Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.214
Peer-review started: September 19, 2023
First decision: October 9, 2023
Revised: October 20, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: January 15, 2024
Processing time: 113 Days and 22.1 Hours
The effectiveness of neoadjuvant therapy in esophageal cancer (EC) treatment is still a subject of debate.
To compare the clinical efficacy and toxic side effects between neoadjuvant chemoradiotherapy (nCRT) and neoadjuvant chemotherapy (nCT) for locally advanced EC (LAEC).
A comprehensive search was conducted using multiple databases, including PubMed, EMBASE, MEDLINE, Science Direct, The Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, Chinese Science and Technology Journal Database, and Chinese Biomedical Literature Database Article. Studies up to December 2022 comparing nCRT and nCT in patients with EC were selected.
The analysis revealed significant differences between nCRT and nCT in terms of disease-free survival. The results indicated that nCRT provided better outcomes in terms of the 3-year overall survival rate (OSR) [odds ratio (OR) = 0.95], complete response rate (OR = 3.15), and R0 clearance rate (CR) (OR = 2.25). However, nCT demonstrated a better 5-year OSR (OR = 1.02) than nCRT. Moreover, when compared to nCRT, nCT showed reduced risks of cardiac complications (OR = 1.15) and pulmonary complications (OR = 1.30).
Overall, both nCRT and nCT were effective in terms of survival outcomes for LAEC. However, nCT exhibited better performance in terms of postoperative complications.
Core Tip: Neoadjuvant chemoradiotherapy (nCRT) significantly improves the overall survival rate, pathological complete response rate, and R0 clearance rate for esophageal cancer. However, neoadjuvant chemotherapy (nCT) offers advantages in reducing postoperative cardiopulmonary complications and perioperative mortality. Esophageal squamous cell carcinoma patients benefit more from nCRT in terms of survival rates. The choice between nCRT and nCT should consider the patient's individual conditions and sensitivity to radiotherapy and chemotherapy. Careful consideration is necessary to achieve optimal long-term survival outcomes while minimizing complications.
- Citation: Yuan MX, Cai QG, Zhang ZY, Zhou JZ, Lan CY, Lin JB. Application of neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy in curative surgery for esophageal cancer: A meta-analysis. World J Gastrointest Oncol 2024; 16(1): 214-233
- URL: https://www.wjgnet.com/1948-5204/full/v16/i1/214.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i1.214
Esophageal cancer (EC) is one of the eight most common malignant tumors worldwide, ranking sixth in terms of incidence and exhibiting a low survival rate alongside high invasiveness[1]. In China, the incidence and mortality rates of EC rank sixth and fourth globally, with adenocarcinoma and squamous cell carcinoma accounting for over 95% of cases[1]. Early stages of EC often lack noticeable symptoms, and patients may only experience mild dysphagia or significant unexplained weight loss, leading to underdiagnosis of locally advanced disease or distant metastases in more than two-thirds of cases. Precancerous lesions and early EC are generally addressed through endoscopic procedures or simple surgeries[2]. While simple surgical interventions are suitable for early EC, patients with locally advanced disease have an average five-year overall survival rate (OSR) of just 25% and a bleak prognosis. Notably, surgical treatment alone often results in high local recurrence rates and increased distant metastasis, particularly when cervical or mediastinal lymph nodes are involved, with a local failure rate of up to 60%[3]. Consequently, a simple surgical approach does not suffice for locally advanced EC (LAEC), necessitating a multimodal combination approach for improved outcomes.
With prolonged survival among tumor patients, the side effects of treatment become more apparent. Domestic and international studies have substantiated the superior prognosis associated with preoperative neoadjuvant therapy for advanced EC, which encompasses preoperative chemotherapy and preoperative radiochemotherapy, compared to traditional postoperative radiochemotherapy[4]. Specifically, neoadjuvant chemoradiotherapy (nCRT) has demonstrated long-term survival advantages over surgical treatment alone and included triple therapy as the standard treatment[5]. In the case of LAEC, nCRT enhances efficacy and prognostic outcomes[6]. Another neoadjuvant therapy modality is neoadjuvant chemotherapy (nCT), which, when combined with surgery, significantly improves survival rates for patients with operable EC compared to surgery alone[7].
This study was designed to comprehensively investigate the efficacy and safety of these two neoadjuvant therapies in EC patients by conducting a meta-analysis of relevant data. The findings will provide an evidence-based medical basis for the treatment of EC patients.
PubMed, EMBASE, MEDLINE, Science Direct, The Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, Chinese Science and Technology Journal Database, and Chinese Biomedical Literature Database were searched for published studies on the efficacy of nCRT vs nCT in EC patients from inception to December 1, 2022. The search keywords included "esophageal or esophageal or gastro-esophageal junction" and "cancer or carcinoma or neoplasm" and "neoadjuvant or preoperative or induction" and "chemoradiotherapy or radiotherapy or radiation" and "chemotherapy". The search strategies were determined after multiple studies, and professional journals were searched manually to avoid omissions. In addition, the research objects of literature retrieval were all human. The search process used the combination of subject words and free words for multiple searches to obtain the references that could be included and then used the search engine to track down each document. The literature quality was assessed using RevMan 5.3.
Criteria for enrolling related literature: (1) There was a clear pathological diagnosis; (2) Prospective randomized controlled trials, case control study and cohort study, which were divided into two groups: nCRT and nCT; (3) Advanced radiotherapy techniques were used: three-dimensional conformal or IMRT; and (4) The primary study endpoints were included. Criteria for excluding related literature: (1) The patients were found to be conjunction with other therapeutic interventions; (2) The patients suffered from severe aphasia or cognitive impairment; (3) The baseline was not comparable or the baseline was not reported; (4) We were unable to extract valid outcome data from the literature, or unable to obtain the full text after contacting the author; (5) The studies were poorly designed or involved incorrect statistical methods; (6) The articles with undefined diagnostic criteria and duration of intervention; and (7) The articles which were case reports, protocols, conference abstracts, animal experiments and reviews, etc.
Two professionals used unified Microsoft Excel to screen the relevant literature independently and extract the data in strict accordance with the included and excluded annotations. They cross-checked the inclusion of the results and discussed the differences to obtain agreement. Data extraction mainly included the nationality and name of the first author, publication year of the literature, study time, number of patients in the nCT group and the nCT radiotherapy group, tissue type and percentage, stage, radiotherapy technique, radiotherapy dose, target range, and chemotherapy regimen. Primary and secondary endpoints were extracted, including overall survival, progression-free survival, R0 clearance rates (CR), p CR, postoperative mortality, and cardiopulmonary complications.
The Cochrane risk bias assessment tool was used to assess the risk of bias in data from randomized controlled studies included in the literature. First, two researchers independently assessed the bias risk of each literature study, and then a third researcher was added. A total of three researchers finally resolved the dispute through discussion. The sources of bias included: (1) Selection bias; (2) Implementation bias; (3) Tracking bias; (4) Measurement bias; and (5) Reporting bias and other biases. Deviation risk was divided into low risk, unclear risk, and high risk. The Newcastle Ottawa Scale (NOS) was used to assess the risk of bias in data from case-control studies (CCSs) and cohort studies. Three aspects were used to evaluate the risk of bias in CCSs and cohort studies: (1) Selection of study population; (2) Comparability of study groups; and (3) The result of measurement. The total score was 9 points. If the final score was greater than or equal to 7 points, the risk of bias was low; results with a score of 4 to 6 had a moderate risk of bias; and studies that end up with a score of less than 4 had a high risk of bias.
RevMan5.3 and Stata software were employed. The mean difference was selected as an indicator of the effect of the continuity variable. Each effect indicator gave a point estimate and a 95% confidence interval (95%CI). Heterogeneity was analyzed by the X2 test (test level = 0.1) and quantitatively determined by I2. P ≥ 0 and I2 ≤ 50% indicated no obvious heterogeneity, and a fixed effects model could be adopted for analysis. If P < 0.05 and I2 > 50%, the heterogeneity was remarkable, and a random effects model was utilized. In addition, subgroup analyses were utilized to explore the possible sources of heterogeneity. The meta-analysis test level was set at = 0.05. The forest plot and asymmetric linear regression funnel plot are given. Funnel plots of different treatment indices were utilized to test publication bias and analyze it.
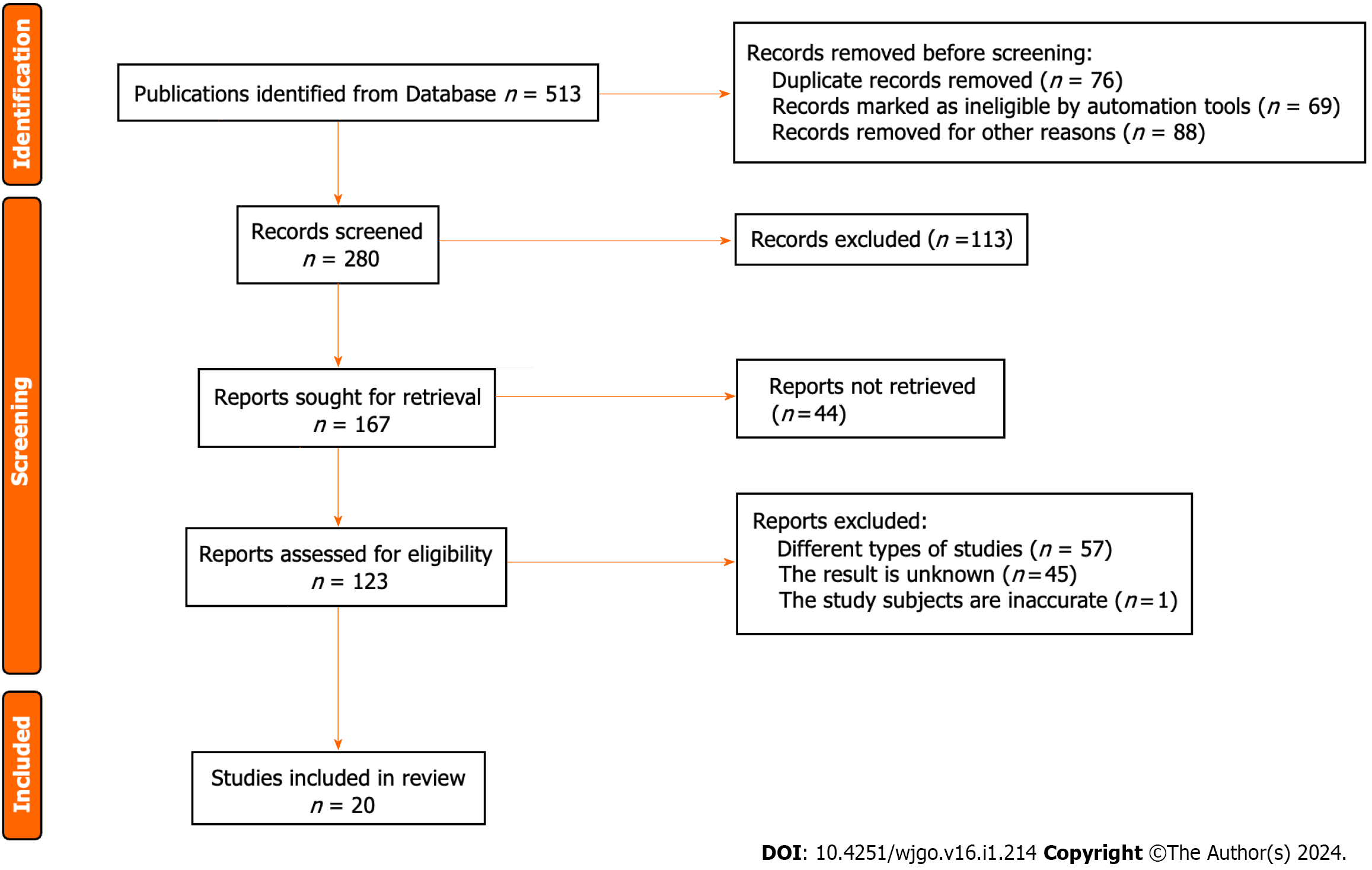
A total of 513 Literature sources were initially obtained from the aforementioned database search. First, 76 duplicate publications, 69 irrelevant studies, and 88 articles with other reasons for exclusion were removed, resulting in a preliminary selection of 280 sources. Through the assessment of abstracts and titles, 113 articles were excluded, leaving 167 articles for further evaluation. Following the exclusion of 44 research reports and review articles, 123 articles remained. The full text of all the remaining articles was thoroughly reviewed, leading to the exclusion of 57 studies that focused on other types of research. Moreover, 45 articles were excluded due to incomplete or unobtainable treatment results. One publication included nonhuman subjects, leaving 20 articles that met the criteria for inclusion in this study. The retrieval process for the relevant literature is depicted in Figure 1.
The relevant information from the literature was extracted upon thorough reading. The 20 included publications had varying sample sizes, ranging from 38 to 7388 participants. Specifically, three papers[8-10] utilized randomized controlled trials (RCTs), while the remaining 17 papers[11-27] employed CCSs and cohort studies. These papers provided detailed descriptions of the treatment process involving nCRT and nCT, documenting the changes in patients before and after treatment. The specific characteristics of the RCTs can be found in Table 1, while those of the CCSs and cohort studies are presented in Table 2.
| Ref. | Case | TNM |
| Anderegg et al[11], 2017 | 313 | Stage I-III |
| Esophageal Cancer Study Group Participating Centers[12], 2019 | 268 | Stage I-III |
| Gawad et al[13], 2015 | 370 | Stage II-IV |
| Markar et al[14], 2017 | 608 | Stage II-III |
| Tian et al[15], 2020 | 827 | Stage I-IV |
| Nakashima[16], 2018 | 120 | Stage II-IV |
| Park et al[17], 2020 | 38 | Stage II-III |
| Pucher et al[18], 2020 | 284 | Stage I-III |
| Samson et al[19], 2016 | 7388 | Stage I-III |
| Spicer et al[20], 2016 | 214 | Stage IIIA |
| Stiles et al[21], 2019 | 338 | Stage I-IV |
| Swisher et al[22], 2010 | 157 | Stage I-III |
| Tang et al[23], 2023 | 264 | Stage II-IV |
| Tercioti et al[24], 2011 | 97 | Stage I-III |
| Tiesi et al[25], 2017 | 297 | Stage II-IV |
| Jiang et al[26], 2022 | 299 | Stage II-III |
| Zhou et al[27], 2020 | 423 | NA |
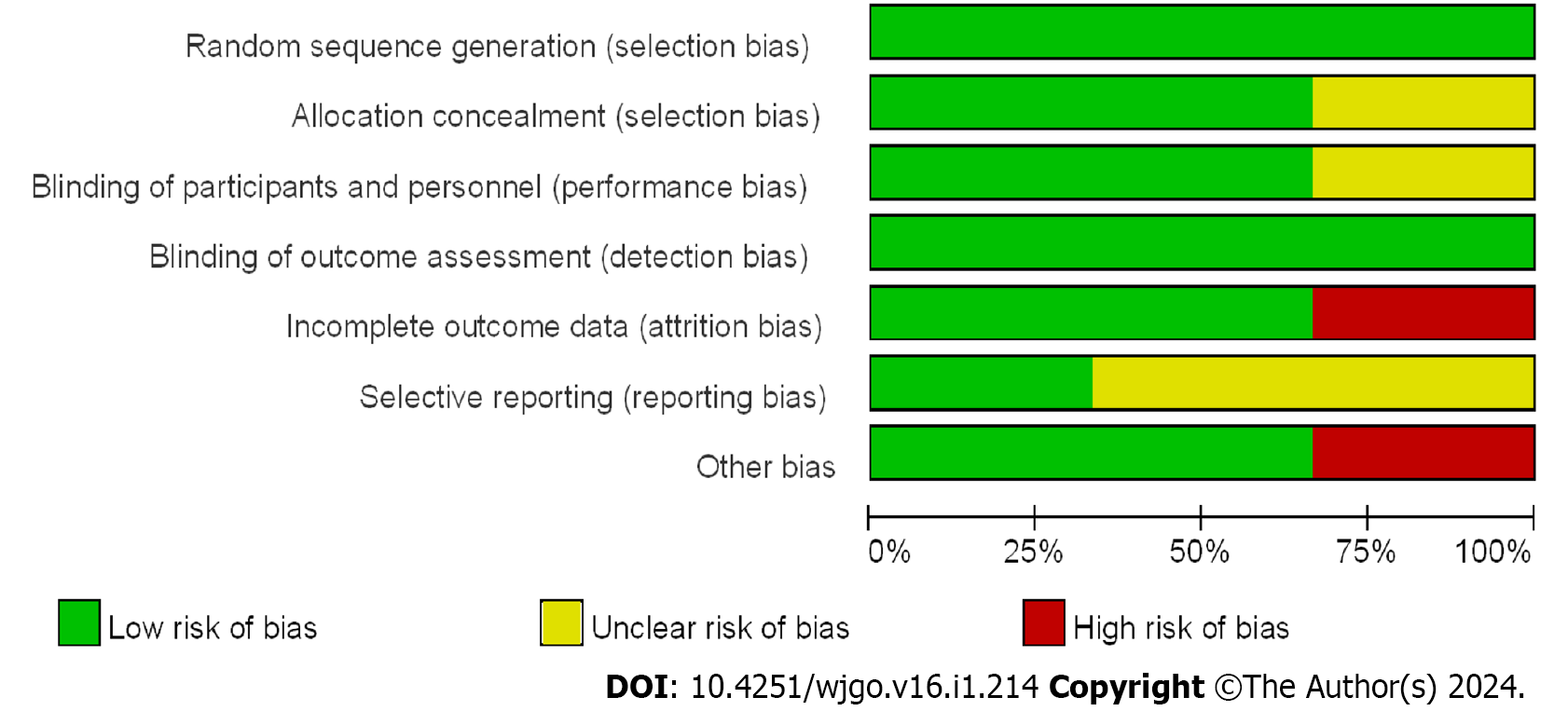
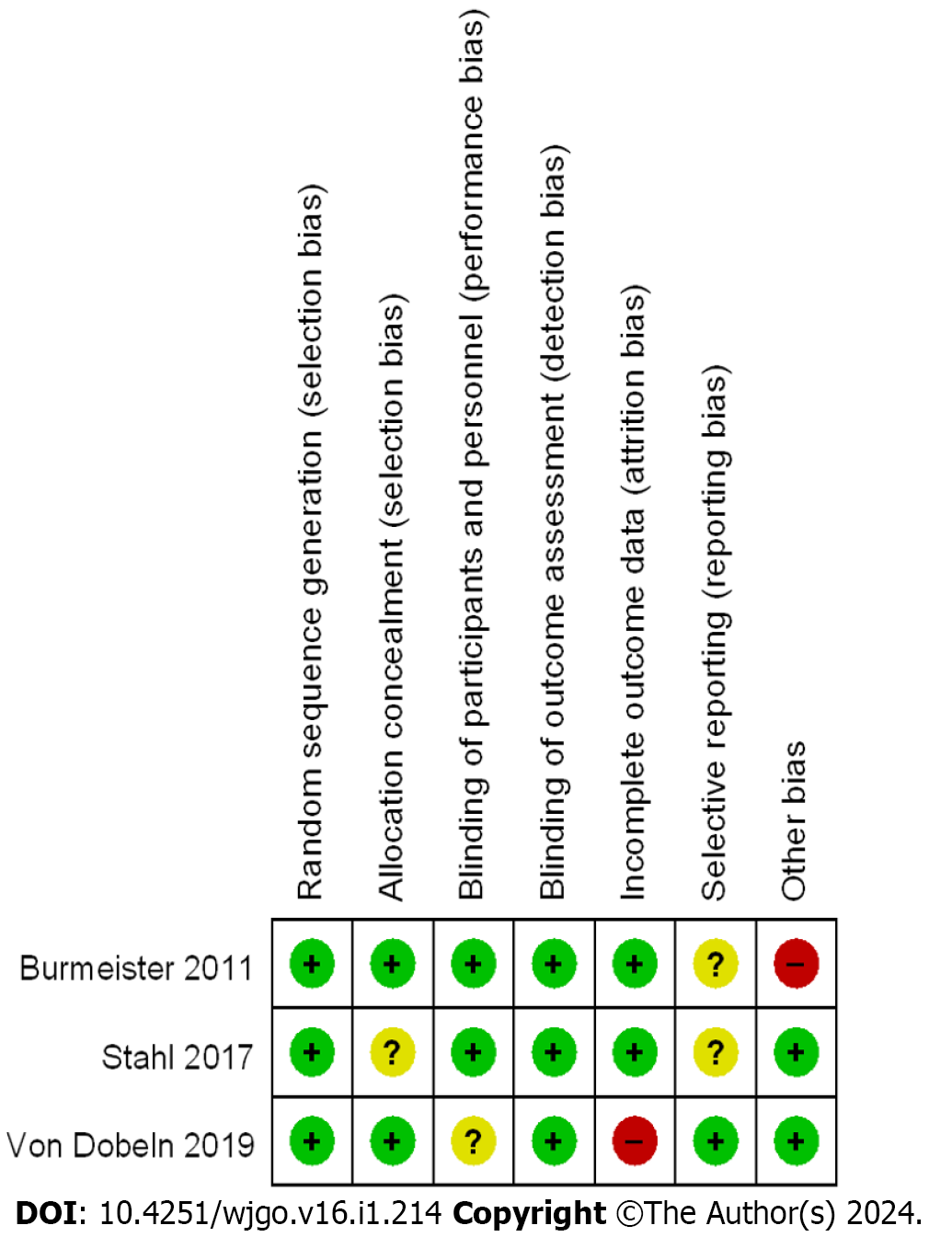
For the assessment of bias risk, the Cochrane risk Bias Assessment tool table was implemented in the analysis of RCTs. Among the three RCTs included in this study, low bias was observed. The NOS was employed to evaluate the bias risk in the CCSs and cohort studies. All 17 studies were found to have evaluation scores greater than 5 points, with 6 categorized as having a moderate bias risk and 11 classified as having a low bias risk. The risk bias evaluation charts for the RCTs are presented in Figure 2, while the summary charts of the risk bias are illustrated in Figure 3. A comprehensive quality evaluation of the CCSs and cohort studies can be found in Table 3.
| Ref. | Research type | Study endpoint | NOS scoring |
| Anderegg et al[11], 2017 | CCS | 1-9 | 7 |
| Esophageal Cancer Study Group Participating Centers[12], 2019 | CCS | 3, 4, and 6 | 7 |
| Gawad et al[13], 2015 | CCS | 4 | 6 |
| Markar et al[14], 2017 | CCS | 3, 5, 7, 8, and 9 | 7 |
| Tian et al[15], 2020 | CCS | 3, 4, 5, and 7 | 7 |
| Nakashima[16], 2018 | CCS | 3-8 | 7 |
| Park et al[17], 2020 | CCS | 5, 7, 8, 9 | 6 |
| Pucher et al[18], 2020 | CCS | 1-7 and 9 | 6 |
| Samson et al[19], 2016 | CCS | 5, 6, and 7 | 6 |
| Spicer et al[20], 2016 | CCS | 1-5, 7, 8, and 9 | 7 |
| Stiles et al[21], 2019 | CCS | 1-4, 6-9 | 7 |
| Swisher et al[22], 2010 | CCS | 3, 4, and 6-9 | 7 |
| Tang et al[23], 2023 | CCS | 5 | 6 |
| Tercioti et al[24], 2011 | CCS | 6, 7, and 9 | 6 |
| Tiesi et al[25], 2017 | CCS | 3-8 | 7 |
| Jiang et al[26], 2022 | CCS | 4 | 7 |
| Zhou et al[27], 2020 | CS | 3 and 4 | 7 |
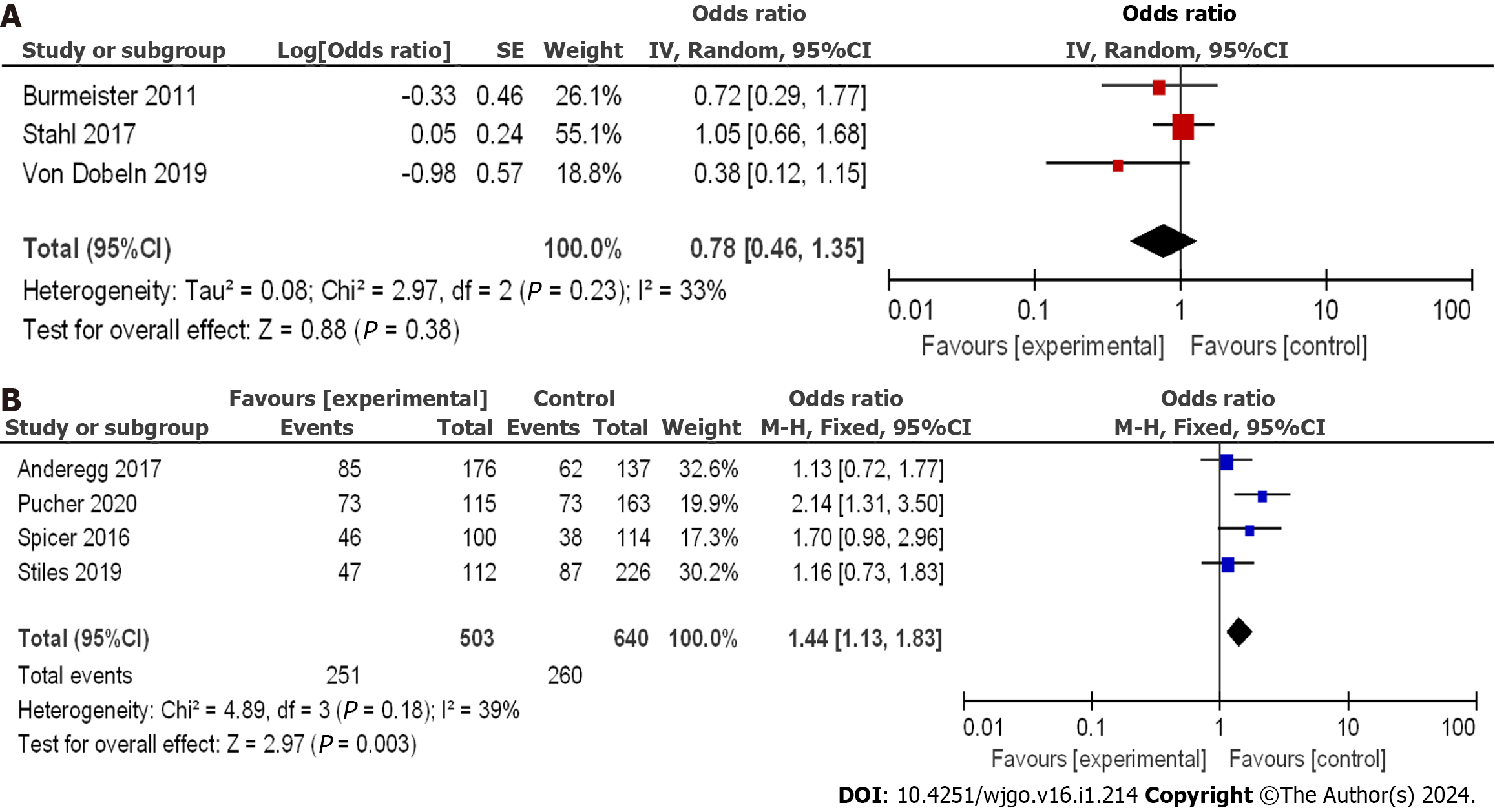
Using the OR as a clinical outcome indicator as depicted in Figure 4A, the OR scores of the three included RCTs were 0.78 with a 95%CI of 0.46 to 1.35. The P value was 0.23, and the I2 statistic indicated a low heterogeneity of 33%. The odds ratio (OR) values suggested no significant difference in the 3-year disease-free survival (DFS) among the various studies, with low heterogeneity observed. The lowest and highest OR values were 0.38 and 1.05, respectively, and their corresponding 95%CI values were 0.12 to 1.15 and 0.66 to 1.68, respectively.
The literature included a total of 1143 patients with EC, with 503 patients in the nCRT group and 640 patients in the nCT group. Using the OR as a clinical outcome indicator as illustrated in Figure 4B, the aggregated OR score from four CCSs was 1.44, with a 95%CI of 1.13 to 1.83. The corresponding p value was 0.18, and the I2 statistic indicated a heterogeneity of 39%. The OR values indicated no significant difference in the 3-year DFS among the different studies, with low heterogeneity observed. The lowest and highest OR values were 1.13 and 2.14, respectively, with 95%CI values of 0.72 to 1.77 and 1.31 to 3.50, respectively.
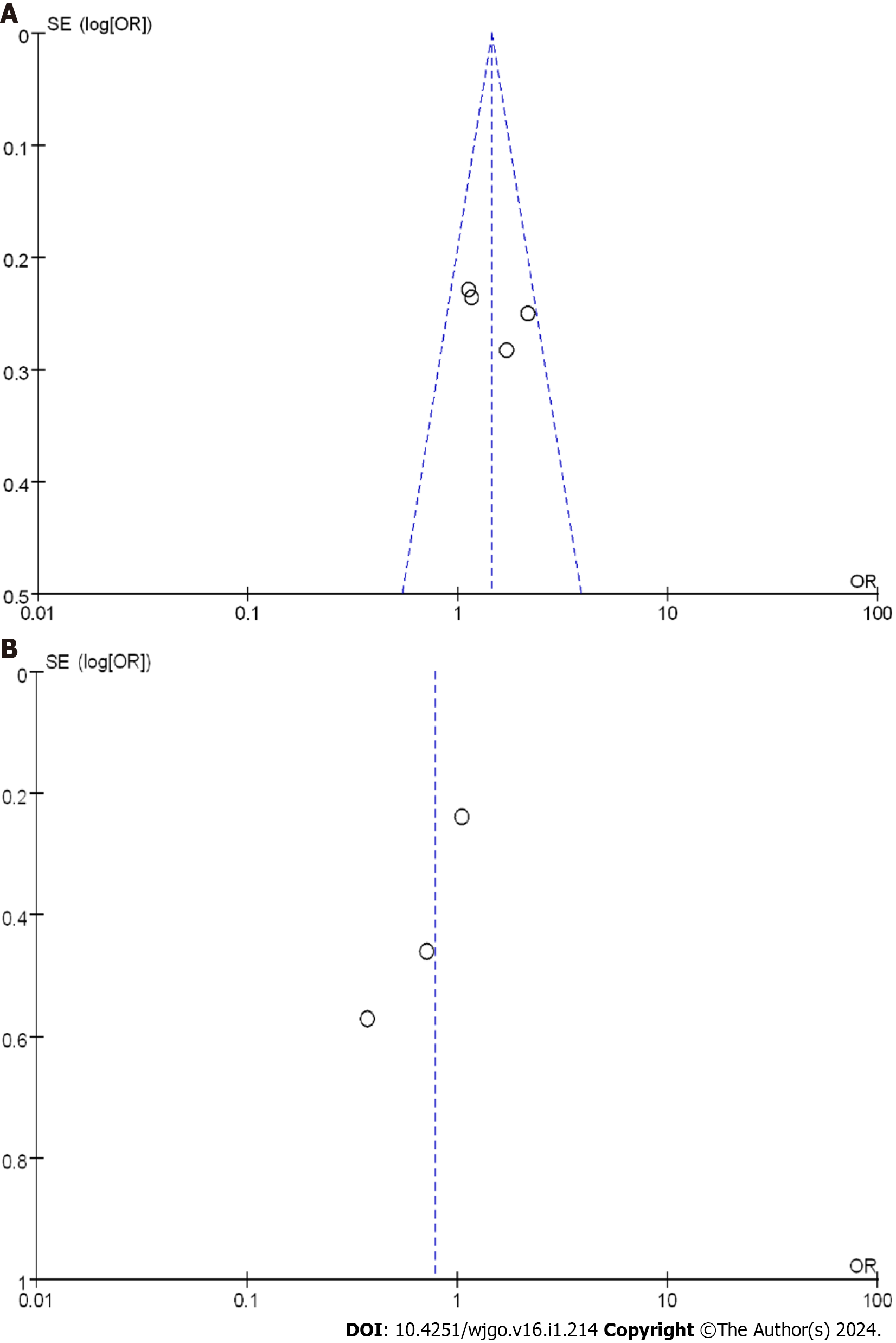
A comprehensive analysis of the 3-year DFS after treatment was performed to provide a better understanding of treatment efficacy. Funnel plots for the two types of research are displayed in Figure 5. These plots indicated that all studies demonstrated minimal bias risk and no research deviation. Based on these findings, both nCRT and nCT showed improvements in the 3-year DFS. In RCTs, nCT had a superior effect compared to nCRT, while in CCSs, the effect was reversed.
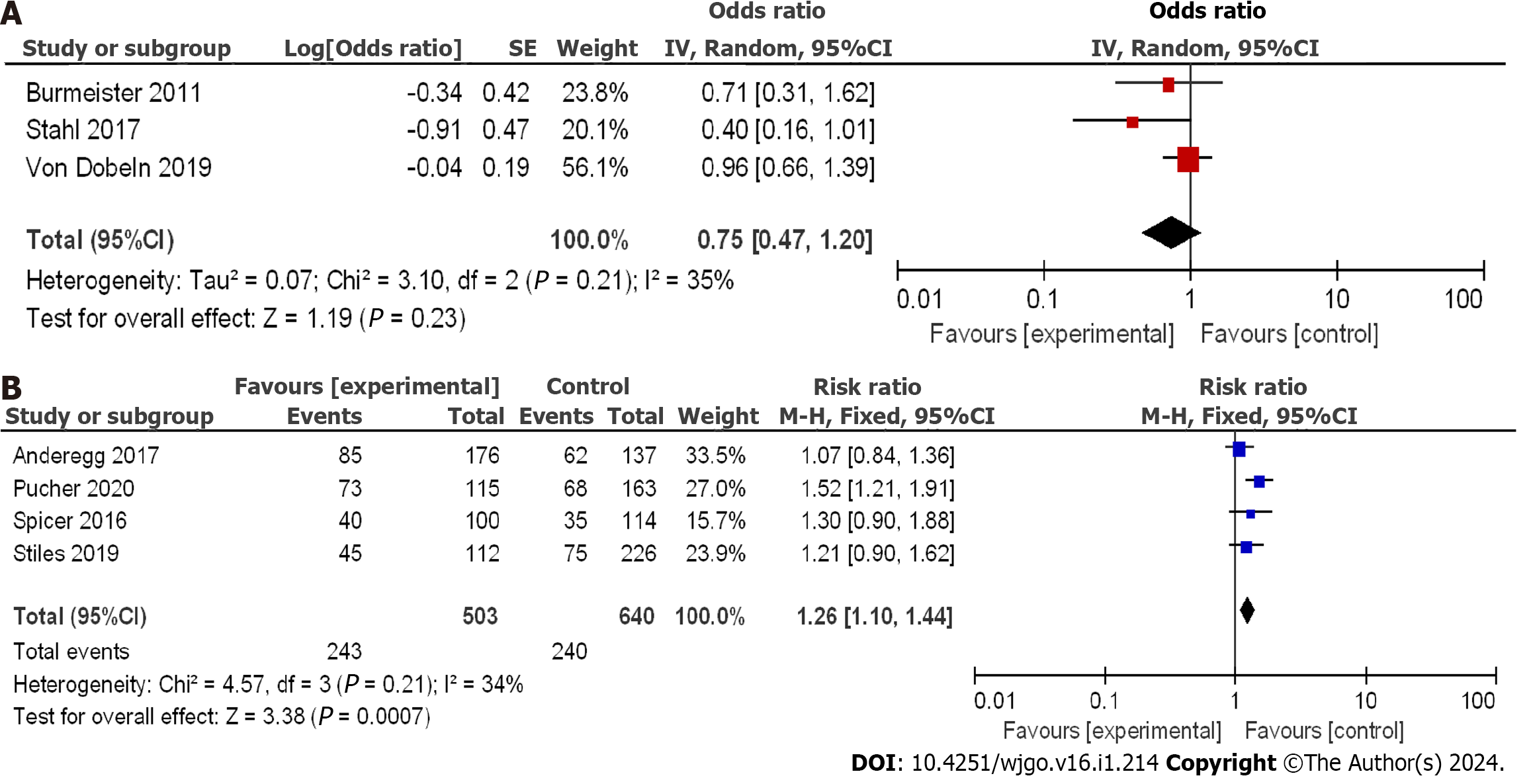
Using the OR as an indicator of clinical outcome, displayed in Figure 6A, the aggregated OR score from three RCTs was 0.75, with a 95%CI of 0.47 to 1.20. The corresponding P value was 0.21, and the I2 statistic indicated a heterogeneity of 35%. The OR values suggested no substantial difference in the 5-year DFS among the studies, with low heterogeneity observed. The lowest and highest OR values were 0.40 and 0.96, respectively, with 95%CI values of 0.16 to 1.01 and 0.66 to 1.39, respectively.
The mentioned studies included a total of 1143 patients with EC, with 503 patients in the nCRT group and 640 patients in the nCT group. Taking OR as a clinical outcome indicator as depicted in Figure 6B, the aggregated OR score from four CCSs was 1.26, with a 95%CI of 1.10 to 1.44. The corresponding P value was 0.21, and the I2 statistic indicated a heterogeneity of 34%. The OR values suggested that the difference in 5-year DFS was not significant, indicating low heterogeneity. The lowest and highest OR values were 1.07 and 1.52, respectively, with 95%CI values of 0.84 to 1.36 and 1.21 to 1.91, respectively.
A systematic analysis of the 5-year DFS after treatment was conducted to better understand treatment efficacy. Funnel plots for the two types of studies are displayed in Figure 7. These plots indicated a small bias risk, and the research deviation could be ignored. Based on these results, both nCRT and nCT were found to enhance 5-year DFS. Specifically, nCRT demonstrated superior efficacy in RCTs, while the opposite trend was observed in CCSs.
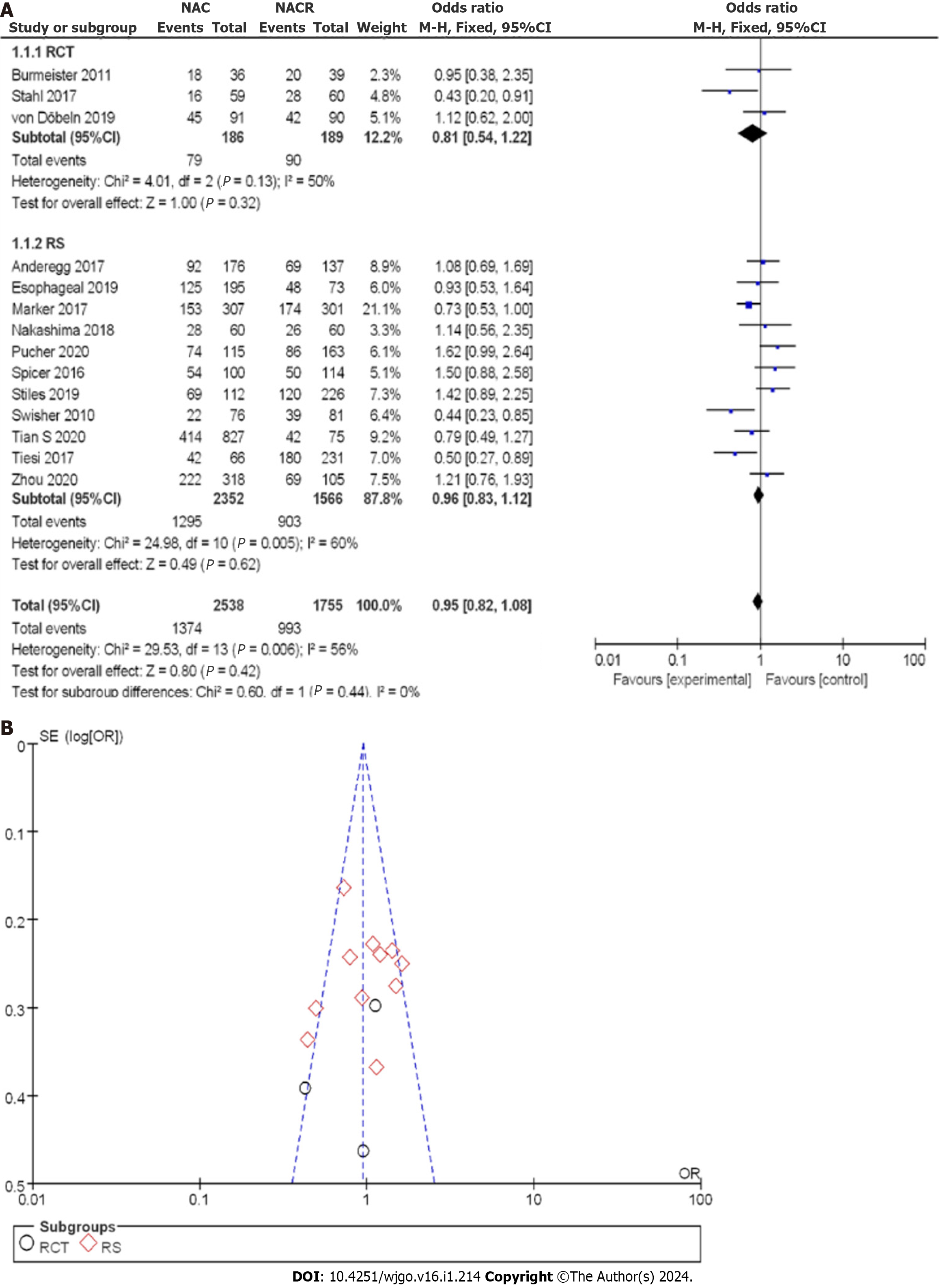
Figure 8A demonstrates the enrollment and analysis of 4337 patients with EC, with 186 patients in the nCRT group and 189 patients in the nCT group within the RCTs. Utilizing the OR as a clinical outcome indicator, the combined OR score from three RCTs was 0.81, with a corresponding 95%CI of 0.54 to 1.22. The resulting p value was 0.13, and the I2 statistic indicated a moderate heterogeneity of 50%. The OR values indicated no significant difference in the 3-year OSR among all the studies, with moderate heterogeneity observed. Within the CCS, a total of 2352 cases in the nCRT group and 1566 cases in the nCT group were analyzed. OR was again selected as a clinical outcome indicator. The combined OR score from eleven CCSs was 0.96, with a resulting 95%CI of 0.83 to 1.12. The corresponding P value was less than 0.05, and the I2 statistic indicated a moderate heterogeneity of 60%. Thus, moderate heterogeneity was observed in the 3-year OSR.
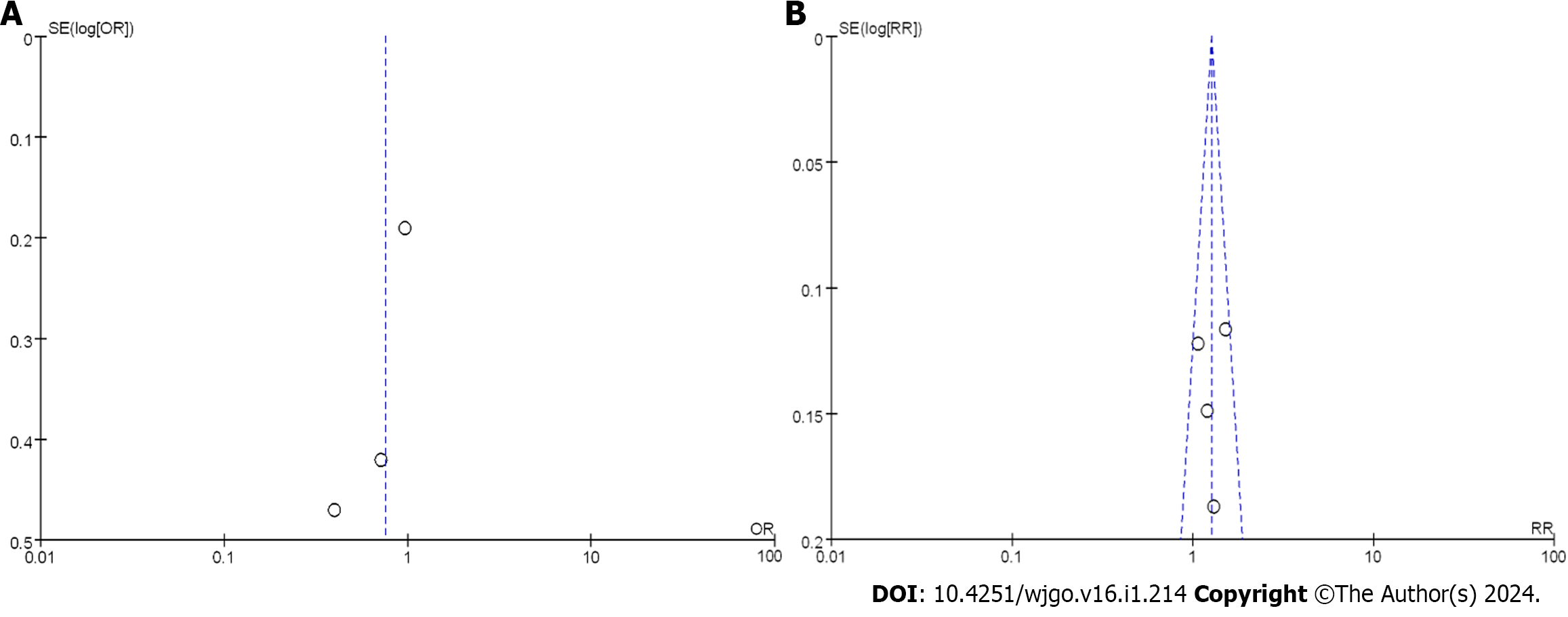
To better understand the treatment effect, a comprehensive analysis of the 3-year OSR after treatment was conducted. Figure 8B displays the funnel plot of the 3-year OSR. The results suggested that the bias risk was not evident, and the research deviation could be disregarded. Based on the above results, nCRT showed improvement in the 3-year OSR compared to nCT.
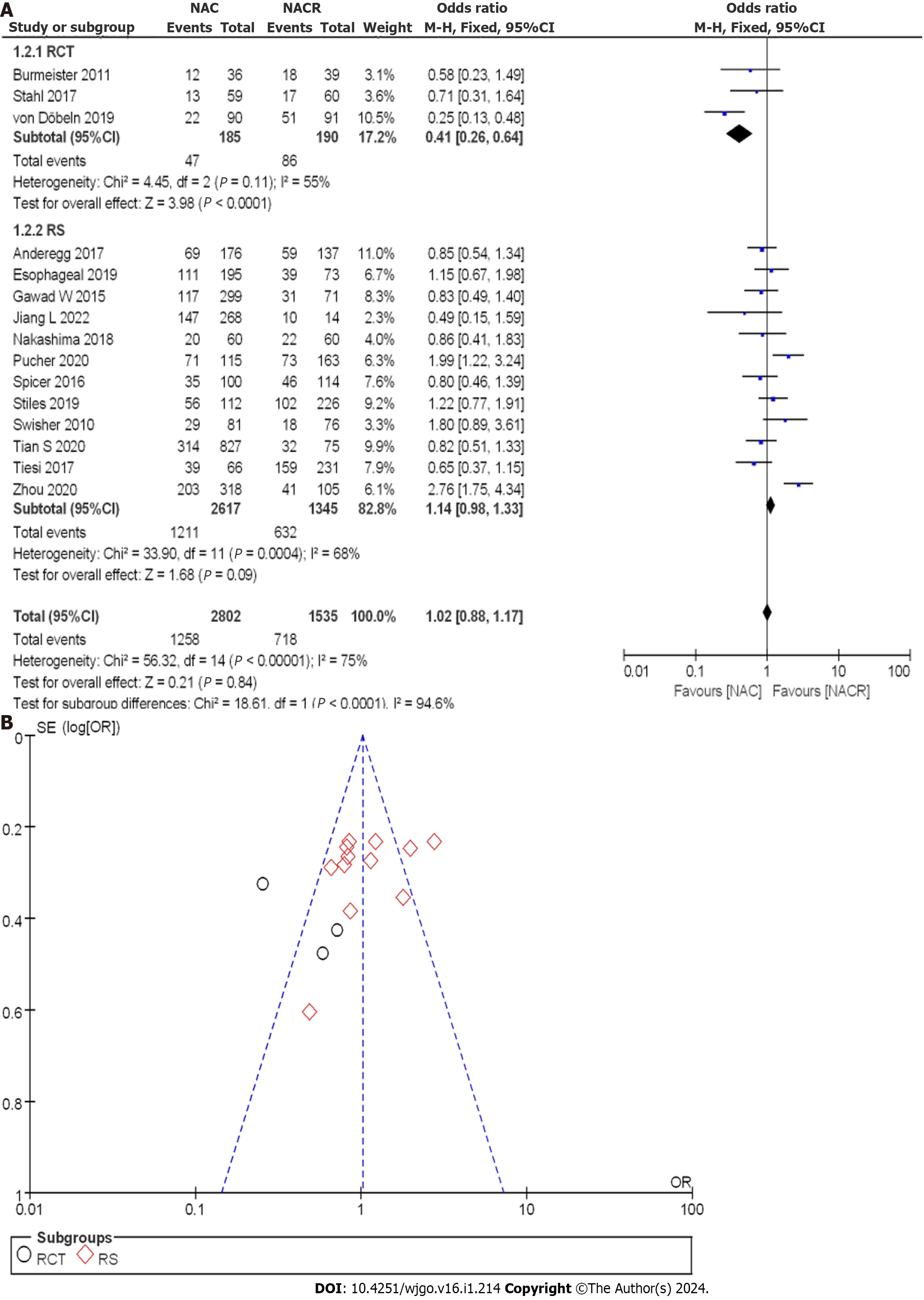
Figure 9A illustrates the enrollment of 4337 patients with EC, with 185 patients in the nCRT group and 190 patients in the nCT group within the RCTs. Employing the OR as a clinical outcome indicator, the combined OR score from three RCTs was 0.41, with a 95%CI of 0.26 to 0.64. The corresponding P value was 0.11, and the I2 statistic indicated a moderate heterogeneity of 55%. The OR values indicated that the reviewed literature exhibited no significant difference in the 5-year OSR, with moderate heterogeneity observed.
Within the CCS, a total of 2,617 cases were analyzed in the nCRT group, and 1345 cases were mentioned in the nCT group. The combined OR score from twelve CCSs was 1.14, with a 95%CI of 0.83 to 1.12. The resulting p value was less than 0.05, and the I2 statistic indicated a moderate heterogeneity of 68%. Therefore, moderate heterogeneity was observed among all the included studies in the 5-year OSR.
A comprehensive analysis of the 5-year OSR after treatment was conducted to observe the treatment effect. Figure 9B displays the funnel plot of the 5-year OSR subgroup analysis. The enrolled literature exhibited a low bias risk. Based on the above results, nCT was found to improve the 5-year OSR compared to nCRT.
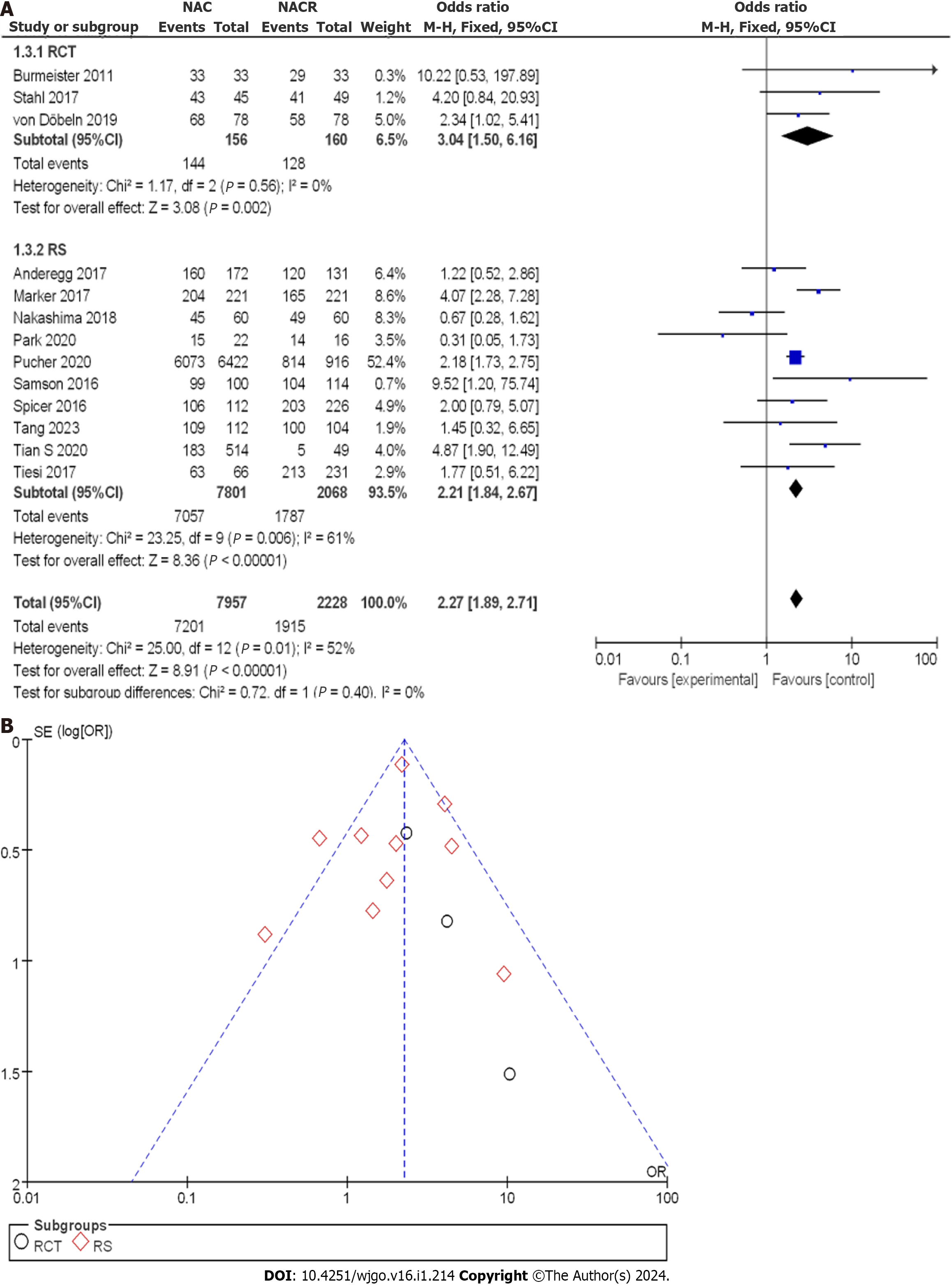
As shown in Figure 10A, 10185 patients with EC were enrolled, with 156 patients in the nCRT group and 160 patients in the nCT group within the RCTs. The OR score from three RCTs was 3.04, with a corresponding 95%CI of 1.50 to 6.16. The resulting P value was 0.561, and the I2 statistic indicated no heterogeneity with a value of 0%. The OR value indicated no observable difference in R0 CR among the different studies, and no heterogeneity was observed. Within the CCS, there were 7801 cases in the nCRT group and 2068 cases in the nCT group. The combined OR score from ten CCSs was 2.20, with a 95%CI of 1.83 to 2.65. The corresponding P value was less than 0.05, and the I2 statistic indicated a moderate heterogeneity of 60%. The OR values suggested moderate heterogeneity in R0 CR within each study.
To gain a clearer understanding of the treatment effect, a comprehensive analysis of R0 CR was conducted. Figure 10B displays the funnel plot of the R0 CR subgroup analysis. The bias risk in all the studies was small, with only two studies deviating from the expected results. Based on the above results, it can be concluded that nCRT had a higher R0 removal rate than nCT.
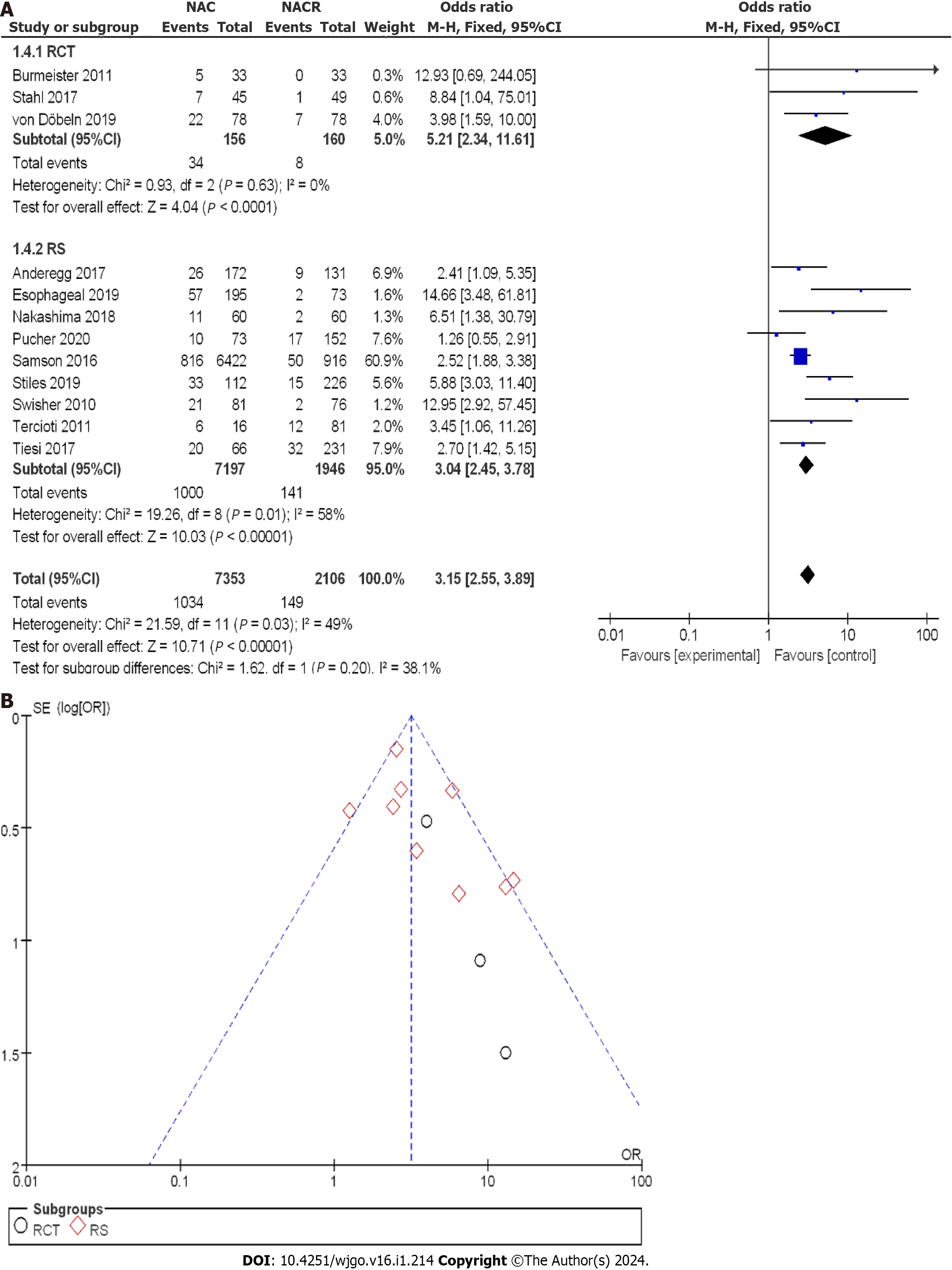
Figure 11A illustrates the enrollment of 9459 patients with EC, with 156 patients in the nCRT group and 160 patients in the nCT group within the RCTs. The OR score from three RCTs was 5.21, with a corresponding 95%CI of 2.34 to 11.61. The resulting p value was 0.63, and the I2 statistic indicated no heterogeneity with a value of 0%. The OR values revealed no significant difference in pCR among the different studies, and no heterogeneity was observed. Within the CCS, there were 7197 cases in the nCRT group and 1946 cases in the nCT group. The combined OR score from nine CCSs was 3.04, with a 95%CI of 2.45 to 3.78. The corresponding p value was less than 0.05, and the I2 statistic indicated a moderate heterogeneity of 58%. The OR values showed moderate heterogeneity in p CR among all the enrolled studies.
A comprehensive analysis of p CR was performed to gain a clearer understanding of the treatment effect. Figure 11B displays the funnel plot of the p CR subgroup analysis. The bias risk in each study was minimal, and no study deviation was observed. Based on the above results, it can be concluded that nCRT significantly improved the pathological complete response rate compared to nCT.
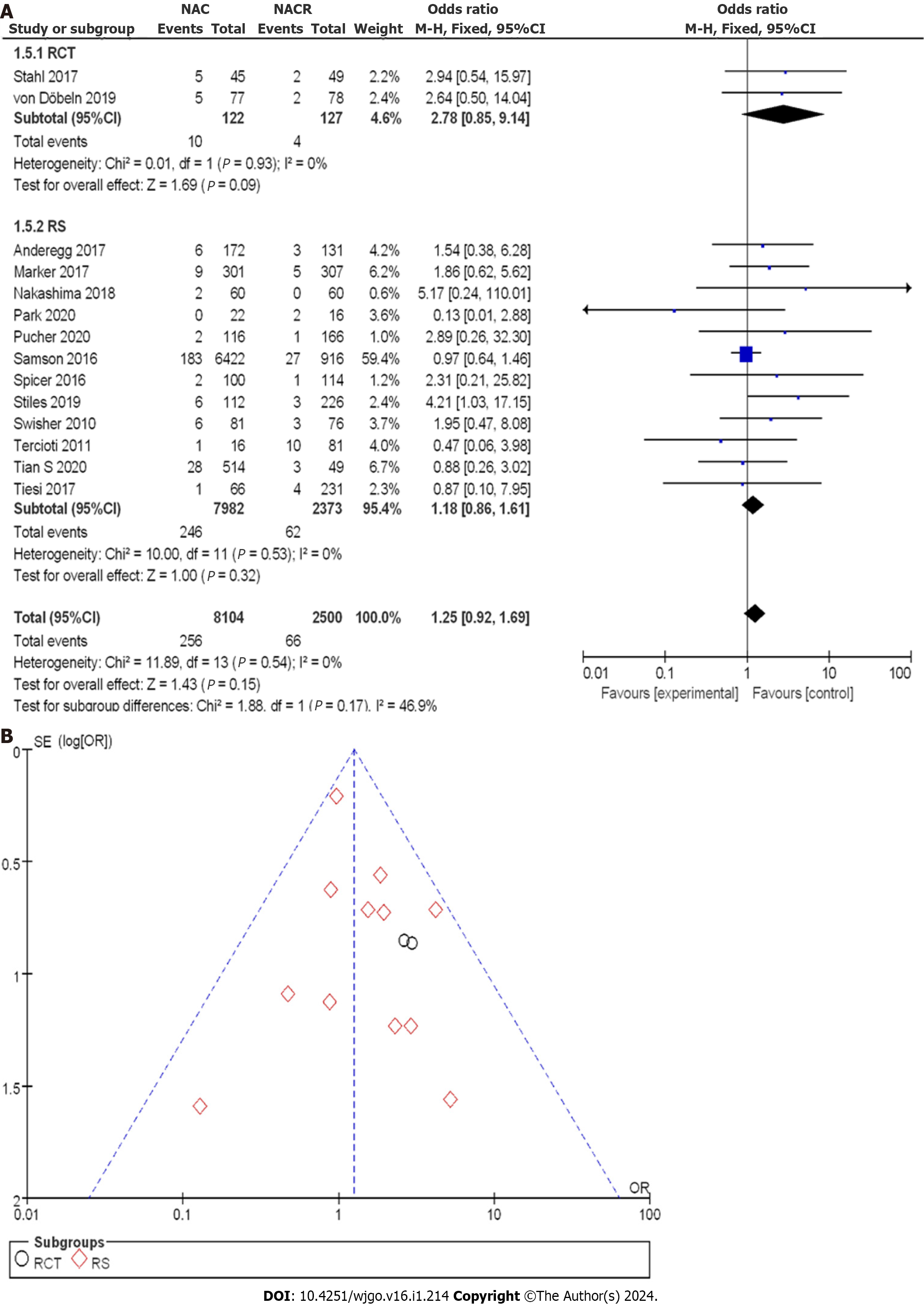
In Figure 12A, the enrollment involved 10604 patients with EC, with 122 patients in the nCRT group and 127 patients in the nCT group within the RCTs. The OR score from two RCTs was 2.78, with a 95%CI of 0.85 to 9.14. The resulting P value was 0.93, and the I2 statistic indicated no heterogeneity with a value of 0%. The OR values suggested no significant difference in postoperative mortality among the studies, indicating no heterogeneity. Within the CCS, there were 7982 cases in the nCRT group and 2373 cases in the nCT group. The combined OR score from twelve CCSs was 1.18, with a corresponding 95%CI of 0.86 to 1.61. The p value was 0.53, and the I2 statistic indicated no heterogeneity.
A comprehensive analysis of postoperative mortality was conducted to observe the efficacy more effectively. Figure 12B displays the funnel plot of the postoperative mortality subgroup analysis. This indicated a small bias risk in all the studies, with no research deviation. Based on the above results, there was no significant difference in mortality between nCT and nCRT.
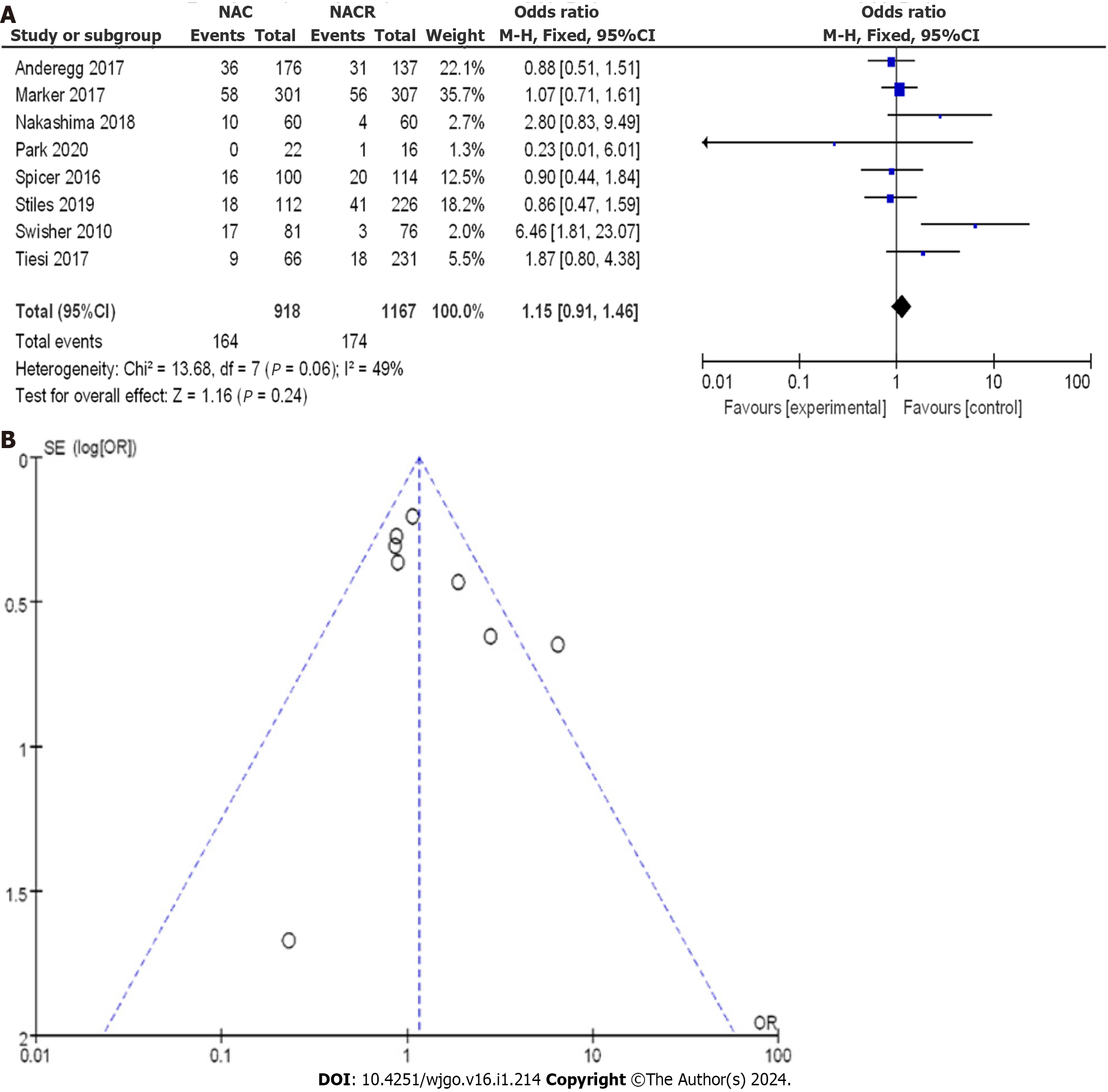
A total of 2085 patients with EC were enrolled, with 918 patients in the nCRT group and 1167 patients in the nCT group. As shown in Figure 13A, the OR score from eight CCSs was 1.15, with a 95%CI of 0.91 to 1.46. The resulting P value was 0.06, and the I2 statistic indicated a moderate heterogeneity of 49%. Based on the OR values, there was no significant difference in cardiac complications among the different studies, and a low level of heterogeneity was observed. The OR values ranged from 0.86 to 6.46, with corresponding 95%CI values of 0.47 to 1.59 and 1.81 to 23.07, respectively.
A comprehensive analysis of cardiac complications after treatment was performed. Figure 13B displays the funnel plot, showing a small bias risk in all the studies, with only one study deviating. Based on the above results, it can be concluded that nCRT increased the incidence of cardiac complications compared to nCT.
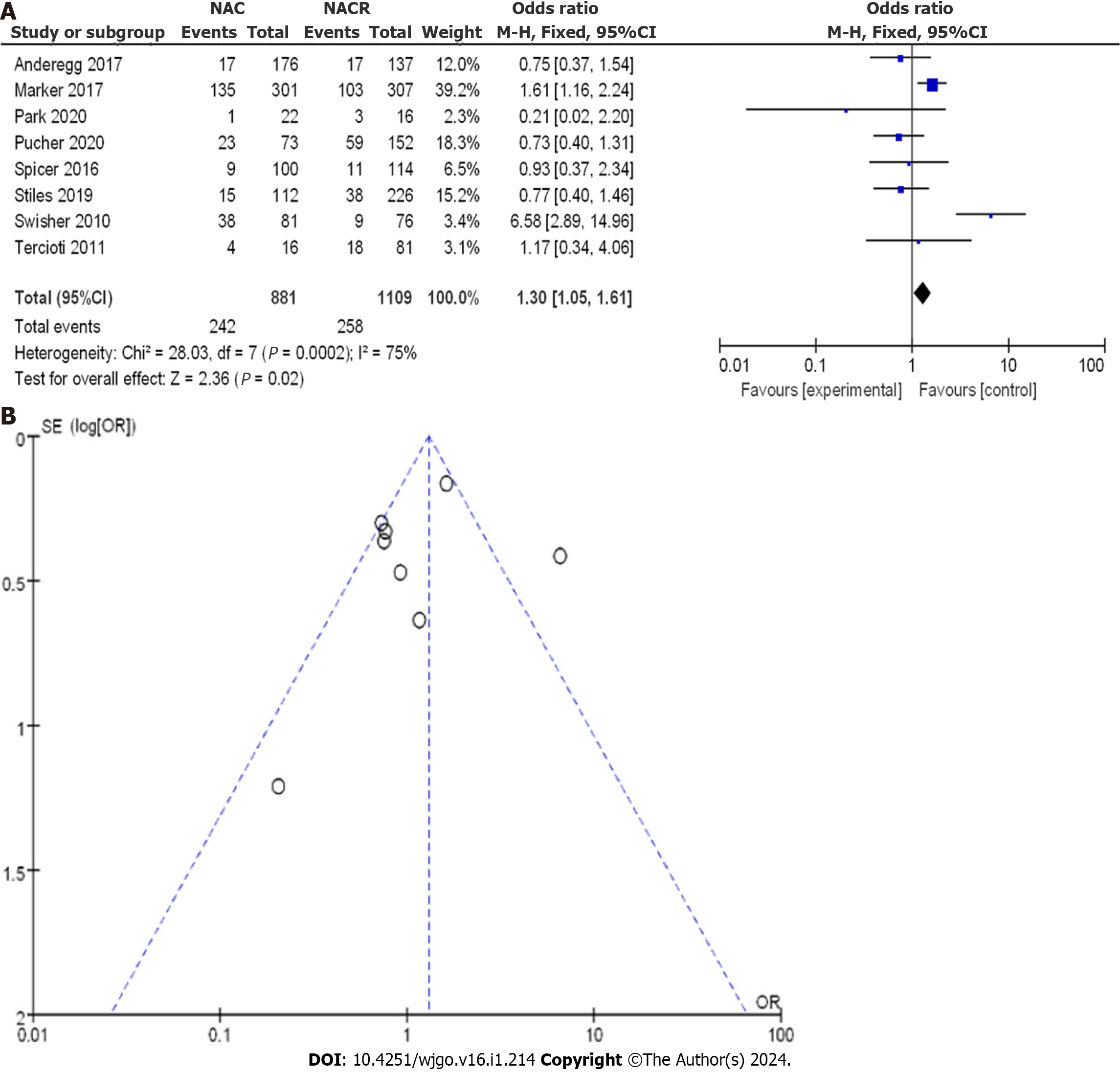
A total of 1990 patients with EC were enrolled, with 881 patients in the nCRT group and 1109 patients in the nCT group. As shown in Figure 14A, the OR score from eight CCSs was 1.30, with a corresponding 95%CI of 1.05 to 1.61. The resulting p value was less than 0.05, and the I2 statistic indicated a moderate heterogeneity of 75%. The OR values suggested no significant difference in lung complications among the studies, with a moderate level of heterogeneity. The OR values ranged from 0.21 to 6.58, with corresponding 95%CI values of 0.02 to 2.20 and 2.89 to 14.96, respectively.
A comprehensive analysis of lung complications after treatment was conducted to gain a clearer understanding of the treatment efficacy. Figure 14B displays the funnel plot of the complications. It revealed a small bias risk in all the studies, with only one study deviating. Based on the above results, it can be concluded that nCRT increased the incidence of lung complications compared to nCT.
A sensitivity analysis was carried out, and the results indicated no significant change in the outcomes of different analysis models, demonstrating the stability of the enrolled literature. The funnel asymmetric linear regression analysis also showed better consistency in the verification.
The present meta-analysis was designed to compare the efficacy and reliability of nCRT and nCT in the prevention and treatment of EC. Among the 20 included studies, the sample sizes of EC ranged from 38 to 7388. The analysis incorporated three RCTs and 17 case-control and cohort studies, reports from which provided a detailed description of the treatment course with nCRT and nCT and documented changes in patients pre- and posttreatment. The meta-analysis findings revealed that nCRT outperformed nCT in terms of the 3-year OSR, pathological complete response rate, and R0 clearance rate for EC. In particular, the improvement in the 3-year OSR was more pronounced in cases of esophageal squamous cell carcinoma. On the other hand, patients receiving nCT experienced a lower incidence of postoperative cardiopulmonary complications and perioperative mortality than those undergoing nCRT, while other indicators showed no statistically significant differences.
Scholars in several studies have suggested that neoadjuvant therapy holds theoretical advantages in controlling the micrometastasis of the disease[28,29]. It can reduce the tumor stage, thus increasing the success rate of focal excision for curable patients. Furthermore, research has consistently shown that the outcomes of neoadjuvant therapy combined with surgery are significantly superior to those of simple surgical excision[30,31]. However, it is worth noting that neoadjuvant treatments may also have detrimental effects on the body's immune system. Additionally, radiotherapy and chemotherapy may lead to body edema, inflammation, and even irreversible fibrosis, resulting in increased postoperative complications and potential mortality[32,33]. The combined application of radiotherapy and chemotherapy, although synergistic in enhancing local efficacy and addressing micrometastasis, increases the toxicity burden associated with both treatments[34]. Recent studies have highlighted that while nCRT significantly improves the R0 removal rate and achieves more pathological complete responses than nCT, it lacks clear survival advantages and may contribute to more postoperative complications.
Therefore, the optimal choice for neoadjuvant therapy remains a subject of debate[35,36]. From previous meta-analyses, scholars have also concluded that nCRT provides a statistically significant survival advantage over nCT. However, some researchers have argued that although nCRT offers advantages in terms of tumor growth control, the difference is not significant[37,38]. Furthermore, data have shown that compared to patients with esophageal adenocarcinoma, those with esophageal squamous cell carcinoma demonstrate greater lesion complete remission and longer average survival rates with adjuvant chemotherapy[39]. In summary, when compared to other traditional adjuvant chemotherapy options for EC, nCRT exhibits certain survival advantages, particularly in cases of squamous cell carcinoma, while nCT performs better in mitigating postoperative complications. This meta-analysis also uncovered the favorable performance of nCT in terms of 5-year overall survival, which differs from prior studies, possibly due to inadequate inclusion of subgroup analysis or a limited number of studies[40,41]. Ultimately, the selection of appropriate neoadjuvant therapy for achieving long-term survival outcomes while reducing perioperative mortality and postoperative complication rates depends on the patient's specific systemic conditions and sensitivity to radiotherapy and chemotherapy.
Of course, this meta-analysis has a few limitations. Most of the included studies involved observational designs spanning a considerable timeframe, potentially introducing variations in surgical selection, dose selection, race, and timing. In addition, all 20 included studies were in English, and some indices in these studies may not have been completely referenced, which could have influenced the results.
No significant difference was observed in 3-year DFS or 5-year DFS when comparing the two treatment approaches and the nCT approach. On the other hand, nCRT showed improved outcomes in terms of the 3-year OSR, lesion complete response rate, and R0 CR for EC. Conversely, better 5-year OSR results were exhibited with nCT. Furthermore, compared to other nCRT, nCT demonstrates a lower incidence of cardiopulmonary complications and perioperative mortality.
Esophageal cancer (EC) treatment using neoadjuvant therapy has shown potential advantages in controlling micrometastasis and improving surgical outcomes. However, the optimal choice between neoadjuvant chemoradiotherapy (nCRT) and neoadjuvant chemotherapy (nCT) remains debated.
The study aimed to compare the efficacy and reliability of nCRT and nCT in the prevention and treatment of EC, addressing the lack of consensus in the literature and providing guidance for future research in this field.
The study aimed to analyze various outcomes such as overall survival rates (OSR), pathological complete response rates, R0 clearance rates (CR), and the incidence of complications to determine the advantages and disadvantages of nCRT and nCT in EC treatment.
A comprehensive meta-analysis was conducted, incorporating three randomized controlled trials and 17 case-control and cohort studies. The analysis involved statistical calculations, including odds ratios, confidence intervals, P values, and I2 statistics, to assess the differences and heterogeneity among the studies.
The study found that nCRT outperformed nCT in terms of the 3-year OSR, pathological complete response rate, and R0 CR. However, nCT showed a lower incidence of postoperative cardiopulmonary complications and perioperative mortality. Other outcomes did not show statistically significant differences.
The study concludes that nCRT is more effective in terms of 3-year survival outcomes and tumor response, particularly in esophageal squamous cell carcinoma cases. On the other hand, nCT is associated with lower postoperative complications and mortality rates. The choice of neoadjuvant therapy should consider the patient's specific conditions and treatment sensitivities.
Future research should focus on comparing specific subgroups, such as esophageal squamous cell carcinoma, and explore tailored approaches to neoadjuvant therapy to optimize survival outcomes while minimizing complications. Addi
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elfaham E, Italy; Mueller B, Germany S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Hipp J, Thomaschewski M, Hummel R, Hoeppner J. Complete response after neoadjuvant therapy for esophageal cancer : Implications for surgery. Chirurg. 2022;93:132-137. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Logarajah S, Jeyarajah P, Darwish M, Moslim M, Jureller M, Osman H, Jeyarajah DR. Does chemotherapy regimen matter in the neoadjuvant treatment of esophageal cancer? J Gastrointest Oncol. 2022;13:2713-2720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Fukaya M, Yokoyama Y, Usui H, Fujieda H, Sakatoku Y, Takahashi T, Miyata K, Niikura M, Sugimoto T, Asahara T, Nagino M, Ebata T. Impact of synbiotics treatment on bacteremia induced during neoadjuvant chemotherapy for esophageal cancer: A randomised controlled trial. Clin Nutr. 2021;40:5781-5791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Phillips AL, Reeves DJ, Storey S. Impact of diabetes (type 2) and glycemic control on health-related outcomes of patients receiving chemotherapy for non-metastatic breast cancer: a retrospective analysis. Support Care Cancer. 2023;31:114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Depypere L, De Hertogh G, Moons J, Provoost AL, Lerut T, Sagaert X, Coosemans W, Van Veer H, Nafteux P. Importance of Lymph Node Response After Neoadjuvant Chemoradiotherapy for Esophageal Adenocarcinoma. Ann Thorac Surg. 2021;112:1847-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Yamada M, Tanaka K, Yamasaki M, Yamashita K, Makino T, Saito T, Takahashi T, Kurokawa Y, Motoori M, Kimura Y, Nakajima K, Eguchi H, Doki Y. Neutrophil-to-lymphocyte ratio after neoadjuvant chemotherapy as an independent prognostic factor in patients with esophageal squamous cell carcinoma. Oncol Lett. 2023;25:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Koca D, Oztop I, Yavuzsen T, Ellidokuz H, Yilmaz U. Evaluation of the efficacy of modified De Gramont and modified FOLFOX4 regimens for adjuvant therapy of locally advanced rectal cancer. Asian Pac J Cancer Prev. 2011;12:3181-3186. [PubMed] |
| 8. | Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, Barbour AP, Gotley DC, Smithers BM. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, Wilke H, Budach W. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 10. | von Döbeln GA, Klevebro F, Jacobsen AB, Johannessen HO, Nielsen NH, Johnsen G, Hatlevoll I, Glenjen NI, Friesland S, Lundell L, Yu J, Nilsson M. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 11. | Anderegg MCJ, van der Sluis PC, Ruurda JP, Gisbertz SS, Hulshof MCCM, van Vulpen M, Mohammed NH, van Laarhoven HWM, Wiezer MJ, Los M, van Berge Henegouwen MI, van Hillegersberg R. Preoperative Chemoradiotherapy Versus Perioperative Chemotherapy for Patients With Resectable Esophageal or Gastroesophageal Junction Adenocarcinoma. Ann Surg Oncol. 2017;24:2282-2290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Esophageal Cancer Study Group Participating Centers. Predictors of staging accuracy, pathologic nodal involvement, and overall survival for cT2N0 carcinoma of the esophagus. J Thorac Cardiovasc Surg. 2019;157:1264-1272.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Gawad W, Fakhr I, Lotayef M, Mansour O, Mokhtar N. Sphincter saving and abdomino-perineal resections following neoadjuvant chemoradiation in locally advanced low rectal cancer. J Egypt Natl Canc Inst. 2015;27:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Markar SR, Noordman BJ, Mackenzie H, Findlay JM, Boshier PR, Ni M, Steyerberg EW, van der Gaast A, Hulshof MCCM, Maynard N, van Berge Henegouwen MI, Wijnhoven BPL, Reynolds JV, Van Lanschot JJB, Hanna GB. Multimodality treatment for esophageal adenocarcinoma: multi-center propensity-score matched study. Ann Oncol. 2017;28:519-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Tian S, Jiang R, Madden NA, Ferris MJ, Buchwald ZS, Xu KM, Cardona K, Maithel SK, McDonald MW, Lin JY, Curran WJ, El-Rayes BF, Behera M, Patel PR. Survival outcomes in patients with gastric and gastroesophageal junction adenocarcinomas treated with perioperative chemotherapy with or without preoperative radiotherapy. Cancer. 2020;126:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Nakashima Y, Saeki H, Hu Q, Tsuda Y, Hisamatsu Y, Ando K, Oki E, Maehara Y. Neoadjuvant Chemotherapy Versus Chemoradiotherapy for Patients with Esophageal Squamous Cell Carcinoma. Anticancer Res. 2018;38:6809-6814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Park SY, Hong MH, Kim HR, Lee CG, Cho JH, Cho BC, Kim DJ. The feasibility and safety of radical esophagectomy in patients receiving neoadjuvant chemoradiotherapy with pembrolizumab for esophageal squamous cell carcinoma. J Thorac Dis. 2020;12:6426-6434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Pucher PH, Rahman SA, Walker RC, Grace BL, Bateman A, Iveson T, Jackson A, Rees C, Byrne JP, Kelly JJ, Noble F, Underwood TJ. Outcomes and survival following neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus: Inverse propensity score weighted analysis. Eur J Surg Oncol. 2020;46:2248-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Samson P, Robinson C, Bradley J, Lockhart AC, Puri V, Broderick S, Kreisel D, Krupnick AS, Patterson GA, Meyers B, Crabtree T. Neoadjuvant Chemotherapy versus Chemoradiation Prior to Esophagectomy: Impact on Rate of Complete Pathologic Response and Survival in Esophageal Cancer Patients. J Thorac Oncol. 2016;11:2227-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Spicer JD, Stiles BM, Sudarshan M, Correa AM, Ferri LE, Altorki NK, Hofstetter WL. Preoperative Chemoradiation Therapy Versus Chemotherapy in Patients Undergoing Modified En Bloc Esophagectomy for Locally Advanced Esophageal Adenocarcinoma: Is Radiotherapy Beneficial? Ann Thorac Surg. 2016;101:1262-9; discussion 1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Stiles BM, Kamel MK, Harrison SW, Rahouma M, Lee B, Nasar A, Port JL, Altorki NK. Neoadjuvant Therapy for Locally Advanced Esophageal Cancer Should Be Targeted to Tumor Histology. Ann Thorac Surg. 2019;107:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Swisher SG, Hofstetter W, Komaki R, Correa AM, Erasmus J, Lee JH, Liao Z, Maru D, Mehran R, Patel S, Rice DC, Roth JA, Vaporciyan AA, Walsh GL, Ajani JA. Improved long-term outcome with chemoradiotherapy strategies in esophageal cancer. Ann Thorac Surg. 2010;90:892-8; discussion 898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Tang H, Wang H, Fang Y, Zhu JY, Yin J, Shen YX, Zeng ZC, Jiang DX, Hou YY, Du M, Lian CH, Zhao Q, Jiang HJ, Gong L, Li ZG, Liu J, Xie DY, Li WF, Chen C, Zheng B, Chen KN, Dai L, Liao YD, Li K, Li HC, Zhao NQ, Tan LJ. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol. 2023;34:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 24. | Tercioti Junior V, Lopes LR, Coelho Neto Jde S, Carvalheira JB, Andreollo NA. Local effectiveness and complications of neoadjuvant therapy in esophageal squamous cell carcinoma: radiotherapy versus chemoradiotherapy. Rev Col Bras Cir. 2011;38:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Tiesi G, Park W, Gunder M, Rubio G, Berger M, Ardalan B, Livingstone A, Franceschi D. Long-term survival based on pathologic response to neoadjuvant therapy in esophageal cancer. J Surg Res. 2017;216:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Jiang L, Zhu J, Chen X, Wang Y, Wu L, Wan G, Han Y, Leng X, Peng L, Wang Q. Safety and efficacy of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in neoadjuvant chemoradiotherapy for patients with locally advanced esophageal carcinoma: a retrospective study. Radiat Oncol. 2022;17:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Zhou HY, Zheng SP, Li AL, Gao QL, Ou QY, Chen YJ, Wu ST, Lin DG, Liu SB, Huang LY, Li FS, Zhu HY, Qiao GB, Lanuti M, Yao HR, Yu YF. Clinical evidence for association of neoadjuvant chemotherapy or chemoradiotherapy with efficacy and safety in patients with resectable esophageal carcinoma (NewEC study). EClinicalMedicine. 2020;24:100422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, Dómine M, Majem M, Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, Cobo M, López Vivanco G, Del Barco E, Bernabé Caro R, Viñolas N, Barneto Aranda I, Viteri S, Pereira E, Royuela A, Calvo V, Martín-López J, García-García F, Casarrubios M, Franco F, Sánchez-Herrero E, Massuti B, Cruz-Bermúdez A, Romero A. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol. 2022;40:2924-2933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 233] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 29. | Chen LL, Lu L, Gong XLi, Xu YC, Chu XY, Huang GC. Gastric Cancer with Bone Marrow Invasion and Disseminated Intravascular Coagulation: A Case Report. Oncologie. 2022;24:599-604. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, Li Z, Cui F, Du Z, Zeng Y, Jiang S, He P, Gu X, Chen H, Zhang H, Lin X, Huang H, Lv W, Cai W, Liang W, Liang H, Jiang W, Wang W, Xu K, Wu K, Lerut T, Fu J, He J. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J Cancer. 2022;151:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 31. | Li MJ, Wei J, Xu GH, Liu Y, Zh JN. Surgery Combined with Molecular Targeted Therapy Successfully Treated Giant Esophageal Gastrointestinal Stromal Tumor. Oncologie. 2022;24:349-356. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Ma W, Xue R, Zhu Z, Farrukh H, Song W, Li T, Zheng L, Pan CX. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp Hematol Oncol. 2023;12:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 33. |
Matani H, Wegner R E, Paskewicz M, Sahu D, Omstead A, Jobe B, Kelly R J, Goel A, Zaidi A: Novel Predictive and Prognostic Gene Signatures for Chemoradiotherapy in Locally Advanced Esophageal Adenocarcinoma.
|
| 34. | Al-Jumayli M, Choucair K, Al-Obaidi A, Park R, Bansal A, Baranda J, Sun W, Saeed A. Pre-operative Carboplatin/Paclitaxel Versus 5-Fluorouracil (5-FU)-based Chemoradiotherapy for Older Adults With Esophageal Cancer. Anticancer Res. 2022;42:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Kato T, Oshikiri T, Goto H, Urakawa N, Hasegawa H, Kanaji S, Yamashita K, Matsuda T, Nakamura T, Suzuki S, Kakeji Y. Preoperative neutrophil-to-lymphocyte ratio predicts the prognosis of esophageal squamous cell cancer patients undergoing minimally invasive esophagectomy after neoadjuvant chemotherapy. J Surg Oncol. 2021;124:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Huihui Zhang, Jing Yan, Xiaoyong Ren, Ying Sheng, Zhenghui Wang, Jianmin Liang, Yan Yan, Yangyang Jia, Zhihui Li, Jin Hou. Nimotuzumab Combined with Neoadjuvant or Induction Chemotherapy for Head and Neck Squamous Cell Carcinoma: A Retrospective Study. Oncologie. 2022;24:707-716. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Chan KKW, Saluja R, Delos Santos K, Lien K, Shah K, Cramarossa G, Zhu X, Wong RKS. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: A network meta-analysis. Int J Cancer. 2018;143:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 38. | Ma HF, Lv GX, Cai ZF, Zhang DH. Comparison of the prognosis of neoadjuvant chemoradiotherapy treatment with surgery alone in esophageal carcinoma: a meta-analysis. Onco Targets Ther. 2018;11:3441-3447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Montagnani F, Fornaro L, Frumento P, Vivaldi C, Falcone A, Fioretto L. Multimodality treatment of locally advanced squamous cell carcinoma of the oesophagus: A comprehensive review and network meta-analysis. Crit Rev Oncol Hematol. 2017;114:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Pasquali S, Yim G, Vohra RS, Mocellin S, Nyanhongo D, Marriott P, Geh JI, Griffiths EA. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Ann Surg. 2017;265:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 41. | Zhao X, Ren Y, Hu Y, Cui N, Wang X, Cui Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PLoS One. 2018;13:e0202185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |