Published online Oct 15, 2024. doi: 10.4251/wjgo.v16.i10.4264
Revised: August 30, 2024
Accepted: September 6, 2024
Published online: October 15, 2024
Processing time: 163 Days and 7.7 Hours
Rare liver tumors (RLTs) have an extremely low likelihood of forming, and some have been recorded only in isolated cases. The lack of normal clinical symptoms in RLTs makes preoperative diagnosis extremely challenging, which results in frequent misinterpretation. The present case report helps enhance our ability to recognize and treat uncommon liver tumor disorders.
We describe four distinct examples of rare liver tumor diseases. These cases were all true cases with no conventional clinical signs or imaging findings. In all patients, hepatic occupancy was discovered on physical examination, which raised the preoperative suspicion of hepatic cancer. All tumors were surgically removed, and postoperative histology and immunohistochemistry were per
The number of patients with RLTs is small, and the clinical and imaging results are vague. Preoperative diagnosis is challenging, and patients are sometimes mistakenly diagnosed with liver cancer, which leads to unnecessary surgical therapy in certain individuals.
Core Tip: To exclude rare liver tumors in patients with atypical liver tumors, imaging should be performed multiple times before surgery. If necessary, preoperative positron emission tomography/computed tomography may be performed to persuade the patient to undergo a liver puncture biopsy. If there is still no evidence of cancer in the patient, then liver surgery should be avoided, and short-term follow-up may be considered.
- Citation: Zhao Y, Bie YK, Zhang GY, Feng YB, Wang F. Rare and lacking typical clinical symptoms of liver tumors: Four case reports. World J Gastrointest Oncol 2024; 16(10): 4264-4273
- URL: https://www.wjgnet.com/1948-5204/full/v16/i10/4264.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i10.4264
Rare liver tumors (RLTs) are primary benign and malignant tumors that occur with a low incidence and very rare clinical occurrence, in addition to common benign and malignant tumors, such as primary hepatocellular carcinoma, cholangiocarcinoma, liver cysts, liver abscesses, and hepatic cavernous hemangiomas. Primary hepatic fibrosarcoma (PHF) is a rare and extremely malignant tumor that originates from hepatic fibroblasts[1]. According to the United States Surveillance, Epidemiology, and End Results Cancer Data Bank, of the 54709 cases of primary hepatic malignancy, only two patients were diagnosed with PHF. Primary hepatic neuroendocrine tumors (PHNETs) are rare neuroendocrine tumors (NETs) that account for 0.3% of all NETs and 0.11% of primary liver tumors[2,3]. Although the global prevalence of non-alcoholic fatty liver disease is 25.24%[4], little is known about focal hepatic steatosis (FHS) compared to diffuse hepatic steatosis. Single necrotic nodule in the liver (SNNL) is a rare benign tumor of the liver, and few cases of SNNL have been reported in the national and international literature. SNNL was first described by Shepherd and Lee in 1983[5]. These four cases of liver tumors and their rarity make preoperative and differential diagnoses very difficult, and there are no treatment guidelines for this type of disease. The aim of these case reports is to increase the attention of hepatobiliary surgeons, increase their experience in the diagnosis and treatment of rare diseases, reduce misdiagnosis and avoid inappropriate treatment.
Case 1: A 64-year-old male patient whose body mass index (BMI) was 22.1. A computed tomography (CT) scan revealed intrahepatic occupation for 18 months.
Case 2: A 40-year-old male patient with a BMI of 22.0. A liver tumor was detected via CT examination during the treatment of a right ureteral stone.
Case 3: A 63-year-old male patient with a BMI of 26.0. CT examination revealed liver occupancy for 5 months.
Case 4: A 60-year-old female patient with a BMI of 25.2. Ultrasound examination revealed liver occupancy for 6 days.
Case 1: There were no symptoms of abdominal pain or bloating during the course of the disease.
Case 2: The hepatic tumor was solid mass that appeared as an infected-like lesion on CT, which led to a lack of attention at the first visit. Two weeks later, a follow-up enhanced CT of the epigastric region revealed the same lesion, but the tumor was considered a neoplastic lesion. There were no clinical symptoms, such as fever, jaundice or abdominal pain, during the course of the disease.
Case 3: There were no symptoms of abdominal pain or bloating during the course of the disease.
Case 4: She had no abdominal pain or distension.
Case 1: He had a history of "hepatitis B virus (HBV) carrier" and "right lung middle lobe squamous cell carcinoma resection".
Case 2: There was no previous history of hepatitis or alcohol consumption.
Case 3: He denied a history of hepatitis and no smoking or alcohol habits.
Case 4: She had a history of hypertension for one year, was hospitalized for conservative treatment of hypertensive cerebral hemorrhage, and underwent surgery for left kidney stones in 2019.
Case 1: He was not addicted to smoking or alcohol and denied a family history of cancer.
Case 2: There was no alcohol consumption.
Case 3: The patient had a family history of liver disease and liver cancer.
Case 4: She denied any family history of liver disease or liver cancer.
Case 1: Physical examination revealed negative abdominal signs.
Case 2: Physical examination revealed that vital signs were normal, there was no yellowing of the skin or sclera, and the results of the abdominal examination were negative for all signs.
Case 3: The results of the physical examination revealed normal vital signs and negative abdominal signs.
Case 4: Physical examination revealed that vital signs were normal, and abdominal signs were negative.
Case 1: The laboratory test results revealed a platelet count of 60 × 109/L (reference range, 125-350 × 109/L), and blood cell morphology in which platelets were easily observed (average 5.4/oil microscope). The high-sensitivity HBV deoxyribonucleic acid assay revealed a titer of 48.6 IU/mL (reference range, < 20 IU/mL). The infection series found a hepatitis B surface antigen (HBsAg) of 88.76 IU/mL (reference range, 0-0.05 IU/mL). Tumor marker levels, hepatic fibrosis indices, liver function and other laboratory test results were normal.
Case 2: The laboratory findings revealed c-reactive protein of 13.76 mg/L (reference range, < 10.0 mg/L) and procalcitonin of 0.136 ng/mL (reference range, 0-0.052 ng/mL). The biochemical indices revealed normal bilirubin, alanine aminotransferase of 750.0 U/L (reference range, 0-50 U/L), and azelaic aminotransferase of 750.0 U/L (reference range, 17-59 U/L). Tumor marker levels and routine blood tests were normal.
Case 3: The laboratory test results were as follows: Hepatitis B series: HBsAg, 44.28 IU/mL (reference range, 0-0.05 IU/mL); hepatitis B E antibody, 9.75 PEIU/mL (reference range, 0-0.4 PEIU/mL); and hepatitis B core antibody, 93.03 PEIU/mL (reference range, 0–0.7 PEIU/mL). The results of the quantitative HBV nucleic acid assay revealed a titer of 1.55E+04 IU/mL (reference range, < 200 IU/mL). Carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), and carbohydrate antigen 19-9 (CA19-9) levels were within the normal range. Liver function, liver fibrosis indices, coagulation series and other laboratory tests were normal.
Case 4: The laboratory test results were unremarkable, and the CEA, AFP and CA19-9 levels were all within the normal range.
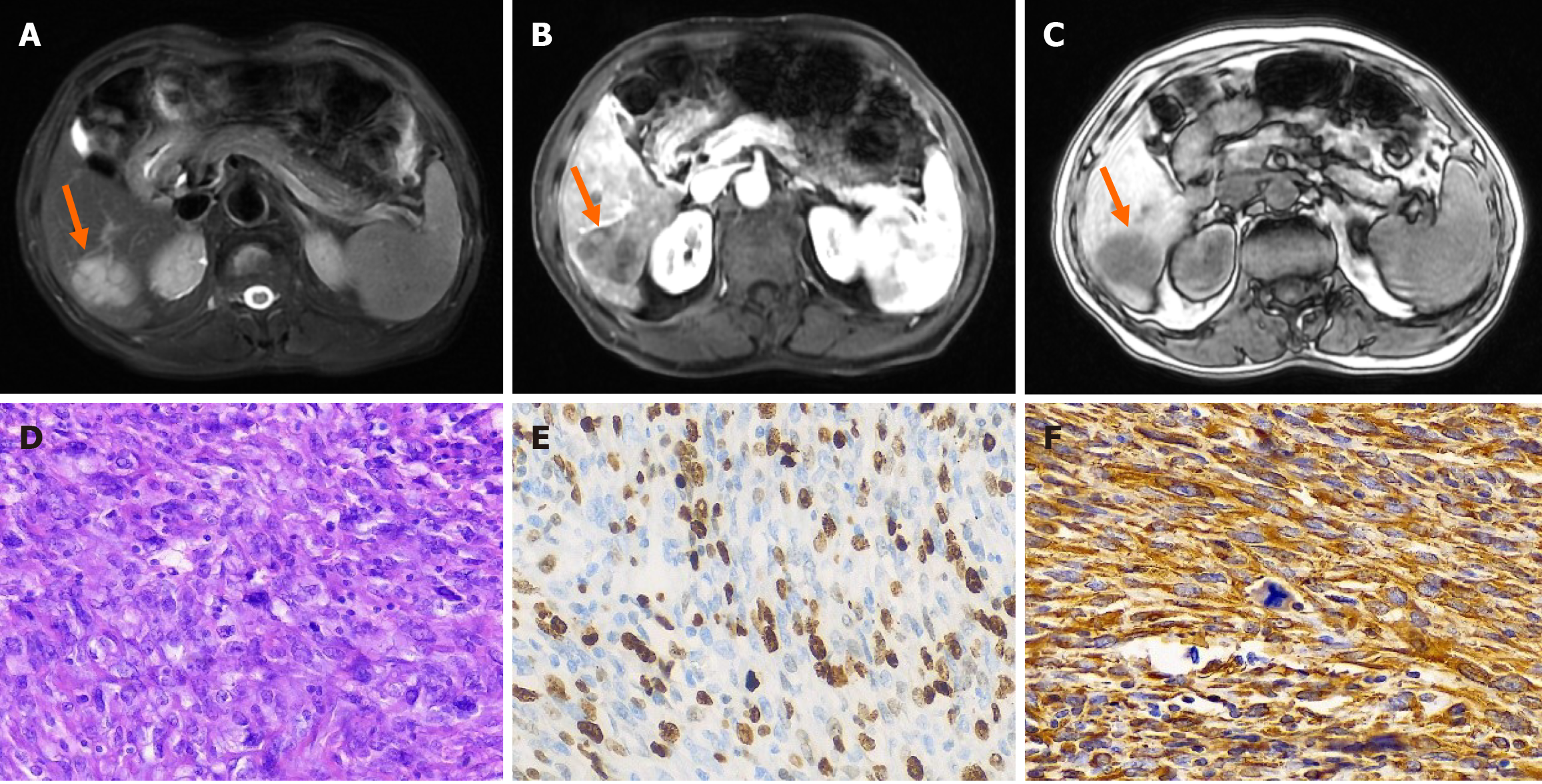
Case 1: Magnetic resonance imaging (MRI) enhancement of the upper abdomen revealed a 4.1 cm × 3.0 cm mass occupying the lower segment of the right posterior lobe of the liver, which suggested a neoplastic lesion with possible metastasis, and a primary hepatocellular carcinoma to be drained (Figure 1).
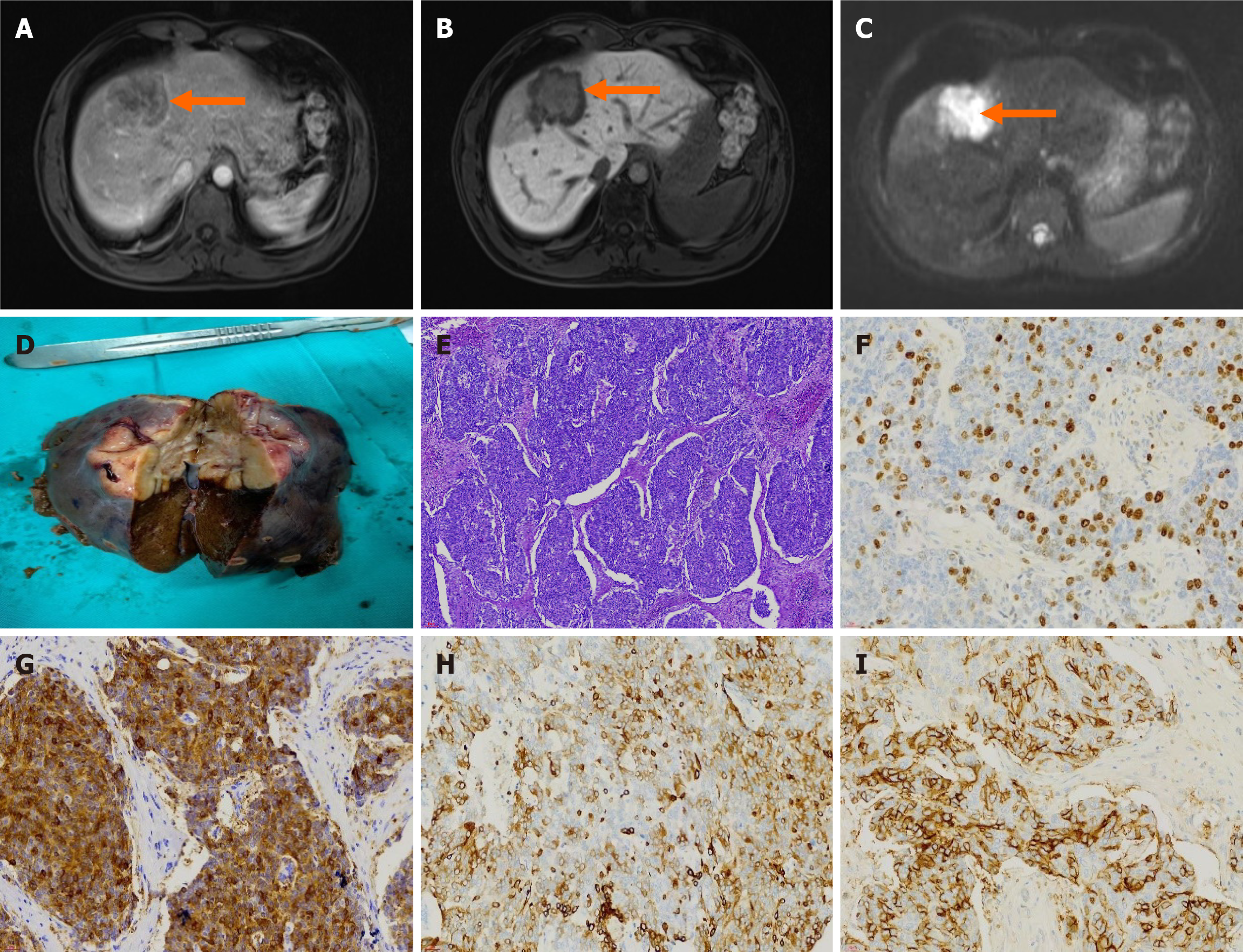
Case 2: MRI enhancement examination of the upper abdomen suggested the presence of a neoplastic lesion (malignant possibility), which was accompanied by a reduced signal in the hepatobiliary phase of the adjacent liver parenchyma (Figure 2).
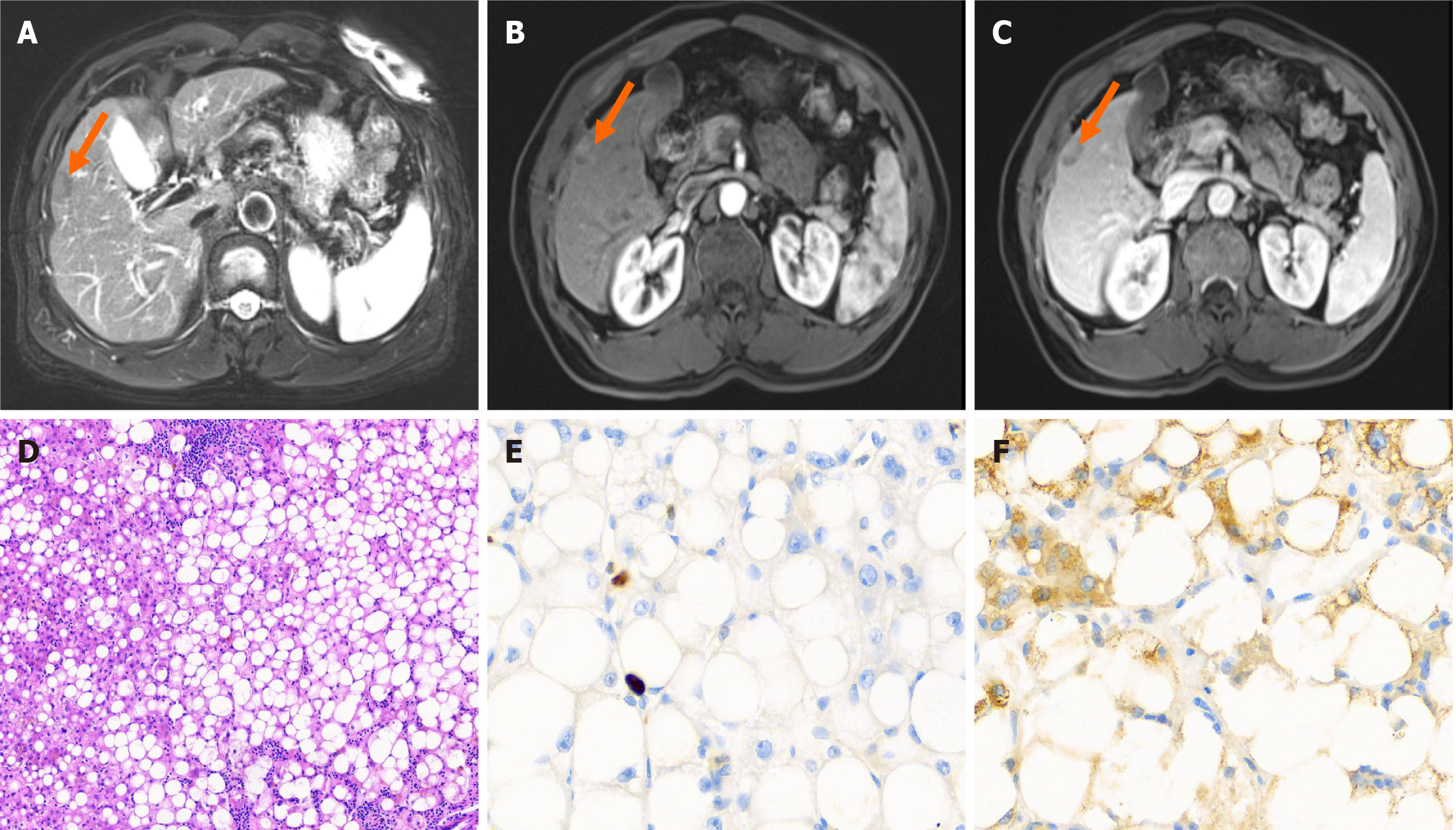
Case 3: Upper abdominal CT enhancement revealed a hepatic S5 subperitoneal strengthening nodule, which was considered a tumorigenic lesion, and did not exclude the possibility of hepatocellular carcinoma. The surface of the liver was not smooth, and the patient was referred to the clinic to exclude cirrhosis. MRI enhancement of the upper abdomen revealed a subperitoneal nodular shadow of liver S5, which indicated a tumor lesion (possible liver cancer) (Figure 3).
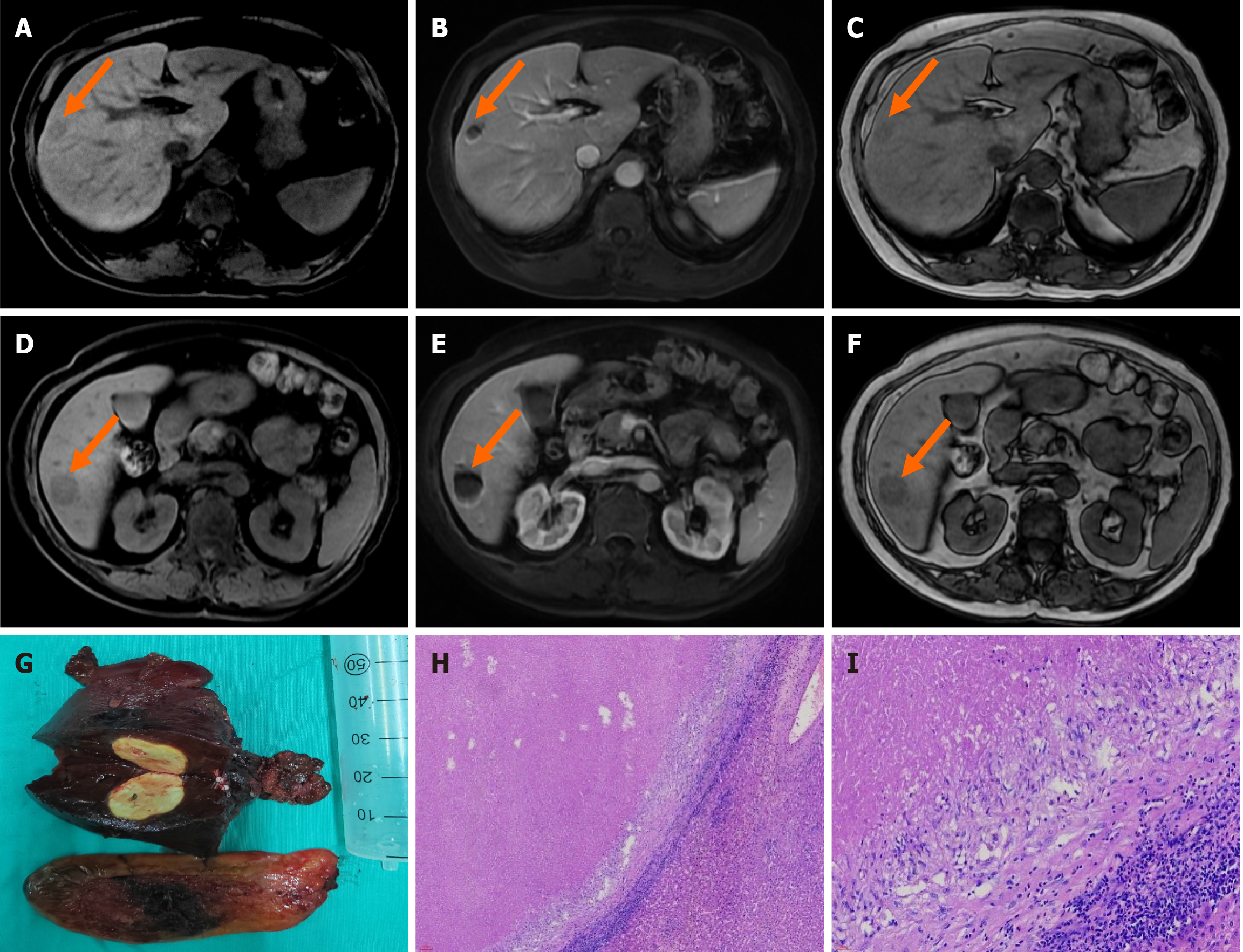
Case 4: CT enhancement examination of the upper abdomen revealed point-like and small patchy enhancement foci in the upper right anterior lobe and lower right posterior lobe in the arterial phase, and rounded low-density shadows were observed in the upper right anterior lobe and lower right posterior lobe in the venous phase, with clear boundaries. The size of the larger foci was approximately 2.2 cm × 2.0 cm, and the range of enhancement in the foci of the upper right anterior lobe and lower right posterior lobe was slightly enlarged in the delayed phase. For lesions in the upper part of the right anterior lobe and lower part of the right posterior lobe of the liver, the possibility of neoplastic lesions and inflammatory lesions to be excluded should be considered, and MRI examination should be recommended. MRI enhancement of the upper abdomen revealed equal and slightly long T1 and equal and slightly long T2 signals were observed in the S6 and S8 segments of the liver, with a slightly high signal on diffusion weighted imaging (DWI) and a high apparent diffusion coefficient. Foci in the S8 segment showed progressive irregular enhancement of the edges, foci in the S6 segment showed ring enhancement, and foci in the hepatobiliary stage exhibited a low signal (Figure 4). Alterations in the S6 and S8 segments of the liver suggest a neoplastic lesion (possible myofibroblastoma), and puncture examination should be performed if necessary.
After discussion with a preoperative multidisciplinary team (MDT), a malignant hepatic tumor was considered with a high possibility of metastasis.
After a preoperative MDT discussion, liver cancer was considered highly likely, and laparoscopic hepatic tumor resection was performed.
After a preoperative MDT discussion, the possibility of primary liver cancer was not excluded. The patient refused to undergo liver puncture biopsy, and he requested liver tumor resection.
The preoperative diagnosis was a high possibility of hepatic myofibroblastoma. The patient refused to undergo puncture biopsy, and hepatic tumor resection was performed.
Postoperative histopathology revealed an "S6 liver tumor" spindle cell tumor with morphology and immunohistochemistry that suggested a fibrosarcoma (Figure 1). The immunohistochemistry results were as follows: cytokeratin (CK) wide (-), vimin (+), Melan A (-), specific monoclonal antibody against melanoma (-melanoma) (-), smooth muscle actin (-), S-100 (-), signal transducer and activator of transcription 6 (-), CD34 (vascular +), Ki-67 (+, approximately 60% positive) (Figure 1E and F), CK5/6 (-), anaplastic lymphoma kinase (-), electroretinography (ERG) (-), CD117 (-), DOG-1 (-), CK5/6 (-), ERG (-), CD117 (-), DOG-1 (-), and epithelial membrane antigen (-).
Postoperative histopathology and immunohistochemistry revealed a malignant tumor, which, combined with immunohistochemistry, was consistent with a high-grade neuroendocrine carcinoma (G3), but no cancerous tissue was observed on the stripped hepatic surface (Figure 2). The immunohistochemistry results were as follows: CK7 (-), CK20 (-), villin (+), CDX2 (-), CK19 (+), CA19-9 (-), hepatocyte (-), glypican-3 (-), GS (-), CD56 (partially +), chromogranin A (-), synaptophysin (+), thyroid transcription factor-1 (-), Ki-67 (+, positivity rate of approximately 30%), CD34 (-), CK wide (+), insulinoma associated protein 1 (+), phosphorylated histone H3 (+, scattered), Rb (-), P53 (+, wild-type), and somatostatin receptor 2A (partial +) (Figure 2F-I).
Postoperative pathological diagnosis of the liver and tumor tissue revealed that the structure of the liver lobules in the lesion was discernible, patches of hepatocytes with large vesicular lipoatrophy, some swollen and degenerated hepatocytes, and a few dilatations of capillary bile ducts (containing bile plugs) were observed locally (Figure 3). The immunostaining results were as follows: CK7 (small number of hepatocytes around the confluent area in the lesion +), CK19 (bile ducts +), hepatocyte (+), glypican-3 (-), GS (mottled +), P53 (scattered weak +, suggesting wild-type), CD10 (capillary bile ducts +), CD34 (partial + in the peripheral band of the confluent area in the lesion), D2-40 (lymphatic vessels +), and hepatitis B core antigen (HbcAg) (+), HbcAg (-), HbsAg (a few hepatocytes within the lesion +, peripheral hepatic liver patchy +), and Ki-67 (+, < 5%) (Figure 3E and F). Morphology combined with immunostaining and special stains suggested a benign lesion, which was considered focal steatosis and chronic viral hepatitis B (G1/G2).
Pathological findings were reported 1 week after surgery and included (partial liver tissue) isolated necrotic nodules. Chronic cholecystitis with cholesterol polyp formation (Figure 4). Special stains included antacid stain (-), silver hexamine (-), periodic acid-Schiff preparation with diastase (-), and Gram (-) (Figure 4H and I).
One cycle of hepatic artery perfusion therapy (lopatin 50 mg) + embolization + docetaxel intravenous chemotherapy was given. Three cycles of intravenous chemotherapy, "albumin paclitaxel + nedaplatin", were recommended every 3 weeks. Ten days prior, the liver tumor was enlarged, and laparoscopic liver tumor resection was performed under general anesthesia after further discussion. During the surgery, the surface of the liver was uneven, with blunted edges and nodular cirrhosis, and the liver was enlarged. The liver tumor was located under the peritoneum of the S6 segment of the liver, and the tumor size was approximately 3.8 cm × 3.0 cm, grayish-white, solid, and hard. The S6 segment and the tumor were completely resected.
Intraoperatively, a mass approximately 6.0 cm × 5.0 cm in size was observed at the junction of S4, S5, and S8 of the liver, with a crater-like depression in the center and protruding from the hepatic envelope. R0 resection of the tumor was performed. Two weeks after discharge, he underwent intravenous chemotherapy with the ‘EP regimen’ (etoposide 170 mg D1-3 + cisplatin 40 mg D1-3), which was repeated once every 3 weeks for a total of 6 hospitalized chemotherapy sessions.
Laparoscopic hepatic tumor resection and cholecystectomy were performed under general anesthesia. During the surgery, the liver showed nodular cirrhosis, and the tumor was located on the surface of the S5 segment. The tumor was approximately 1.8 cm × 1.2 cm × 1.0 cm in size and grayish yellow with a medium texture. The hepatic S5 segment was routinely resected.
Laparoscopic hepatic tumor resection and cholecystectomy were performed under general anesthesia. During the surgery, no mass was observed on the surface of the liver, and resection of segments S5, S6 and S8 was performed using intraoperative ultrasonic localization. A mass of approximately 3.5 cm × 2.2 cm was observed in segment S6, and a yellow, round, hard and solid mass with a size of approximately 2.2 cm × 2.0 cm was observed in segment S8.
The patient recovered well without postoperative complications and was discharged. After discharge, the patient refused further treatment, such as chemotherapy. After outpatient follow-up for 2 years, the patient experienced no distant complications, tumor recurrence or metastasis.
The patient recovered well without postoperative complications and was discharged. Outpatient gastroenteroscopy and positron emission tomography/CT examinations were performed to exclude extrahepatic primary lesions. After one year of outpatient follow-up, the patient experienced no long-term complications, tumor recurrence or metastasis.
The patient recovered well without postoperative complications and was discharged. The outpatient follow-up was 22 months, and the patient reported no symptoms or tumor recurrence.
The patient recovered well without postoperative complications and was discharged from the hospital. One month later, the patient was followed up in the outpatient clinic, and she reported no symptoms or physical discomfort.
Hepatic carcinoma is the sixth most common cancer worldwide in terms of incidence and the fourth leading cause of cancer-related mortality[6]. Although the pathogenesis of hepatic carcinoma is closely associated with inflammation and immune responses in the tumor microenvironment, the specific etiology and pathogenesis of most RLTs is not clear. A review of the literature is necessary to understand the potential causes of RLTs. HF accounts for approximately 1% to 2% of primary malignant tumors of the liver. Pro-related studies have shown that PHF is associated with hypoglycemia, the mechanism of which may involve high insulin-like growth factor II expression[7]. Acquired immune deficiency syndrome may also be a risk factor for hepatic fibrosarcoma[8]. Relevant studies have indicated that primary benign fibrous tumors are at risk of malignant transformation into fibrosarcoma[9]. Since Endmonson first reported PHNETs in 1958[10], more than 150 cases have been reported in the English literature[11,12]. Some scholars believe that these tumors are caused by ectopic pancreatic tissue, whereas others believe that they originate from progenitor cells in the intrahepatic bile ducts, but no final consensus has been reached due to their rarity[2,13]. Hepatic steatosis with focal fat deposition leads to FHS. It is a risk factor for FHS promotion in obesity, diabetes, alcoholism, genetic or metabolic disorders, and in chemotherapy-treated cancer patients[14]. Hepatic steatosis has been reported in up to 47% of cancer patients[15]. SNNL is prevalent in middle-aged and elderly males, with a prevalence of single nodules and occasional multiple nodules, and lesions are mostly located on the surface of the right lobe of the liver. The etiology of SNNL is primarily related to infection, parasites, hepatic trauma, vascular cirrhosis, and allergic reactions.
RLTs lack typical clinical manifestations and imaging features, and their imaging characteristics were studied retrospectively in these cases. PHF patients had no previous history of hepatitis or cirrhosis, and the AFP assay was normal. On imaging, there was a single solid or cystic solid mass shadow with irregular morphology, large cystic areas appeared when the tumor was large, and the enhancement was not obvious or was weakly inhomogeneous, which was sometimes difficult to differentiate from atypical hepatocellular carcinoma. Yu et al[16] explored the diagnostic value of CT for primary hepatic sarcoma in 12 cases of primary hepatic sarcoma that were diagnosed correctly in only 2 cases using CT preoperatively. According to the 2019 World Health Organization Classification of Neuroendocrine Tumors, NETs are classified as highly, moderately, or poorly differentiated (G1, G2, and G3, respectively). G3, which is a neuroendocrine carcinoma, has a nuclear schizophrenic image of > 20/10 HPF and/or a Ki-67 index > 20%[17]. Nuclear schizophrenia and the Ki-67 index are valuable for assessing the malignancy of PHNETs and its prognosis[18]. Our patient had a Ki-67 index of approximately 30%, which is a poorly differentiated neuroendocrine carcinoma according to the World Health Organization Classification of Neuroendocrine Tumors, which suggests a poorer prognosis. PHNETs may differ in imaging and pathological manifestations according to their grade. The PHNETs showed a single lesion on MRI, with inhomogeneous signals on T1 weighted imaging (WI) and T2WI, enhancement of the margins of the lesion in the arterial phase, and mild enhancement of the middle lobe in a segregated pattern. The lesion in the hepatobiliary phase showed a low or slightly low signal, and the lesion on DWI showed a high signal shadow. Wang et al[19] studied 40 patients with PHNETs and showed that higher tumor grade, AE1/AE3 negativity, and elevated Ki-67 expression correlated with poorer patient survival rates. Although parameters, such as treatment modalities, do not significantly affect overall survival, they are effective in controlling tumor progression and alleviating symptoms.
In some patients, FHS and SNNL are difficult to distinguish from hepatic malignant tumors on imaging, and some patients have a background of hepatitis or cirrhosis. In our two patients, the lesions showed progressive marginal irregular enhancement or ring enhancement on MRI, with a low signal in the hepatobiliary phase, and the degree of late enhancement was lower than the adjacent hepatic parenchyma, which is similar to the manifestation of malignant tumors that are "fast in and fast out". Moreover, the patient refused liver puncture biopsy and requested direct surgical resection. Therefore, the preoperative diagnosis was difficult, and the diagnosis was confirmed only by postoperative histopathology and immunohistochemistry. Shan et al[20] reported that quantitative analysis of contrast-enhanced ultrasound images improved the diagnostic performance of atypical benign FHS and malignant FHS in the context of fatty liver. It is widely recognized that SNNLs are formed via infection and degeneration in the final stage of the natural course of the lesion[21]. According to recent reports, Kondi-Pafiti et al[22] suggested that SNNL was associated with metastatic cancer and that "isolated" necrotic nodules may not occur in isolation; moreover, the possibility of metastatic necrotic neoplastic lesions should be considered in the preoperative diagnosis[23].
For the treatment of RLTs, preoperative MDT should be used to help develop an individualized plan[24]. Hepatic tumor resection remains the primary treatment for patients with difficult preoperative diagnoses and surgical resectability, especially patients who do not wish to undergo hepatic puncture biopsy. The margins of malignant tumors should preferably be larger than 1 cm, and complete resection of the tumor and R0 resection should be ensured to prolong patient survival time[25]. Case 1 and Case 2 patients were confirmed to have hepatic malignant tumors using histopathological and immunohistochemical examinations, and postoperative chemotherapy was recommended to reduce the recurrence of tumors and prolong their survival time. Case 3 and Case 4 patients were confirmed to have benign hepatic tumors, and no progressive treatment was needed after surgery, with regular follow-up. These two cases were difficult to diagnose preoperatively and could have undergone short-term observation if liver puncture biopsy had been performed preoperatively. However, physicians should respect the patient's choice, and surgical resection is needed for patients with suspected malignant tumors.
RLTs have the common characteristics of being clinically rare, lacking typical symptoms and clinical manifestations. For RLTs, it is difficult to distinguish benign and malignant tumors on imaging, and it is difficult to diagnose preoperatively. When we diagnose and treat liver tumors, we consider the type of RLT as much as possible preoperatively, and we cannot completely rely on postoperative pathological examination and immunohistochemistry to confirm the diagnosis. Comprehensive clinical data combined with detailed imaging and tumor puncture biopsy are used to improve the preoperative diagnosis and avoid overtreatment of benign liver tumors. Moreover, individualized treatment plans for rare malignant tumors of the liver have been developed to maximize the prognosis of patients.
| 1. | Augsburger D, Nelson PJ, Kalinski T, Udelnow A, Knösel T, Hofstetter M, Qin JW, Wang Y, Gupta AS, Bonifatius S, Li M, Bruns CJ, Zhao Y. Current diagnostics and treatment of fibrosarcoma -perspectives for future therapeutic targets and strategies. Oncotarget. 2017;8:104638-104653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Luchini C, Pelosi G, Scarpa A, Mattiolo P, Marchiori D, Maragliano R, Sessa F, Uccella S. Neuroendocrine neoplasms of the biliary tree, liver and pancreas: a pathological approach. Pathologica. 2021;113:28-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Elayan A, Batah H, Badawi M, Saadeh A, Abdel Hafez S. Primary Hepatic Neuroendocrine Tumor: A Case Report and Literature Review. Cureus. 2022;14:e22370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 5. | Shepherd NA, Lee G. Solitary necrotic nodules of the liver simulating hepatic metastases. J Clin Pathol. 1983;36:1181-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Deng Z, Xu XY, Yunita F, Zhou Q, Wu YR, Hu YX, Wang ZQ, Tian XF. Synergistic anti-liver cancer effects of curcumin and total ginsenosides. World J Gastrointest Oncol. 2020;12:1091-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Kotani K, Tsuji M, Oki A, Kashihara T, Yamada K, Kawakami F, Tako H, Okuno G, Hizuka N, Aiba M. IGF-II producing hepatic fibrosarcoma associated with hypoglycemia. Intern Med. 1993;32:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Ninane J, Moulin D, Latinne D, De Bruyere M, Scheiff JM, Duchateau J, Cornu G. AIDS in two African children--one with fibrosarcoma of the liver. Eur J Pediatr. 1985;144:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Huang ML, Wu H, Chen JS, Lv ZL. Hepatic fibrosarcoma in a middle-aged man. Int J Clin Exp Pathol. 2019;12:3555-3559. [PubMed] |
| 10. | Parray A, Patkar S, Goel M. Primary hepatic neuroendocrine tumours of liver- a rarity: Single centre analysis of 13 patients. Ann Hepatobiliary Pancreat Surg. 2020;24:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Lambrescu IM, Martin S, Cima L, Herlea V, Badiu C, Fica S. Primary hepatic neuroendocrine tumor after 4 years tumor-free follow-up. J Gastrointestin Liver Dis. 2015;24:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Quartey B. Primary Hepatic Neuroendocrine Tumor: What Do We Know Now? World J Oncol. 2011;2:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Wang LX, Liu K, Lin GW, Jiang T. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging. 2015;15:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Metin NO, Karaosmanoğlu AD, Metin Y, Karçaaltıncaba M. Focal hypersteatosis: a pseudolesion in patients with liver steatosis. Diagn Interv Radiol. 2019;25:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Peppercorn PD, Reznek RH, Wilson P, Slevin ML, Gupta RK. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008-2011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Yu R, Wei J, Li R, Zhang S. [CT manifestations of primary hepatic sarcoma]. Zhonghua Yixue Zazhi. 2002;82:541-545. [PubMed] |
| 17. | Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2021;145:664-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 18. | He Z, Shi C, Wen H, Li F, Wang B, Wang J. The potential of carcinoembryonic antigen, p53, Ki-67 and glutathion Stransferase-π as clinico-histopathological markers for colorectal cancer. J Biomed Res. 2010;24:51-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wang HH, Liu ZC, Zhang G, Li LH, Li L, Meng QB, Wang PJ, Shen DQ, Dang XW. Clinical characteristics and outcome of primary hepatic neuroendocrine tumors after comprehensive therapy. World J Gastrointest Oncol. 2020;12:1031-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Shan QY, Chen LD, Zhou LY, Wang Z, Liu GJ, Huang Y, Li W, Liu JY, Xie XY, Lu MD, Liu J, Wang W. Focal Lesions in Fatty Liver: If Quantitative Analysis Facilitates the Differentiation of Atypical Benign from Malignant Lesions. Sci Rep. 2016;6:18640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Zhou YM, Li B, Xu F, Wang B, Li DQ, Liu P, Yang JM. Clinical features of solitary necrotic nodule of the liver. Hepatobiliary Pancreat Dis Int. 2008;7:485-489. [PubMed] |
| 22. | Kondi-Pafiti AI, Grapsa DS, Kairi-Vasilatou ED, Voros DK, Smyrniotis VE. "Solitary" necrotic nodule of the liver: an enigmatic entity mimicking malignancy. Int J Gastrointest Cancer. 2006;37:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Deniz K, Coban G. Solitary necrotic nodule of the liver: always benign? J Gastrointest Surg. 2010;14:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Ye X, Tang X, Li F, Lin Y. A giant malignant solitary fibrous tumor in the liver: A case report. Asian J Surg. 2023;46:3920-3923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Chen N, Slater K. Solitary fibrous tumour of the liver-report on metastasis and local recurrence of a malignant case and review of literature. World J Surg Oncol. 2017;15:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |