Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.133
Peer-review started: October 3, 2023
First decision: October 8, 2023
Revised: October 14, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: January 15, 2024
Processing time: 99 Days and 11.7 Hours
Gastric cancer (GC) and colorectal cancer (CRC) are the fifth and third most common cancer worldwide, respectively. Nowadays, GC is reported to have a potential predictive value for CRC, especially for advanced CRC.
To evaluate the necessity of colonoscopy for gastric neoplasm (GN) patients.
Four databases, including PubMed, EMBASE, the Cochrane Library, and Ovid, were used to perform the search strategy on May 2, 2023. The prevalence of colorectal neoplasms (CRN) and baseline characteristics were compared between the neoplasm group and the control group. Continuous variables are expressed as the mean difference and standard deviation. Relationships of categorical variables in the two groups are expressed as odds ratios (OR) and 95% confidence intervals (95%CIs). Subgroup analysis according to different kinds of GNs was conducted for more in-depth analysis. The results of this study are represented by forest plots. Publication bias was evaluated by a funnel plot. All data analyses were performed by STATA SE 16.0 software.
A total of 3018 patients with GNs and 3905 healthy controls (age and sex matched) were enrolled for analysis. After comparing the prevalence of CRNs between the two groups, CRNs were detected significantly more frequently in GN patients than in controls (OR = 1.69, 95%CI = 1.28 to 2.23, I2 = 85.12%, P = 0.00), especially in patients with GC (OR =1.80, 95%CI = 1.49 to 2.18, I2 = 25.55%, P < 0.1). Moreover, other risk factors including age (OR = 1.08, 95%CI = 1.00 to 1.17, I2 = 90.13%, P = 0.00) and male sex (OR = 2.31, 95%CI = 1.26 to 4.22, I2 = 87.35%, P = 0.00), were related to the prevalence of CRNs. For patients in the GN group, body mass index (BMI, OR = 0.88, 95%CI = 0.80 to 0.98, I2 = 0.00%, P = 0.92) and smoking (OR = 1.03, 95%CI = 1.01 to 1.05, I2 = 0.00%, P = 0.57) were protective and risk factors for CRNs, respectively.
Patients are recommended to undergo colonoscopy when diagnosed with GNs, especially GC patients with a low BMI and a history of smoking.
Core Tip: Gastric cancer (GC) is currently the fifth largest malignant tumor worldwide and the second largest cause of cancer-related deaths in the world. Synchronous and homologous neoplasms are common in gastric neoplasm (GN) patients, and the colorectal neoplasm (CRN) is the main neoplasm type. The prevalence of CRN in GN patients is a concern. Some studies reported that GN was not a risk factor for CRN. Therefore, the purpose of this pooling up analysis was to explore whether colonoscopy was needed for GN patients to detecting CRN. A total of ten case-control studies were included, involving 6923 patients. In conclusion, GN patients had higher risk of CRN, especially for GC patients. Therefore, colonoscopy was recommended when patients diagnosed with GN.
- Citation: Liu XR, Wen ZL, Liu F, Li ZW, Liu XY, Zhang W, Peng D. Colonoscopy plays an important role in detecting colorectal neoplasms in patients with gastric neoplasms. World J Gastrointest Oncol 2024; 16(1): 133-143
- URL: https://www.wjgnet.com/1948-5204/full/v16/i1/133.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i1.133
According to the International Agency for Research on Cancer, gastric cancer (GC) is the fifth most common cancer worldwide, accounting for 1.1 million new cancer cases[1,2]. Helicobacter pylori (H. pylori) infection is the greatest known risk factor for GC and shows a positive association with gastric polyps[3,4]. Gastric polyps are asymptomatic lesions found incidentally during endoscopy that may develop into GC. Gastric neoplasm (GN) is a general term for gastric adenoma and GC.
Similar to GC, colorectal cancer (CRC) is a gastrointestinal malignant disease that develops from colorectal polyps[5]. Early colorectal polyps can be removed under colonoscopy, which significantly decreases the incidence of CRC[6,7]. Some characteristics, including age, male sex, family history, obesity, and red meat intake, have been reported to have predictive value for colorectal neoplasms (CRNs) (including colorectal polyps and CRC)[8-10]. Therefore, regular colonoscopy in high-risk patients with CRN is important to improve their survival.
Recently, GN was also reported to have potential predictive value for CRN, especially for advanced CRN[11-15]. However, some other studies demonstrated that colonoscopy surveillance is not recommended for all GN patients[16-18]. Therefore, this study sought to investigate whether it is necessary for GN patients to receive colonoscopy.
This current analysis was conducted by the PRISMA statement[19].
Two items including colonoscopy and GN were used for searching articles studying on the necessity of colonoscopy for GN patients. The text words of colonoscopy included colonoscopy, colonoscopies, and colonoscopic. The text words of GN included GC, gastric carcinoma, GNs, stomach cancer, stomach carcinoma, and stomach neoplasms. The search scope was limited to titles, abstracts, and author keywords. Only English was allowed.
The inclusion criteria were as follows: (1) Patients were divided into the GN group (gastric adenoma or cancer) and the control group; and (2) prevalence of CRN (colorectal adenoma, polyp or cancer) was reported. The exclusion criteria were as follows: (1) No comparison or insufficient data; and (2) the study types were conferences abstract, trail, review, meta-analysis, case report, letters to the editor, or comments.
Eligible studies were searched in four databases including PubMed, EMBASE, the Cochrane Library, and Ovid. After conducting the search strategy, duplicates records were removed at first. Then, records in ineligible study types were excluded. Finally, full-texts were screened and studies were selected according to the inclusion and exclusion criteria.
Baseline information of included studies and patients were collected for analysis. As for included studies, author, year, country, study date, study type, sample size, patients in the study group, evaluation of outcomes, conclusion, and the Newcastle-Ottawa Scales (NOS) score were collected. As for patients, age, sex, body mass index (BMI), diabetes, hypertension, alcohol, and smoking were collected. Moreover, for patients with CRNs, size, location, pathology, and number of CRN were also collected. Variables including age, male, BMI, smoking, drinking, and diabetes were collected to find whether there was a potential predictive value for CRNs in the whole patients and in the GN patients.
We used NOS score to assess the quality of included studies[20]. All the studies were case-control studies, which were assessed in selection, comparability and exposure. Nine score was regarded as high-quality, eight or seven score was regarded as median-quality, and lower than seven score was regarded as low-quality.
Continuous variables were expressed as mean difference (MD) and standardized deviation (SD), and the relationship of categorical variables in two groups were expressed as odds ratios (ORs) and 95% confidence intervals (95%CIs). All the variables were pooled up for a pooling up analysis using the random-effects model and DerSimonian-Laird method. When P < 0.1, the results was considered statistically significant. The chi-squared test and the I2 value were used to evaluate the statistical heterogeneity[21,22]. When the I² < 30%, the statistical heterogeneity was considered non-important. When the I² = 30%-60%, the statistical heterogeneity was considered moderate. When the I² > 60%, the statistical heterogeneity was considered substantial. The funnel plot was used to evaluate the publication bias. STATA SE V16.0 software was used for data analysis.
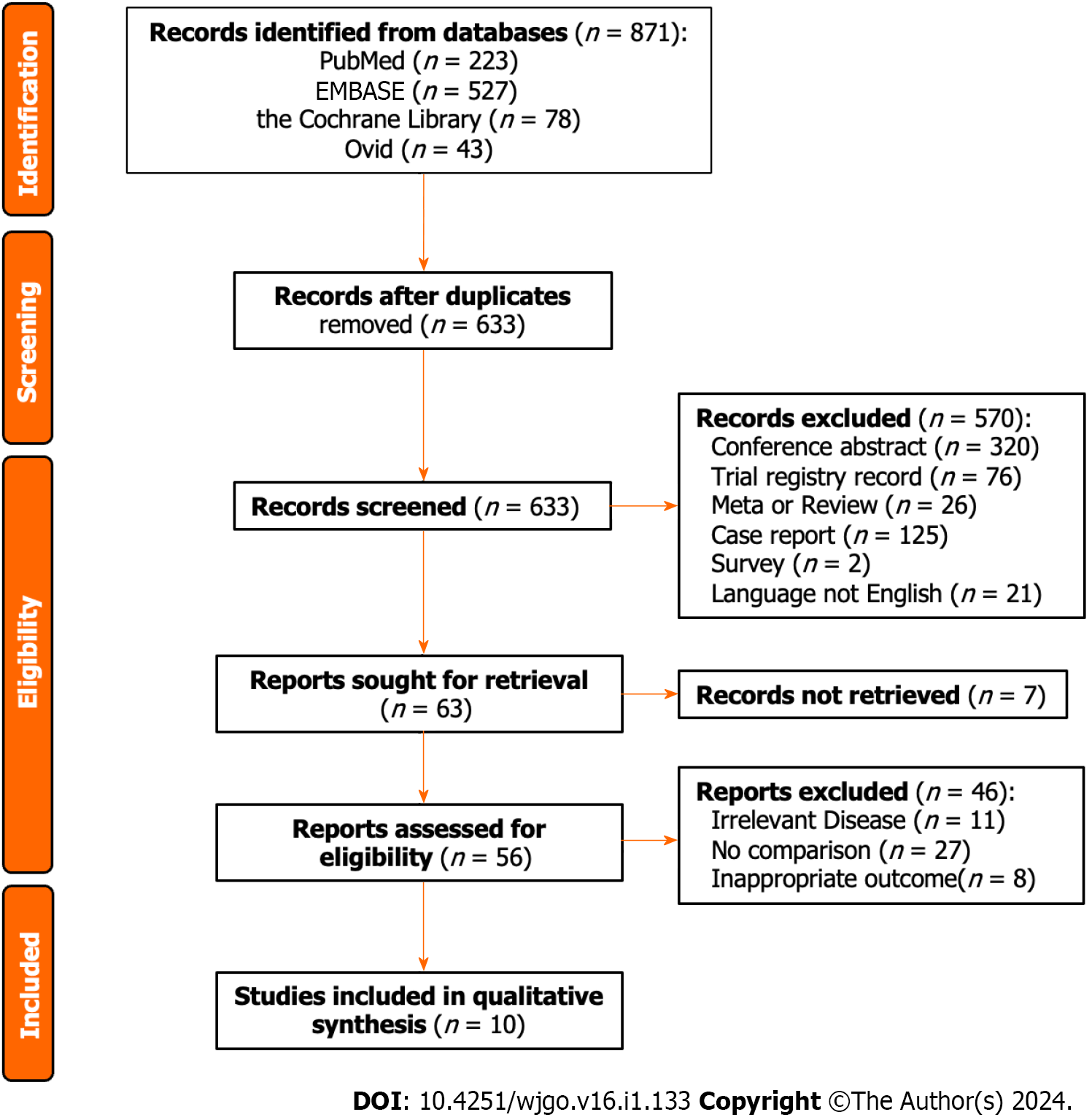
There were 871 studies after conducting the search strategy in four databases (223 studies in PubMed, 527 studies in EMBASE, 78 studies in the Cochrane Library, and 43 studies in Ovid). Duplicate records and records in ineligible study type were removed by Endnote software, and the left 63 records were ready for screening. Excluded for seven studies without unavailable full-text, 56 studies were carefully selected by two authors according to the inclusion and exclusion criteria. Finally, this current analysis enrolled ten studies (Figure 1).
Except for one study conducting in Japan, the other nine studies were conducted in Korea. The ten included studies were all case-control studies, and five were retrospectively conducted, the other five were prospectively conducted. As for patients in the case group, four studies reported GN, three studies reported GC, two studies reported early GC (EGC), and the other one reported early GN (EGN). After receiving colonoscopy, CRNs including colorectal adenoma, high-risk adenoma, cancerous adenoma, and CRC were reported. More information including author, year, study date, patients, conclusion, and the NOS score were shown in Table 1.
| Ref. | Country | Study date | Study type | Patients | Patients in the study group | Evaluation of outcomes | Conclusion | NOS |
| Chung et al[16], 2017 | Korea | January 2009-December 2012 | Retrospective case-control study | 402 | EGC | Colorectal neoplasm and advanced polyps | Colonoscopy plays an important role with respect to the detection of synchronous advanced colorectal neoplasm in patients with EGC | 7 |
| Imai et al[11], 2017 | Japan | January 2010-December 2012 | Retrospective case-control study | 390 | EGC | High-risk adenomas | Patients with EGC had a significant risk for colorectal cancer | 6 |
| Joo et al[12], 2010 | Korea | January 2002-December 2008 | Retrospective case-control study | 372 | GN | Adenomatous and cancerous colon polyps | Endoscopists should consider performing routine fiberoptic colonoscopy in patients undergoing endoscopic removal of GNs | 7 |
| Kim et al[13], 2022 | Korea | January 2015-December 2016 | Prospective case-control study | 220 | EGN | Colorectal adenoma | More stringent colonoscopy surveillance should be considered in elderly patients with EGN | 6 |
| Kim et al[17], 2013 | Korea | September 2005-August 2010 | Prospective case-control study | 832 | Gastric adenoma or cancer | Colorectal adenoma or cancer | Screening colonoscopy should be considered for gastric adenoma or cancer patients | 8 |
| Koh et al[20], 2022 | Korea | January 2010-July 2018 | Retrospective case-control study | 1505 | Gastric adenoma or cancer | Adenoma and cancerous colon polyps | Patients with GN are regarded as a high-risk group for colorectal cancer and are recommended for screening colonoscopy at the time of diagnosis | 8 |
| Lee et al[14], 2011 | Korea | October 2008-September 2010 | Prospective case-control study | 214 | GN | Colorectal neoplasm and high-risk colorectal neoplasm | A screening colonoscopy should be considered in patients with EGN undergoing endoscopic submucosal dissection | 6 |
| Lee et al[15], 2011 | Korea | July 2005-June 2010 | Retrospective case-control study | 369 | GC | Colorectal neoplasms | Patients with stomach cancer should be regarded as a high-risk group for colorectal neoplasms, and colonoscopy should be recommended for screening | 7 |
| Park et al[31], 2010 | Korea | November 2004-October 2006 | Prospective case-control study | 1629 | GC | Colorectal neoplasia including colorectal cancer and adenoma | There is a higher prevalence and risk of colorectal cancer in patients diagnosed with GC | 9 |
| Yoo et al[32], 2013 | Korea | January 2009-December 2010 | Prospective case-control study | 990 | GC | Colorectal neoplasm | Preoperative colonoscopy is strongly indicated in patients with GC | 8 |
After comparing the baseline characteristics between the GN group and the control group, we found that patients with GNs had lower BMI (MD = -0.38, 95%CI = -0.73 to -0.03, I2 = 8.00%, P = 0.03). There was no significant difference in age, sex, diabetes, hypertension, alcohol, and smoking (P > 0.1). As for patients who were detected to have CRNs in the two groups, there was no significant difference in size, location, pathology, and number > 3 (P > 0.1, Table 2).
| Characteristics | Studies | Patients (GN group/control group) | Odds ratio/mean difference (95%CI) | Heterogeneity |
| Age | 9 | 2888/3645 | 0.73 (0.25, 1.21); P = 0.77 | I2 = 0.00%; P = 0.00 |
| Sex | ||||
| Female | 10 | Reference | Reference | Reference |
| Male | 10 | 3012/3776 | 1.01 (0.91, 1.12); P = 0.85 | I2 = 0.00%; P = 1.00 |
| BMI | 6 | 2100/2857 | -0.38 (-0.73, -0.03); P = 0.03 | I2 = 8%; P=0.03 |
| Diabetes | 8 | 2181/2847 | 1.08 (0.86, 1.34); P = 0.51 | I2 = 35.08%; P = 0.15 |
| Hypertension | 3 | 494/494 | 0.93 (0.68, 1.27); P = 0.64 | I2 = 18.56%; P = 0.29 |
| Alcohol | 7 | 1273/1526 | 0.99 (0.71, 1.38); P = 0.94 | I2 = 75.73%; P = 0.00 |
| Smoking | 8 | 1816/2612 | 1.32 (0.89, 1.95); P = 0.17 | I2 = 86.15%; P = 0.00 |
| Colorectal neoplasms | ||||
| Size | 2 | 807/897 | 1.69 (0.77, 2.61); P = 0.22 | I2 = 33.22%; P = 0.00 |
| Location | ||||
| Rectum | 5 | Reference | Reference | Reference |
| Colon | 5 | 841/915 | 1.02 (0.59, 1.77); P = 0.94 | I2 = 75.16%; P = 0.00 |
| Pathology | ||||
| Tubular adenoma | 4 | Reference | Reference | Reference |
| Tubulovillous/villous adenoma | 4 | 1066/941 | 0.54 (0.03, 10.69); P = 0.68 | I2 = 97.24%; P = 0.00 |
| Serrated adenoma | 4 | 1066/941 | 0.23 (0.03, 2.03); P = 0.19 | I2 = 83.15%; P = 0.00 |
| Adenocarcinoma | 4 | 1066/941 | 3.15 (0.25, 39.30); P = 0.37 | I2 = 71.58%; P = 0.01 |
| Number > 3 | 2 | 460/460 | 1.50 (0.95, 2.36); P = 0.11 | I2 = 0.00%; P = 0.08 |
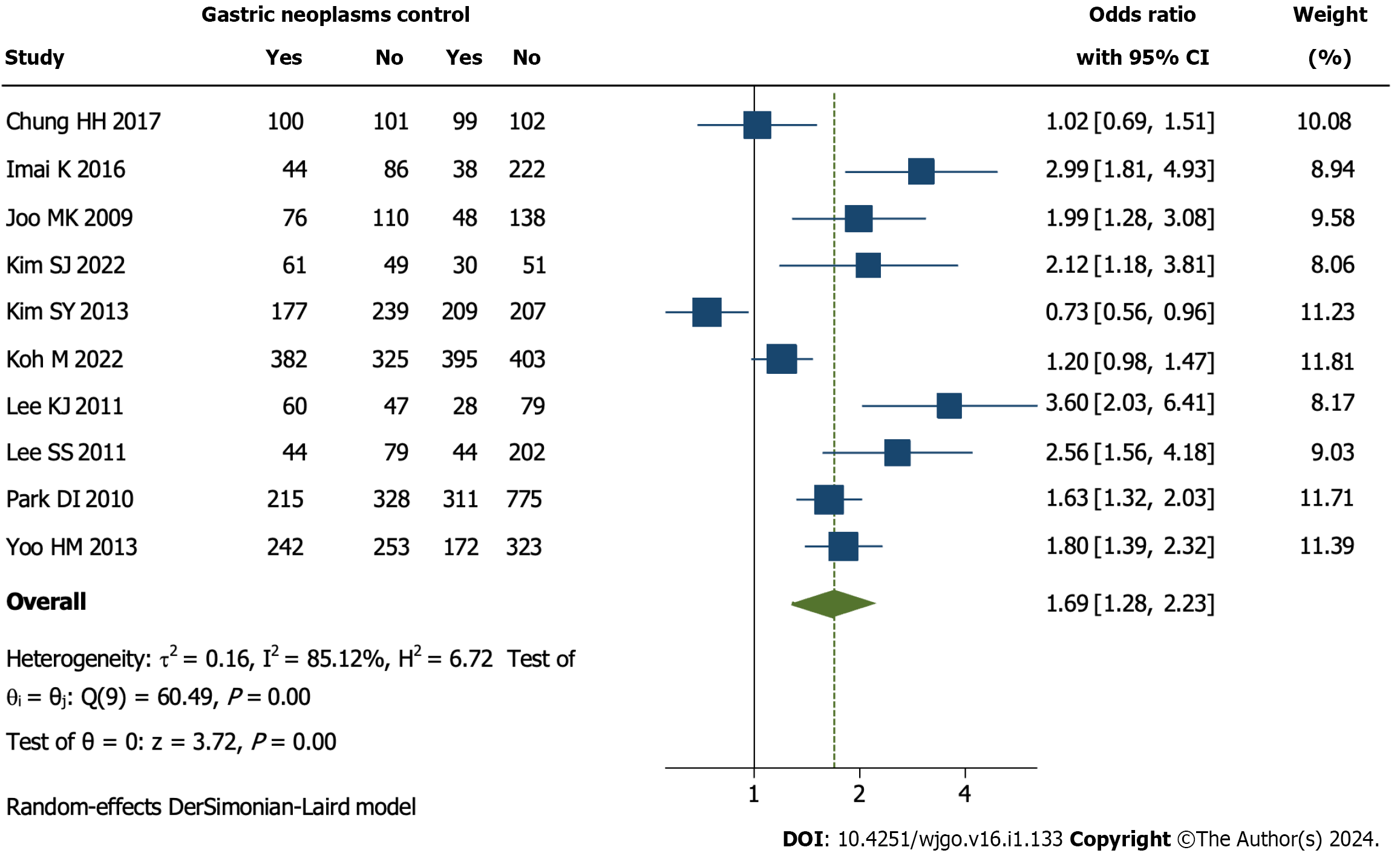
The prevalence of CRN was pooled, and it was found that the detection of CRN was significantly more in the GN group than the control group (OR = 1.69, 95%CI = 1.28 to 2.23, I2 = 85.12%, P = 0.00, Figure 2).
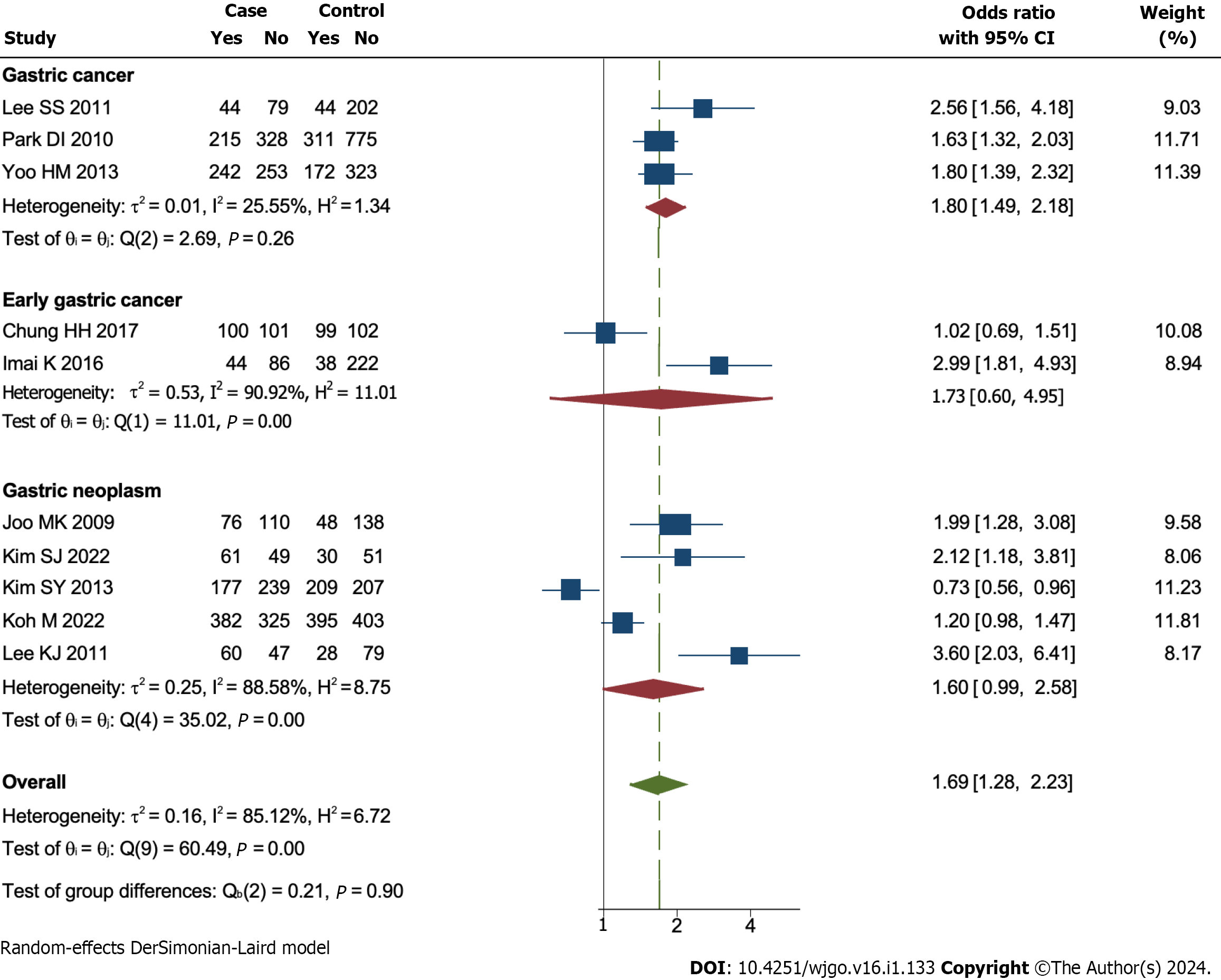
Subgroup analysis according to patients with different kinds of GNs was conducted. The results showed that GC patients (OR = 1.80, 95%CI = 1.49 to 2.18, I2 = 25.55%, P < 0.1) had a higher prevalence of CRN compared to patients with EGC (OR = 1.73, 95%CI = 0.60 to 4.95, I2 = 90.92%, P > 0.1) or EGN (OR = 1.60, 95%CI = 0.99 to 2.23, I2 = 85.12%, P > 0.1, Figure 3).
As for the whole patients included in this study, the analysis showed that age (OR = 1.08, 95%CI = 1.00 to 1.17, I2 = 90.13%, P = 0.00) and male (OR = 2.31, 95%CI = 1.26 to 4.22, I2 = 87.35%, P = 0.00) were independent risk factors for CRN. Other variables including BMI, smoking, drinking, and diabetes had no predictive value (P > 0.1). As for patients in the GN group, the analysis showed that BMI (OR = 0.88, 95%CI = 0.80 to 0.98, I2 = 0.00%, P = 0.92) was a protective factor and smoking (OR = 1.03, 95%CI = 1.01 to 1.05, I2 = 0.00%, P = 0.57) was a risk factor for CRN. Other variables including age, male, and drinking had no predictive value (P > 0.1, Table 3)
| Variables | Studies | Participants (GN group/control group) | Odds ratio (95%CI) | Heterogeneity |
| Whole group | ||||
| Age | 6 | 1735/1956 | 1.08 (1.00, 1.17); P = 0.04 | I2 = 90.13%; P = 0.00 |
| Male | 5 | 1625/1846 | 2.31 (1.26, 4.22); P = 0.01 | I2 = 87.35%; P = 0.00 |
| BMI | 3 | 944/1165 | 1.04 (0.82,1.32); P = 0.73 | I2 = 0.00%; P = 0.44 |
| Smoking | 2 | 237/367 | 1.16 (0.70, 1.91); P = 0.57 | I2 = 0.00%; P = 0.72 |
| Drinking | 2 | 237/367 | 1.23 (0.79, 1.92); P = 0.97 | I2 = 0.00%; P = 0.35 |
| Diabetes | 2 | 293/293 | 1.16 (0.40, 3.36); P = 0.79 | I2 = 76.42%; P = 0.04 |
| GN group | ||||
| Age | 4 | 933/1063 | 2.17 (0.91, 5.17); P = 0.08 | I2 = 83.37%; P = 0.00 |
| Male | 4 | 933/1063 | 1.85 (0.88, 3.90); P = 0.10 | I2 = 68.71%; P = 0.02 |
| BMI | 3 | 438/568 | 0.88 (0.80, 0.98); P = 0.02 | I2 =0.00%; P = 0.92 |
| Smoking | 3 | 438/568 | 1.03 (1.01, 1.05); P = 0.02 | I2 = 0.00%; P = 0.57 |
| Drinking | 2 | 237/367 | 1.36 (0.71, 2.62); P = 0.36 | I2 = 0.00%; P = 0.79 |
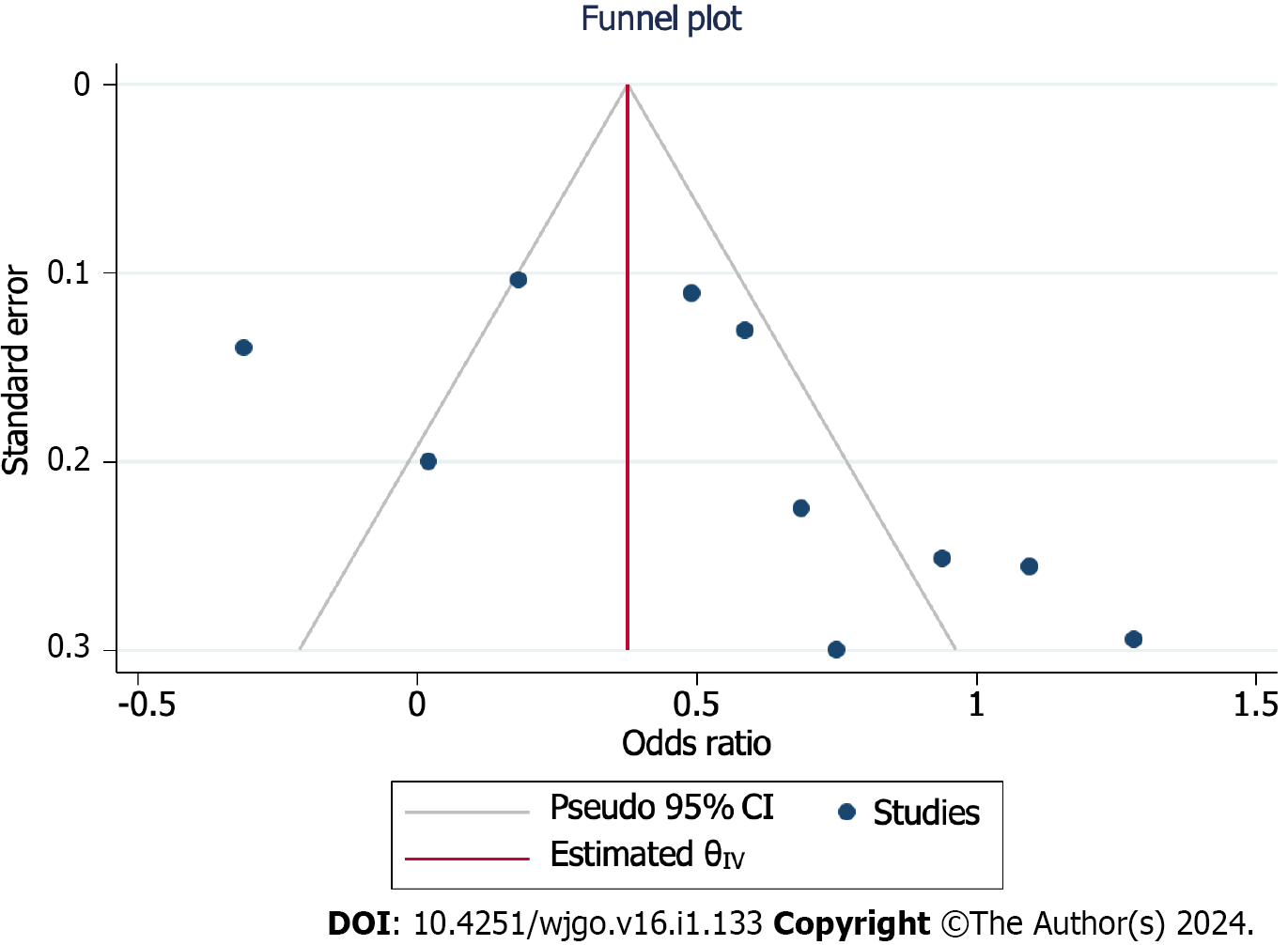
The funnel plot was used for evaluating the publication bias. The plot was not relatively symmetrical, and four plots were outside the 95%CIs, which meant that the results were affected by some publication bias (Figure 4).
This study evaluated the sensitivity by duplicate analysis of excluding each study at a time. The results of every time analysis were not significantly different, which meant that the results were relatively robust.
The current analysis included 6923 patients and found that GN patients had a higher risk of CRN, especially GC patients. Moreover, age and male sex were found to be independent risk factors for CRN in all patients, and BMI and smoking were protective and risk factors in GN patients, respectively.
Other primary neoplasms are common in GN patients, with the incidence ranging from 3.4% to 42.2%[23-26]. CRN is the main neoplasm type of synchronous and homologous neoplasms[23,24]. Although early CRN and EGN share many similarities, they have different tumor immune signatures and drug responses, which pose significant challenges for advanced CRN and GN[27-30]. Early detection of neoplasms is obviously an important way to improve patient prognosis; therefore, regular medical checkups are needed for GN patients.
Several previous studies have revealed an association between GN and CRN. Imai et al[11] reported that EGC is a risk factor for CRC. Others have demonstrated that GC patients are at high risk for not only CRC but also all CRN[15,31,32]. Moreover, colonoscopy might be considered for patients with benign GN[12-14]. However, both Chung et al[16] and Koh et al[18] revealed that the prevalence of CRN was not significantly different between patients with and without GN. Based on the above findings, our study was designed to address the current controversy and provide more valuable suggestions for GN patients.
In addition to GN, H. pylori is thought to promote the development of CRN[33,34]. H. pylori can not only increase the risk of GN and GC by damaging the mucosal barrier but also affect intestinal mucosa through the secretion of gastrin[35,36]. Moreover, H. pylori can alter the immune signature by reducing T cells, pro-carcinogenic STAT3 signaling, and goblet cells, which have proinflammatory and degrading microbial effects, contributing to neoplasm development[37,38]. Reducing the incidence of GN and CRN through eradication of H. pylori has been demonstrated in both mice and humans[37].
Another hypothesis is associated with genetic alteration and microsatellite instability[39,40]. Mutations in the hMSH2 and hMLH1 genes, which mainly participate in repair of base-pair mismatches during DNA replication, play an important role in the occurrence of GN and CRN[39]. In addition, the same K-ras, p53, and APC genes mutations are detected in both GN and CRN[40]. These genetic correlations between CRN and GN support the higher risk of CRN in GN patients, as indicated in the current analysis.
This study addresses a current pressing question and provides reliable evidence for GN patients to receive regular colonoscopy. Since almost all the patients were Korean, the results are particularly applicable to Korea. Although there were important discoveries revealed by this study, there are some limitations. The results are limited in terms of region and ethnicity, and there is some publication bias. Therefore, more prospective case-control studies conducted worldwide are needed for further investigation.
Patients are recommended to receive colonoscopy when diagnosed with GN, especially those diagnosed with GC.
Gastric cancer (GC) and colorectal cancer (CRC) are the fifth and third most common cancer worldwide, respectively. Nowadays, GC is reported to have a potential predictive value for CRC, especially for advanced CRC.
Colonoscopy is not commonly received by GC patients. Whether colonoscopy is necessary for GC patients is unclear.
The objectives of this study are patients diagnosed with gastric neoplasms (GNs).
This study conducted a pooling-up analysis and subgroup analysis by STATA SE 16.0 software.
Colorectal neoplasm (CRN) was detected significantly more frequently in GN patients than controls.
GC patients were suggested to receive colonoscopy before surgery.
This study first systematically reviewed the prevalence of CRNs in patients with and without GNs.
We acknowledged to all the authors in this article, and we thank Xun Lei for the substantial work in the statistical methods.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotelevets SM, Russia; Senchukova M, Russia; Samy Azer, Saudi Arabia S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Kang B, Liu XY, Cheng YX, Tao W, Peng D. Factors associated with hypertension remission after gastrectomy for gastric cancer patients. World J Gastrointest Surg. 2022;14:743-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Nam SY, Park BJ, Ryu KH, Nam JH. Effect of Helicobacter pylori infection and its eradication on the fate of gastric polyps. Eur J Gastroenterol Hepatol. 2016;28:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Cho YS, Nam SY, Moon HS, Kim TH, Kim SE, Jung JT. Helicobacter pylori eradication reduces risk for recurrence of gastric hyperplastic polyp after endoscopic resection. Korean J Intern Med. 2023;38:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Qaderi SM, Vromen H, Dekker HM, Stommel MWJ, Bremers AJA, de Wilt JHW. Development and implementation of a remote follow-up plan for colorectal cancer patients. Eur J Surg Oncol. 2020;46:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 907] [Article Influence: 113.4] [Reference Citation Analysis (2)] |
| 7. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;159:1916-1934.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 8. | Sninsky JA, Shore BM, Lupu GV, Crockett SD. Risk Factors for Colorectal Polyps and Cancer. Gastrointest Endosc Clin N Am. 2022;32:195-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Zhou E, Rifkin S. Colorectal Cancer and Diet: Risk Versus Prevention, Is Diet an Intervention? Gastroenterol Clin North Am. 2021;50:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, Chen H, Dai M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021;522:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 11. | Imai K, Hotta K, Yamaguchi Y, Kawata N, Kakushima N, Tanaka M, Takizawa K, Matsubayashi H, Shimoda T, Mori K, Ono H. Clinical impact of colonoscopy for patients with early gastric cancer treated by endoscopic submucosal dissection: A matched case-control study. Dig Liver Dis. 2017;49:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Joo MK, Park JJ, Lee WW, Lee BJ, Hwang JK, Kim SH, Jung W, Kim JH, Yeon JE, Kim JS, Byun KS, Bak YT. Differences in the prevalence of colorectal polyps in patients undergoing endoscopic removal of gastric adenoma or early gastric cancer and in healthy individuals. Endoscopy. 2010;42:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kim SJ, Lee J, Baek DY, Lee JH, Hong R. Early gastric neoplasms are significant risk factor for colorectal adenoma: A prospective case-control study. Medicine (Baltimore). 2022;101:e29956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Lee KJ, Kim JH, Kim SI, Jang JH, Lee HH, Hong SN, Lee SY, Sung IK, Park HS, Shim CS, Han HS. Clinical significance of colonoscopic examination in patients with early stage of gastric neoplasm undergoing endoscopic submucosal dissection. Scand J Gastroenterol. 2011;46:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Lee SS, Jung WT, Kim CY, Ha CY, Min HJ, Kim HJ, Kim TH. The synchronous prevalence of colorectal neoplasms in patients with stomach cancer. J Korean Soc Coloproctol. 2011;27:246-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Chung HH, Kim KO, Lee SH, Jang BI, Kim TN. Frequency and risk factors of colorectal adenoma in patients with early gastric cancer. Intern Med J. 2017;47:1184-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Kim SY, Jung SW, Hyun JJ, Koo JS, Choung RS, Yim HJ, Lee SW, Choi JH. Is colonoscopic screening necessary for patients with gastric adenoma or cancer? Dig Dis Sci. 2013;58:3263-3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Koh M, Kim MC, Jang JS. Difference in the prevalence of advanced colon adenoma between patients with gastric neoplasm and healthy people: A STROBE-compliant study. Medicine (Baltimore). 2022;101:e29308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 1308] [Article Influence: 327.0] [Reference Citation Analysis (1)] |
| 20. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12634] [Article Influence: 842.3] [Reference Citation Analysis (0)] |
| 21. | Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 460] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 22. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 23. | Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, Lee KU. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008;98:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Raszeja-Wyszomirska J, Białek A, Karpińska-Kaczmarczyk K, Starzyńska T. Synchronous and metachronous neoplasms in gastric cancer patients: a 23-year study. World J Gastroenterol. 2014;20:7480-7487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Aoyama T, Ju M, Komori K, Tamagawa H, Tamagawa A, Maezawa Y, Hashimoto I, Kano K, Hara K, Cho H, Morita J, Segami K, Onodera A, Endo K, Onuma S, Oshima T, Yukawa N, Rino Y. The Clinical Impact of Synchronous and Metachronous Other Primary Cancer in Gastric Cancer Patients Who Receive Curative Treatment. In Vivo. 2022;36:2514-2520. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Zhang W, Peng D. The outcome of young vs. old gastric cancer patients following gastrectomy: a propensity score matching analysis. BMC Surg. 2021;21:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Suzuki A, Koide N, Takeuchi D, Okumura M, Ishizone S, Suga T, Miyagawa S. Prevalence of synchronous colorectal neoplasms in surgically treated gastric cancer patients and significance of screening colonoscopy. Dig Endosc. 2014;26:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Cheng YX, Tao W, Kang B, Liu XY, Yuan C, Zhang B, Peng D. Impact of Preoperative Type 2 Diabetes Mellitus on the Outcomes of Gastric Cancer Patients Following Gastrectomy: A Propensity Score Matching Analysis. Front Surg. 2022;9:850265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 29. | Yang W, Zhao Y, Ge Q, Wang X, Jing Y, Zhao J, Liu G, Huang H, Cheng F, Ye Y, Song W, Liu X, Du J, Sheng J, Cao X. Genetic mutation and tumor microbiota determine heterogenicity of tumor immune signature: Evidence from gastric and colorectal synchronous cancers. Front Immunol. 2022;13:947080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Peng D, Zou YY, Cheng YX, Tao W, Zhang W. Effect of Time (Season, Surgical Starting Time, Waiting Time) on Patients with Gastric Cancer. Risk Manag Healthc Policy. 2021;14:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Park DI, Park SH, Yoo TW, Kim HS, Yang SK, Byeon JS, Koh BM, Kim JO, Shim KN, Jeen YT, Lee BI, Choi KY, Lee HL, Han DS, Baek I, Park CH, Park SJ. The prevalence of colorectal neoplasia in patients with gastric cancer: a Korean Association for the Study of Intestinal Disease (KASID) Study. J Clin Gastroenterol. 2010;44:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Yoo HM, Gweon TG, Seo HS, Shim JH, Oh SI, Choi MG, Song KY, Jeon HM, Park CH. Role of preoperative colonoscopy in patients with gastric cancer: a case control study of the prevalence of coexisting colorectal neoplasms. Ann Surg Oncol. 2013;20:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Chen XZ, Schöttker B, Castro FA, Chen H, Zhang Y, Holleczek B, Brenner H. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget. 2016;7:17182-17193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Fujimori S. Progress in elucidating the relationship between Helicobacter pylori infection and intestinal diseases. World J Gastroenterol. 2021;27:8040-8046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 35. | Bornschein J, Malfertheiner P. Helicobacter pylori and gastric cancer. Dig Dis. 2014;32:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Kim HO, Hwang SI, Yoo CH, Kim H. Preoperative colonoscopy for patients with gastric adenocarcinoma. J Gastroenterol Hepatol. 2009;24:1740-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Ralser A, Dietl A, Jarosch S, Engelsberger V, Wanisch A, Janssen KP, Middelhoff M, Vieth M, Quante M, Haller D, Busch DH, Deng L, Mejías-Luque R, Gerhard M. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut. 2023;72:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 38. | Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol. 2022;12:1023177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 205] [Reference Citation Analysis (0)] |
| 39. | Schulmann K, Reiser M, Schmiegel W. Colonic cancer and polyps. Best Pract Res Clin Gastroenterol. 2002;16:91-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Ohtani H, Yashiro M, Onoda N, Nishioka N, Kato Y, Yamamoto S, Fukushima S, Hirakawa-Ys Chung K. Synchronous multiple primary gastrointestinal cancer exhibits frequent microsatellite instability. Int J Cancer. 2000;86:678-683. [PubMed] [DOI] [Full Text] |