Published online Oct 15, 2024. doi: 10.4251/wjgo.v16.i10.4104
Revised: August 18, 2024
Accepted: September 6, 2024
Published online: October 15, 2024
Processing time: 191 Days and 18.7 Hours
The colon cancer prognosis is influenced by multiple factors, including clinical, pathological, and non-biological factors. However, only a few studies have focused on computed tomography (CT) imaging features. Therefore, this study aims to predict the prognosis of patients with colon cancer by combining CT imaging features with clinical and pathological characteristics, and establishes a nomogram to provide critical guidance for the individualized treatment.
To establish and validate a nomogram to predict the overall survival (OS) of patients with colon cancer.
A retrospective analysis was conducted on the survival data of 249 patients with colon cancer confirmed by surgical pathology between January 2017 and December 2021. The patients were randomly divided into training and testing groups at a 1:1 ratio. Univariate and multivariate logistic regression analyses were performed to identify the independent risk factors associated with OS, and a nomogram model was constructed for the training group. Survival curves were calculated using the Kaplan–Meier method. The concordance index (C-index) and calibration curve were used to evaluate the nomogram model in the training and testing groups.
Multivariate logistic regression analysis revealed that lymph node metastasis on CT, perineural invasion, and tumor classification were independent prognostic factors. A nomogram incorporating these variables was constructed, and the C-index of the training and testing groups was 0.804 and 0.692, respectively. The calibration curves demonstrated good consistency between the actual values and predicted probabilities of OS.
A nomogram combining CT imaging characteristics and clinicopathological factors exhibited good discrimination and reliability. It can aid clinicians in risk stratification and postoperative monitoring and provide important guidance for the individualized treatment of patients with colon cancer.
Core Tip: It is necessary to establish an accurate survival prediction model for colon cancer to improve patient prognosis. This study combined computed tomography imaging features and clinicopathological factors to identify independent risk factors associated with overall survival using univariate and multivariate logistic regression analyses. A nomogram model was constructed, and it demonstrated high accuracy.
- Citation: Hu ZX, Li Y, Yang X, Li YX, He YY, Niu XH, Nie TT, Guo XF, Yuan ZL. Constructing a nomogram to predict overall survival of colon cancer based on computed tomography characteristics and clinicopathological factors. World J Gastrointest Oncol 2024; 16(10): 4104-4114
- URL: https://www.wjgnet.com/1948-5204/full/v16/i10/4104.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i10.4104
Colon cancer is the third most common malignant tumor worldwide and the second leading cause of cancer death[1]. The survival rate of colon cancer has gradually improved over the last 30 years[2]. However, the overall survival (OS) rate of patients with colon cancer remains low. The five-year survival rate for colon cancer in China is 0.62[3]. Consequently, an accurate predictive model must be established for the survival of patients with colon cancer to improve their prognosis in clinical settings.
Currently, the American Joint Committee on Cancer (AJCC) pathological TNM staging remains the traditional method for staging colon cancer[4]. Despite being in the same stage, patients can have different prognoses due to multiple factors. Previous studies have investigated the OS of colon cancer by combining non-biological factors and clinicopathological characteristics[5]. Other studies have also predicted the prognosis of tumor-related genes and clinicopathological features[6]. However, non-biological factors and tumor-related genes are difficult to obtain and expensive with certain limitations. Computed tomography (CT) imaging characteristics also play a crucial role in colon cancer staging. Recent studies have indicated that CT imaging characteristics, such as tumor size, extramural vascular invasion on CT (ctEMVI), and tumor enhancement ratio, are related to colon cancer prognosis[7-9]. Meanwhile, few studies have combined CT imaging characteristics with clinicopathological factors to predict colon cancer prognosis; however, due to lower efficacy and a limited number of CT imaging characteristics, only some of these studies have been validated[10]. Consequently, this study included more CT imaging characteristics and combined them with clinicopathological factors to construct a nomogram for a more accurate prediction of one-, three-, and five-year OS in patients with colon cancer.
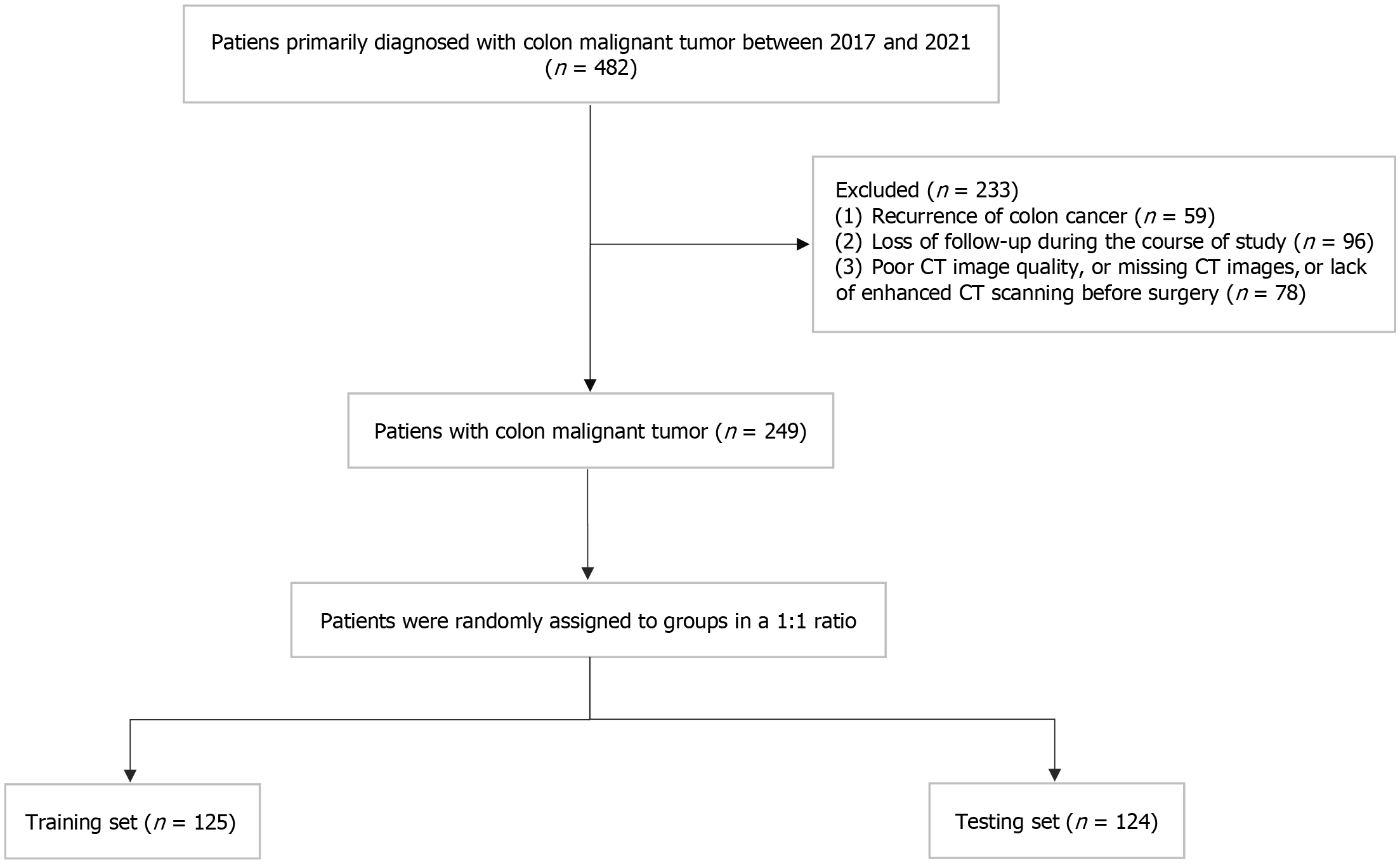
This study was approved by the Ethics Committee of Hubei Cancer Hospital. From January 2017 to December 2021, 482 patients with colorectal cancer who received surgical treatment at the Hubei Cancer Hospital were screened. The inclusion criteria were as follows: (1) Pathologically confirmed colon cancer after surgery; (2) Complete preoperative medical records and CT imaging data; and (3) No anti-tumor therapy before CT. The exclusion criteria were as follows: (1) Colon cancer recurrence during follow-up; (2) Loss to follow-up during the study; and (3) Poor CT image quality, missing CT images, or lack of enhanced CT scanning before surgery. Finally, 249 patients were enrolled and randomly divided into training and testing groups in a 1:1 ratio. In this study, 153 male and 96 female patients aged 23-84 years were enrolled. A flowchart of patient selection is displayed in Figure 1.
All patients were instructed to fast for 8 hours, drink 800-1000 mL of water, and practice holding their breath before the CT examination. All CT examinations were performed using a 64-slice multi-detector spiral CT system (SOMOTOM Definition AS+, Siemens or Light Speed-XT, GE Medical System). Patients were administered scopolamine hydrochloride intramuscularly before the examination to reduce gastrointestinal motility artifacts. A dose of 1.5 mL/kg ioversol contrast agent (320-370 mg/mL) was injected using an automatic high-pressure syringe at a speed of 3.0 mL/s. Arterial and venous phase images were acquired with 30-35 seconds and 70-75 seconds delays after injecting contrast material. Patients were positioned supine, with a scanning range including the entire abdominal and pelvic regions. All images were acquired and reconstructed with a tube voltage of 120 kV, slice thickness and spacing of 5 mm, and auto-current tube modulation.
Two radiologists (with five and three years of experience in abdominal radiology, respectively) blinded to the clinical outcomes and pathology results reviewed all CT images and reached a consensus. This study included 12 CT imaging characteristics, including tumor location, length, and thickness, imaging T stage (rT), lymph node metastasis on CT (ctLNM), partition of lymph node metastasis (MLN_partition), ctEMVI, and tumor attenuation value on plain (N), arterial (A) and venous (V), arterial (AER), and venous (VER) enhancement rates. Tumor locations included the ascending colon (ascending colon, ascending hepatic flexure, ascending colonic ileum, and ascending colonic appendix), transverse colon (transverse colon and splenic flexure of the transverse colon), descending colon (descending colon and splenic flexure of the descending colon), and the sigmoid colon. Tumor length was the longest longitudinal diameter in the coronal or sagittal planes. The thickness of tumor infiltration was defined as the maximum diameter on the axial plane perpendicular to the colonic wall. The rT stage was determined based on the eighth edition of the TNM staging system for colon cancer using AJCC. According to the third edition of the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma[11], MLN_partition was classified into N1, N2, and N3 groups. N1 was defined as within 5 cm of the tumor edge, N2 was defined as between N1 and N3, and N3 was defined as around the root of the superior mesenteric or celiac artery. ctEMVI was defined as the tumor tissue in the adjacent blood vessels on contrast-enhanced CT. Considering the uncertainty of the size criteria, the combination of internal heterogeneity and irregular outer borders of the lymph nodes was defined as ctLNM (+)[12]. The N value was measured at the maximum solid portion of the tumor before contrast injection. The A and V values were measured at the maximum solid portion of the tumor during the arterial and venous phases after contrast injection. The AER was calculated as A-N/N, whereas the VER was calculated as V-N/N.
The collected clinicopathological data included nine variables: Gender, age, pathological T stage (pT), pathological N stage (pN), number of lymph node metastases (MLN-number), lymphovascular invasion (LVI), vessel invasion, perineural invasion (PNI), and tumor classification. Experienced gastrointestinal pathologists evaluated pT and pN stage, tumor classification, LVI, MLN-numbers, vascular invasion, and PNI in biopsy tissues. Patient age was divided into ≤ 35, 36–59, 60–74, and ≥ 75 years[13]. Tumors were classified into four categories based on tumor classification: Poorly differe
The Kaplan-Meier (K-M) method was used to calculate the OS of the study population and compare survival curves using the log-rank test. Multivariable logistic regression analysis was performed to identify the independent prognostic factors for OS. On this basis, nomograms were constructed to predict one-, three-, and five-year OS and validated using the concordance index (C-index) and calibration curve. A C-index > 0.6 was considered indicative of good discrimination[14]. In the calibration curve, the closer the distribution of points and error lines are to the diagonal, the higher the accuracy of the chart. The data were analyzed using the R software. All tests were two-sided, and P < 0.05 was considered statistically significant.
This study included 249 patients, 125 in the training and 124 in the testing groups. Table 1 presents the clinicopathological and CT imaging characteristics of the patients with colon cancer. Of the patients, 153 (61.4%) were males and 96 (38.6%) were females. The tumor was mostly located in the ascending colon (108 cases, 43.4%), followed by the sigmoid colon (89 cases, 35.7%). Among the CT imaging characteristics, 76 (30.5%) and 107 (43.0%) patients had ctLNM and ctEMVI, respectively. Regarding clinicopathological features, 83 (33.3%), 89 (35.7%), and 46 (18.5%) patients had lymphovascular, vessel, and PNI, respectively. The rT and pT stages were mainly at the T3 stage, accounting for 107 (43.0%) and 177 (71.1%) cases, respectively. The most common tumor classification was moderately differentiated adenocarcinoma (174 cases, 69.9%).
| Variable | Overall (n = 249) | Train (n = 125) | Test (n = 124) | |||
| n | % | n | % | n | % | |
| Sex | ||||||
| Female | 153 | 61.4 | 77 | 61.6 | 76 | 61.3 |
| Male | 96 | 38.6 | 48 | 38.4 | 48 | 38.7 |
| Age (years) | ||||||
| ≤ 35 | 11 | 4.4 | 6 | 4.8 | 5 | 4.0 |
| 36-59 | 105 | 42.2 | 50 | 42.4 | 52 | 41.9 |
| 60-74 | 107 | 43.0 | 53 | 40.0 | 57 | 46.0 |
| ≥ 75 | 26 | 10.4 | 16 | 12.8 | 10 | 8.1 |
| Tumor_location | ||||||
| Ascending colon | 108 | 43.4 | 59 | 47.2 | 49 | 39.5 |
| Transverse colon | 20 | 8.0 | 10 | 8.0 | 10 | 8.1 |
| Descending colon | 32 | 12.9 | 11 | 8.8 | 21 | 16.9 |
| Sigmoid colon | 89 | 35.7 | 45 | 36.0 | 44 | 35.5 |
| rT | ||||||
| T1 | 4 | 1.6 | 3 | 2.4 | 1 | 0.8 |
| T2 | 18 | 7.2 | 11 | 8.8 | 7 | 5.6 |
| T3 | 107 | 43.0 | 52 | 41.6 | 55 | 44.4 |
| T4 | 120 | 48.2 | 59 | 47.2 | 61 | 49.2 |
| ctLNM | ||||||
| (-) | 173 | 69.5 | 86 | 68.8 | 87 | 70.2 |
| (+) | 76 | 30.5 | 39 | 31.2 | 37 | 29.8 |
| ctEMVI | ||||||
| (-) | 142 | 57.0 | 70 | 56.0 | 72 | 58.1 |
| (+) | 107 | 43.0 | 55 | 44.0 | 52 | 41.9 |
| pT | ||||||
| T1 | 9 | 3.6 | 4 | 3.2 | 5 | 4.0 |
| T2 | 16 | 6.4 | 9 | 7.2 | 7 | 5.6 |
| T3 | 177 | 71.1 | 85 | 68.0 | 92 | 74.2 |
| T4 | 47 | 18.9 | 27 | 21.6 | 20 | 16.1 |
| pN | ||||||
| N0 | 142 | 57.0 | 74 | 59.2 | 68 | 54.8 |
| N1 | 74 | 29.7 | 34 | 27.2 | 40 | 32.3 |
| N2 | 33 | 13.3 | 17 | 13.6 | 16 | 12.9 |
| MLN-number | ||||||
| 0 | 156 | 62.7 | 83 | 66.4 | 973 | 58.9 |
| 1-3 | 60 | 24.1 | 25 | 20.0 | 35 | 28.2 |
| 4-6 | 13 | 5.2 | 6 | 4.8 | 7 | 5.6 |
| ≥ 7 | 20 | 8.0 | 11 | 8.8 | 9 | 7.3 |
| MLN_partition | ||||||
| No MLN | 156 | 62.7 | 83 | 66.4 | 73 | 58.9 |
| N1 | 5 | 2.0 | 2 | 1.6 | 3 | 2.4 |
| N2 | 81 | 32.5 | 36 | 28.8 | 45 | 36.3 |
| N3 | 7 | 2.8 | 4 | 3.2 | 3 | 2.4 |
| LVI | ||||||
| (-) | 166 | 66.7 | 83 | 66.4 | 83 | 66.9 |
| (+) | 83 | 33.3 | 42 | 33.6 | 41 | 33.1 |
| Vessel | ||||||
| (-) | 160 | 64.3 | 80 | 64.0 | 80 | 64.5 |
| (+) | 89 | 35.7 | 45 | 36.0 | 44 | 35.5 |
| Perineural | ||||||
| (-) | 203 | 81.5 | 101 | 80.8 | 102 | 82.3 |
| (+) | 46 | 18.5 | 24 | 19.2 | 22 | 17.7 |
| Tumor classification | ||||||
| Mucinous carcinoma | 25 | 10.0 | 18 | 14.4 | 7 | 5.6 |
| Poorly differentiated adenocarcinoma | 33 | 13.3 | 18 | 14.4 | 15 | 12.1 |
| Moderately differentiated adenocarcinoma | 174 | 69.9 | 80 | 64.0 | 94 | 75.8 |
| Well-differentiated adenocarcinoma | 17 | 6.8 | 9 | 7.2 | 8 | 6.5 |
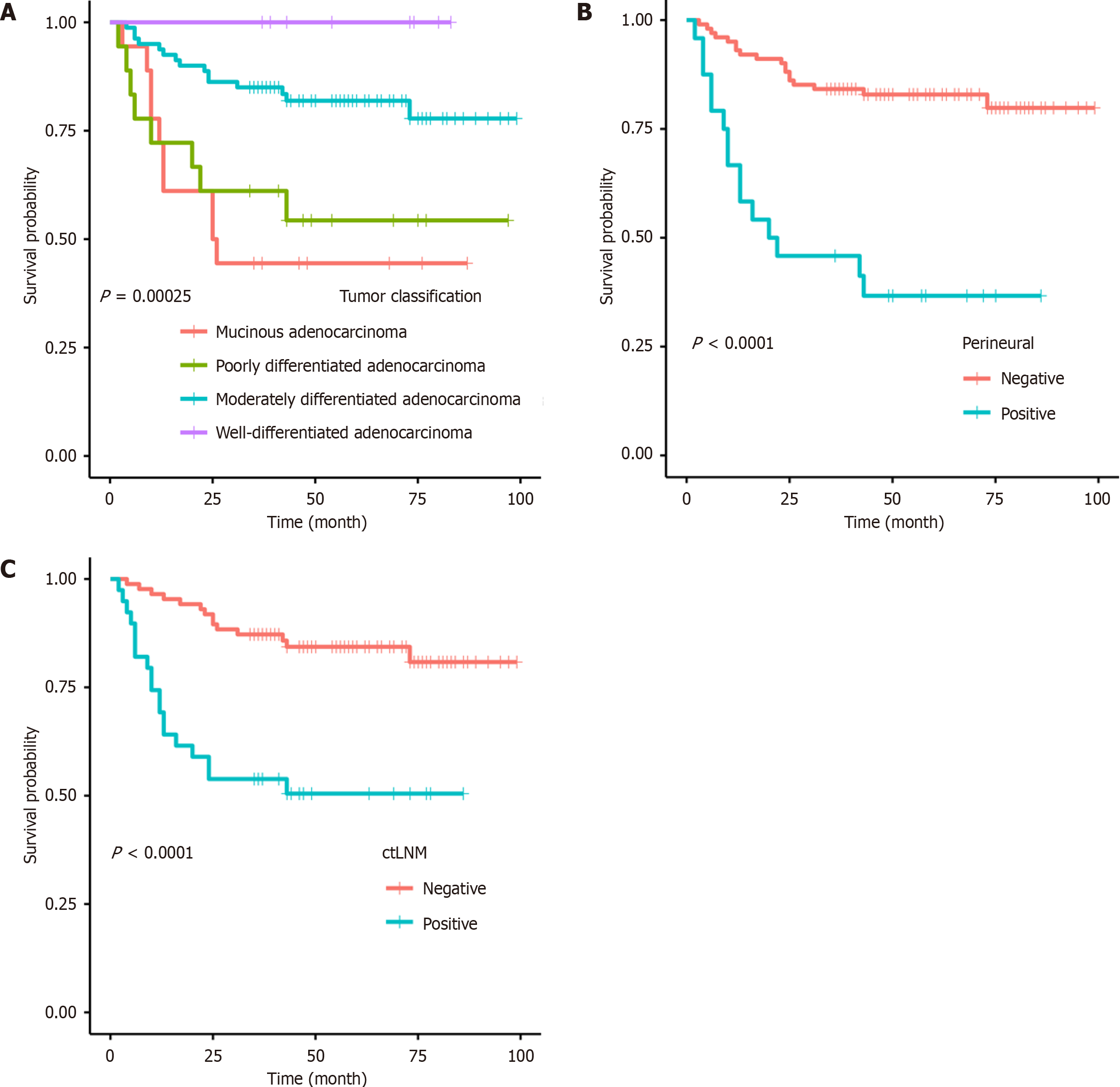
Logistic regression analysis was conducted to examine the variables predicting OS using univariate and multivariate approaches, and the independent prognostic factors for patients with colon cancer were determined based on the training group. Univariate analysis results displayed that rT, ctLNM, ctEMVI, pT, pN, MLN-number, MLN_partition, LVI, vessel invasion, PNI, and tumor classification were significantly associated with OS. Furthermore, multivariate analysis revealed that ctLNM [hazard ratio (HR) 3.4387; 95% confidence interval (CI): 1.6982–6.963; P = 0.000601], PNI (HR 4.3407; 95%CI: 2.1293-8.8489; P < 0.001), and differentiation (HR 0.45; 95%CI: 0.3002-0.6745; P = 0.000111) were independent prognostic factors for OS (Table 2). The K-M analysis using the log-rank test (Figure 2) yielded results similar to those of the above analysis.
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex | ||||||
| Female | 0.911 | 0.448-1.853 | 0.796 | |||
| Age (years) | 1.070 | 0.879-1.303 | 0.498 | |||
| Tumor_location | ||||||
| Transverse colon | 0.929 | 0.274-3.155 | 0.906 | |||
| Descending colon | 0.931 | 0.274-3.162 | 0.909 | |||
| Sigmoid colon | 0.638 | 0.286-1.421 | 0.271 | |||
| Length | 1.035 | 0.924-1.160 | 0.556 | |||
| rT | 3.428 | 1.690-6.954 | < 0.001b | |||
| Thickness | 1.124 | 0.943-1.341 | 0.191 | |||
| ctLNM | ||||||
| (+) | 4.165 | 2.082-8.333 | < 0.001b | 3.439 | 1.698-6.963 | < 0.001b |
| ctEMVI | ||||||
| (+) | 3.541 | 1.684-7.446 | < 0.001b | |||
| pT | 2.702 | 1.454-5.021 | 0.002a | |||
| pN | 3.016 | 1.975-4.606 | < 0.001b | |||
| MLN_number | 2.106 | 1.583-2.802 | < 0.001b | |||
| MLN_partition | 1.738 | 1.267-2.385 | < 0.001b | |||
| LVI | ||||||
| (+) | 2.204 | 1.113-4.366 | 0.023a | |||
| Vessel | ||||||
| (+) | 2.251 | 1.136-4.459 | 0.020a | |||
| Perineural | ||||||
| (+) | 5.224 | 2.619-10.420 | < 0.001b | 4.341 | 2.129-8.849 | < 0.001b |
| Tumor classification | 0.466 | 0.323-0.671 | < 0.001b | 0.450 | 0.300-0.675 | < 0.001b |
| N | 0.936 | 0.869-1.008 | ||||
| A | 0.978 | 0.950-1.006 | ||||
| V | 1.005 | 0.978-1.033 | ||||
| AER | 0.637 | 0.199-2.041 | ||||
| VER | 2.601 | 0.946-7.152 | ||||
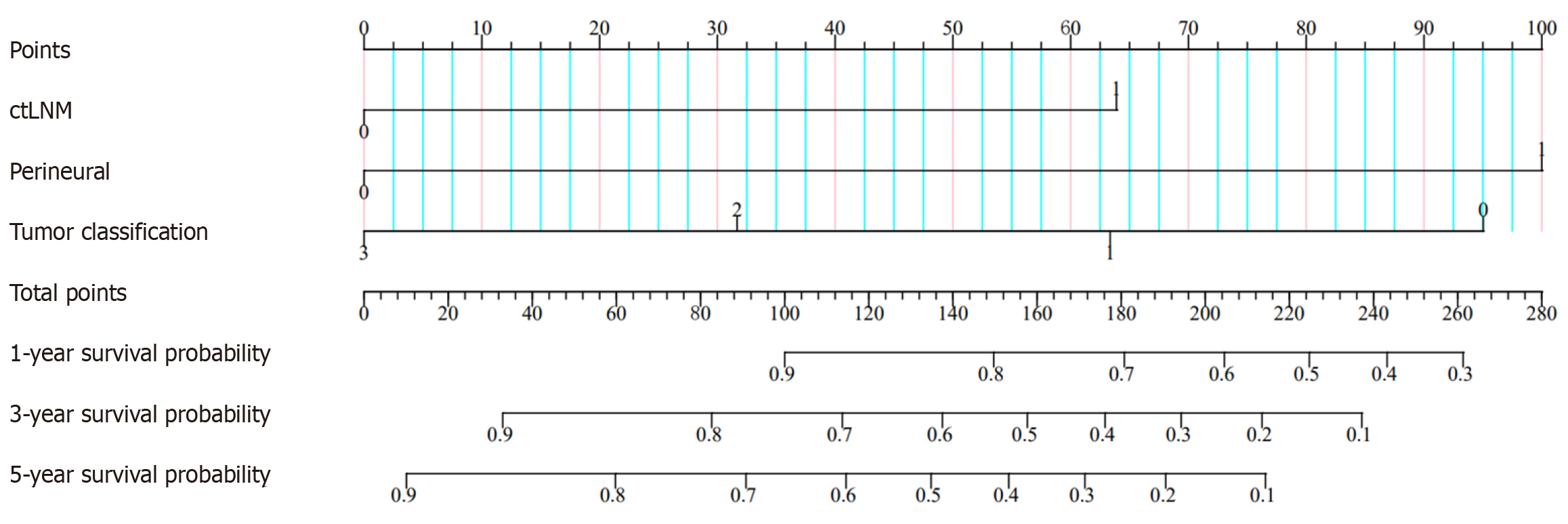
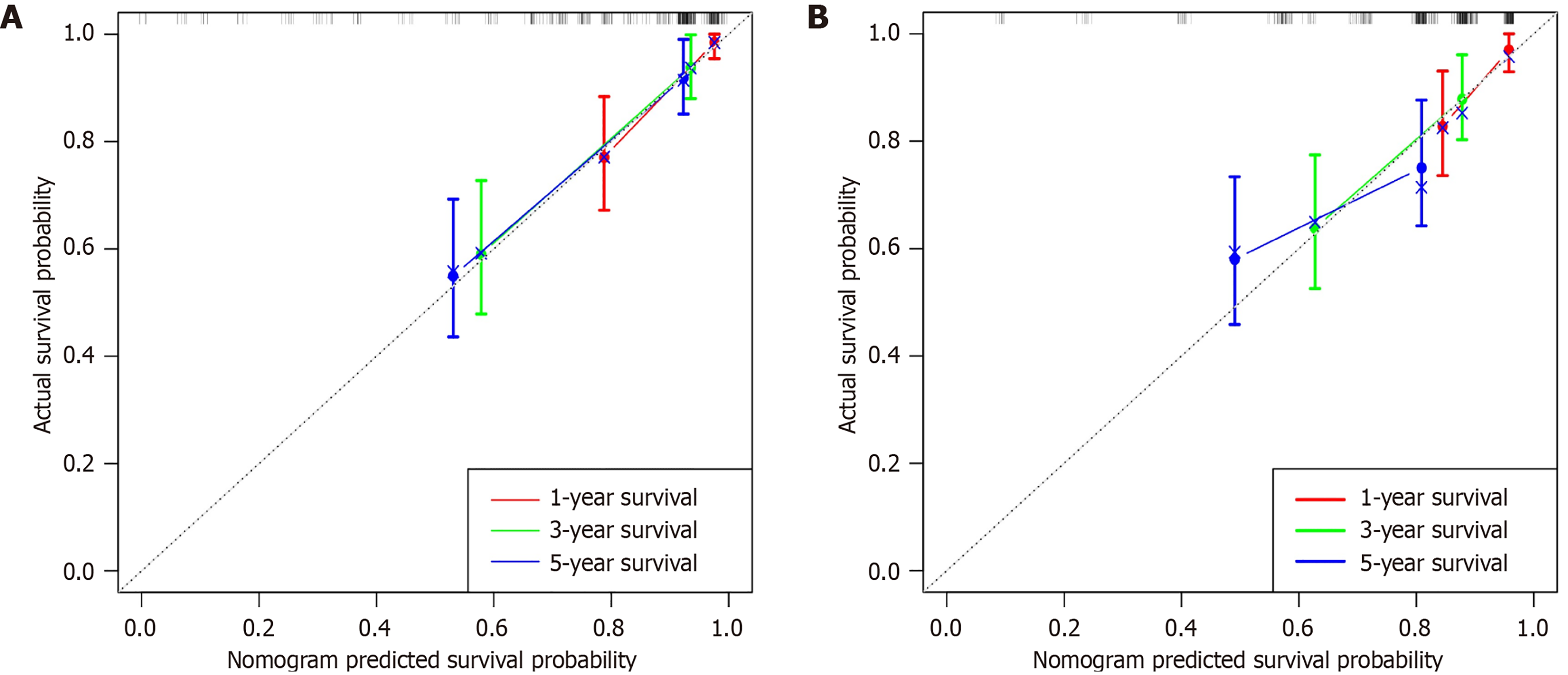
A nomogram was established in the training group (Figure 3) using the independent prognostic factors mentioned above (ctEMVI, tumor classification, and PNI) to predict one-, three-, and five-year OS in patients with colon cancer. The imaging and clinicopathological feature points for each patient were calculated and summed to obtain the total score corresponding to the probabilities of one-, three-, and five-year OS. The nomogram was validated for the testing group. The C-index of the training and testing groups were 0.804 and 0.692, respectively. The calibration plots in both groups demonstrated the good predictive accuracy of the nomogram at multiple time points (Figure 4).
This study included 12 CT image characteristics and nine clinicopathological factors and utilized univariate and multivariate analyses to identify independent risk factors associated with OS in patients with colon cancer. A nomogram was constructed to predict one-, three-, and five-year OS probabilities. The results revealed that ctLNM, PNI, and tumor classification were independent risk factors for OS in colon cancer, and the C-index of the training and testing groups for the developed nomogram were 0.804 and 0.692, respectively.
Many studies have reported non-biological factors, clinicopathological characteristics, and genes related to colon cancer prognosis. Liu et al[15] found a close association between household income, marital status, and prognosis of colon cancer. Some researchers[16,17] have identified T and N stages as important predictive factors for colon cancer prognosis. Yao et al[17] developed a radiomic nomogram that integrates radiomic signature and clinicopathological features to predict the prognosis of colon cancer and believed that pN stage, pT stage, and radiomic features were significant independent variables with prognostic value. The results revealed that the radiomic signature was more effective in predicting disease-free survival (DFS) than the TNM staging system, with a C-index of 0.780 and 0.738, respectively, lower than our study's C-index (0.804). Huang et al[6] found that the PLEKHA8P1 gene was an independent risk factor affecting the five-year survival rate of patients with colon cancer. However, few studies have incorporated CT imaging characteristics to predict the OS of patients with colon cancer. Yuan et al[13] and Yao et al[10] have discussed this topic. According to Yuan et al[13], the depth of intestinal wall infiltration and the number of metastatic lymph nodes are the most important factors affecting the prognosis of colon cancer, with several metastatic lymph nodes increasing the relative risk of death in patients with colon cancer. Yao et al[10] believed that ascites, enlarged mesenteric lymph nodes at the root, liver metastases, and nerve invasion were potential factors affecting the prognosis of patients with colon cancer. However, these studies included relatively few CT imaging characteristics.
This study included 12 CT imaging characteristics, and the results revealed that ctLNM is an independent risk factor for predicting OS in patients with colon cancer. Huang et al[18] suggested that the lymph node status reported on CT correlates with the actual lymph node status and is an independent risk factor for predicting preoperative lymph node metastasis. However, their study only demonstrated the relationship between the state of lymph nodes on CT images and actual pathology in predicting preoperative lymph node metastasis. They did not address the relationship between lymph node metastasis and colon cancer prognosis. However, Chen et al[19] considered lymph node metastasis to be the most important pathological feature for colon cancer prognosis. These findings are consistent with our study results, presenting that lymph node metastasis can be determined on CT by combining internal heterogeneity and irregular outer borders of the lymph nodes, thereby reflecting the actual pathological status of lymph node metastasis. Since actual lymph node metastasis is frequently associated with tumor recurrence and prognosis, accurate evaluation of lymph node metastasis in patients with colon cancer on preoperative CT imaging can predict their prognosis. However, unlike previous studies, the pN in this study was not an independent influencing factor due to two possible reasons. First, the limited data collected may have introduced some bias in the results. Second, the number of lymph nodes evaluated in resected specimens may vary among patients, surgeons, pathologists, and tumor or treatment-related factors[16], leading to discrepancies in the statistical results. Although pN was not an independent prognostic factor for colon cancer in this study, univariate analysis results indicated that pN might be an important factor affecting the prognosis of colon cancer
This study also suggests that the PNI is an independent risk factor for colon cancer prognosis. This is consistent with the findings of Liebig et al[20], who argued that PNI should be considered when stratifying patients with colon cancer for adjuvant therapy. This study suggests that PNI is associated with the advanced stages of colon cancer. Furthermore, this study concluded that PNI plays a role in disease progression and tumor metastasis. This further confirms the strong prognostic significance of PNI in colon cancer. However, Zhang et al[21] argued that LVI is a better prognostic factor than PNI and that PNI status can only predict three-year DFS, not three-year OS. This disparity may be due to differences in the study subjects. The former study focused on patients with colon cancer who underwent surgical resection, while the latter study focused on patients with stage III colon cancer who underwent curative treatment. The variation in disease staging among patients may result in different degrees of tumor infiltration and surrounding involvement, potentially leading to bias in LVI and PNI analyses.
The logistic regression model also revealed that tumor classification was an independent prognostic factor for colon cancer, with mucinous adenocarcinoma having the worst prognosis. This supports the findings of Wu et al[22] and Zhou et al[23], who concluded that mucinous adenocarcinoma has a worse prognosis than other non-specific adenocarcinomas. Wu et al[22] suggested that mucinous adenocarcinoma is more likely to reach advanced stages (T4, N2, M1, III, and IV) with a higher progression grade and younger patient population. Mucinous adenocarcinoma is associated with various clinical and pathological characteristics, such as younger age, poorer differentiation, increased metastatic potential, and advanced stage. Moreover, histologically, mucinous adenocarcinoma is distinct from other non-specific adenocarcinomas, defined by the World Health Organization, with > 50% extracellular mucin pools and malignant epithelial or tumor cells. This classification indicates that its prognosis may differ from other types and is generally worse.
Ren et al[24] developed a prediction model using age, differentiation degree, N stage, CA19-9, PNI, and postoperative chemotherapy as variables to predict the three- and five-year OS rates of patients who underwent curative surgery for stage II/III colon cancer, with a C-index of 0.780. Wang et al[25] extracted data from 10 clinicopathological variables and developed a prognosis graph to predict the OS of patients with respectable colon cancer, with a C-index of 0.71. In our study, the combination of CT imaging characteristics and clinicopathological factors resulted in a well-performing model, with a training group C-index of 0.804, higher than the aforementioned studies. Our model demonstrated good discrimi
In this study, the C-index, K-M curve, and nomogram were used to evaluate the resolution, reliability, and clinical application value of the final model. The C-index estimates the probability of the predicted result and agrees with the observed results. In this study, the C-index of the training and testing groups were 0.804 and 0.692, respectively, presenting that the nomogram had a high degree of differentiation. Additionally, nomograms have better clinical benefits, and personalized survival prognosis predictions can be easily obtained using this novel, easy-to-implement scoring system. For example, a colon cancer patient with ctLNM (approximately 52 points), PNI (approximately 62 points), and moderately differentiated adenocarcinoma (approximately 32 points) had a score of 146 points. The patient OS values at one, three, and five years were 67%, 39%, and 28%, respectively. Consequently, through the above examples, doctors can better score patients and develop beneficial individualized treatment and follow-up plans for patients with different prognoses.
This study has several limitations. First, although this was a single-center study, it used a moderate sample size and yielded valuable results. In the future, we will conduct multi-center studies to validate the effectiveness of the model. Second, this study included only Chinese patients; further external validation is needed to determine whether the results apply to patients from other countries. Finally, this study did not have new prognostic biomarkers, such as KRAS and BRAF mutations, microsatellite instability status, and clinical blood indicators, due to the difficulty of obtaining them through invasive procedures. In addition, the features currently introduced are not comprehensive enough, and more features, such as lymph node-related pathological features, will be included in the future to enhance the effectiveness of the model and ensure its comprehensiveness and effectiveness.
This study successfully established and validated a model based on ctLNM, PNI, and cell differentiation. This model demonstrates high accuracy and facilitates clinical doctors in rapidly and accurately assessing prognosis and survival. Besides, the nomogram constructed based on CT imaging characteristics and clinicopathological features can be a useful low-cost risk stratification tool for formulating more effective personalized treatment strategies.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Jiang Y, Yuan H, Li Z, Ji X, Shen Q, Tuo J, Bi J, Li H, Xiang Y. Global pattern and trends of colorectal cancer survival: a systematic review of population-based registration data. Cancer Biol Med. 2021;19:175-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Wang R, Lian J, Wang X, Pang X, Xu B, Tang S, Shao J, Lu H. Survival rate of colorectal cancer in China: A systematic review and meta-analysis. Front Oncol. 2023;13:1033154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4397] [Article Influence: 549.6] [Reference Citation Analysis (4)] |
| 5. | Zhou Z, Mo S, Dai W, Xiang W, Han L, Li Q, Wang R, Liu L, Zhang L, Cai S, Cai G. Prognostic nomograms for predicting cause-specific survival and overall survival of stage I-III colon cancer patients: a large population-based study. Cancer Cell Int. 2019;19:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Huang C, Zhao J, Zhu Z. Prognostic Nomogram of Prognosis-Related Genes and Clinicopathological Characteristics to Predict the 5-Year Survival Rate of Colon Cancer Patients. Front Surg. 2021;8:681721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Mou A, Li H, Chen XL, Fan YH, Pu H. Tumor size measured by multidetector CT in resectable colon cancer: correlation with regional lymph node metastasis and N stage. World J Surg Oncol. 2021;19:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Guan Z, Zhang XY, Li XT, Sun RJ, Lu QY, Wu AW, Sun YS. Correlation and prognostic value of CT-detected extramural venous invasion and pathological lymph-vascular invasion in colon cancer. Abdom Radiol (NY). 2022;47:1232-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Ye Y, Lu W, Deng Q, Chen Y, Han S, Dai S, Chen Z, Li J, Song Y, Wang Z, Ding K. Tumor enhancement ratio on preoperative abdominal contrast-enhanced CT scan for predicting recurrence risk in stage II colon cancer. Abdom Radiol (NY). 2022;47:1265-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 10. | Yao L, Zhang H, Wang W, An X, Cheng Z, Zhang X, Wang K, Zhang B. Clinical characteristics and prognosis of 196 Chinese patients with colon cancer. Front Surg. 2022;9:1008149. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon. 2019;3:175-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 436] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 12. | Rollvén E, Abraham-Nordling M, Holm T, Blomqvist L. Assessment and diagnostic accuracy of lymph node status to predict stage III colon cancer using computed tomography. Cancer Imaging. 2017;17:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Yuan Y, Li MD, Hu HG, Dong CX, Chen JQ, Li XF, Li JJ, Shen H. Prognostic and survival analysis of 837 Chinese colorectal cancer patients. World J Gastroenterol. 2013;19:2650-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Ling M, Gao B, Yin W, Wei J, Li S, Pan B. Prognostic Factors and a Nomogram Predicting Overall Survival in Muscle-invasive Bladder Cancer. ijSciences. 2022;11:24-28. [DOI] [Full Text] |
| 15. | Liu Q, Zhang R, Li Q, Li X. Clinical Implications of Nonbiological Factors With Colorectal Cancer Patients Younger Than 45 Years. Front Oncol. 2021;11:677198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Manilich EA, Kiran RP, Radivoyevitch T, Lavery I, Fazio VW, Remzi FH. A novel data-driven prognostic model for staging of colorectal cancer. J Am Coll Surg. 2011;213:579-588, 588.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Yao X, Sun C, Xiong F, Zhang X, Cheng J, Wang C, Ye Y, Hong N, Wang L, Liu Z, Meng X, Wang Y, Tian J. Radiomic signature-based nomogram to predict disease-free survival in stage II and III colon cancer. Eur J Radiol. 2020;131:109205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, Ma ZL, Liu ZY. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol. 2016;34:2157-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1320] [Article Influence: 146.7] [Reference Citation Analysis (0)] |
| 19. | Chen K, Wang H, Collins G, Hollands E, Law IYJ, Toh JWT. Current Perspectives on the Importance of Pathological Features in Prognostication and Guidance of Adjuvant Chemotherapy in Colon Cancer. Curr Oncol. 2022;29:1370-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, Berger DH, Albo D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131-5137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 21. | Zhang L, Deng Y, Liu S, Zhang W, Hong Z, Lu Z, Pan Z, Wu X, Peng J. Lymphovascular invasion represents a superior prognostic and predictive pathological factor of the duration of adjuvant chemotherapy for stage III colon cancer patients. BMC Cancer. 2023;23:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Wu X, Lin H, Li S. Prognoses of different pathological subtypes of colorectal cancer at different stages: A population-based retrospective cohort study. BMC Gastroenterol. 2019;19:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Zhou C, Lu L, Huang Q, Tang Z, Tang R, Xiao Z, Xiao S. The effects of chemotherapy, primary tumor location and histological subtype on the survival of stage III colon cancer patients. BMC Gastroenterol. 2023;23:110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Ren D, Wang WL, Wang G, Chen WW, Li XK, Li GD, Bai SX, Dong HM, Chen WH. Development and Internal Validation of a Nomogram-Based Model to Predict Three-Year and Five-Year Overall Survival in Patients with Stage II/III Colon Cancer. Cancer Manag Res. 2022;14:225-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Wang S, Liu Y, Shi Y, Guan J, Liu M, Wang W. Development and external validation of a nomogram predicting overall survival after curative resection of colon cancer. J Int Med Res. 2021;49:3000605211015023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |