Published online Oct 15, 2024. doi: 10.4251/wjgo.v16.i10.4045
Revised: May 11, 2024
Accepted: June 4, 2024
Published online: October 15, 2024
Processing time: 195 Days and 20.7 Hours
Colorectal cancer (CRC) is a leading global health concern, and early identification and precise prognosis play a vital role in enhancing patient results. Endoscopy is a minimally invasive imaging technique that is crucial for the screening, diag
Core Tip: Endoscopy is a vital tool for the early identification and accurate diagnosis of colorectal cancer (CRC). Advanced endoscopy techniques, such as endoscopic sub
- Citation: Li SW, Liu X, Sun SY. Advances in endoscopic diagnosis and management of colorectal cancer. World J Gastrointest Oncol 2024; 16(10): 4045-4051
- URL: https://www.wjgnet.com/1948-5204/full/v16/i10/4045.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i10.4045
In this editorial, we would like to comment on an article entitled “Colorectal cancer screening: A review of current knowledge and progress in research”[1]. Colorectal cancer (CRC) represents a substantial health concern worldwide, contributing significantly to the global burden of cancer-related mortality[2]. CRC prognosis is closely associated with the stage at diagnosis, with early-stage disease having a higher likelihood of successful treatment and better survival rates[3]. Early detection and accurate diagnosis of CRC are therefore crucial for improving outcomes. Endoscopy is a minimally invasive imaging technique that has become an essential tool for CRC screening, diagnosis, and management. It enables direct visualization of the colorectal mucosa and precise tissue sampling for histological examination[4,5].
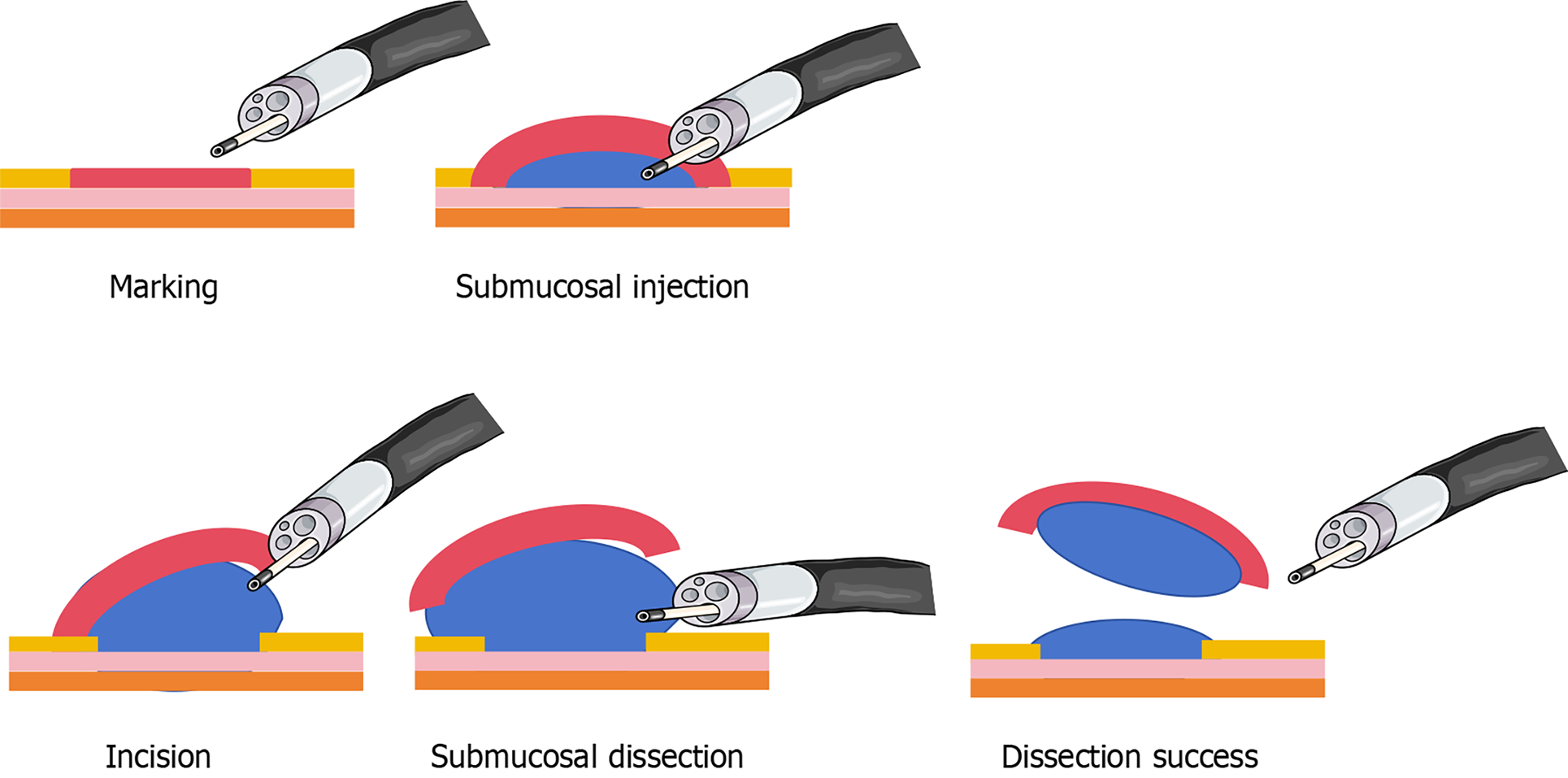
In recent decades, significant advances in endoscopic techniques have greatly enhanced the detection and management of CRC[6]. Narrow-band imaging (NBI) and autofluorescence endoscopy have been widely used to improve the visualization of mucosal surface patterns and microvascular architecture, particularly in the detection of early-stage CRC[7]. In addition to diagnostic imaging techniques, advanced endoscopic treatment procedures such as endoscopic submucosal dissection (ESD) (Figure 1) and full-thickness resection (FTR) have transformed the treatment of early-stage CRC[8,9]. These minimally invasive procedures facilitate en bloc resection of large colorectal lesions, providing curative treatment options for patients who were previously deemed ineligible for surgery due to advanced age, comorbidities, or patient preference. With careful patient selection and skilled operators, ESD and FTR have yielded promising oncological outcomes comparable to surgical resection, with reduced morbidity, shorter hospital stays, and improved quality of life[10-12].
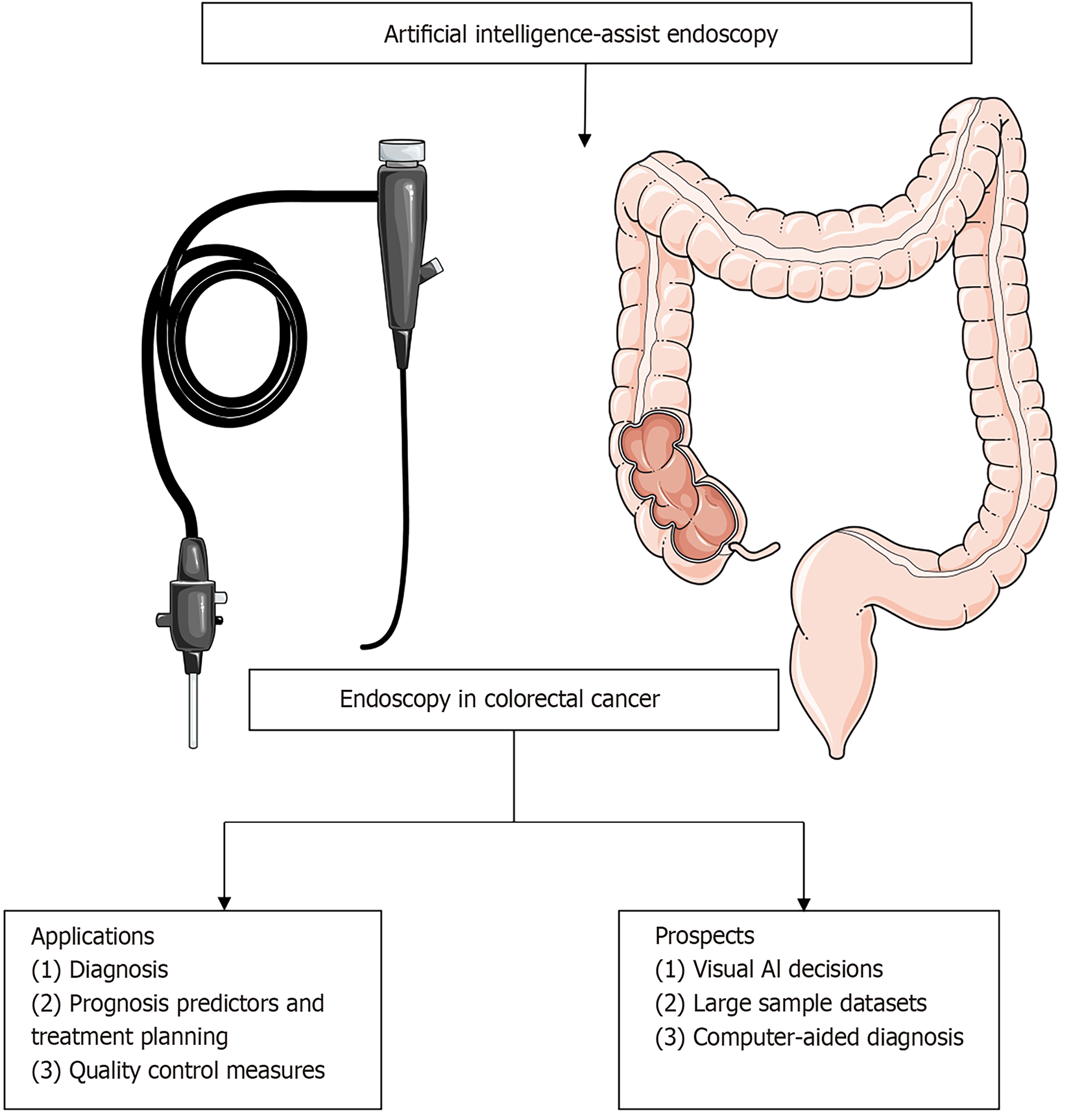
Artificial intelligence (AI) and machine learning techniques have become key focal points in the realm of endoscopic research, and have the potential to revolutionize diagnostic accuracy and procedural outcomes (Figure 2)[13]. AI algorithms can identify suspicious lesions via analysis of endoscopic images and videos, provide real-time feedback to endoscopists, and reduce the risk of missed diagnosis[14,15]. Machine learning algorithms, a subset of AI, have the capacity to learn from large datasets, enabling them to identify complex patterns and features that may be undetectable to the human eye[16]. These algorithms can be trained to recognize subtle changes in the colorectal mucosa that indicate the possible presence of cancerous or precancerous lesions[17]. In doing so, they help endoscopists make more informed decisions regarding treatment planning and prognostication, as well as select the most appropriate biopsy sampling strategies[18].
The integration of AI into computer-aided diagnosis (CAD) systems is particularly transformative, as these systems can automatically identify suspicious areas on endoscopic images, reducing interobserver variability and improving the overall diagnostic yield of endoscopy[19]. AI-driven CAD systems also have the potential to enhance the characterization of lesions, estimating their likelihood of malignancy based on morphological and architectural features[20]. This capability facilitates more precise risk stratification, enabling endoscopists to tailor their interventions and therapeutic strategies to the individual needs of the patient[21]. By optimizing biopsy sampling, AI can ensure that the most informative samples are obtained, leading to more accurate histological diagnosis and timely treatment decisions[22].
AI/ML demonstrates remarkable potential in diagnosing CRC. However, it is not without limitations and drawbacks[23]. The algorithms employed are typically based on historical data, potentially resulting in inaccurate predictions for novel or unrepresented cases. Furthermore, the safety of AI/ML applications is a pressing concern among expert physicians[24]. While these systems undergo rigorous testing and validation, errors or misdiagnoses could have severe consequences in extreme cases. Radiologists are apprehensive about the potential burden of extensive data collection and training that machine learning models necessitate. Additionally, these models may lack the intuitive judgement and experiential knowledge possessed by clinical practitioners[25]. Consequently, a subset of physicians propose that AI/ML results should be considered as supplementary references to, rather than substitutes for, the judgement of clinical physicians[26]. Further research and development are imperative to address these concerns and maximize the benefits of AI/ML in the field of CRC management.
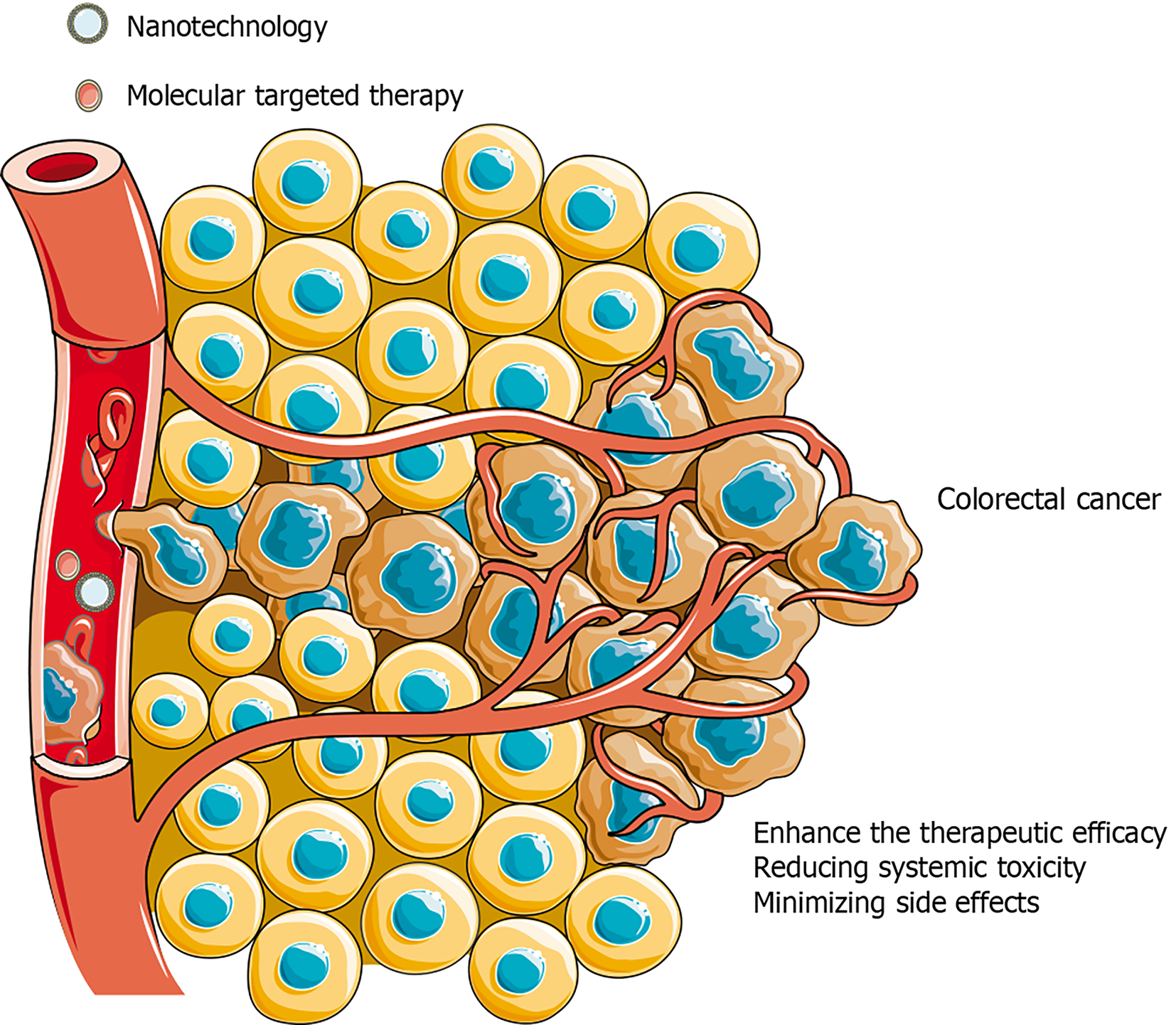
Novel innovations such as nanotechnology and molecular targeted therapy present exciting opportunities for personalized CRC treatment strategies (Figure 3)[27,28]. Nanotechnology has the capacity to manipulate matter at the atomic, molecular, and supramolecular levels, and is poised to revolutionize drug delivery systems in CRC[29]. Nanoparticle based drug delivery systems can enhance the therapeutic efficacy of anticancer agents by targeting the delivery of drugs directly to the site of the tumor, thereby reducing systemic toxicity and minimizing side effects[30]. Nanoparticles can be engineered to preferentially accumulate in tumor cells, thereby increasing the concentration of therapeutics at the site of action and potentially enhancing treatment efficacy[30]. Nanotechnology can also facilitate the delivery of multiple drugs simultaneously, potentially mediating the administration of combination therapies that can address the complex biology of CRC[31].
Molecular targeted therapy represents another rapidly advancing frontier in the treatment of CRC. The approach focuses on specific genetic mutations and signaling pathways involved in CRC development and progression[28]. In so doing, molecular targeted therapies can inhibit the growth and spread of cancer cells while sparing normal cells, thereby minimizing the adverse effects associated with traditional chemotherapy[28]. Personalized medicine in CRC can be achieved by identifying the specific genetic alterations present in an individual patient’s tumor and then selecting the most appropriate targeted therapy[32]. This tailored treatment strategy has the potential to enhance treatment response rates, improve outcomes, and lower the risk of disease recurrence and metastasis[26].
The use of sensors in CRC diagnosis has significantly evolved over the years. Advanced imaging sensors, such as optical coherence tomography and Raman spectroscopy, allow for the detection of abnormal tissue characteristics, thereby contributing to early diagnosis of lesions[33,34]. These sensors not only enhance visualization but also provide real-time feedback during endoscopic procedures, enabling more targeted and informed decision-making. Additionally, sensors are being integrated into existing endoscopic systems, providing clinicians with valuable information to facilitate more personalized treatment plans[35].
Similarly, endoscopic devices have witnessed significant development in the diagnosis and treatment of CRC. High-definition cameras equipped with NBI technology allow for better visualization of mucosal structures, thereby enhancing lesion detection rates[36]. Furthermore, robotic systems and automated modules have been integrated into endoscopic devices, streamlining complex procedures and reducing procedural time, thereby minimizing discomfort for patients[37].
Moreover, the integration of these devices with workflow processes has significantly improved the efficiency of CRC care. Automated data collection and analysis systems have facilitated seamless communication between healthcare providers, enabling comprehensive and coordinated care. Telemedicine platforms have also emerged as a viable option for remote consultations, allowing specialists to provide expert guidance and opinions in real-time, even in areas with limited access to specialized providers.
Despite these advancements, challenges persist. Standardization and validation of these technologies are crucial to ensure reliable and accurate results. Furthermore, ensuring patient privacy and data security must be adequately addressed to avoid any breaches in patient confidentiality. Additionally, further research is needed to identify novel sensors and endoscopic devices that can further enhance the accuracy and efficiency of CRC diagnosis and treatment.
Endoscopy has become an essential tool in the diagnosis and treatment of CRC, facilitating minimally invasive diagnostic and therapeutic options. Rapid technological advances have resulted in enhanced diagnostic accuracy, improved treatment modalities, and personalized treatment strategies. Integration of AI and novel technologies in endoscopic practice exhibits great promise with respect to transforming CRC management and improving outcomes. Continued development of endoscopic techniques, knowledge sharing, and collaboration among healthcare providers, researchers, and industry partners are required to further enhance the capabilities of endoscopy in CRC management.
The authors extend the deepest appreciation to Dr. Si-Yu Sun and Dr. Xiang Liu, who have made genuine contributions to the manuscript and endorsed the conclusion.
| 1. | Lopes SR, Martins C, Santos IC, Teixeira M, Gamito É, Alves AL. Colorectal cancer screening: A review of current knowledge and progress in research. World J Gastrointest Oncol. 2024;16:1119-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2289] [Article Influence: 208.1] [Reference Citation Analysis (1)] |
| 3. | Woolf SH. The best screening test for colorectal cancer--a personal choice. N Engl J Med. 2000;343:1641-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Gellad ZF, Weiss DG, Ahnen DJ, Lieberman DA, Jackson GL, Provenzale D. Colonoscopy withdrawal time and risk of neoplasia at 5 years: results from VA Cooperative Studies Program 380. Am J Gastroenterol. 2010;105:1746-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Vanella G, Bronswijk M, Arcidiacono PG, Larghi A, Wanrooij RLJV, de Boer YS, Rimbas M, Khashab M, van der Merwe SW. Current landscape of therapeutic EUS: Changing paradigms in gastroenterology practice. Endosc Ultrasound. 2023;12:16-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Deshmukh A, Elmeligui AM, Okasha HH, Parsa N, Tejedor-Tejada J, Nieto J. EUS-guided fiducial gold marker placement in metastatic colon cancer to the spleen. Endosc Ultrasound. 2022;11:79-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow Band Imaging, Magnifying Chromoendoscopy, and Gross Morphological Features for the Optical Diagnosis of T1 Colorectal Cancer and Deep Submucosal Invasion: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Ohata K, Kobayashi N, Sakai E, Takeuchi Y, Chino A, Takamaru H, Kodashima S, Hotta K, Harada K, Ikematsu H, Uraoka T, Murakami T, Tsuji S, Abe T, Katagiri A, Hori S, Michida T, Suzuki T, Fukuzawa M, Kiriyama S, Fukase K, Murakami Y, Ishikawa H, Saito Y. Long-term Outcomes After Endoscopic Submucosal Dissection for Large Colorectal Epithelial Neoplasms: A Prospective, Multicenter, Cohort Trial From Japan. Gastroenterology. 2022;163:1423-1434.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 9. | Kuellmer A, Mueller J, Caca K, Aepli P, Albers D, Schumacher B, Glitsch A, Schäfer C, Wallstabe I, Hofmann C, Erhardt A, Meier B, Bettinger D, Thimme R, Schmidt A; FTRD study group. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc. 2019;89:1180-1189.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Zwager LW, Bastiaansen BAJ, van der Spek BW, Heine DN, Schreuder RM, Perk LE, Weusten BLAM, Boonstra JJ, van der Sluis H, Wolters HJ, Bekkering FC, Rietdijk ST, Schwartz MP, Nagengast WB, Ten Hove WR, Terhaar Sive Droste JS, Rando Munoz FJ, Vlug MS, Beaumont H, Houben MHMG, Seerden TCJ, de Wijkerslooth TR, Gielisse EAR, Hazewinkel Y, de Ridder R, Straathof JA, van der Vlugt M, Koens L, Fockens P, Dekker E; Dutch eFTR Group. Endoscopic full-thickness resection of T1 colorectal cancers: a retrospective analysis from a multicenter Dutch eFTR registry. Endoscopy. 2022;54:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Li P, Ma B, Li W. What is the true effect of endoscopic full-thickness resection on early colorectal cancer? Gastrointest Endosc. 2019;90:539-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Gupta N, Rodríguez-Ruiz G, Siddiqui UD, Chapman CG, Donboli K, Hart J, Xiao SY, Waxman I. Endoscopic submucosal dissection for colorectal lesions: outcomes from a United States experience. Surg Endosc. 2022;36:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Kuntz S, Krieghoff-Henning E, Kather JN, Jutzi T, Höhn J, Kiehl L, Hekler A, Alwers E, von Kalle C, Fröhling S, Utikal JS, Brenner H, Hoffmeister M, Brinker TJ. Gastrointestinal cancer classification and prognostication from histology using deep learning: Systematic review. Eur J Cancer. 2021;155:200-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 14. | Wallace MB, Sharma P, Bhandari P, East J, Antonelli G, Lorenzetti R, Vieth M, Speranza I, Spadaccini M, Desai M, Lukens FJ, Babameto G, Batista D, Singh D, Palmer W, Ramirez F, Palmer R, Lunsford T, Ruff K, Bird-Liebermann E, Ciofoaia V, Arndtz S, Cangemi D, Puddick K, Derfus G, Johal AS, Barawi M, Longo L, Moro L, Repici A, Hassan C. Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia. Gastroenterology. 2022;163:295-304.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 141] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 15. | Foersch S, Glasner C, Woerl AC, Eckstein M, Wagner DC, Schulz S, Kellers F, Fernandez A, Tserea K, Kloth M, Hartmann A, Heintz A, Weichert W, Roth W, Geppert C, Kather JN, Jesinghaus M. Multistain deep learning for prediction of prognosis and therapy response in colorectal cancer. Nat Med. 2023;29:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 16. | Nemlander E, Ewing M, Abedi E, Hasselström J, Sjövall A, Carlsson AC, Rosenblad A. A machine learning tool for identifying non-metastatic colorectal cancer in primary care. Eur J Cancer. 2023;182:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Sharma A, Kumar R, Yadav G, Garg P. Artificial intelligence in intestinal polyp and colorectal cancer prediction. Cancer Lett. 2023;565:216238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Wang R, Dai W, Gong J, Huang M, Hu T, Li H, Lin K, Tan C, Hu H, Tong T, Cai G. Development of a novel combined nomogram model integrating deep learning-pathomics, radiomics and immunoscore to predict postoperative outcome of colorectal cancer lung metastasis patients. J Hematol Oncol. 2022;15:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 19. | Nemoto D, Guo Z, Katsuki S, Takezawa T, Maemoto R, Kawasaki K, Inoue K, Akutagawa T, Tanaka H, Sato K, Omori T, Takanashi K, Hayashi Y, Nakajima Y, Miyakura Y, Matsumoto T, Yoshida N, Esaki M, Uraoka T, Kato H, Inoue Y, Peng B, Zhang R, Hisabe T, Matsuda T, Yamamoto H, Tanaka N, Lefor AK, Zhu X, Togashi K. Computer-aided diagnosis of early-stage colorectal cancer using nonmagnified endoscopic white-light images (with videos). Gastrointest Endosc. 2023;98:90-99.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Takeda K, Kudo SE, Mori Y, Misawa M, Kudo T, Wakamura K, Katagiri A, Baba T, Hidaka E, Ishida F, Inoue H, Oda M, Mori K. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy. 2017;49:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Nazarian S, Glover B, Ashrafian H, Darzi A, Teare J. Diagnostic Accuracy of Artificial Intelligence and Computer-Aided Diagnosis for the Detection and Characterization of Colorectal Polyps: Systematic Review and Meta-analysis. J Med Internet Res. 2021;23:e27370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Repici A, Spadaccini M, Antonelli G, Correale L, Maselli R, Galtieri PA, Pellegatta G, Capogreco A, Milluzzo SM, Lollo G, Di Paolo D, Badalamenti M, Ferrara E, Fugazza A, Carrara S, Anderloni A, Rondonotti E, Amato A, De Gottardi A, Spada C, Radaelli F, Savevski V, Wallace MB, Sharma P, Rösch T, Hassan C. Artificial intelligence and colonoscopy experience: lessons from two randomised trials. Gut. 2022;71:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 23. | Hann A, Troya J, Fitting D. Current status and limitations of artificial intelligence in colonoscopy. United European Gastroenterol J. 2021;9:527-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Kudo SE, Mori Y, Abdel-Aal UM, Misawa M, Itoh H, Oda M, Mori K. Artificial intelligence and computer-aided diagnosis for colonoscopy: where do we stand now? Transl Gastroenterol Hepatol. 2021;6:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Taghiakbari M, Mori Y, von Renteln D. Artificial intelligence-assisted colonoscopy: A review of current state of practice and research. World J Gastroenterol. 2021;27:8103-8122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (5)] |
| 26. | Antonelli G, Rizkala T, Iacopini F, Hassan C. Current and future implications of artificial intelligence in colonoscopy. Ann Gastroenterol. 2023;36:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 27. | Kasi PB, Mallela VR, Ambrozkiewicz F, Trailin A, Liška V, Hemminki K. Theranostics Nanomedicine Applications for Colorectal Cancer and Metastasis: Recent Advances. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 28. | Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125:4139-4147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 29. | Gogoi P, Kaur G, Singh NK. Nanotechnology for colorectal cancer detection and treatment. World J Gastroenterol. 2022;28:6497-6511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 30. | Titu S, Grapa CM, Mocan T, Balacescu O, Irimie A. Tetraspanins: Physiology, Colorectal Cancer Development, and Nanomediated Applications. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Vinchhi P, Patel MM. Triumph against cancer: invading colorectal cancer with nanotechnology. Expert Opin Drug Deliv. 2021;18:1169-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Ducreux M, Chamseddine A, Laurent-Puig P, Smolenschi C, Hollebecque A, Dartigues P, Samallin E, Boige V, Malka D, Gelli M. Molecular targeted therapy of BRAF-mutant colorectal cancer. Ther Adv Med Oncol. 2019;11:1758835919856494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Noothalapati H, Iwasaki K, Yamamoto T. Non-invasive diagnosis of colorectal cancer by Raman spectroscopy: Recent developments in liquid biopsy and endoscopy approaches. Spectrochim Acta A Mol Biomol Spectrosc. 2021;258:119818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Luo H, Li S, Zeng Y, Cheema H, Otegbeye E, Ahmed S, Chapman WC Jr, Mutch M, Zhou C, Zhu Q. Human colorectal cancer tissue assessment using optical coherence tomography catheter and deep learning. J Biophotonics. 2022;15:e202100349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Nguyen KT, Kim HY, Park JO, Choi E, Kim CS. Tripolar Electrode Electrochemical Impedance Spectroscopy for Endoscopic Devices toward Early Colorectal Tumor Detection. ACS Sens. 2022;7:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 36. | Matsumura T, Ebigbo A, Römmele C, Ikematsu H, Ishigami H, Suzuki T, Harada H, Yada T, Takatori Y, Takeuchi M, Okimoto K, Akizue N, Maruoka D, Kitagawa Y, Minamide T, Iwaki T, Amano Y, Matsusaka K, Nagashima K, Maehata T, Yahagi N, Messmann H, Kato N. Diagnostic Value of Adding Magnifying Chromoendoscopy to Magnifying Narrow-Band Imaging Endoscopy for Colorectal Polyps. Clin Gastroenterol Hepatol. 2023;21:2551-2559.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 37. | Zhang Q, Prendergast JM, Formosa GA, Fulton MJ, Rentschler ME. Enabling Autonomous Colonoscopy Intervention Using a Robotic Endoscope Platform. IEEE Trans Biomed Eng. 2021;68:1957-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |