Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.102
Peer-review started: September 3, 2023
First decision: October 30, 2023
Revised: November 12, 2023
Accepted: December 8, 2023
Article in press: December 8, 2023
Published online: January 15, 2024
Processing time: 130 Days and 4.8 Hours
While gelatin sponge particles and calibrated microspheres are commonly used as embolic materials in conventional transarterial chemoembolization (cTACE), direct comparisons between these embolic agents are rare.
To compare the efficacy and safety of superselective cTACE using Embosphere® or Marine gel® in patients with early-stage hepatocellular carcinoma (HCC).
This retrospective study included 70 patients with small (< 4 cm) HCC who underwent cTACE with Embosphere® (n = 33) or Marine gel® (n = 37) as the embolic agent at a single center between March 2021 and July 2022. The radiologic images and clinical data were retrospectively reviewed, with an emphasis on tumor response, procedure-related complications, and local tumor recurrence. The primary index tumor was assessed on a 1-mo follow-up image, and local progression-free survival was obtained using the Kaplan-Meier method and was compared by the log-rank test.
The median tumor size of both groups was 1.5 cm, and 69 patients achieved a complete response one month after cTACE. The cumulative local recurrence rate at 12 mo was 15.5% in the Embosphere® group and 14.4% in the Marine gel® group. The local progression-free survival was not significantly different between the two groups (P = 0.83). In the multivariate analysis, high serum alpha-fetoprotein was the only significant poor prognostic factor for local tumor progression (P = 0.01). Postembolization syndrome occurred in 36.4% of the Embosphere® group and 35.1% of the Marine gel® group, and there were no cases of biloma, biliary duct dilation, or liver abscess in either group.
Calibrated gelatin sponge particles (Marine gel®) and calibrated microspheres (Embosphere®) have similar outcomes in terms of tumor response for superselective cTACE of small HCC.
Core Tip: Conventional chemoembolization, a main treatment option for unresectable hepatocellular carcinoma (HCC), involves delivery of anticancer drug and embolic materials. The current study aimed to assess the tumor response of superselective chemoembolization using calibrated gelatin sponge particles and calibrated microspheres as embolic materials. There is no significant difference in tumor response between the use of calibrated gelatin sponge particles and calibrated microspheres for conventional chemoembolization in small HCC.
- Citation: Kim HC, Choi JW. Comparative study between Embosphere® and Marine gel® as embolic agents for chemoembolization of hepatocellular carcinoma. World J Gastrointest Oncol 2024; 16(1): 102-109
- URL: https://www.wjgnet.com/1948-5204/full/v16/i1/102.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i1.102
Transarterial chemoembolization (TACE) is a mainstay treatment option for patients with unresectable hepatocellular carcinoma (HCC)[1]. In cases where curative modalities are not feasible for various reasons, such as tumor location and comorbidities, TACE may be indicated as an alternative treatment option for early-stage HCC[1]. The two main elements of TACE involve intra-arterial delivery of a chemotherapeutic agent and blockage of the hepatic artery. In conventional TACE (cTACE), iodized oil (Lipiodol; Laboratoire Andre Guerbet, Aulnay-sous-Bois, France) and gelatin sponge particles are used as a drug carrier and embolic material, respectively, while drug-loaded microspheres such as DC beads (Boston Scientific, Natick, MA, United States) and HepaSphere (Merit Medical) are used as both a drug carrier and embolic material in drug-eluting embolic TACE[2].
Hand-cut or crushed gelatin sponge particles have been used as embolic materials in Asian countries for several decades[3,4]. However, these particles may have some limitations. For instance, their wide range of sizes and tendency of aggregation may induce premature occlusion of the proximal tumor-feeding vessel, which can reduce the efficacy of the treatment[4]. On the other hand, microspheres such as Embosphere® are commonly used in Western countries for cTACE due to their ability to penetrate deeper into fine intratumoral vessels and their predictable size[5,6]. Additionally, the temporary nature of gelatin sponge particles and the permanent nature of Embosphere® can also affect the duration and efficacy of the treatment.
Recently, calibrated gelatin sponge particles made from fish, known as Marine gel® (PL Micromed, Yangsan, Korea), have been introduced as a potential alternative. These particles are expected to offer the benefits of both temporary embolic material and calibrated microspheres. The objective of this study was to compare the efficacy and safety of superselective cTACE employing either Embosphere® or Marine gel® as the embolic agent in individuals diagnosed with early-stage HCC.
The institutional review board approved this retrospective study and permitted the waiving of informed consent. This study encompassed HCC patients who received cTACE using either Embosphere® or Marine gel® as the embolic agent at a single center between March 2021 and July 2022. The cohort consisted of a total of 70 patients, of whom 33 were treated with Embosphere® and 37 with Marine gel®. The study population was comprised of 55 men and 15 women [mean age: 67.4 ± 10.5 years (range: 27-90 years)]. The inclusion criteria were: (1) At least one treatment-naïve HCC ≥ 10 mm; (2) Nodular HCC; (3) Successful superselective cTACE (all subsegmental tumor-feeding arteries were catheterized, lipiodol and the embolic agent were adequately infused, and there was no untreated tumor-feeding artery); and (4) Child-Pugh score ≤ 8. The exclusion criteria were: (1) Primary index tumor > 4 cm; and (2) Tumor number > 4.
One author (17 years of experience in interventional oncology) performed all chemoembolization procedures, which were previously described in detail[7-9]. Celiac and superior mesenteric angiograms were obtained using a 5-Fr catheter, and a cone-beam computed tomography (CT) scan was performed at the level of the proper hepatic artery or its equivalent to investigate the entire liver. The cone-beam CT images were thoroughly reviewed by the operators to determine the tumor number and size, as well as the tumor-feeding vessels and their origin. Subsegmental tumor-feeding arteries were catheterized using a 1.7-Fr microcatheter (Progreat lamda; Terumo, Tokyo, Japan, or Radiostar 1.7; Taewoong, Koyang, Korea). An emulsion of iodized oil and doxorubicin hydrochloride (Adriamycin RDF; Ildong, Seoul, Korea) was infused via the subsegmental tumor-feeding arteries until dense accumulation of iodized oil in the tumor was observed under fluoroscopy. Additional embolization was performed using Embosphere® (40-120 μm) or Marine gel® (100-150 μm) to achieve complete stasis of tumor-feeding arteries. The use of Embosphere® and Marine gel® was determined by the operator in a random fashion without any preference. Follow-up enhanced cross-sectional imaging was performed 1 mo after TACE and then every 2-3 mo thereafter.
Radiologic images and clinical data were retrospectively reviewed with a focus on tumor response, procedure-related complications, and local tumor recurrence. The primary index tumor was defined as the largest tumor, and the tumor response of the primary index tumor was assessed using the modified Response Evaluation Criteria in Solid Tumors on the 1-mo follow-up image[10]. Complications related to chemoembolization were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0[11].
Statistical analysis was performed to compare the demographic and clinical variables between the two groups using Fisher’s exact test, χ2 test, or Student’s t test. Local progressive disease was defined as either the regrowth of the primary index tumor or the appearance of new viable tumors within the primary index tumor. For patients who underwent surgical resection or liver transplantation, local progression-free survival was censored on the day of operation. The Kaplan-Meier method was used to obtain local progression-free survival, and it was compared by the log-rank test. Treatment effects between the two groups were compared using univariable analysis and multivariable Cox proportional hazard models. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 27.0 software (SPSS Inc., Chicago, IL, United States).
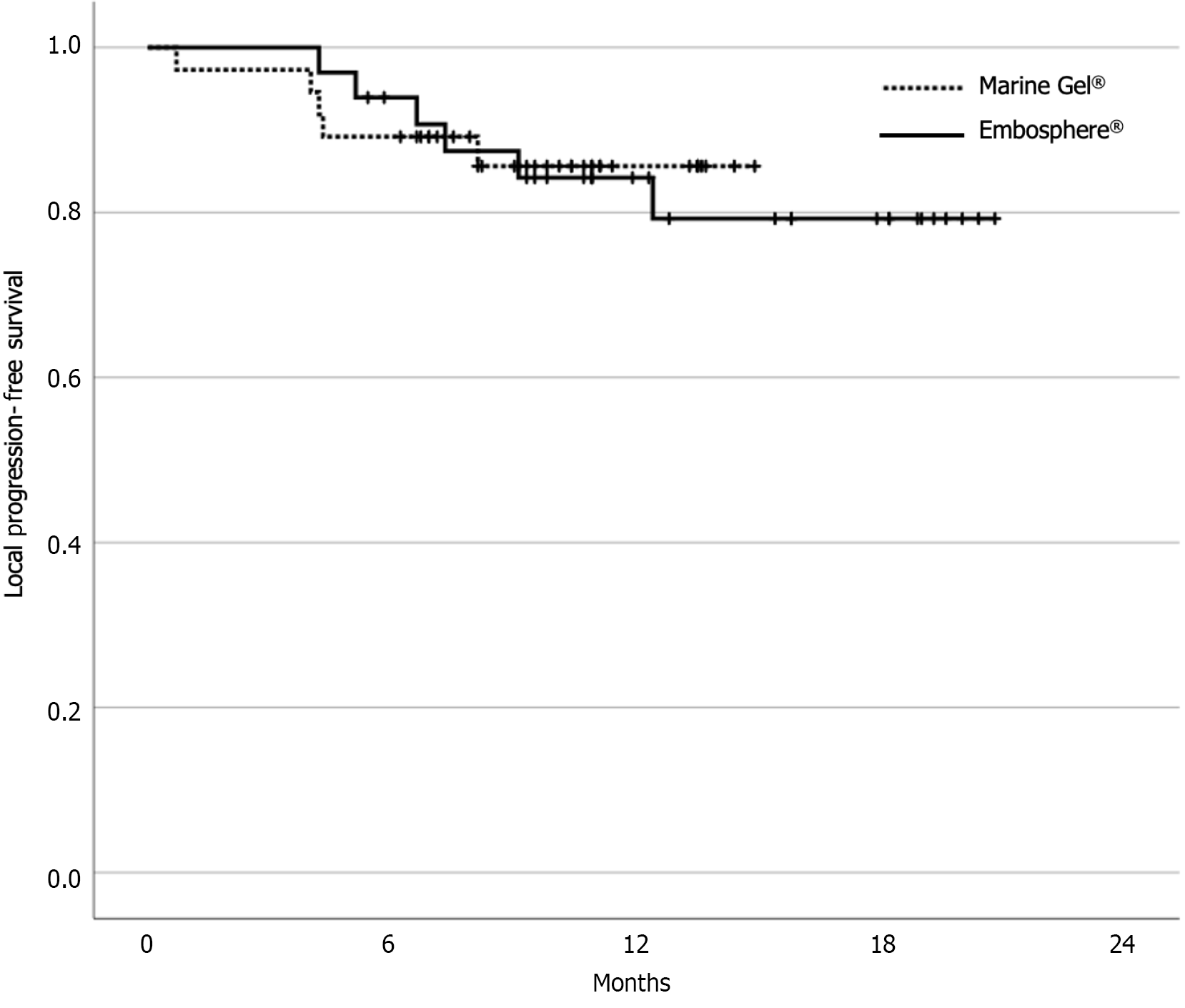
The baseline characteristics of the two study groups are summarized in Table 1. The median tumor size in both groups was 1.5 cm. The median follow-up period was 12.9 mo and 10.7 mo in the Embosphere® and Marine gel® groups, respectively. The data-cutoff date was July 31, 2023. Tumor response 1 mo after cTACE was a complete response in 69 patients and a partial response in one patient in the Marine gel® group. During the follow-up period, local progression was observed in six patients in the Embosphere® group and five patients in the Marine gel® group. There was no significant difference in local progression-free survival between the two groups (P = 0.83) (Figure 1). At the 12-mo mark, the cumulative local recurrence rates were 15.5% in the Embosphere® group and 14.4% in the Marine gel® group. In the multivariate analysis, only high serum alpha-fetoprotein emerged as a significant poor prognostic factor for local tumor progression (P = 0.01) (Table 2).
| Total (n = 70) | Embosphere (n = 33) | Marine gel (n = 37) | P value | |
| Age | 67.4 ± 10.5 | 68.4 ± 8.5 | 66.4 ± 12.1 | 0.44 |
| Sex | 0.53 | |||
| Male | 55 | 27 | 28 | |
| Female | 15 | 6 | 9 | |
| Etiology | 0.99 | |||
| HBV | 48 | 24 | 24 | |
| HCV | 2 | 1 | 1 | |
| HBV + HCV | 5 | 1 | 4 | |
| Non-viral | 15 | 7 | 8 | |
| Platelet (103/mm3) | 126.1 ± 58.9 | 119.4 ± 65.7 | 132.1 ± 52.3 | 0.37 |
| Serum albumin(g/dL) | 4.05 ± 0.47 | 3.97 ± 0.52 | 4.12 ± 0.41 | 0.20 |
| Total bilirubin (mg/dL) | 0.77 ± 0.60 | 0.74 ± 0.54 | 0.80 ± 0.64 | 0.70 |
| PT (INR) | 1.08 ± 0.14 | 1.10 ± 0.13 | 1.07 ± 0.15 | 0.32 |
| Creatinine (mg/dL) | 1.09 ± 0.92 | 1.14 ± 1.03 | 1.04 ± 0.82 | 0.67 |
| ALT | 27.7 ± 14.7 | 30.5 ± 16.8 | 25.1 ± 12.2 | 0.13 |
| AST | 34.1 ± 16.7 | 38.0 ± 19.6 | 30.7 ± 12.9 | 0.07 |
| Child-Pugh class | 0.49 | |||
| A | 67 | 31 | 36 | |
| B | 3 | 2 | 1 | |
| Previous Tx | 0.55 | |||
| RFA | 18 | 11 | 7 | |
| Resection | 5 | 1 | 4 | |
| AFP (≤ 100 ng/mL/> 100 ng/mL) | 62/8 | 30/3 | 32/5 | 0.56 |
| PIVKA (≤ 100 mAU/mL/> 100 mAU/mL) | 59/11 | 30/3 | 29/8 | 0.15 |
| Tumor size (cm) | 0.50 | |||
| mean ± SD | 1.7 ± 0.6 | 1.8 ± 0.7 | ||
| Median | 1.5 | 1.5 | ||
| Range | 1.0-3.2 | 1.0-3.6 |
| Parameter | Univariable analysis | Multivariable analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Group (embosphere vs marine gel) | 1.121 | 0.339-3.707 | 0.851 | |||
| Age (> 65 vs ≤ 65) | 1.444 | 0.383-5.448 | 0.58 | |||
| Sex (male vs female) | 2.628 | 0.336-20.55 | 0.34 | |||
| HBV (positive vs negative) | 1.911 | 0.558-6.540 | 0.29 | |||
| Platelet (≤ 120 vs > 120) (103/mm3) | 1.939 | 0.567-6.630 | 0.28 | |||
| Serum albumin (≤ 3.5 vs > 3.5) (g/dL) | 25.03 | 0.012-5251.5 | 0.20 | |||
| Total bilirubin (> 1.0 vs ≤ 1.0) (mg/dL) | 26.63 | 0.026-2756.8 | 0.14 | |||
| PT (> 1.2 vs ≤ 1.2) (INR) | 26.33 | 0.024-2905.2 | 0.15 | |||
| ALT (> 40 vs ≤ 40) (IU/L) | 1.088 | 0.235-5.040 | 0.91 | |||
| AST (> 40 vs ≤ 40) (IU/L) | 3.528 | 0.451-27.62 | 0.20 | |||
| AFP (> 100 ng/mL vs ≤ 100 ng/mL) | 5.407 | 1.572-18.60 | 0.003 | 5.130 | 1.479-17.79 | 0.01 |
| PIVKA (> 100 mAU/mL vs 100 mAU/mL) | 1.233 | 0.266-5.719 | 0.79 | |||
| Tumor size (> 2 cm vs ≤ 2 cm) | 1.040 | 0.275-3.935 | 0.953 | |||
The incidence of adverse events is summarized in Table 3. Postembolization syndrome occurred in 36.4% and 35.1% of the Embosphere® group and Marine gel® groups, respectively. Two patients had unscheduled hospital visits due to abdominal pain, and one of them had focal hepatic parenchymal infarction, which was managed by conservative treatment including painkillers. There were no cases of biloma, biliary duct dilation, or liver abscess.
| Total (n = 70) | Embosphere (n = 33) | Marine gel (n = 37) | P value | |
| Postembolization syndrome | 25 | 12 (36.4) | 13 (35.1) | 0.915 |
| Fever | 1 | 1 | 0 | 0.286 |
| Nausea/vomiting | 6 | 2 | 4 | 0.479 |
| Pain | 23 | 11 | 12 | 0.448 |
| Unscheduled hospital visit | 2 | 1 | 1 | 0.935 |
| Laboratory toxicity (90 d) | ||||
| PT-INR (grade 0/1/2) | 69/1/0 | 32/1/0 | 37/0/0 | 0.286 |
| Albumin (grade 0/1/2) | 67/3/0 | 32/1/0 | 35/2/0 | 0.624 |
| Total bilirubin (grade 0/1/2) | 63/5/2 | 30/2/1 | 33/3/1 | 0.811 |
| AST (grade 0/1/2/3) | 58/11/0/1 | 26/7/0/0 | 32/4/0/1 | 0.394 |
| ALT (grade 0/1/2/3) | 63/5/1/1 | 30/2/1/0 | 33/3/0/1 | 0.811 |
| Alkaline phosphatase (grade 0/1/2) | 67/3/0 | 33/0/0 | 34/3/0 | 0.095 |
| Biloma | 0 | 0 | 0 | 1.0 |
| Liver abscess | 0 | 0 | 0 | 1.0 |
| Biliary duct dilation | 0 | 0 | 0 | 1.0 |
| Focal hepatic parenchymal infarct | 1 | 1 | 0 | 0.286 |
In this study, the effectiveness of two embolic materials, calibrated gelatin sponge particles (Marine gel®) and calibrated microspheres (Embosphere®), was compared for superselective cTACE of small HCC. The study aimed to minimize the bias from patient and technical factors on tumor response by including only patients with small HCCs that were completely treated by superselective cTACE. All patients, except for one treated with Marine gel®, had a complete response at the 1-mo follow-up CT, and there was no statistically significant difference in local progression-free survival between the two groups. Cumulative local recurrence was less than 20% at 1 year for both embolic materials.
The results of this study are consistent with a previous report that used crushed gelatin sponge particles smaller than 200 μm as an embolic material[12]. Although various embolic materials have been used in cTACE, the advantages of each embolic agent remain controversial, and direct comparisons between embolic agents are rare. In Asian countries, temporary embolic agents such as gelatin sponge particles have been used for several decades due to their desirable characteristics in terms of maintaining hepatic artery patency and preventing biliary injury. However, the potential disadvantage of premature occlusion of tumor-feeding vessels due to particle aggregation and early tumor recurrence due to early recanalization of the tumor-feeding artery should be considered. Marine gel® is an alternative embolic material made of fish-derived calibrated gelatin sponge particles that can be used in Islamic countries.
In this study, one patient (Embosphere® group) had a focal hepatic parenchymal infarct, which may have been associated with excessive embolization of the hepatic artery. However, there were no cases of biloma or biliary duct dilation. The reported incidence of biliary complications is quite variable (4%-35%), depending on the embolic material used, the degree of superselection, and the presence of liver cirrhosis[12-14]. The low incidence of biliary complications in this study may be due to the inclusion of only patients with small tumors that were superselectively treated. Further studies, including larger tumors (> 4 cm), are needed to investigate the incidence of biliary complications.
This study has several limitations, including the relatively short follow-up period and the lack of comparison of overall survival. All patients had early-stage HCC and were alive at the time of data collection. Long-term follow-up studies are necessary to investigate survival differences between embolic materials. Additionally, all cTACE procedures were performed by one experienced interventional radiologist, which may limit the reproducibility of the excellent tumor response if cTACE is performed in a less selective fashion by inexperienced practitioners.
In conclusion, the study suggests that there are comparable outcomes in terms of tumor response for superselective cTACE of small HCC when using either calibrated gelatin sponge particles or calibrated microspheres. However, the choice of embolic material should be carefully considered based on patient and tumor characteristics, as well as the experience of the practitioner.
Hand-cut or crushed gelatin sponge particles have been used in Asian countries for decades as embolic materials in conventional transarterial chemoembolization (cTACE), but they have limitations, including a wide range of sizes and a tendency to aggregate, which can reduce treatment efficacy. In Western countries, microspheres like Embosphere® are preferred due to their ability to penetrate fine intratumoral vessels and their predictable size. The choice between these embolic agents, with gelatin sponge particles being temporary and Embosphere® being permanent, can impact the duration and effectiveness of the cTACE treatment, although direct comparisons between them are rare.
Lately, there has been an introduction of calibrated gelatin sponge particles derived from fish, marketed under the name Marine gel® (PL Micromed, Yangsan, Korea), which are being considered as a possible alternative. These particles are anticipated to provide the advantages of both temporary embolic materials and calibrated microspheres.
The aim of this study was to evaluate the effectiveness and safety of superselective cTACE when utilizing either Embosphere® or Marine gel® as the embolic agent in individuals with early-stage hepatocellular carcinoma (HCC).
This retrospective analysis involved 70 patients diagnosed with small HCC, measuring less than 4 cm in size. These patients underwent chemoembolization procedures at a single medical center between March 2021 and July 2022, with Embosphere® (n = 33) or Marine gel® (n = 37) serving as the chosen embolic agents. The study involved a comprehensive review of radiological images and clinical data, focusing on factors such as tumor response, complications associated with the procedure, and instances of local tumor recurrence. The effectiveness of the primary index tumor treatment was assessed using a 1-mo follow-up imaging analysis. The study also calculated local progression-free survival using the Kaplan-Meier method and compared the outcomes using the log-rank test.
Both groups had a median tumor size of 1.5 cm, and after one month of undergoing cTACE, 69 patients achieved a complete response. The cumulative local recurrence rate at the 12-mo mark was 15.5% in the Embosphere® group and 14.4% in the Marine gel® group. Notably, there was no significant difference in local progression-free survival between these two groups (P = 0.83). In a multivariate analysis, high serum alpha-fetoprotein emerged as the sole significant adverse prognostic factor for local tumor progression (P = 0.01).
Superselective cTACE for small HCC yields comparable tumor response outcomes when employing calibrated gelatin sponge particles (Marine gel®) and calibrated microspheres (Embosphere®).
Multicenter prospective study is needed to confirm the adequate embolic agents in chemoembolization for HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bi JF, China; Niu ZS, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2547] [Article Influence: 849.0] [Reference Citation Analysis (59)] |
| 2. | Lee EW, Khan S. Recent advances in transarterial embolotherapies in the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:265-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (39)] |
| 3. | Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (32)] |
| 4. | Miyayama S, Matsui O. Superselective Conventional Transarterial Chemoembolization for Hepatocellular Carcinoma: Rationale, Technique, and Outcome. J Vasc Interv Radiol. 2016;27:1269-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (34)] |
| 5. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (30)] |
| 6. | Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, Vaidya S, Monsky WL, Park JO, Bhattacharya R, Hippe DS, Harris WP. Segmental Yttrium-90 Radioembolization versus Segmental Chemoembolization for Localized Hepatocellular Carcinoma: Results of a Single-Center, Retrospective, Propensity Score-Matched Study. J Vasc Interv Radiol. 2017;28:777-785.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (37)] |
| 7. | Miyayama S, Yamashiro M, Hashimoto M, Hashimoto N, Ikuno M, Okumura K, Yoshida M, Matsui O. Identification of small hepatocellular carcinoma and tumor-feeding branches with cone-beam CT guidance technology during transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2013;24:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (17)] |
| 8. | Miyayama S. Ultraselective conventional transarterial chemoembolization: When and how? Clin Mol Hepatol. 2019;25:344-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (34)] |
| 9. | Kim HC, Miyayama S, Choi JW, Kim GM, Chung JW. Hepatocellular Carcinoma Supplied by the Inferior Phrenic Artery or Cystic Artery: Anatomic and Technical Considerations. Radiographics. 2023;43:e220076. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3287] [Article Influence: 219.1] [Reference Citation Analysis (36)] |
| 11. | U.S. Department of Health and Human Services. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. [cited 15 July 2023]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. |
| 12. | Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, Takeda T, Yoneda N, Notsumata K, Toya D, Tanaka N, Mitsui T. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Lee IJ, Lee JH, Lee YB, Kim YJ, Yoon JH, Yin YH, Lee M, Hur S, Kim HC, Jae HJ, Chung JW. Effectiveness of drug-eluting bead transarterial chemoembolization versus conventional transarterial chemoembolization for small hepatocellular carcinoma in Child-Pugh class A patients. Ther Adv Med Oncol. 2019;11:1758835919866072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Guiu B, Deschamps F, Aho S, Munck F, Dromain C, Boige V, Malka D, Leboulleux S, Ducreux M, Schlumberger M, Baudin E, de Baere T. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol. 2012;56:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |