice daily) for one week was used for eradication therapy, and urea breath test results were subsequently negative for H. pylori. A repeat endoscopy approximately 1 year later revealed no significant changes. Two years later (at the age of 70), slight erosions appeared on the mucosa of the greater curvature of the gastric body, and nine months later, the lesion had changed to multiple ulcers (Figure 2C). The lesion was biopsied and found to be consistent with MALT lymphoma, and H. pylori was negative histopathologically. Furthermore, 6 months later, a whitish mucosal lesion was observed in the fundus of the stomach (Figure 2D), and a biopsy of this lesion also suggested MALT lymphoma. One year later (4 years and 3 months after H. pylori eradication), the multiple ulcer lesions on the greater curvature of the gastric body were deeper and more widespread (Figure 2E); the biopsy suggested DLBCL.
There are no noteworthy points.
The patient had few subjective symptoms, and his performance status was 0. No abnormal findings were observed physically, including in the abdomen.
Blood cell counts and soluble interleukin-2 receptor levels were within the normal range (443 U/mL), and the anti-H. pylori antibody levels were slightly elevated at 10 U/mL.
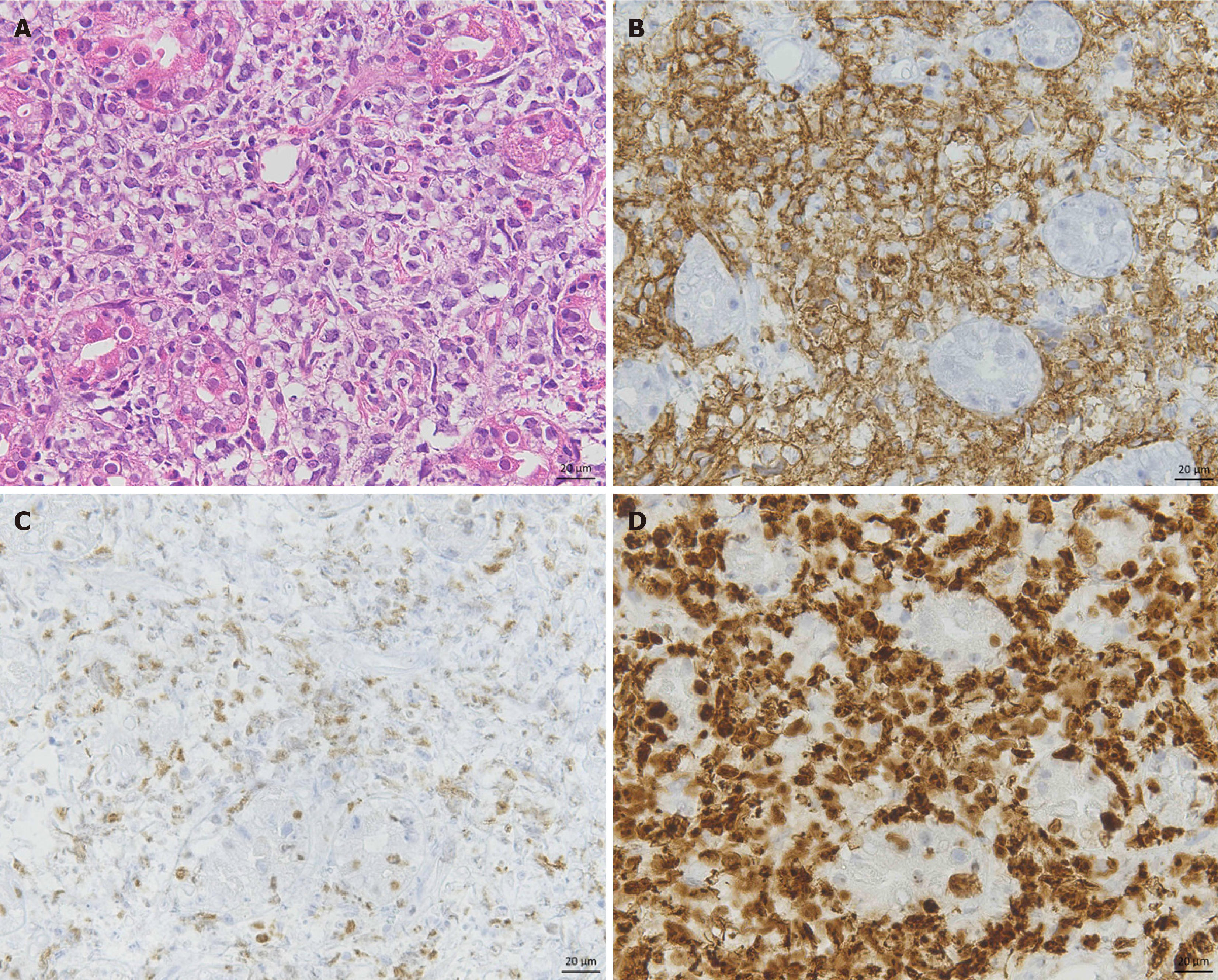
Esophagogastroduodenoscopy and colonoscopy were performed in our department, and the lesions in the fundus of the stomach and ascending colon were almost unchanged from when they were first discovered. A biopsy revealed that the lesion was compatible with MALT lymphoma. In contrast, the lesions in the gastric body had progressed (Figure 2F) within 1 month. Histological findings from the biopsy revealed large atypical lymphoid cells with enlarged nuclei proliferating into the glandular ducts (Figure 3A). Immunostaining revealed that these abnormal cells were positive for CD20 (Figure 3B) and c-myc (Figure 3C), and the MIB-1 index was positive in more than 90% (Figure 3D), indicating that they had DLBCL. Microscopic examination revealed that H. pylori was negative. PET revealed strong accumulation in the gastric body and in swollen lymph nodes around the stomach, with a maximum standardized uptake value (SUVmax) of 10-19 (not shown), which is consistent with DLBCL. The hepatic curvature of the colon had a SUVmax of 6.9, which was unchanged over the previous 6 years, suggesting MALT lymphoma.
On the basis of the above findings, the lesions in the colon and gastric fundus were diagnosed as MALT lymphoma, and the lesion in the gastric body was diagnosed as DLBCL derived from MALT (Lugano-Stage II1, IPI: Low, revised IPI: Very good).
A total of 6 courses of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy were administered. The treatment was completed in 3-week cycles without any adverse events.
All lesions, including those in the stomach, colon, and lymph nodes, disappeared, resulting in complete remission (CR). More than 3 years have passed since the end of treatment, and the patient has maintained CR to date. He is now 76 years old and leads a healthy daily life.
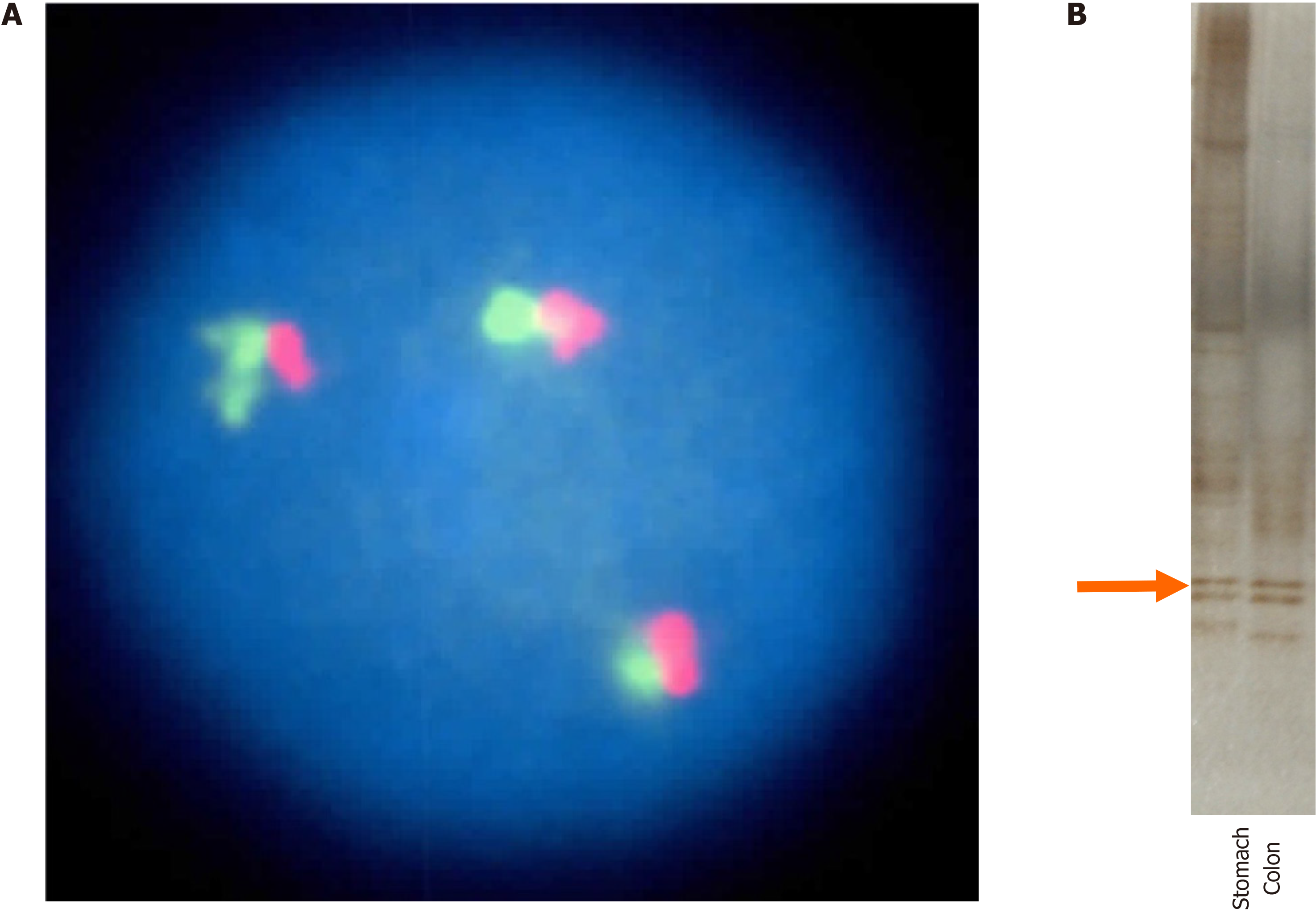
Fluorescence in situ hybridization (FISH) revealed MALT1-positive lymphoma cells in both the stomach and colon, and 3 copies of the MALT1 gene were found in the nucleus of a small number of tumor cells (Figure 4A). The FISH results for p53 and c-myc were negative. We also investigated the IgH gene rearrangement of tumor cells in lymphoma lesions in the stomach and colon by polymerase chain reaction-based single-strand conformational polymorphism (PCR-SSCP) analysis[14]. As a result, the bands indicating monoclonality matched, revealing that the two lesions had the same clonality (Figure 4B).
Considering the comprehensive safety of the treatment itself, its effectiveness against colonic MALT lymphoma[12,13], and its ability to reduce the risk of developing gastric adenocarcinoma later in life[15], we believe that H. pylori eradication is a reasonable treatment option. However, it was not only ineffective against colonic MALT lymphoma but also failed to suppress the development of gastric MALT lymphoma and subsequent DLBCL. It is also possible that MALT lymphoma was already occurring histologically at the time of eradication. Gastric MALT lymphomas that are positive for MALT1 translocation, as in this case, are not associated with H. pylori infection, and eradication is often ineffective[3,5,6]. In addition, it was believed that the development of DLBCL approximately 2 years later was not de novo but rather was due to transformation from H. pylori-independent MALT lymphoma.
Extra copies of chromosome 3 or 18 (trisomy) are chromosomal abnormalities that may be associated with DLBCL transformation in patients with gastric MALT lymphoma[7]. Additionally, there is a report[16] that extra copies of MALT1 (18q21 trisomy), as in our case, are an independent factor for poor event-free survival in patients with gastric MALT lymphoma. Although our patient did not exhibit c-myc translocation by FISH, the c-myc protein was expressed. “MYC-driven B-cell lymphoma” has been proposed as a general term for lymphomas whose pathogenesis is presumed to involve the activation of MYC[17]. Magnoli et al[18] reported that high c-myc protein expression is a poor prognostic factor in extranodal DLBCL[18]. The patient was reexamined at age 76 (10 years after MALT lymphoma was first found) and confirmed to be in CR in both the stomach and colon more than 3 years after the end of treatment. We intend to continue to monitoring him closely.
Owing to the rarity of colonic MALT lymphoma, there are few comprehensive reports on this disease[9-11]. Its treat
Previously, it was reported that out of 9 patients with colonic MALT lymphoma, 3 had synchronous MALT lymphoma in the stomach[10]. To the best of our knowledge, our patient is the first reported case in which the clinical course of MALT lymphoma, which first developed in the colon and then metachronously developed in the stomach, was followed in real time, and analysis via the PCR-SSCP method demonstrated that both lesions had the same clonality.
In this study, eradication treatment for H. pylori was not only ineffective against newly diagnosed colonic MALT lymphoma but also failed to suppress the onset of gastric MALT lymphoma from chronic atrophic gastritis and the subsequent development of transformed DLBCL. Because these lesions had MALT1 translocations accompanied by trisomy 18q21, it was thought that MALT lymphoma developed independently of H. pylori infection and further worsened. Furthermore, although both the colonic and gastric lesions had metachronous onset, PCR-SSCP proved that they had the same clonality.
The authors would like to thank Ikuo Sato (Sapporo Clinical Laboratory Inc.) and Kyoko Fujii (Department of Cancer Pathology, Faculty of Medicine, Hokkaido University) for the technical support provided in the laboratory aspects of this study and Dr. Sosuke Kato (Department of Gastroenterology, NTT Medical Center Sapporo) for providing detailed information as the attending physician at the hospital where the patient was previously followed.
| 1. | Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53:2515-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Nakamura S, Müller-Hermelink HK, Delabie J. Lymphoma of the stomach. In: Bosman FD, Carneiro F, Hruban RH, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon, France: IARC press, 2010: 69–73. |
| 3. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T, Kamoshida T, Watanabe N, Chiba T, Origasa H, Asaka M; JAPAN GAST Study Group. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 4. | Lemos FFB, de Castro CT, Calmon MS, Silva Luz M, Pinheiro SLR, Faria Souza Mendes Dos Santos C, Correa Santos GL, Marques HS, Delgado HA, Teixeira KN, Souza CL, Oliveira MV, Freire de Melo F. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J Gastroenterol. 2023;29:2202-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Nakamura S, Matsumoto T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: recent progress in pathogenesis and management. World J Gastroenterol. 2013;19:8181-8187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Remstein ED, Kurtin PJ, James CD, Wang XY, Meyer RG, Dewald GW. Mucosa-associated lymphoid tissue lymphomas with t(11;18)(q21;q21) and mucosa-associated lymphoid tissue lymphomas with aneuploidy develop along different pathogenetic pathways. Am J Pathol. 2002;161:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Gay ND, Chen A, Okada CY. Colorectal Lymphoma: A Review. Clin Colon Rectal Surg. 2018;31:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Won JH, Kim SM, Kim JW, Park JH, Kim JY. Clinical features, treatment and outcomes of colorectal mucosa-associated lymphoid tissue (MALT) lymphoma: literature reviews published in English between 1993 and 2017. Cancer Manag Res. 2019;11:8577-8587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Hollie N, Asakrah S. MALT lymphoma of the colon: a clinicopathological review. J Clin Pathol. 2020;73:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Tannoury J, Amiot A, Lemonnier F, Dupuis J, Gagnière C, Belhadj K, Bras FL, Sobhani I, Haioun C, Copie-Bergman C, Lévy M. Colonic mucosa-associated lymphoid tissue lymphoma: a case series. Leuk Lymphoma. 2020;61:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Matsumoto T, Iida M, Shimizu M. Regression of mucosa-associated lymphoid-tissue lymphoma of rectum after eradication of Helicobacter pylori. Lancet. 1997;350:115-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Nakase H, Okazaki K, Ohana M, Ikeda K, Uchida K, Uose S, Itoh T, Iwano M, Watanabe N, Yazumi S, Kawanami C, Inoue F, Chiba T. The possible involvement of micro-organisms other than Helicobacter pylori in the development of rectal MALT lymphoma in H. pylori-negative patients. Endoscopy. 2002;34:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Orba Y, Tanaka S, Nishihara H, Kawamura N, Itoh T, Shimizu M, Sawa H, Nagashima K. Application of laser capture microdissection to cytologic specimens for the detection of immunoglobulin heavy chain gene rearrangement in patients with malignant lymphoma. Cancer. 2003;99:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Feng Y, Duan TJ, Huang Q, Li ZY, Liu YP, Luo MS, Lu GF, Shi W, Zhang ZY, Li HX. The clinicopathological characteristics of gastric cancer and precancerous conditions in gastric DLBCL and MALT lymphoma patients: a multi-center retrospective study. Ann Med. 2023;55:2193423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Nakamura S, Ye H, Bacon CM, Goatly A, Liu H, Banham AH, Ventura R, Matsumoto T, Iida M, Ohji Y, Yao T, Tsuneyoshi M, Du MQ. Clinical impact of genetic aberrations in gastric MALT lymphoma: a comprehensive analysis using interphase fluorescence in situ hybridisation. Gut. 2007;56:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Hematology Am Soc Hematol Educ Program. 2013;2013:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Magnoli F, Bernasconi B, Vivian L, Proserpio I, Pinotti G, Campiotti L, Mazzucchelli L, Sessa F, Tibiletti MG, Uccella S. Primary extranodal diffuse large B-cell lymphomas: Many sites, many entities? Clinico-pathological, immunohistochemical and cytogenetic study of 106 cases. Cancer Genet. 2018;228-229:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Saito M, Tsukamoto S, Ishio T, Yokoyama E, Izumiyama K, Mori A, Morioka M, Kondo T, Sugino H. Multiple Colorectal Mucosa-Associated Lymphoid Tissue Lymphoma Successfully Treated with Chemotherapy. Case Rep Oncol. 2021;14:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Niwa T, Watahiki M, Kosugi T, Kusama D, Tamakoshi H, Takinami M, Kaneko J, Takahashi Y, Nishino M, Yamada T. Endoscopic ultrasound-guided fine needle biopsy diagnosis of circumferentially extraluminal mucosa-associated lymphoid tissue lymphoma in the transverse colon: a case report. Clin J Gastroenterol. 2024;17:461-465. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |