Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.118
Peer-review started: August 4, 2023
First decision: October 23, 2023
Revised: November 20, 2023
Accepted: December 12, 2023
Article in press: December 12, 2023
Published online: January 15, 2024
Processing time: 159 Days and 22.8 Hours
The TGF-β/SMAD3 and VEGFR-1 signaling pathways play important roles in gastric cancer metastasis. SMAD3 phosphorylation is a crucial prognostic marker in gastric cancer.
To determine the prognostic value and relationship of SMAD3 phospho-isoforms and VEGFR-1 in gastric cancer.
This was a single-center observational study which enrolled 98 gastric cancer patients and 82 adjacent normal gastric tissues from patients aged 32-84 years (median age 65) between July 2006 and April 2007. Patients were followed up until death or the study ended (median follow-up duration of 28.5 mo). The samples were used to generate tissue microarrays (TMAs) for immunohistochemical (IHC) staining. The expressions of TGF-β1, pSMAD3C(S423/425), pSMAD3L(S204), and VEGFR-1 in gastric cancer (GC) tumor tissue and normal tissue were measured by IHC staining using TMAs obtained from 98 GC patients. Prognosis and survival information of the patients was recorded by Outdo Biotech from May 2007 to July 2015. The relationship between TGF-β1, pSMAD
TGFβ-1 and VEGFR-1 expression was significantly upregulated in gastric cancer tissue compared to adjacent non-cancerous tissue. The positive expression of phosphorylated isoforms of Smad3 varied depending on the phosphorylation site [pSMAD3C(S423/425): 51.0% and pSMAD3L(S204): 31.6%]. High expression of pSMAD
Co-upregulation of pSMAD3L(S204) and VEGFR-1 can serve as a predictive marker for poor gastric cancer prognosis, and pSMAD3L(204) may be involved in enhanced gastric cancer metastasis in a VEGFR-1-dependent manner.
Core Tip: This study investigated the prognostic value and relationship between SMAD3 phospho-isoforms and VEGFR-1 in gastric cancer. The results showed that high expression of both pSMAD3L(S204) and VEGFR-1 was associated with poor overall survival in gastric cancer patients. Co-upregulation of pSMAD3L(S204) and VEGFR-1 can serve as a predictive marker for poor gastric cancer prognosis.
- Citation: Lv SL, Guo P, Zou JR, Chen RS, Luo LY, Huang DQ. Prognostic significance and relationship of SMAD3 phospho-isoforms and VEGFR-1 in gastric cancer: A clinicopathological study. World J Gastrointest Oncol 2024; 16(1): 118-132
- URL: https://www.wjgnet.com/1948-5204/full/v16/i1/118.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i1.118
Gastric cancer (GC) is one of the leading causes of cancer-related deaths worldwide[1]. Despite improvements in therapeutic strategies, GC, especially advanced GC, has a high mortality rate[2,3]. Tumor metastasis, in which angio
VEGFR-1 (Vascular endothelial growth factor receptor-1) is a tyrosine kinase receptor for VEGF-A, VEGF-B, and placental growth factor (PlGF)[6]. VEGFR-1 is mainly expressed in endothelial cells, smooth muscle cells, and macro
TGF-β is a pleiotropic cytokine that regulates various biological functions, including angiogenesis, tumorigenesis, proliferation, differentiation, and fibrosis[12]. The TGF-β/SMAD axis is a primary signaling pathway that promotes epithelial-mesenchymal transition (EMT)[13]. TGF-β can mediate SMAD2 and SMAD3 phosphorylation, allowing them to affect the transcription of genes related to tumor invasiveness[14,15]. SMAD3, a key component of this pathway, contains highly conserved MAD homology domains (MH1 and MH2) connected by a less conserved junction region (LR). This linker region contains four proline-directed kinase phosphorylation sites: Thr179, Ser204, Ser208, and Ser213. Phosphorylation of SMAD3 leads to the formation of three phosphorylated forms: Carboxyl-terminal phosphorylated SMAD3 (pSMAD3C), linker-phosphorylated SMAD3 (pSMAD3L), and carboxyl-linker-dual phosphorylated SMAD3 (pSMAD3L/C)[16].
The carboxyl-terminal phosphorylation of SMAD3 is essential for TGF-β-mediated signal transduction. Blocking or attenuating carboxyl-terminal phosphorylation of SMAD3 at Ser423/425 significantly inhibits TGF-β expression levels in cancer cells[17,18]. In lung cancer, TGF-β-induced epithelial cell transformation depends on pSMAD3C[19]. High levels of pSMAD3L have been considered a primary risk factor in the clinical management of liver cancer, and inhibitors targeting the JNK/pSMAD3L axis have been shown to suppress hepatocellular carcinoma (HCC) progression[20]. Mutations in the phosphorylation sites of SMAD3 LR and carboxyl-terminus can impair primary tumor growth and metastasis, respectively, in breast cancer[21]. Furthermore, TGF-β promotes cancer cell growth in a SMAD3L/C-dependent manner[22]. These observations highlight the importance of SMAD3 phosphorylation in cancer cell behavior, suggesting a need for better understanding of SMAD3 phospho-isoforms in GC.
It has been shown that TGF-β1 elevates VEGF and VEGFR-1 expression through the SMAD pathway in mouse dendritic cells[23]. Additionally, PGF1 expressed on the surface of lung cancer cells can bind to VEGFR-1 on macrophages, resulting in increased TGF-β1 production and enhanced lung cancer angiogenesis[24]. These findings suggest that the TGF-β1/SMAD3 pathway is involved in VEGFR-1-promoted tumor angiogenesis. Moreover, the phosphorylation status at both the carboxyl-terminus and LR determines the activity of SMAD3[25]. For instance, TGF-β can phosphorylate SMAD3 at its LR, and consequently regulate its function[26]. However, the association of pSMAD3L with VEGFR-1 is unclear in GC.
To examine the relationship between SMAD3 phospho-isoforms and VEGFR-1, we measured the expression levels of pSMAD3C(S423/425), pSMAD3L(S204), VEGFR-1, and TGF-β1 in GC and investigated how their expression was related to the clinicopathological features and prognosis of patients with GC. Moreover, to investigate the association between TGF-β/SMADs signaling and VEGFR-1 protein expression, we analyzed the correlation between pSMAD3C(S423/425), pSMAD3L(S204), TGF-β1, and VEGFR-1 expression, and explored the significance of pSMAD3L(S204) and VEGFR-1 co-expression in GC.
Ninety-eight GC tissue samples (n = 98) and adjacent normal gastric tissues (n = 82) were postoperatively collected from patients aged 32-84 years (median age of 65 years) between July 2006 and April 2007 by Outdo Biotech (Shanghai, China). Samples were used to generate the tissue microarrays for immunohistochemical (IHC) staining. Prognosis and survival information were recorded by Outdo Biotech from May 2007 to July 2015. TNM staging was conducted according to the 7th edition criteria of the American Joint Committee on Cancer[27]. Histological grading of tumors followed the WHO guidance on digestive system tumor classification[28]. The study protocol was approved by the hospital ethics committee.
IHC was performed as previously described[29]. Briefly, paraffin-embedded sections (4 µm) were dewaxed and hydrated, followed by heat-induced antigen retrieval in citrate buffer (pH 6.0). Subsequently, endogenous peroxidases were blocked with H2O2, and sections were incubated with primary antibodies at 4°C overnight: anti-TGF-β1 (Abcam, 66043, 1:150), anti-pSMAD3C(S423/425) (Abcam, 52903, 1:150), anti-pSMAD3L(S204) (Thermo-Fisher Scientific, 2816414, 1:150), anti-VEGFR-1 (Abcarta, PA359, 1:100). The next day, sections were washed with PBS three times and incubated with anti-rabbit HRP-conjugated IgG secondary antibody (Zsbio, China) for 1 h at 37°C. Sections stained with PBS were used as the negative control. Subsequently, sections were washed and incubated with peroxidase substrate (Zsbio, China) for 20 min at 37°C. Finally, nuclei were counterstained with hematoxylin, and sections were processed for dehydration and covered with a mounting medium.
Two pathologists blinded to the study scored the degree of staining according to the German semi-quantitative statistical methods[30]. The overall staining intensity and percent of positively stained cells were used to reflect protein expression levels.
Overall staining intensity was divided into four categories: No staining (0); light yellow staining (1); yellow staining (2); and dark yellow/brown staining (3). The percent positive staining was defined as the percent of glandular epithelial cells within the tissue that were positively stained on the slide. The corresponding scoring criteria are as follows: < 5% (0); 5%-25% (1); 25%-50% (2); 50%-75% (3); > 75% (4). The overall staining intensity and percent positive staining scores were combined to describe the level of protein expression: negative (0-2), + (3-5), ++ (6-8), +++ (9-12). Ultimately, we classified all of the tissues into a low expression group (- or +) and high expression group (++ or +++)[29].
Statistical analyses were performed using SPSS 11.5 (SPSS Inc, Chicago, United States). The relationship between protein expression levels [TGF-β1, pSMAD3C (S423/425), pSMAD3L (S204), and VEGFR-1] and clinicopathological parameters were analyzed using the Chi-squared test. A survival curve was generated using the Kaplan-Meier survival analysis. Univariate and multivariate survival analyses were performed using the Cox proportional hazards regression model. The relationship between TGF-β1, pSMAD3C(S423/425), pSMAD3L(S204), and VEGFR-1 protein expression levels was analyzed using Pearson’s correlation coefficient. The receiver operating characteristic (ROC) curve was generated using the R package “pROC”. Nomograms and calibration plots were constructed using the R package “rms”. A P value of < 0.05 was considered statistically significant.
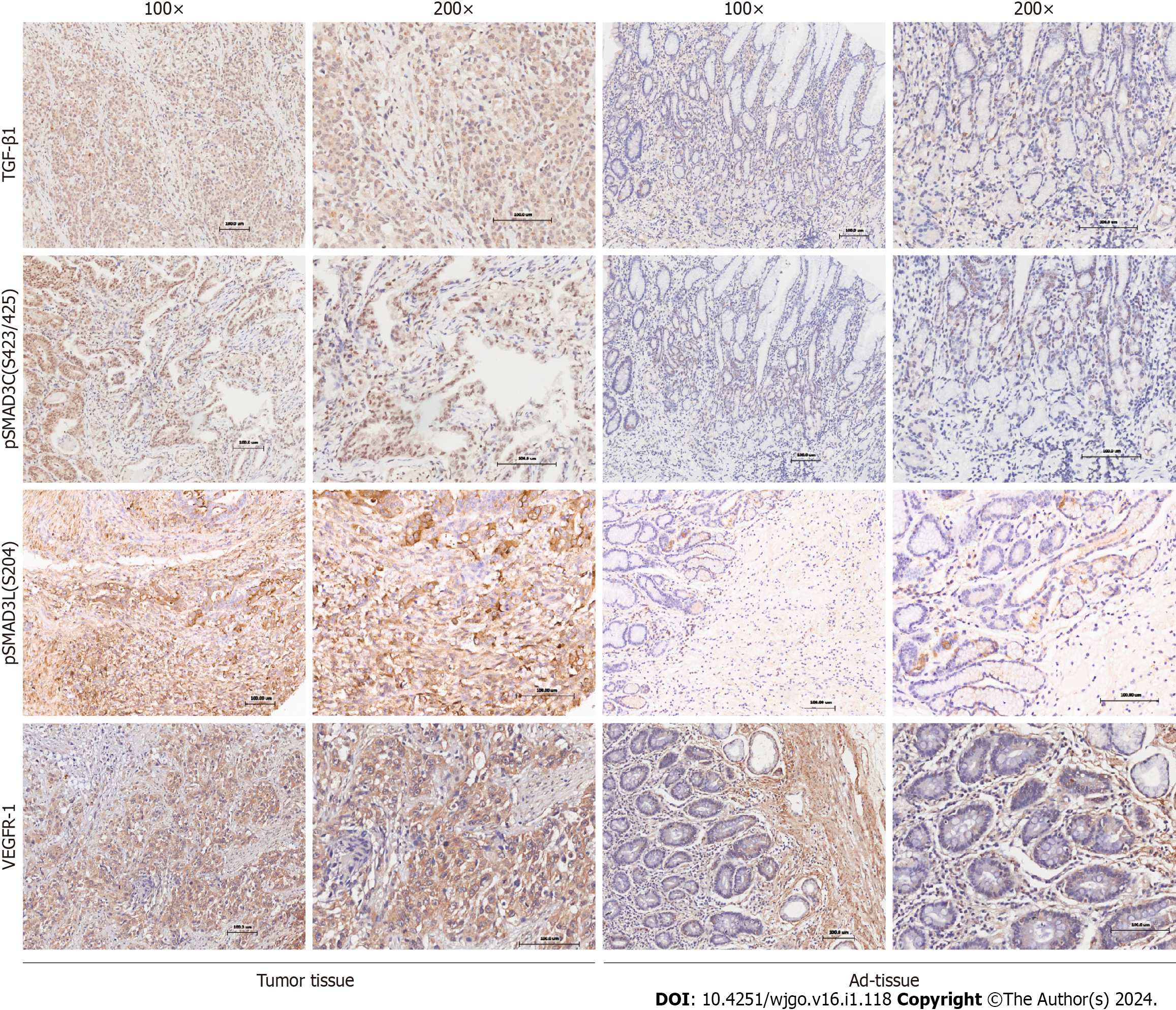
The expression and localization of phosphorylated SMAD3 isoforms, TGF-β1, and VEGFR-1 were evaluated in GC and adjacent normal tissues (Figure 1). TGF-β1 exhibited dominant cytoplasmic localization, while the pSMAD3C(S423/425) was predominantly observed in the nucleus. Statistical results showed significantly higher levels of pSMAD3C(S423/425) (P < 0.05) and TGF-β1 (P < 0.001) in GC tissues compared to adjacent normal tissues (Table 1). Notably, minimal pSMAD3L(S204) was detected in normal gastric tissue, but its immune reactivity was observed exclusively in the nuclei of cancer cells. IHC analysis revealed that 31.6% (31/98) of the GC samples exhibited pSMAD3L(S204) positivity. Moreover, VEGFR-1 displayed immune reactivity in both the cell membrane and cytoplasm of tumor cells, as well as tumor stromal vessels. Semi-quantitative analysis demonstrated significantly higher VEGFR-1 expression in GC compared to adjacent normal tissues (P < 0.001) (Table 1).
| IHC grading | pSmad3(S423/425) | TGF-β1 | VEGFR-1 | ||||||
| Tumor | Ad-tissue | P value | Tumor | Ad-tissue | P value | Tumor | Ad-tissue | P value | |
| - | 5 | 4 | < 0.05 | 3 | 3 | < 0.001 | 13 | 12 | < 0.001 |
| + | 43 | 38 | 31 | 58 | 27 | 65 | |||
| ++ | 41 | 40 | 56 | 21 | 50 | 3 | |||
| +++ | 9 | 0 | 8 | 0 | 8 | 2 | |||
| n | 98 | 82 | 98 | 82 | 98 | 82 | |||
We investigated the correlation of pSMAD3C(S423/425), TGF-β1, pSMAD3L(S204), and VEGFR-1 and clinicopathological factors of the 98 GC patients (Table 2). The results revealed significant associations between the expression levels of these proteins and specific clinicopathological features. Elevated expression of pSMAD3C(S423/425) was significantly associated with distant metastasis (M staging) (P = 0.042). High expression of TGF-β1 was correlated with advanced T stage (P = 0.042) and tumors located in the gastric antrum (P = 0.022). Furthermore, higher expression of pSMAD3L(S204) was positively associated with larger tumors (P = 0.038) and advanced N stage (P = 0.035). In addition, increased expression levels of VEGFR-1 were associated with larger tumors (P = 0.015) and higher tumor pathological grades (P = 0.013). However, no significant correlations were found between the expression levels of these proteins (pSMAD3C(S423/425), TGF-β1, pSMAD3L(S204), and VEGFR-1) and other clinicopathological parameters (P = > 0.05).
| Clinicopathological factors | Total (n = 98) | pSMAD3C (S423/425) | TGF-β1 | pSMAD3L (S204) | VEGFR-1 | ||||||||
| Low (n = 48) | High (n = 50) | P value | Low (n = 34) | High (n = 64) | P value | Low (n = 67) | High (n = 31) | P value | Low (n = 40) | High (n = 58) | P value | ||
| Gender | 0.878 | 0.822 | 0.239 | 0.470 | |||||||||
| Female | 36 (36.7) | 18 (37.5) | 18 (36) | 13 (38.2) | 23 (35.9) | 22 (32.8) | 14 (45.2) | 13 (32.5) | 23 (39.7) | ||||
| Male | 62 (63.3) | 30 (62.5) | 32 (64) | 21 (61.8) | 41 (64.1) | 45 (67.2) | 17 (54.8) | 27 (67.5) | 35 (60.3) | ||||
| Age (yr) | 0.225 | 0.515 | 0.796 | 0.818 | |||||||||
| ≤ 60 | 33 (33.7) | 19 (39.6) | 14 (28) | 10 (29.4) | 23 (35.9) | 22 (32.8) | 11 (35.5) | 14 (35) | 19 (32.8) | ||||
| > 60 | 65 (66.3) | 29 (60.4) | 36 (72) | 24 (70.6) | 41 (64.1) | 45 (67.2) | 20 (64.5) | 26 (65) | 39 (67.2) | ||||
| Pathological grade | 0.556 | 0.459 | 0.402 | 0.013 | |||||||||
| I/II | 44 (44.9) | 23 (47.9) | 21 (42) | 17 (50) | 27 (42.2) | 32 (47.8) | 12 (38.7) | 24 (60) | 20 (34.5) | ||||
| III/IV | 54 (55.1) | 25 (52.1) | 29 (58) | 17 (50) | 37 (57.8) | 35 (52.2) | 19 (61.3) | 16 (40) | 38 (65.5) | ||||
| T Stage | 0.946 | 0.042 | 0.946 | 0.248 | |||||||||
| T1 | 6 (6.1) | 3 (6.2) | 3 (6) | 4 (11.8) | 2 (3.1) | 4 (6) | 2 (6.5) | 1 (2.5) | 5 (8.6) | ||||
| T2 | 9 (9.2) | 5 (10.4) | 4 (8) | 4 (11.8) | 5 (7.8) | 6 (9) | 3 (9.7) | 4 (10) | 5 (8.6) | ||||
| T3 | 64 (65.3) | 30 (62.5) | 34 (68) | 24 (70.6) | 40 (62.5) | 45 (67.2) | 19 (61.3) | 30 (75) | 34 (58.6) | ||||
| T4 | 19 (19.4) | 10 (20.8) | 9 (18) | 2 (5.9) | 17 (26.6) | 12 (17.9) | 7 (22.6) | 5 (12.5) | 14 (24.1) | ||||
| N Stage | 0.640 | 0.344 | 0.035 | 0.421 | |||||||||
| N0/N1 | 37 (37.8) | 17 (35.4) | 20 (40) | 15 (44.1) | 22 (34.4) | 30 (44.8) | 7 (22.6) | 17 (42.5) | 20 (34.5) | ||||
| N2/N3 | 61 (62.2) | 31 (64.6) | 30 (60) | 19 (55.9) | 42 (65.6) | 37 (55.2) | 24 (77.4) | 23 (57.5) | 38 (65.5) | ||||
| Metastasis | 0.042 | 0.233 | 0.623 | 0.404 | |||||||||
| No | 89 (90.8) | 47 (97.9) | 42 (84) | 33 (97.1) | 56 (87.5) | 62 (92.5) | 27 (87.1) | 38 (95) | 51 (87.9) | ||||
| Yes | 9 (9.2) | 1 (2.1) | 8 (16) | 1 (2.9) | 8 (12.5) | 5 (7.5) | 4 (12.9) | 2 (5) | 7 (12.1) | ||||
| Cancer stage | 0.105 | 0.444 | 0.338 | 0.119 | |||||||||
| I | 9 (9.2) | 4 (8.3) | 5 (10) | 4 (11.8) | 5 (7.8) | 5 (7.5) | 4 (12.9) | 2 (5) | 7 (12.1) | ||||
| II | 30 (30.6) | 17 (35.4) | 13 (26) | 11 (32.4) | 19 (29.7) | 24 (35.8) | 6 (19.4) | 17 (42.5) | 13 (22.4) | ||||
| III | 50 (51.0) | 26 (54.2) | 24 (48) | 18 (52.9) | 32 (50) | 33 (49.3) | 17 (54.8) | 19 (47.5) | 31 (53.4) | ||||
| IV | 9 (9.2) | 1 (2.1) | 8 (16) | 1 (2.9) | 8 (12.5) | 5 (7.5) | 4 (12.9) | 2 (5) | 7 (12.1) | ||||
| Tumor size (cm) | 0.791 | 0.829 | 0.038 | 0.015 | |||||||||
| ≤ 5 | 62 (63.3) | 31 (64.6) | 31 (62) | 22 (64.7) | 40 (62.5) | 47 (70.1) | 15 (48.4) | 31 (77.5) | 31 (53.4) | ||||
| > 5 | 36 (36.7) | 17 (35.4) | 19 (38) | 12 (35.3) | 24 (37.5) | 20 (29.9) | 16 (51.6) | 9 (22.5) | 27 (46.6) | ||||
| Tumor location | 0.427 | 0.022 | 0.739 | 0.501 | |||||||||
| Antrum | 53 (54.1) | 24 (50) | 29 (58) | 13 (38.2) | 40 (62.5) | 37 (55.2) | 16 (51.6) | 20 (50) | 33 (56.9) | ||||
| Others | 45 (45.9) | 24 (50) | 21 (42) | 21 (61.8) | 24 (37.5) | 30 (44.8) | 15 (48.4) | 20 (50) | 25 (43.1) | ||||
Quantification of protein levels revealed that expression of TGF-β1, pSMAD3C(S423/425), and pSMAD3L(S204) was positively correlated with VEGFR-1 (r = 0.220, P = 0.029; r = 0.302, P = 0.002; r = 0.201, P = 0.047, respectively). These results indicate that SMAD3 phosphor-isoforms may regulate TGF-β1-mediated tumor migration in a VEGFR-1-dependent manner (Table 3).
| VEGFR-1 | pSMAD3-S425 | TGF-β1 | pSMAD3-S204 | |||||||||||||||
| - | + | ++ | +++ | r value | P value | - | + | ++ | +++ | r value | P value | - | + | ++ | +++ | r value | P value | |
| - | 0 | 10 | 3 | 0 | 0.302 | 0.002 | 0 | 8 | 5 | 0 | 0.22 | 0.029 | 1 | 10 | 1 | 1 | 0.201 | 0.047 |
| + | 3 | 12 | 11 | 1 | 1 | 8 | 15 | 3 | 4 | 18 | 5 | 0 | ||||||
| ++ | 2 | 20 | 22 | 6 | 2 | 14 | 32 | 2 | 6 | 24 | 14 | 6 | ||||||
| +++ | 0 | 1 | 5 | 2 | 0 | 1 | 4 | 3 | 1 | 3 | 3 | 1 | ||||||
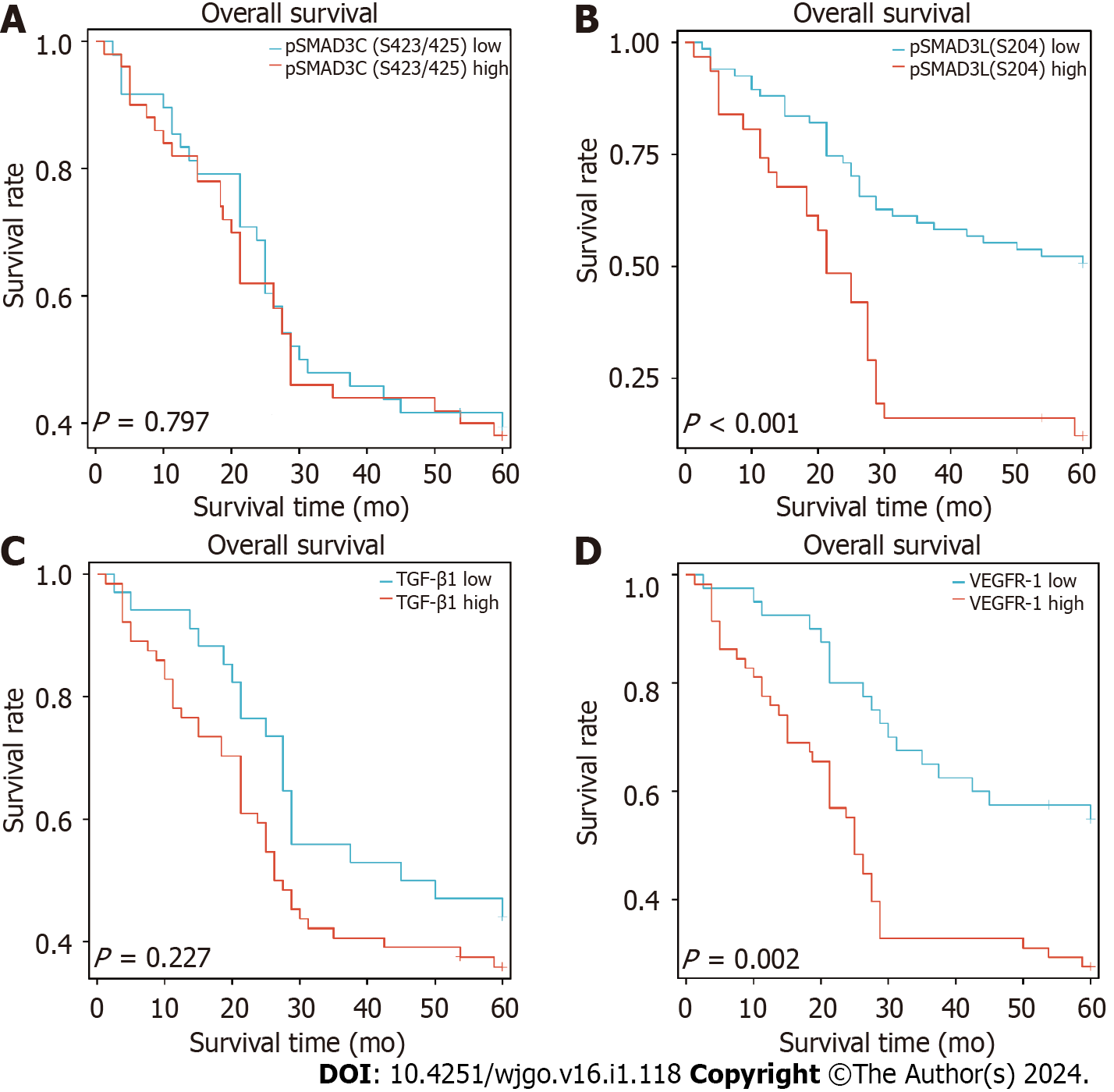
We assessed the overall survival (OS) of 98 GC patients and found that the 3-year and 5-year OS rates were 45.9% and 38.8%, respectively. Kaplan-Meier analysis revealed a significant association between higher expression of pSMAD3L(S204) and reduced OS (P < 0.001), and a significant correlation between higher expression of VEGFR-1 and decreased OS (P = 0.002). Notably, although patients with higher expression of TGF-β1 exhibited a trend of decreased OS, this trend did not reach statistical significance (P = 0.227). Interestingly, the expression of pSMAD3C(S423/425) did not significantly impact patient prognosis (Figure 2).
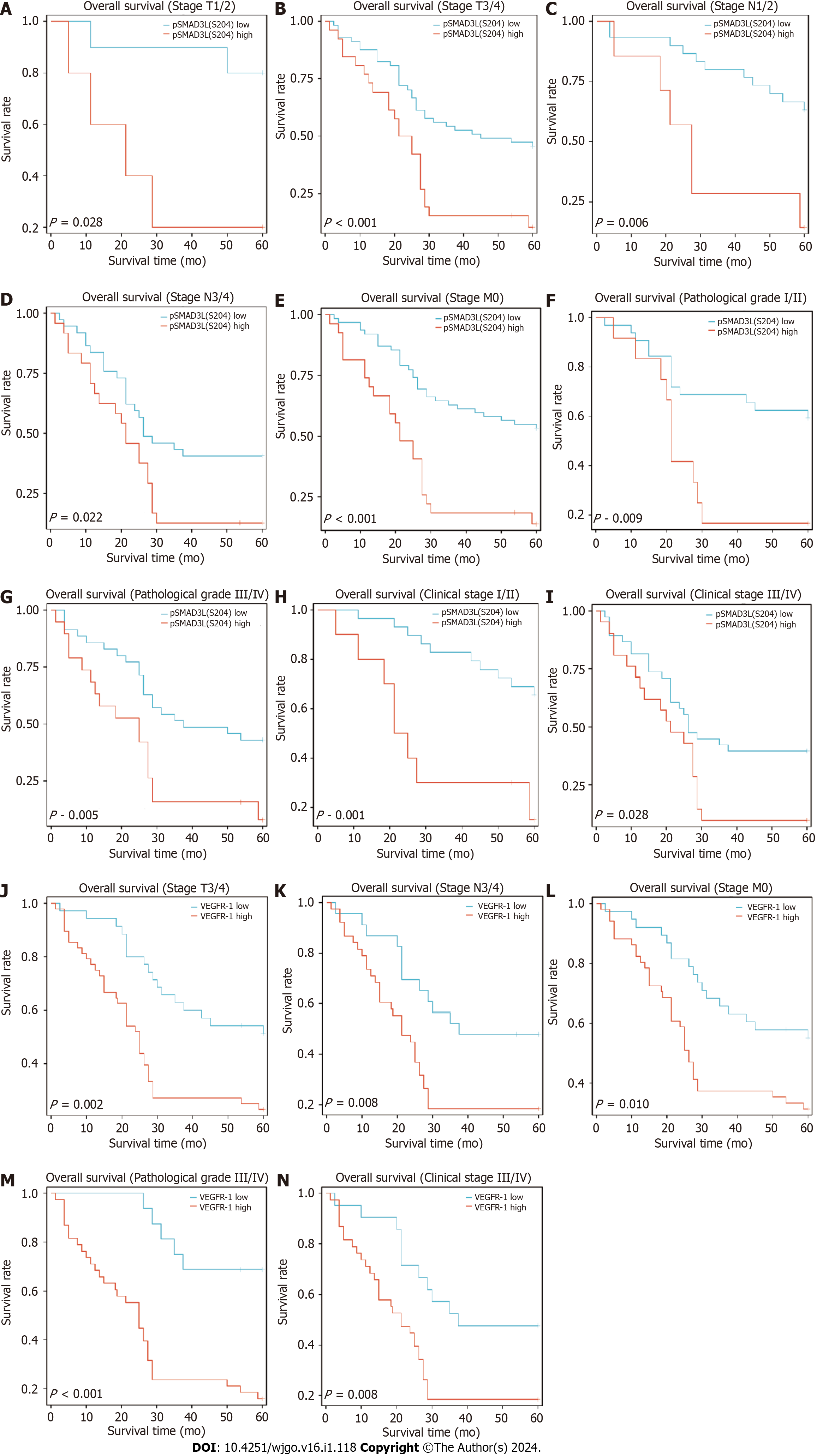
To further investigate the impact of pSMAD3L(S204) and VEGFR-1 expression on other prognostic factors, subgroup survival analyses were performed. The results showed that elevated pSMAD3L(S204) was significantly associated with OS in different cancer stages (I/II stage, P = 0.001; III/IV stage, P = 0.028), TNM stages (M0, P < 0.001; T1/T2, p=0.028; T3/T4, P < 0.001; N1/N2, P = 0.006; N3/N4, P = 0.022), and pathological grades (Grade I/II, p=0.009; Grade III/IV, P = 0.005) (Figure 3A-I). The results also showed that elevated VEGFR-1 was significantly associated with OS of cancer stages (III/IV stage, P = 0.008), TNM stages (M0, P = 0.01; T3/T4, P = 0.002; N3/N4, P = 0.008), and pathological grades (Grade III/IV, P < 0.001) (Figure 3J-N).
In addition, we investigated the impact of pSMAD3L(S204) and VEGFR-1 expression on GC prognosis through univariate and multivariate Cox regression analyses. The results showed that tumor size, N stage, M stage, pSMAD3L(S204) expression, VEGFR-1 expression, and TNM stage were significantly associated with poor prognosis (P < 0.05) (Table 4). Furthermore, multivariate survival analysis revealed that pSMAD3L(S204) and VEGFR-1 expression, as well as tumor size, were independent prognostic factors in GC patients (P < 0.05) (Table 4).
| Characteristics | HR (95%CI) univariate analysis | P value univariate analysis | HR (95%CI) multivariate analysis | P value multivariate analysis |
| Age (> 60 vs ≤ 60 yr) | 1.332 (0.767-2.314) | 0.309 | ||
| Sex (female vs male) | 0.995 (0.588-1.682) | 0.985 | ||
| Pathological Grade (III/IV vs I/II) | 1.490 (0.885-2.511) | 0.134 | ||
| Clinical T stage (T3–T4 vs T1–T2) | 1.974 (0.848-4.594) | 0.084 | 1.597 (0.644-3.964) | 0.312 |
| Clinical N stage (N2–N3 vs N0-N1) | 2.287 (1.299-4.026) | 0.004 | 1.469 (0.578-3.736) | 0.419 |
| Clinical M stage (M1 vs M0) | 2.664 (1.258-5.643) | 0.01 | 1.316 (0.586-2.954) | 0.506 |
| Clinical stage (stage III–IV vs stage I-II) | 2.304 (1.322-4.017) | 0.003 | 1.210 (0.472-3.106) | 0.691 |
| Tumor size (cm) (> 5 vs ≤ 5) | 2.616 (1.570-4.359) | < 0.001 | 2.033 (1.193-3.465) | 0.009 |
| Tumor location (antrum vs others) | 0.922 (0.555-1.530) | 0.753 | ||
| VEGFR-1 (High vs Low) | 2.360 (1.354-4.111) | 0.002 | 1.858 (1.033-3.339) | 0.038 |
| pSMAD3C (S423/425) (high vs low) | 1.069 (0.644-1.774) | 0.797 | ||
| TGF-β1 (high vs low) | 1.399 (0.811-2.411) | 0.227 | ||
| pSMAD3L(S204) (high vs low) | 2.884 (1.715-4.851) | < 0.001 | 1.943 (1.095-3.448) | 0.023 |
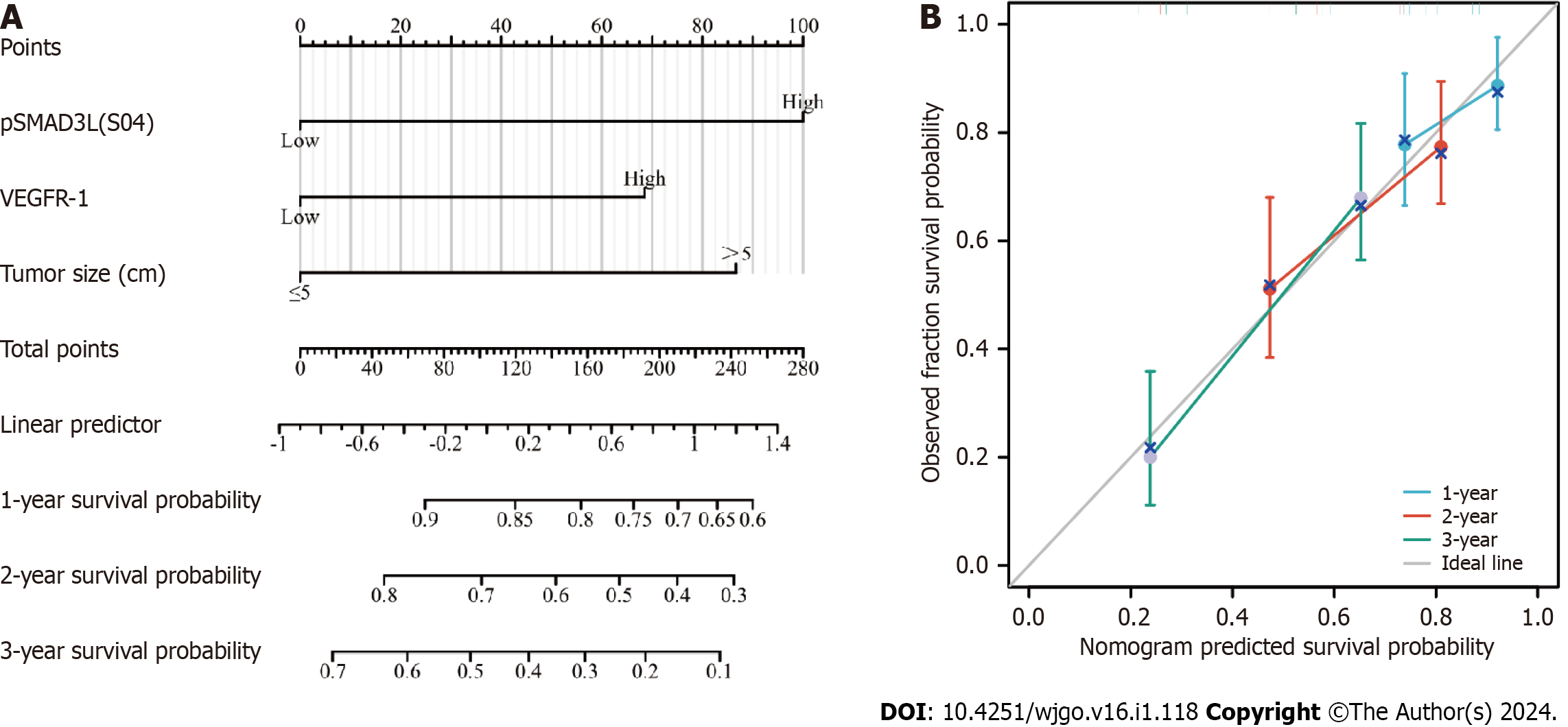
To predict OS, a prognostic nomogram was constructed incorporating pSMAD3L(S204) and VEGFR-1 expression levels, as well as tumor size, based on the statistically significant independent prognostic factors identified by the Cox regression analysis. The efficiency of the nomogram was assessed using calibration curves, which demonstrated optimal predictions for 1-, 2-, and 3-year clinical outcomes (Figure 4). The C-index was 0.690 (0.656-0.724), indicating its potential as a prognostic tool.
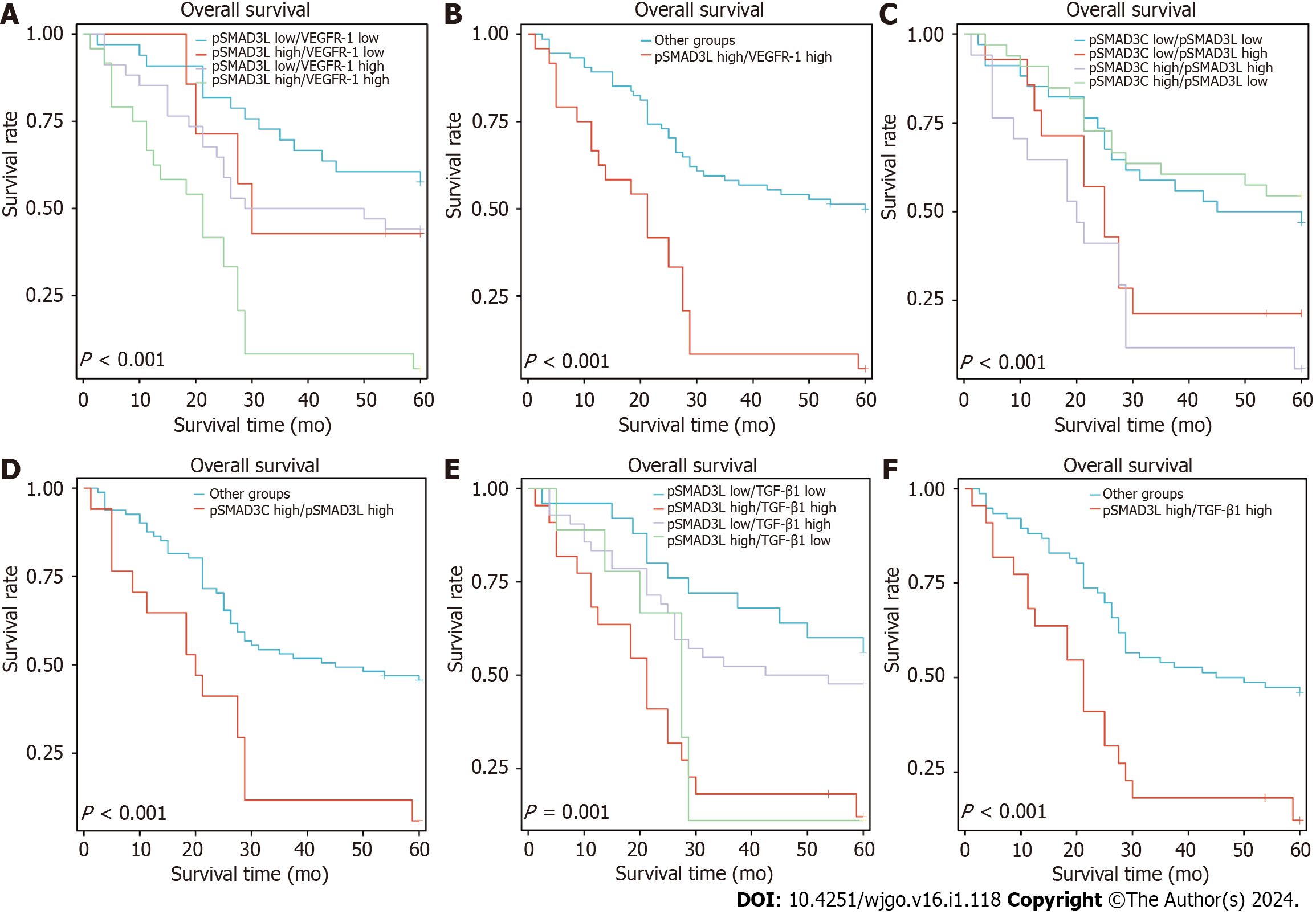
To investigate the prognostic value of co-expression patterns involving pSMAD3C(S204) and TGF-β1, VEGFR-1, or pSMAD3L(S423/425), we conducted a retrospective evaluation of the OS rate in 98 patients, with a median follow-up duration of 28.5 mo. The patients were categorized into four groups based on their expression levels of pSMAD3L(S204) and VEGFR-1: pSMAD3L low/VEGFR-1 low (n = 33), pSMAD3L High/VEGFR-1 low (n = 7), pSMAD3L low/VEGFR-1 high (n = 34), and pSMAD3L high/VEGFR-1 high (n = 24). Our results revealed that patients with high levels of both pSMAD3L(S204) and VEGFR-1 expression had the poorest OS (Figure 5A; P < 0.001). Furthermore, when comparing the OS of this group to that of all other biomarker combinations, the difference remained significant (Figure 5B; P < 0.001).
Similarly, we stratified the cohort based on the levels of pSMAD3L(S204) and pSMAD3C(S423/425) and assessed the OS of each group. Our findings showed that the OS of patients with pSMAD3C High/pSMAD3L High was significantly lower than that of patients with pSMAD3C High/pSMAD3L Low, pSMAD3C Low/pSMAD3L High, or pSMAD3C Low/pSMAD3L Low (Figure 5C; P < 0.001). Additionally, when comparing the OS of this group to that of all other biomarker combinations, the difference remained significant (Figure 5D; P < 0.001).
Furthermore, patients with high expression levels of both pSMAD3L and TGF-β1 (pSMAD3L high/TGF-β1 high) exhibited shorter OS compared to patients with pSMAD3L High/TGF-β1 Low, pSMAD3L Low/TGF-β1 High, or pSMAD3L Low/TGF-β1 Low) (Figure 5E and F).
These findings suggest that the co-expression patterns of pSMAD3C(S204) and TGF-β1, VEGFR-1, or pSMAD3L(S423/425) have prognostic implications and may provide valuable insights into the OS of GC patients.
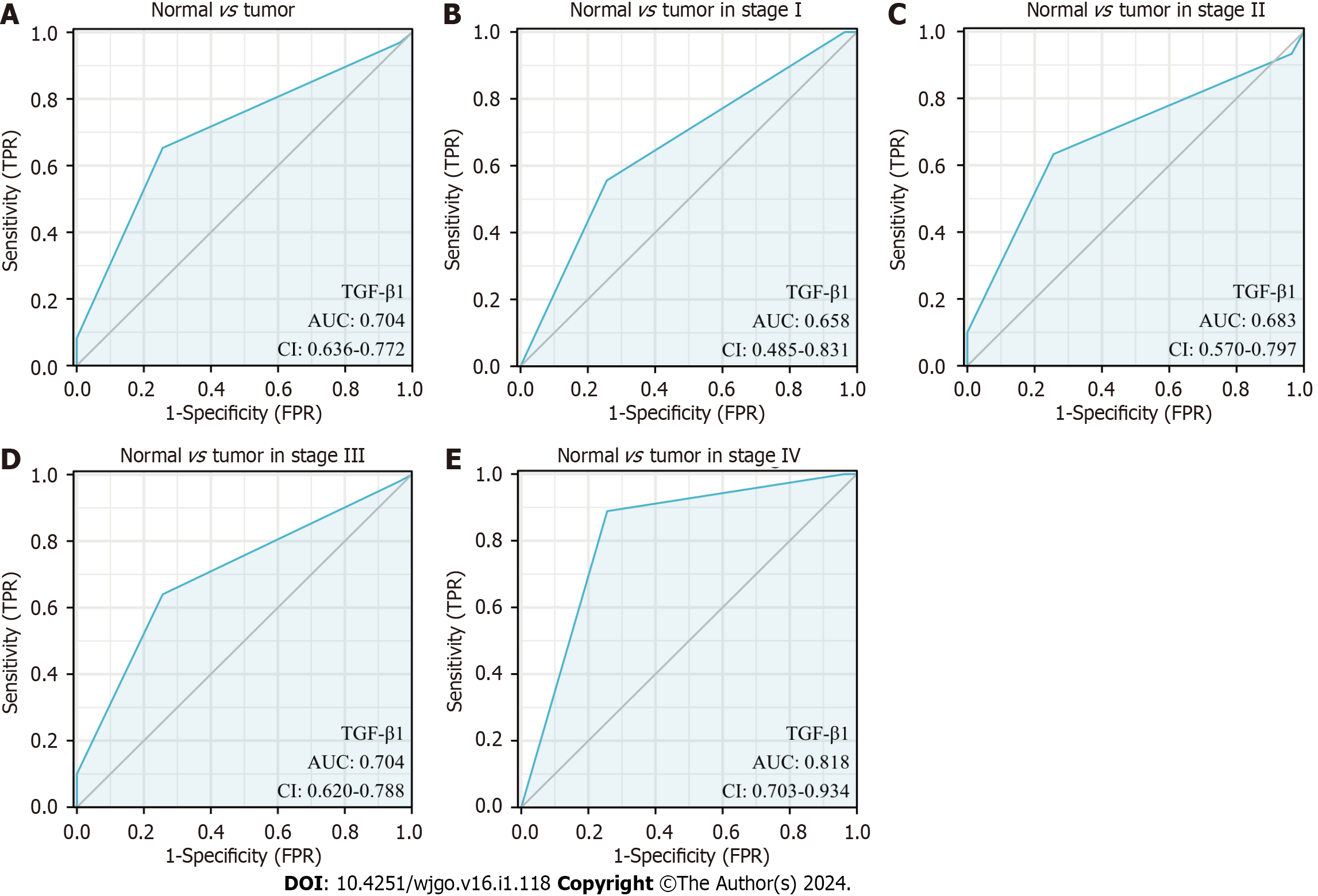
We further interrogated the diagnostic value of TGF-β1 expression in GC by generating a ROC curve. The results show that the area under the curve (AUC) of TGF-β1 is 0.704. We also analyzed the diagnostic power of TGF-β1 expression in different stages and showed that the AUC of TGF-β1 in stages I, II, III, and IV were 0.658, 0.683, 0.704, and 0.818, respectively (Figure 6).
TGF-β signaling can promote the malignant progression of cancer by promoting EMT, which facilitates tumor cell invasion and chemoresistance[31]. TGF-β1 is abundantly expressed in various cancers, and high levels of TGF-β1 usually predict adverse clinical outcomes[32,33]. Therefore, targeting TGF-β production is an important anticancer strategy.
Our results showed that TGF-β1 expression was significantly higher in GC than in normal tissues and that high TGF-β1 expression was associated with T stages (P = 0.042), consistent with the findings of previous studies[32,33]. We also found that TGF-β1 expression is discriminative in all stages of GC, but especially in late-stage tumors (stages III and IV), indicating its prognostic value. TGF-β signaling requires phosphorylation at Ser423/425 in SMAD3. Therefore, inhibiting or attenuating SMAD3(Ser423/425) phosphorylation can significantly inhibit TGF-β1 expression levels in cancer cells. Our study found that pSMAD3C(S423/425) was highly expressed in GC, and its high expression was associated with the M stage (P = 0.042).
The SMAD3 phospho-isoform, pSMAD3L, has been reported to play a pro-cancer role in different types of cancer[34,35], but its clinical significance in GC remains unclear. Based on the results of the IHC staining, we found that 31.6% (31/98) of GC patients expressed a high level of pSMAD3L(S204) and that the increased levels of pSMAD3L(S204) were associated with larger tumors (P = 0.038) and advanced N stage (P = 0.035). Moreover, our univariate and multivariate COX regression analysis revealed, for the first time, that the expression of pSMAD3L(S204) is an independent predictor of GC prognosis. This is consistent with previous studies that have shown that SMAD3 phospho-isomers have also been associated with poor prognosis in colorectal cancer, HCC, and esophageal squamous cell carcinoma by promoting tumor growth and metastasis[35-37]. Our results also showed that high expression of pSMAD3L(S204) was associated with low OS, suggesting that pSMAD3L(S204) is a molecular biomarker for outcome prediction in GC.
The SMAD3 LR has been reported to mediate TGF-β signaling transduction as TGF-β-mediated SMAD3 carboxyl-terminus phosphorylation is a precursor for TGF-β-induced SMAD3 LR phosphorylation[26]. pSMAD3L/C has been demonstrated to promote tumor progression via the facilitation of tumor cell metastasis and proliferation[34]. Our study found that the OS of GC patients was not associated with increased TGF-β1 and pSMAD3C(S423/425), but rather with concomitant elevation of pSMAD3L(S204)/pSMAD3C(S423/425), or pSMAD3L(S204)/TGF-β1. These observations are consistent with previous studies[34,38]. In addition, strong pSMAD3L/C expression was observed in tumors with EMT features[34] and also led to the activation of alternate oncogenic pathways in colorectal cancer. This suggests that the pSMAD3L/C pathway simultaneously promotes tumor-promoting TGF-β signaling and activates invasive behavior[38].
It has been reported that functional VEGFR-1 in cancer cells promotes invasion and growth and that high expression of VEGFR-1 is associated with poor prognosis in HCC[39]. Our study found that VEGFR-1 expression was significantly higher in GC than in normal tissues. We also found that high expression of VEGFR-1 was associated with larger tumors (P = 0.015) and higher tumor pathological grades (P = 0.013). Univariate and multivariate COX regression analyses highlighted that the expression of VEGFR-1 is an independent predictor of prognosis in patients with GC. These results suggest that VEGFR-1-related signaling may play a tumor-promoting role in GC. Of note, TGF-β1 effectively induces VEGFR-1 expression in vascular endothelial cells, thus preventing oxygen-induced vascular loss in vivo[40]. Moreover, upregulated TGF-β was observed in HCC and could induce the secretion of VEGF, the major activator of angiogenesis[41]. However, the relationship between the TGF-β/SMAD3 pathway and VEGFR-1 in GC is unclear. Our results showed that TGF-β1, pSMAD3L(S204), and pSMAD3C(S423/425) were positively correlated with VEGFR-1 expression. These results suggest that VEGFR-1 may be associated with TGF-β1/SMAD3-mediated angiogenesis. Our study revealed a significant association between high expression of VEGFR-1 and poor prognosis in patients with GC. Notably, when both pSMAD3L(S204) and VEGFR-1 are upregulated, OS rates are significantly reduced, providing evidence for a potential interplay between these proteins in cancer progression. Evaluating the expression pattern of these two proteins together may help predict prognosis.
In conclusion, this study is the first to characterize the relationship between pSMAD3L(S204) and VEGFR-1 expression in GC. Our current results suggest that these proteins may lead to GC metastasis by promoting angiogenesis. We also found that the simultaneous upregulation of pSMAD3L(S204) and VEGFR-1 was an unfavorable prognostic factor for GC and that targeting VEGFR-1 and pSMAD3L(S204) could represent a promising treatment strategy for GC. Our study provides novel insights into the tumorigenesis of GC and has identified potential therapeutic targets for this malignancy.
Gastric cancer is a significant health concern and understanding the molecular mechanisms underlying its progression and metastasis is crucial. The TGF-β/SMAD3 and VEGFR-1 signaling pathways have been identified as important players in gastric cancer metastasis, with SMAD3 phospho-isoforms emerging as a critical prognostic marker.
Given the clinical significance of gastric cancer, there is a pressing need to elucidate the prognostic value and interrelationship of SMAD3 phospho-isoforms and VEGFR-1 in gastric cancer. This study aims to address this gap in knowledge.
The primary objective of this study was to determine the prognostic significance and relationship between SMAD3 phospho-isoforms (pSMAD3C(S423/425) and pSMAD3L(S204)) and VEGFR-1 in gastric cancer.
This observational single-center study enrolled 98 gastric cancer patients and 82 adjacent normal gastric tissues. Immunohistochemical staining and tissue microarrays were utilized to measure the expression levels of TGF-β1, pSMAD3C(S423/425), pSMAD3L(S204), and VEGFR-1. Prognosis and survival information of the patients were recorded, and statistical analyses including Pearson's correlation coefficient, Chi-squared test, and Kaplan-Meier survival analysis were employed.
The study revealed that TGF-β1 and VEGFR-1 expression were significantly upregulated in gastric cancer tissue compared to adjacent non-cancerous tissue. High expression of pSMAD3L(S204) and VEGFR-1 was associated with larger tumors, later N stages, tumor size, and pathological grading, and was correlated with unfavorable overall survival outcomes. Multivariate analysis identified high expression of pSMAD3L(S204) and VEGFR-1 as independent risk factors for the prognosis of GC patients.
The co-upregulation of pSMAD3L(S204) and VEGFR-1 may serve as a predictive marker for poor gastric cancer prognosis, suggesting a potential role for pSMAD3L(204) in enhanced gastric cancer metastasis in a VEGFR-1-dependent manner.
Future research could focus on elucidating the underlying molecular mechanisms linking SMAD3 phospho-isoforms and VEGFR-1 in gastric cancer metastasis, potentially paving the way for targeted therapeutic interventions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Muguruma N, Japan; Watanabe T, Japan S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1467] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 2. | Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev. 2020;85:101980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 4. | Du S, Yang Z, Lu X, Yousuf S, Zhao M, Li W, Miao J, Wang X, Yu H, Zhu X, Chen H, Shi L, Xu E, Xia X, Guan W. Anoikis resistant gastric cancer cells promote angiogenesis and peritoneal metastasis through C/EBPβ-mediated PDGFB autocrine and paracrine signaling. Oncogene. 2021;40:5764-5779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Nienhüser H, Schmidt T. Angiogenesis and Anti-Angiogenic Therapy in Gastric Cancer. Int J Mol Sci. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Ceci C, Atzori MG, Lacal PM, Graziani G. Role of VEGFs/VEGFR-1 Signaling and its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 7. | D'Alessio A, Proietti G, Lama G, Biamonte F, Lauriola L, Moscato U, Vescovi A, Mangiola A, Angelucci C, Sica G. Analysis of angiogenesis related factors in glioblastoma, peritumoral tissue and their derived cancer stem cells. Oncotarget. 2016;7:78541-78556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Yuan XH, Yang J, Wang XY, Zhang XL, Qin TT, Li K. Association between EGFR/KRAS mutation and expression of VEGFA, VEGFR and VEGFR2 in lung adenocarcinoma. Oncol Lett. 2018;16:2105-2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Nasir A, Reising LO, Nedderman DM, Fulford AD, Uhlik MT, Benjamin LE, Schade AE, Holzer TR. Heterogeneity of Vascular Endothelial Growth Factor Receptors 1, 2, 3 in Primary Human Colorectal Carcinoma. Anticancer Res. 2016;36:2683-2696. [PubMed] |
| 10. | Hou Y, Wu Y, Farooq SM, Guan X, Wang S, Liu Y, Oblak JJ, Holcomb J, Jiang Y, Strieter RM, Lasley RD, Arbab AS, Sun F, Li C, Yang Z. A critical role of CXCR2 PDZ-mediated interactions in endothelial progenitor cell homing and angiogenesis. Stem Cell Res. 2015;14:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Subarnbhesaj A, Miyauchi M, Chanbora C, Mikuriya A, Nguyen PT, Furusho H, Ayuningtyas NF, Fujita M, Toratani S, Takechi M, Niida S, Takata T. Roles of VEGF-Flt-1 signaling in malignant behaviors of oral squamous cell carcinoma. PLoS One. 2017;12:e0187092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Gao J, Ye J, Ying Y, Lin H, Luo Z. Negative regulation of TGF-β by AMPK and implications in the treatment of associated disorders. Acta Biochim Biophys Sin (Shanghai). 2018;50:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Hua W, Ten Dijke P, Kostidis S, Giera M, Hornsveld M. TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol Life Sci. 2020;77:2103-2123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 14. | Ross S, Hill CS. How the Smads regulate transcription. Int J Biochem Cell Biol. 2008;40:383-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Zhang T, Xia W, Song X, Mao Q, Huang X, Chen B, Liang Y, Wang H, Chen Y, Yu X, Zhang Z, Yang W, Xu L, Dong G, Jiang F. Super-enhancer hijacking LINC01977 promotes malignancy of early-stage lung adenocarcinoma addicted to the canonical TGF-β/SMAD3 pathway. J Hematol Oncol. 2022;15:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Hori Y, Ikeura T, Yamaguchi T, Yoshida K, Matsuzaki K, Ishida M, Satoi S, Okazaki K. Role of phosphorylated Smad3 signal components in intraductal papillary mucinous neoplasm of pancreas. Hepatobiliary Pancreat Dis Int. 2020;19:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Tschernia NP, Gulley JL. Tumor in the Crossfire: Inhibiting TGF-β to Enhance Cancer Immunotherapy. BioDrugs. 2022;36:153-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 797] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 19. | Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, Zhao J, Zhang HT. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Murata M, Matsuzaki K, Yoshida K, Sekimoto G, Tahashi Y, Mori S, Uemura Y, Sakaida N, Fujisawa J, Seki T, Kobayashi K, Yokote K, Koike K, Okazaki K. Hepatitis B virus X protein shifts human hepatic transforming growth factor (TGF)-beta signaling from tumor suppression to oncogenesis in early chronic hepatitis B. Hepatology. 2009;49:1203-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Bae E, Sato M, Kim RJ, Kwak MK, Naka K, Gim J, Kadota M, Tang B, Flanders KC, Kim TA, Leem SH, Park T, Liu F, Wakefield LM, Kim SJ, Ooshima A. Definition of smad3 phosphorylation events that affect malignant and metastatic behaviors in breast cancer cells. Cancer Res. 2014;74:6139-6149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Matsuzaki K. Smad phosphoisoform signals in acute and chronic liver injury: similarities and differences between epithelial and mesenchymal cells. Cell Tissue Res. 2012;347:225-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Nam EH, Park SR, Kim PH. TGF-beta1 induces mouse dendritic cells to express VEGF and its receptor (Flt-1) under hypoxic conditions. Exp Mol Med. 2010;42:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kong X, Bu J, Chen J, Ni B, Fu B, Zhou F, Pang S, Zhang J, Xu S, He C. PIGF and Flt-1 on the surface of macrophages induces the production of TGF-β1 by polarized tumor-associated macrophages to promote lung cancer angiogenesis. Eur J Pharmacol. 2021;912:174550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Prokova V, Mavridou S, Papakosta P, Kardassis D. Characterization of a novel transcriptionally active domain in the transforming growth factor beta-regulated Smad3 protein. Nucleic Acids Res. 2005;33:3708-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Wang G, Matsuura I, He D, Liu F. Transforming growth factor-{beta}-inducible phosphorylation of Smad3. J Biol Chem. 2009;284:9663-9673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6460] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 28. | Fenoglio-Preiser C, Muñoz N, Carneiro F, Powell S. M., Correa P, Rugge M, Guilford P, Sasako M, Lambert R, Stolte M, Megraud F, Watanabe H. Tumours of the Stomach. In: Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press, 2000: 37-52. |
| 29. | Zou J, Li C, Jiang S, Luo L, Yan X, Huang D, Luo Z. AMPK inhibits Smad3-mediated autoinduction of TGF-β1 in gastric cancer cells. J Cell Mol Med. 2021;25:2806-2815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Cheng X, Li D, Qi T, Sun J, Zhou T, Zheng WV. Objective to identify and verify the regulatory mechanism of DTNBP1 as a prognostic marker for hepatocellular carcinoma. Sci Rep. 2022;12:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Huynh LK, Hipolito CJ, Ten Dijke P. A Perspective on the Development of TGF-β Inhibitors for Cancer Treatment. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 32. | Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 2021;14:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 33. | Li J, Shen C, Wang X, Lai Y, Zhou K, Li P, Liu L, Che G. Prognostic value of TGF-β in lung cancer: systematic review and meta-analysis. BMC Cancer. 2019;19:691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Matsuzaki K, Kitano C, Murata M, Sekimoto G, Yoshida K, Uemura Y, Seki T, Taketani S, Fujisawa J, Okazaki K. Smad2 and Smad3 phosphorylated at both linker and COOH-terminal regions transmit malignant TGF-beta signal in later stages of human colorectal cancer. Cancer Res. 2009;69:5321-5330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Sekimoto G, Matsuzaki K, Yoshida K, Mori S, Murata M, Seki T, Matsui H, Fujisawa J, Okazaki K. Reversible Smad-dependent signaling between tumor suppression and oncogenesis. Cancer Res. 2007;67:5090-5096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Cho SY, Ha SY, Huang SM, Kim JH, Kang MS, Yoo HY, Kim HH, Park CK, Um SH, Kim KH, Kim SH. The prognostic significance of Smad3, Smad4, Smad3 phosphoisoform expression in esophageal squamous cell carcinoma. Med Oncol. 2014;31:236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J, Okazaki K, Seki T. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 38. | Kawamata S, Matsuzaki K, Murata M, Seki T, Matsuoka K, Iwao Y, Hibi T, Okazaki K. Oncogenic Smad3 signaling induced by chronic inflammation is an early event in ulcerative colitis-associated carcinogenesis. Inflamm Bowel Dis. 2011;17:683-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Li T, Zhu Y, Qin CY, Yang Z, Fang A, Xu S, Ren W. Expression and prognostic significance of vascular endothelial growth factor receptor 1 in hepatocellular carcinoma. J Clin Pathol. 2012;65:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci U S A. 2003;100:15859-15864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50:1140-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |