Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.79
Peer-review started: September 27, 2023
First decision: October 24, 2023
Revised: November 2, 2023
Accepted: November 29, 2023
Article in press: November 29, 2023
Published online: January 15, 2024
Processing time: 105 Days and 17.6 Hours
Propofol and sevoflurane are commonly used anesthetic agents for maintenance anesthesia during radical resection of gastric cancer. However, there is a debate concerning their differential effects on cognitive function, anxiety, and depression in patients undergoing this procedure.
To compare the effects of propofol and sevoflurane anesthesia on postoperative cognitive function, anxiety, depression, and organ function in patients under
A total of 80 patients were involved in this research. The subjects were divided into two groups: Propofol group and sevoflurane group. The evaluation scale for cognitive function was the Loewenstein occupational therapy cognitive asse
The LOTCA score at 1 d after surgery was significantly lower in the propofol group than in the sevoflurane group. Additionally, the SAS and SDS scores of the sevoflurane group were significantly lower than those of the propofol group. The sevoflurane group showed greater stability in heart rate as well as the mean arterial pressure compared to the propofol group. Moreover, the sevoflurane group displayed better pulmonary function and less lung injury than the propofol group.
Both propofol and sevoflurane could be utilized as maintenance anesthesia during radical resection of gastric cancer. Propofol anesthesia has a minimal effect on patients' pulmonary function, consequently enhancing their postoperative recovery. Sevoflurane anesthesia causes less impairment on patients' cognitive function and mitigates negative emotions, leading to an improved postoperative mental state. Therefore, the selection of anesthetic agents should be based on the individual patient's specific circumstances.
Core Tip: This study compared the effects of propofol and sevoflurane anesthesia on cognitive function, anxiety, and depression in patients undergoing radical resection of gastric cancer. The results demonstrated that both anesthetics significantly decreased cognitive function posttreatment. However, the propofol group had a lower cognitive function score at 1 d after surgery compared to the sevoflurane group. Additionally, the sevoflurane group had lower scores for anxiety and depression compared to the propofol group. These findings suggest that sevoflurane anesthesia may have a greater capacity to alleviate cognitive dysfunction and negative emotions in gastric cancer patients.
- Citation: Li AH, Bu S, Wang L, Liang AM, Luo HY. Impact of propofol and sevoflurane anesthesia on cognition and emotion in gastric cancer patients undergoing radical resection. World J Gastrointest Oncol 2024; 16(1): 79-89
- URL: https://www.wjgnet.com/1948-5204/full/v16/i1/79.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i1.79
Due to the increase in the prevalence of risk factors associated with gastric cancer, such as Helicobacter pylori infection, a diet high in salty or smoked foods, and tobacco use, gastric cancer is becoming one of the foremost causes of cancer-related mortality[1]. Radical resection, a primary treatment modality for localized gastric cancer, is performed to remove the tumor and adjacent lymph nodes, promoting long-term survival and disease control[2]. However, the surgical procedure itself poses additional challenges, including the risk of impacting cognitive function and inducing negative emotions in patients[3,4].
Anesthesia plays a pivotal role in surgical procedures, in terms of ensuring patient comfort and safety. Among the anesthetics commonly employed, propofol and sevoflurane have become widely used due to their favorable pharmacokinetic profiles and efficacy[5,6]. While their effects on immediate postoperative outcomes have been investigated to some extent, the impact on cognitive function and emotional well-being in patients undergoing radical resection of gastric cancer remains inadequately understood[6].
Cognitive function, encompassing memory, attention, and executive functions, is imperative for daily functioning and quality of life[7,8]. Impairments in cognitive performance following surgical intervention can result in delayed recovery, diminished patient satisfaction, and decreased overall postoperative recovery[9,10]. Similarly, negative emotions such as anxiety, depression, and fear may develop postoperatively, adversely affecting patient outcomes and overall prognosis[8].
Propofol and sevoflurane are common anesthetics for radical resection of gastric cancer; the former specifically activates the γ-aminobutyric acid receptor-chloride ionophore complex, with obvious advantages of strong liposolubility and safety; the latter is a new type of anesthetic with a mild neurological influence on patients and relatively simple administration that can effectively control the depth of anesthesia[10,11].
Understanding the specific effects of anesthetics on cognitive function and negative emotions in patients undergoing radical resection of gastric cancer is critical for optimizing perioperative care and enhancing patient outcomes. By elucidating the potential differences in the anesthetic effects of propofol and sevoflurane, health care professionals can better tailor anesthesia regimens to mitigate adverse effects and promote overall patient well-being.
The objective of this study was to thoroughly scrutinize and contrast the anesthetic impacts of propofol and sevoflurane on patients who are undergoing radical resection of gastric cancer, specifically in relation to cognitive function and negative emotion. To achieve this, we assessed multiple aspects of cognitive function, such as memory, attention, and executive functions, utilizing established neuropsychological tests for accurate evaluation. The assessment of negative emotion was accomplished through standardized self-report questionnaires. By collecting and analyzing comprehensive data, we intended to shed light on optimizing anesthesia selection and management strategies to preserve cognitive function and promote positive emotional outcomes in this specific patient population.
The data of 80 patients admitted to our hospital for radical resection of gastric cancer between January 2022 and May 2023 were retrospectively analyzed. Based on the anesthesia method, they were divided into a propofol group (n = 40) and a sevoflurane group (n = 40).
The inclusion criteria were: (1) Patients who were identified as having a primary gastric tumor and who met the diagnostic criteria for gastric cancer[11]; (2) patients with an American Society of Anesthesiologists (ASA) class II or lower; (3) patients with surgical indications and who underwent radical resection of gastric cancer; (4) patients who cooperated with the research; and (5) patients with a complete clinical record.
The exclusion criteria were: (1) Subjects with impaired hearing, language disorders, unclear awareness, or history of psychiatric disorders; (2) patients with preoperative heart, brain, liver, kidney, or other important organ dysfunction; (3) patients with significant abnormal pulmonary function; (4) patients with other primary tumors; (5) patients who had undergone other surgical treatments within the last 6 mo; (6) patients who had taken anti-inflammatory or analgesic drugs, including steroids and nonsteroids, within the last month; and (7) patients who were unable to cooperate with the research.
The study conformed to the Declaration of Helsinki (2013), and the patients as well as their family members were informed about the purpose, significance, content and confidentiality of the research and subsequently signed consent forms.
Both patient groups underwent surgery performed by the same surgical team. Prior to the procedure, venous access was established, and anesthesia was induced with a combination of intravenous injections: 0.1 mg/kg midazolam (H20067041; Yichang Humanwell Pharmaceutical Co., Ltd), 0.4 μg/kg sufentanil (H20054172; Yichang Humanwell Pharmaceutical Co., Ltd), and 0.2 mg/kg etomidate (H20031037; Jiangsu Enhua Pharmaceutical Group Co., Ltd). Oxygen was administered for 1 min using a face mask, and a noninvasive depth of anesthesia monitor (Sichuan Zhineng Electronics Industrial Co., Ltd.; Sichuan Medical Products Administration Certified No. 20062210024) was inserted and connected. The monitoring electrode was positioned in the middle of the forehead as well as the left mastoid, while the reference electrode was placed on the left forehead. Auditory stimulation was applied with headphones at 70 dB and 6.9 Hz. During the surgical procedure, the propofol group received a target-controlled infusion of propofol (H19990282; Xi'an Libang Pharmaceutical Co., Ltd.), while the sevoflurane group received continuous inhalation of sevoflurane (Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu, China), both at an oxygen flow rate of 2 L/min. In both groups, 0.15 mg/kg of both cisatracurium (H20060869; Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu, China) and sufentanil were intermittently administered intraoperatively to maintain the bispectral index within the range of 40-60. After surgery, the endotracheal tube was removed when spontaneous breathing was restored.
General data: The sociodemographic data and clinical data of patients were collected and compared between both groups in terms of sex, age, body mass, body mass index (BMI), ASA classes, lesion location, cancer types, and metastasis.
Physiological stress indices: Heart rate (HR) and mean arterial pressure (MAP) were obtained at specific time points throughout the procedure. These time points included before anesthesia (T0), 30 min after anesthesia (T1), at the conclusion of surgery (T2), and 1 h following surgery (T3). Venous blood was also collected from the patients, with superoxide dismutase (SOD) levels detected by the xanthine oxidase method (ShanghaiHonsun Biological Technology Co., Ltd; CAS No. 9002-17-9) and malondialdehyde (MDA) levels tested by the thiobarbituric acid method (Shanghai Acmec Biochemical Co., Ltd; CAS No. 504-17-6).
Pulmonary function indices: The patients underwent pulmonary function testing at T0, T1, T2, and T3, and the alveolar-arterial oxygen tension difference (A-aDO2), respiratory index (RI), and pulmonary shunt fraction (Qs/Qt) were calculated. The forced expiratory volume in 1 s (FEV1) was recorded by spirometry at T0 and 7 d after surgery (T5).
Determination of cognitive function using the Loewenstein occupational therapy cognitive assessment (LOTCA): The LOTCA comprises a series of validated neuropsychological tests. The assessment encompassed various domains, including orientation (16 points), spatial perception (12 points), visual motor organization (28 points), visual perception (16 points), motor application (12 points), and thinking operation (31 points). Scores on the LOTCA range from 0 to 115, with better scores suggesting better cognitive function. The patients’ LOTCA scores were compared at T0 and 1 d after surgery (T4).
Mini-mental state examination (MMSE) score: The MMSE includes six items, such as orientation and memory. The total score of the scale is 30 points. A higher cognitive function score corresponds to better performance. The patients’ MMSE scores were compared at T0 and T4.
Self-rating anxiety scale (SAS) score and self-rating depression scale (SDS) score: The SAS and SDS each have 20 items associated with anxiety or depression. Each item is scored from 1 to 4 points, and the total score changes on a percentage scale; the higher the score, the more severe the mood. The patients’ SAS scores and SDS scores were compared at T0 and T4.
Data analyses for this study were performed using SPSS 20.0 software. The graphs were created utilizing GraphPad Prism 7, a program developed by GraphPad Software in San Diego, United States. The study involved the analyses of count and measurement data, which were assessed using χ2 tests and t tests, respectively. A P value below 0.05 was used to indicate statistical significance.
No significant differences were observed between the two groups regarding their general characteristics, including age, sex, BMI, and body mass (P > 0.05) (Table 1).
| Group | Propofol (n = 40) | Sevoflurane (n = 40) | χ2/t | P value |
| Sex | 0.05 | 0.823 | ||
| Female | 19 | 20 | ||
| Male | 21 | 20 | ||
| BMI (kg/m2) | 22.85 ± 2.10 | 22.94 ± 2.12 | 0.234 | 0.816 |
| Age (yr) | 47.33 ± 5.20 | 47.82 ± 5.11 | 0.521 | 0.604 |
| Body mass (kg) | 64.98 ± 5.21 | 65.01 ± 5.23 | 0.031 | 0.975 |
| ASA classes | 0.051 | 0.822 | ||
| Class Ⅰ | 22 | 23 | ||
| Class Ⅱ | 18 | 17 | ||
| Cancer type | 2.066 | 0.559 | ||
| Adenocarcinoma | 23 | 22 | ||
| Undifferentiated carcinoma | 12 | 11 | ||
| Mucinous carcinoma | 5 | 5 | ||
| Other | 0 | 2 | ||
| Metastasis | 0.155 | 0.926 | ||
| Bone metastasis | 5 | 4 | ||
| Liver metastasis | 4 | 4 |
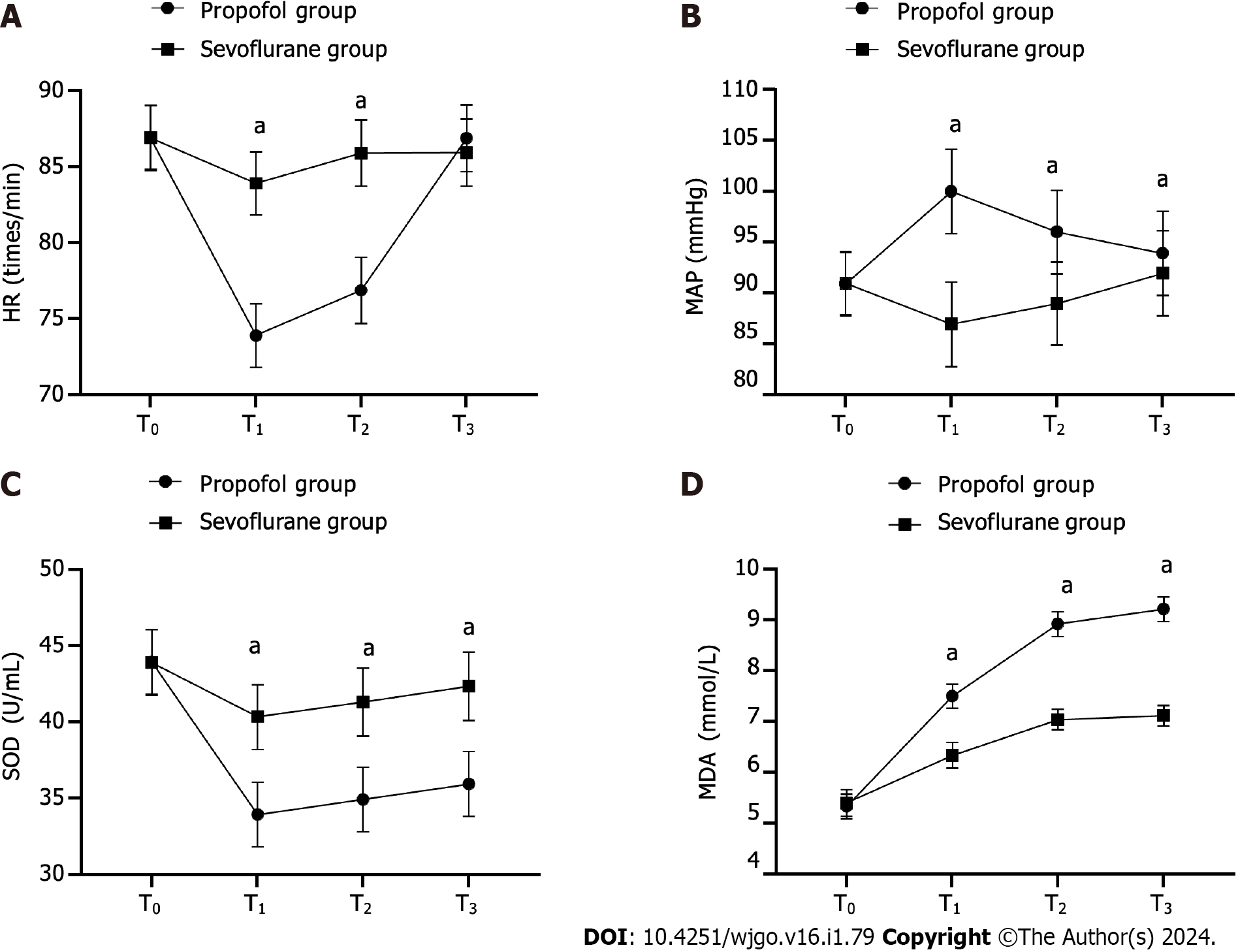
At T1, T2, and T3, the HR in the propofol group was notably lower than that of the sevoflurane group (P < 0.05). Furthermore, the propofol group exhibited significantly higher MAP and MDA levels (P < 0.001) and markedly lower SOD levels (P < 0.001) than the sevoflurane group (Figure 1).
In contrast, at T0, there was no significant difference in HR between the groups (86.92 ± 2.15 vs 86.93 ± 2.09, P > 0.05). At T1, T2, and T3, HR was lower in the propofol group than in the sevoflurane group (73.90 ± 2.09 vs 83.93 ± 2.07, 76.88 ± 2.18 vs 85.92 ± 2.18, 86.90 ± 2.19 vs 85.95 ± 2.21, P < 0.05).
Similarly, there was no significant difference in MAP between the groups at T0 (90.90 ± 3.11 vs. 90.98 ± 3.10, P > 0.05). However, at T1, T2, and T3, the MAP was higher in the propofol group than in the sevoflurane group (99.98 ± 4.15 vs 86.95 ± 4.15, 96.00 ± 4.10 vs 88.98 ± 4.07, 93.90 ± 4.15 vs 91.95 ± 4.18, P < 0.001).
In terms of SOD level, no notable difference between the groups was found at T0 (43.94 ± 2.12 vs 43.91 ± 2.15, P > 0.05); however, at T1, T2, and T3, the propofol group presented lower SOD levels than the sevoflurane group (33.94 ± 2.12 vs 40.35 ± 2.12, 34.94 ± 2.12 vs 41.32 ± 2.24, 35.94 ± 2.12 vs 42.35 ± 2.24, P < 0.001).
Additionally, at T0, MDA level did not differ remarkably between the groups (5.33 ± 0.24 vs 5.40 ± 0.26, P > 0.05). However, at T1, T2, and T3, the MDA levels were higher in the propofol group than in the sevoflurane group (7.50 ± 0.24 vs 6.34 ± 0.25, 8.92 ± 0.24 vs 7.04 ± 0.20, 9.21 ± 0.24 vs 7.12 ± 0.20, P < 0.001).
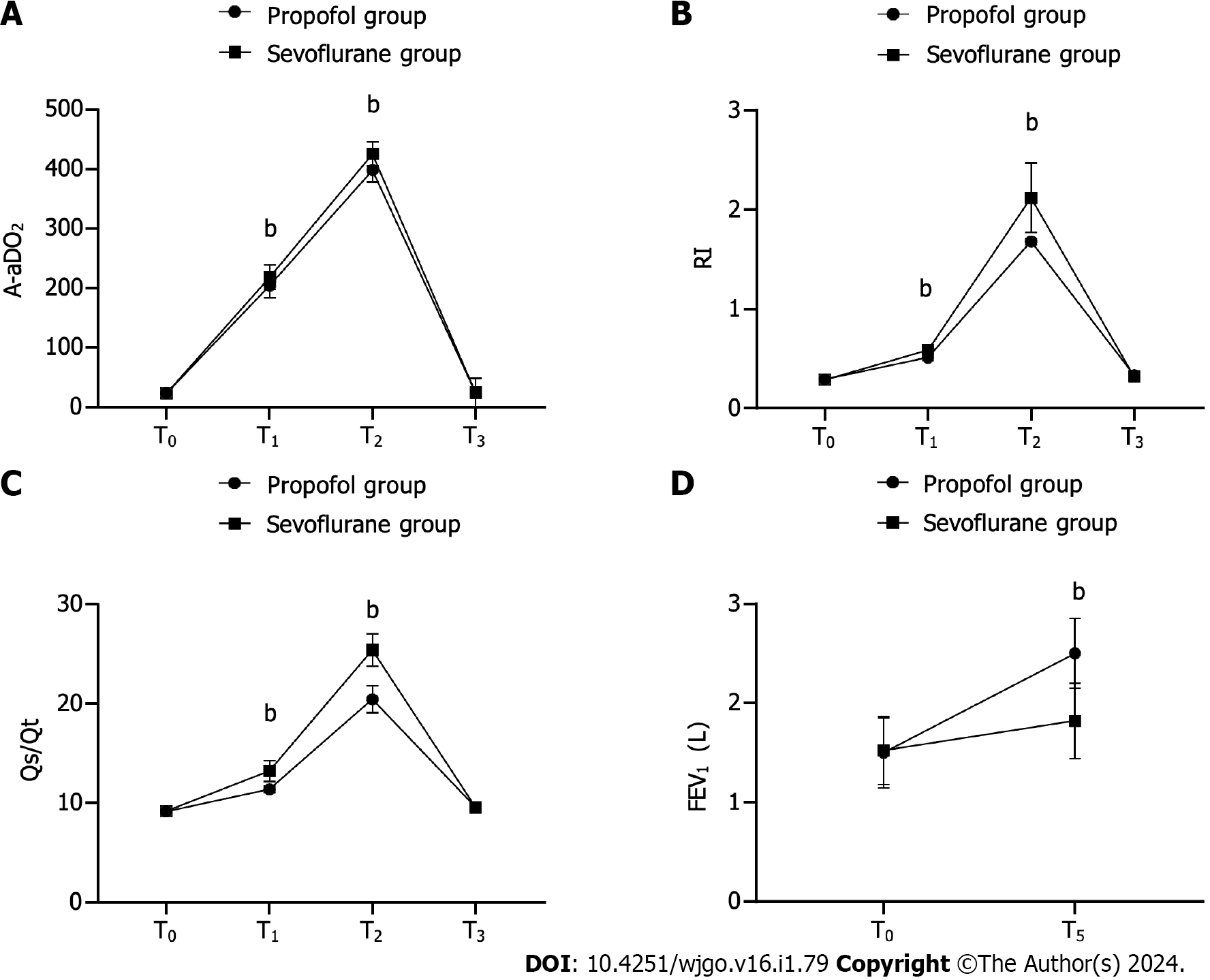
There were no significant differences noted between the two groups in terms of A-aDO2, RI, or Qs/Qt levels at T0 and T3 (P > 0.05). At T1 and T2, the propofol group demonstrated significantly better A-aDO2, RI, and Qs/Qt levels than the sevoflurane group (P < 0.001). Additionally, the FEV1 level at T5 was significantly higher in the propofol group than in the sevoflurane group (P < 0.001) (Figure 2).
At T0 and T3, A-aDO2 did not significantly differ between the two groups (23.32 ± 2.21 vs 23.41 ± 2.21 and 24.15 ± 2.21 vs 24.35 ± 2.05, respectively, P > 0.05). However, at T1 and T2, the propofol group had significantly lower A-aDO2 levels than the sevoflurane group (204.55 ± 20.46 vs 218.50 ± 20.50 and 398.54 ± 20.53 vs 425.53 ± 20.49, respectively, P < 0.001).
Similarly, the RI levels were not significantly different between the groups at T0 and T3 (0.29 ± 0.05 vs 0.29 ± 0.04 and 0.33 ± 0.03 vs 0.32 ± 0.04, respectively, P > 0.05). However, at T1 and T2, significantly lower RI levels were observed in the propofol group than in the sevoflurane group (0.51 ± 0.03 vs 0.59 ± 0.03 and 1.68 ± 0.03 vs 2.12 ± 0.35, respectively, P < 0.001).
For Qs/Qt, there were no significant differences between the groups at T0 and T3 (9.12 ± 0.35 vs 9.15 ± 0.31 and 9.55 ± 0.31 vs 9.57 ± 0.37, respectively, P > 0.05). However, at T1 and T2, significantly lower Qs/Qt levels were observed in the propofol group than in the sevoflurane group (11.37 ± 0.37 vs 13.22 ± 1.05 and 20.45 ± 1.37 vs 25.42 ± 1.65, respectively, P < 0.001).
FEV1 at T0 did not differ significantly between the two groups (1.50 ± 0.35 vs 1.52 ± 0.34, P > 0.05). However, a significantly higher FEV1 level was observed in the propofol group than in the sevoflurane group at T5 (2.50 ± 0.35 vs 1.82 ± 0.38, P < 0.001).
No remarkable difference was found between the groups in the LOTCA score at T0 (P > 0.05). After treatment, the LOTCA scores of both groups were significantly decreased (P < 0.001), and the LOTCA score of the sevoflurane group was significantly higher than that of the propofol group at T4 (P < 0.001) (Table 2).
| Group | T0 | T4 | t | P value |
| Propofol (n = 40) | 111.98 ± 7.53 | 90.30 ± 5.25 | 15.304 | < 0.001 |
| Sevoflurane (n = 40) | 112.03 ± 6.48 | 102.32 ± 5.21 | 7.756 | < 0.001 |
| t | 0.038 | 11.711 | ||
| P value | 0.97 | < 0.001 |
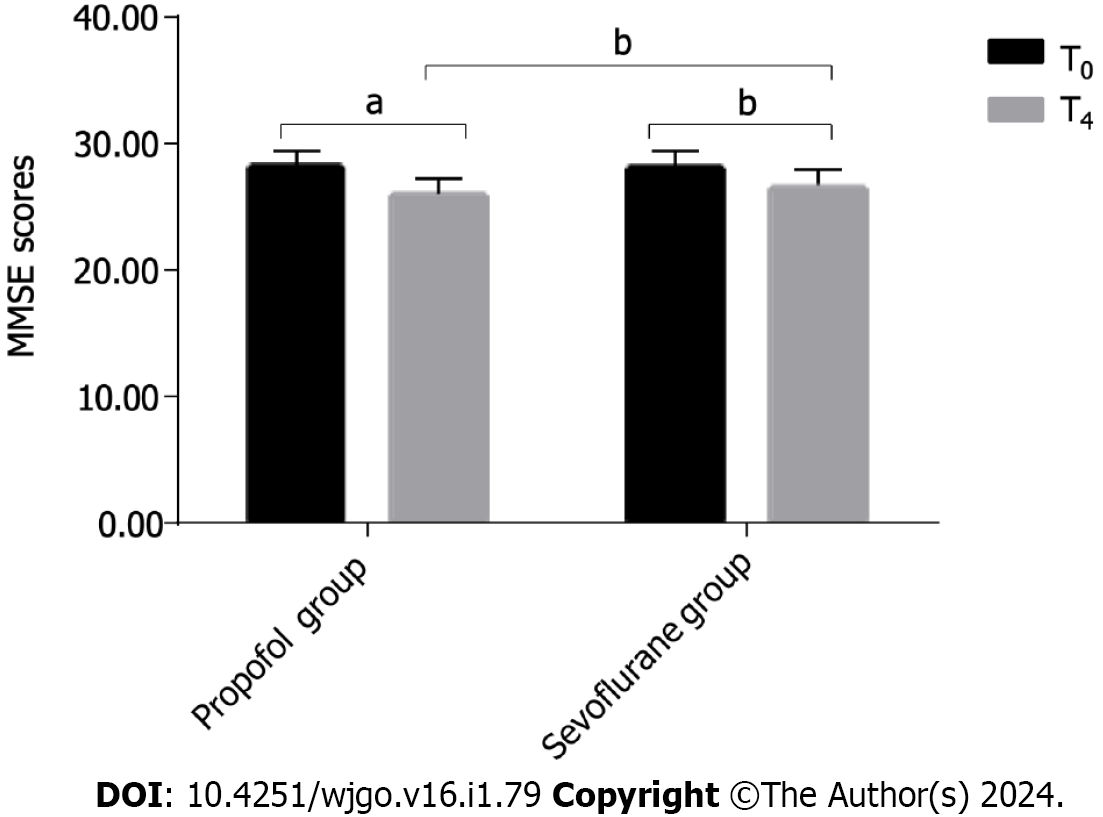
No significant difference was observed between the two groups when comparing the MMSE score at baseline (T0, P > 0.05). Posttreatment, the MMSE scores for both groups significantly decreased (P < 0.05). However, the MMSE score in the sevoflurane group was significantly higher than that of the propofol group at T4 (P < 0.05) (Figure 3).
The MMSE score did not significantly differ between the two groups at T0 (28.18 ± 1.20 vs 28.14 ± 1.25, P > 0.05). At T4, the propofol group exhibited a significantly lower MMSE score than the sevoflurane group (26.02 ± 1.18 vs 26.63 ± 1.30, P = 0.024). Furthermore, there was a significant decrease in the MMSE scores at T4 in both groups compared to those at T0 (26.02 ± 1.18 vs 28.18 ± 1.20, P < 0.001; 26.63 ± 1.30 vs 28.14 ± 1.25, P = 0.046).
No statistically significant differences were observed between the two groups in terms of the SAS and SDS scores at T0 (P > 0.05). However, after treatment, both groups showed a significant increase in SAS and SDS scores (P < 0.05). Notably, at T4, the sevoflurane group exhibited significantly lower SAS and SDS scores than the propofol group (P < 0.05) (Table 3).
Radical resection is the standard treatment for early- and mid-stage gastric cancer[12]. It effectively removes tumor tissue and clears metastatic lymph nodes. However, it also triggers nonspecific reactions in the body, leading to hemodynamic fluctuations, organ dysfunction, and other complications in patients[13]. Postoperative dysfunctions commonly include cognitive dysfunction, anxiety, depression, and other negative emotions. The mechanisms behind these dysfunctions are not fully understood, but they are mainly related to the stress response, hypoxemia, and neuronal damage[14]. Clinical manifestations include impaired perception, memory, and thinking, which, in severe cases, may increase the risk of long-term mortality[14].
Anesthesia directly affects the central nervous system and has a direct impact on the occurrence of postoperative cognitive dysfunction[15]. Therefore, selecting appropriate anesthetics can effectively improve postoperative cognitive function and reduce cognitive dysfunction and negative emotions. Propofol, the most widely used intravenous anesthetic, activates the γ-aminobutyric acid receptor-chloride ionophore complex and accelerates the desensitization of γ-aminobutyric acid receptors[15]. This mechanism exerts sedative effects with relatively low accumulation risk[16]. Previous studies have shown that propofol stabilizes the mitochondrial membrane potential, improves the expression of antioxidant proteins, and protects damaged neurons, suggesting a positive impact on brain function[17].
Propofol exerts its sedative effects through its action on the gamma-aminobutyric acid receptors. By activating the γ-aminobutyric acid receptor-chloride ionophore complex and accelerating the desensitization of γ-aminobutyric acid receptors, propofol enhances inhibitory neurotransmission in the central nervous system, ultimately leading to its sedative effects. Previous studies have shown that propofol can stabilize the mitochondrial membrane potential and improve the expression of antioxidant proteins, suggesting its potential impact on brain function and neuroprotection.
Nevertheless, alternative research studies have suggested that propofol has a higher propensity for inducing cognitive dysfunction than sevoflurane[18]. This may be due to the limited cerebral protective efficacy of propofol[19]. In this study, which included 80 patients undergoing radical resection of gastric cancer, the evaluation scale for cognitive function was the LOTCA. A cutoff score of 26 was used. Significantly lower LOTCA score at T4 was observed in the propofol group than in the sevoflurane group. This suggests that propofol is less effective in reducing cognitive dysfunction than sevoflurane. Moreover, the sevoflurane group exhibited significantly lower SAS and SDS scores than the propofol group. This finding suggests that sevoflurane has a greater capacity to alleviate postoperative anxiety and depression than propofol. These results may be due to the milder influence of sevoflurane on the central nervous system and its better ability to regulate emotions in patients.
Sevoflurane, on the other hand, is a novel inhalation anesthetic that offers unique advantages in terms of its pharmacophysiology. Sevoflurane is known for its stable physicochemical properties and has been shown to provide cerebral protection by guarding against neuroelectrophysiological alterations in the hippocampus. Unlike propofol, sevoflurane does not require intravenous injection, reducing the potential impact of drug solutions on body tissues and ensuring a more stable anesthetic effect. The primary absorption of sevoflurane through the lungs amplifies the body's antioxidant stress capacity and alleviates the physiological stress experienced by patients upon entering the bloodstream. These properties suggest that sevoflurane may have enhanced oxidation resistance and a protective effect on brain tissue[20]. This feature reduces the required anesthetic dosage, eliminates the potential impact of drug solutions on body tissues, and ensures a more stable anesthetic effect[21-23]. A study suggested that sevoflurane possesses stable physicochemical properties and can provide cerebral protection by guarding against neuroelectrophysiological alterations in the hippocampus[21]. Previous research has primarily concentrated on investigating the application of propofol as well as sevoflurane in elderly patients undergoing radical gastric cancer resection, neglecting an analysis of their application effects in gastric cancer patients of various ages[24,25]. Sun et al[26] discovered that sevoflurane can mitigate surgical-induced neurological damage and alleviate postoperative anxiety and depression, consequently enhancing patients' mental well-being. Furthermore, the study findings demonstrated a significantly lower Montreal Cognitive Assessment score in the propofol group than in the sevoflurane group at T4 (24.70 ± 2.28 vs 26.38 ± 1.70, P < 0.001). This observation reinforces the evident protective effect of sevoflurane on brain tissue. Due to its primary absorption through the lungs, sevoflurane amplifies the body's antioxidant stress capacity and alleviates the physiological stress experienced by patients upon entering the bloodstream[27]. Notably, the sevoflurane group showed greater stability in HR and MAP than the propofol group. At T1, T2, and T3, the propofol group exhibited significantly higher MAP and MDA levels (P < 0.001) and lower SOD levels (P < 0.001) than the sevoflurane group. SOD acts as a critical scavenger of free radicals, maintaining the body's metabolic balance and exerting antioxidant and anti-inflammatory effects. On the other hand, MDA, a primary product of membrane lipid peroxidation, reflects the presence of free radicals and lipid peroxidation levels in tissues. Both of these measurements underscore sevoflurane's role in enhancing oxidation resistance and ameliorating stress, indicating its ability to protect tissue function and mitigate the adverse effects of surgical trauma on tissues by stabilizing hemodynamic indicators and attenuating oxidative stress.
In addition to its cerebral protective properties, sevoflurane also exhibits a safeguarding effect on pulmonary function[28,29]. This investigation revealed that the sevoflurane group had significantly better pulmonary function than the propofol group. Specifically, A-aDO2 and RI serve as key indicators for assessing lung function and quantifying the extent of damage, while Qs/Qt indirectly reflects the ventilation-perfusion ratio, enabling the analysis of lung injury. These findings demonstrate that sevoflurane anesthesia offers a robust protective effect on both brain and lung function, thereby addressing the need for perioperative organ function preservation and facilitating improved postoperative recovery conditions for patients, assuming that other factors remain relatively constant. Nevertheless, it is important to note that prior studies primarily focused on elderly patients, while the present study encompassed patients of diverse age groups without specific age stratification. Consequently, the obtained results may be subject to sample size limitations and influenced by other factors, necessitating further research for verification.
Our study possesses several strong points, including a comprehensive data analysis that rigorously compared the effects of propofol and sevoflurane anesthesia on cognitive function, anxiety, and depression in patients undergoing radical resection of gastric cancer. By considering comprehensive data from both groups, we provided a thorough assessment of the impact of these anesthetic agents. The findings of our study have important clinical relevance, as they contribute valuable insights into anesthesia selection and management strategies for optimizing patient outcomes and promoting postoperative recovery.
These study findings have important implications for clinical practice. Anesthesia selection plays a crucial role in perioperative care and can impact patient recovery and overall outcomes. Clinicians should consider the specific needs and conditions of individual patients when choosing between propofol and sevoflurane anesthesia for radical resection of gastric cancer. Patients with preexisting pulmonary conditions or significant risk factors for postoperative pulmonary complications may benefit from propofol anesthesia, which has been shown to have minimal impact on pulmonary function. On the other hand, patients with concerns regarding cognitive function and emotional well-being may be better served by sevoflurane anesthesia, as it showed a milder influence on these factors.
Furthermore, these findings highlight the importance of a multidisciplinary approach in perioperative care. Collaboration between anesthesiologists, surgeons, and other healthcare professionals is necessary to optimize anesthesia selection and tailor the care plan to the individual patient's needs. Future research should focus on refining anesthesia protocols, investigating the long-term outcomes of propofol and sevoflurane anesthesia, and exploring the potential benefits of combining these agents to further enhance patient outcomes.
However, it is important to acknowledge the limitations of our study. First, the sample size of 80 patients might be relatively small, limiting the generalizability of the findings. Future studies with larger sample sizes are warranted to further validate the results. Second, the age distribution of the patient population was not specifically analyzed, which could have introduced confounding effects. Age-related differences in cognitive function and response to anesthesia should be explored in future studies by stratifying the patient population according to age groups. Third, the duration of anesthesia and surgery for the included patients was not reported, potentially impacting the observed cognitive and emotional outcomes. Longer durations of anesthesia and surgery have been associated with increased postoperative complications, including cognitive dysfunction. Hence, it is important to assess the influence of anesthesia duration on the study outcomes in future investigations. Fourth, the differences in administration routes and pharmacokinetics of propofol and sevoflurane should be considered. The intravenous administration of propofol and inhalation administration of sevoflurane may have contributed to the divergent effects on cognitive function and emotional outcomes observed in this study. Further research is needed to elucidate the underlying mechanisms associated with these different administration routes. Lastly, it is crucial to acknowledge that other perioperative factors, such as preoperative anxiety, pain management strategies, and postoperative care protocols, may have influenced the outcomes. Future studies should aim to comprehensively analyze the impact of these factors to provide a more holistic understanding of the effects of propofol and sevoflurane anesthesia.
Both propofol and sevoflurane can be employed as maintenance anesthesia during radical resection of gastric cancer. Propofol anesthesia has minimal influence on patients' pulmonary function, thereby promoting their postoperative recovery. Sevoflurane minimally affects patients' cognitive function and negative emotions, leading to an improved postoperative mental state. Consequently, the selection of anesthetic agents should be based on individual patient considerations and specific circumstances.
Radical resection is the standard treatment for gastric cancer, but it can lead to cognitive dysfunction and negative emotions. The choice of anesthesia can impact these outcomes. The mechanisms behind postoperative dysfunctions are not fully understood but are related to stress response, hypoxemia, and neuronal damage. Previous research has shown conflicting results regarding the effects of propofol and sevoflurane anesthesia on cognitive function and emotions.
Understanding the effects of different anesthesia agents on cognitive function and emotions is crucial for improving postoperative outcomes and patient well-being. The selection of appropriate anesthesia agents can potentially reduce complications and improve recovery for patients undergoing radical resection for gastric cancer.
The aim of this study was to compare the effects of propofol and sevoflurane anesthesia on cognitive function, anxiety, and depression in patients undergoing radical resection of gastric cancer. The study also aimed to determine which anesthesia agent is more effective in reducing cognitive dysfunction and negative emotions in these patients.
This study included 80 patients undergoing radical resection of gastric cancer. Cognitive function was assessed using the Loewenstein occupational therapy cognitive assessment evaluation scale, while anxiety and depression were evaluated using the self-rating anxiety scale and self-rating depression scale, respectively. The patients were divided into a propofol group and a sevoflurane group based on the anesthesia agent used. Statistical analyses were performed to compare the outcomes between the two groups.
The study found that both propofol and sevoflurane anesthesia significantly decreased cognitive function after treatment. However, the propofol group had a lower cognitive function score at T4 compared to the sevoflurane group. Additionally, the sevoflurane group had lower scores for anxiety and depression compared to the propofol group. These results suggest that sevoflurane anesthesia may have a greater capacity to alleviate cognitive dysfunction and negative emotions in gastric cancer patients.
In conclusion, both propofol and sevoflurane can be used as maintenance anesthesia during radical resection of gastric cancer. Propofol anesthesia has minimal influence on pulmonary function, promoting postoperative recovery. Sevoflurane minimally affects cognitive function and negative emotions, leading to an improved postoperative mental state. The choice of anesthesia agents should be based on individual patient considerations and specific circumstances.
Further research with a larger sample size is needed to verify the results of the present study and to explore the effects of anesthesia agents in different age groups. Future studies should also investigate the underlying mechanisms behind the effects of anesthesia on cognitive function and emotions. Additionally, exploring other potential factors that can impact postoperative outcomes and recovery in gastric cancer patients would be valuable.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Kops MS, Germany; Samarghandian S, Iran S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Zhang XD
| 1. | Huang W, Jiang Y, Xiong W, Sun Z, Chen C, Yuan Q, Zhou K, Han Z, Feng H, Chen H, Liang X, Yu S, Hu Y, Yu J, Chen Y, Zhao L, Liu H, Zhou Z, Wang W, Xu Y, Li G. Noninvasive imaging of the tumor immune microenvironment correlates with response to immunotherapy in gastric cancer. Nat Commun. 2022;13:5095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Li T, Liu G, Li J, Cui J, Wang X, Li W, Zhao Z, Zhang K, Liu T. Gastric tumorigenesis after radical resection combined with adjuvant chemotherapy for colorectal cancer: two case reports and a literature review. J Int Med Res. 2021;49:3000605211007050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Qin W, Shang J, Cui H, Chang Z, Yang S. Solitary metachronous adrenal metastasis after radical resection of gastric large cell neuroendocrine carcinoma: a case report and literature review. J Int Med Res. 2023;51:3000605231163709. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Li MJ, Wei J, Xu GH, Liu Y, Zhu J. Surgery Combined with Molecular Targeted Therapy Successfully Treated Giant Esophageal Gastrointestinal Stromal Tumor. Oncologie. 2022;24:349-356. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Liu J, Yang L. Effects of propofol and sevoflurane on blood glucose, hemodynamics, and inflammatory factors of patients with type 2 diabetes mellitus and gastric cancer. Oncol Lett. 2020;19:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ai L, Wang H. Effects of propofol and sevoflurane on tumor killing activity of peripheral blood natural killer cells in patients with gastric cancer. J Int Med Res. 2020;48:300060520904861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Shan GJ, Zhang YX, Cao SJ, Zhu SN, Li HJ, Ma D, Wang DX; First Study of Perioperative Organ Protection (SPOP1) investigators. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth. 2018;121:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 8. | Belrose JC, Noppens RR. Anesthesiology and cognitive impairment: a narrative review of current clinical literature. BMC Anesthesiol. 2019;19:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 9. | O'Bryan LJ, Atkins KJ, Lipszyc A, Scott DA, Silbert BS, Evered LA. Inflammatory Biomarker Levels After Propofol or Sevoflurane Anesthesia: A Meta-analysis. Anesth Analg. 2022;134:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Mei X, Zheng HL, Li C, Ma X, Zheng H, Marcantonio E, Xie Z, Shen Y. The Effects of Propofol and Sevoflurane on Postoperative Delirium in Older Patients: A Randomized Clinical Trial Study. J Alzheimers Dis. 2020;76:1627-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Oh CS, Park HJ, Piao L, Sohn KM, Koh SE, Hwang DY, Kim SH. Expression Profiles of Immune Cells after Propofol or Sevoflurane Anesthesia for Colorectal Cancer Surgery: A Prospective Double-blind Randomized Trial. Anesthesiology. 2022;136:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1336] [Article Influence: 334.0] [Reference Citation Analysis (2)] |
| 13. | Yang Y, Hu J, Ma Y, Chen G, Liu Y. Multivisceral resection for locally advanced gastric cancer: A retrospective study. Am J Surg. 2021;221:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Kanda M. Preoperative predictors of postoperative complications after gastric cancer resection. Surg Today. 2020;50:3-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Evered LA, Silbert BS. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth Analg. 2018;127:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 282] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Choi JY, Lee HS, Kim JY, Han DW, Yang JY, Kim MJ, Song Y. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J Clin Anesth. 2022;82:110955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 17. | Zhong H, Song R, Pang Q, Liu Y, Zhuang J, Chen Y, Hu J, Liu Z, Tang J. Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell Death Dis. 2018;9:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Sun X, Zhang J, Li H, Zhang Z, Yang J, Cui M, Zeng B, Xu T, Cao J, Xu L. Propofol effects on excitatory synaptic efficacy in the CA1 region of the developing hippocampus. Brain Res Dev Brain Res. 2005;157:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Mikkelsen MLG, Ambrus R, Rasmussen R, Miles JE, Poulsen HH, Moltke FB, Eriksen T. The influence of norepinephrine and phenylephrine on cerebral perfusion and oxygenation during propofol-remifentanil and propofol-remifentanil-dexmedetomidine anaesthesia in piglets. Acta Vet Scand. 2018;60:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Sun M, Xie Z, Zhang J, Leng Y. Mechanistic insight into sevoflurane-associated developmental neurotoxicity. Cell Biol Toxicol. 2022;38:927-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 21. | Jafari-Sales A, Shariat A, Baghi HB, Baradaran B, Jafari B. The Presence of Human Papillomavirus and Epstein-Barr Virus Infection in Gastric Cancer: A Systematic Study. Oncologie. 2022;24:413-426. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Franzén S, Semenas E, Taavo M, Mårtensson J, Larsson A, Frithiof R. Renal function during sevoflurane or total intravenous propofol anaesthesia: a single-centre parallel randomised controlled study. Br J Anaesth. 2022;128:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 23. | Liang TY, Peng SY, Ma M, Li HY, Wang Z, Chen G. Protective effects of sevoflurane in cerebral ischemia reperfusion injury: a narrative review. Med Gas Res. 2021;11:152-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Piwowarczyk P, Rypulak E, Sysiak-Sławecka J, Nieoczym D, Socała K, Wlaź A, Wlaź P, Turski W, Czuczwar M, Borys M. Propofol and Sevoflurane Anesthesia in Early Childhood Do Not Influence Seizure Threshold in Adult Rats. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | van Limmen J, Wyffels P, Berrevoet F, Vanlander A, Coeman L, Wouters P, De Hert S, De Baerdemaeker L. Effects of propofol and sevoflurane on hepatic blood flow: a randomized controlled trial. BMC Anesthesiol. 2020;20:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Sun H, Zhang G, Ai B, Zhang H, Kong X, Lee WT, Zheng H, Yan T, Sun L. A systematic review: comparative analysis of the effects of propofol and sevoflurane on postoperative cognitive function in elderly patients with lung cancer. BMC Cancer. 2019;19:1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Chen LL, Lu L, Gong XL, Xu YC, Chu XY, Huang GC. Gastric Cancer with Bone Marrow Invasion and Disseminated Intravascular Coagulation: A Case Report. Oncologie. 2022;24:599-604. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Zheng F, Wu X, Zhang J, Fu Z, Zhang Y. Sevoflurane reduces lipopolysaccharide-induced apoptosis and pulmonary fibrosis in the RAW264.7 cells and mice models to ameliorate acute lung injury by eliminating oxidative damages. Redox Rep. 2022;27:139-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 29. | Fu Z, Wu X, Zheng F, Zhang Y. Sevoflurane anesthesia ameliorates LPS-induced acute lung injury (ALI) by modulating a novel LncRNA LINC00839/miR-223/NLRP3 axis. BMC Pulm Med. 2022;22:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |