Published online Sep 15, 2023. doi: 10.4251/wjgo.v15.i9.1662
Peer-review started: May 31, 2023
First decision: July 23, 2023
Revised: August 4, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 15, 2023
Processing time: 104 Days and 10.8 Hours
Fibrinogen-to-albumin ratio (FAR) has been found to be of prognostic significance for several types of malignant tumors. However, less is known about the association between FAR and survival outcomes in hepatocellular carcinoma (HCC) patients.
To explore the association between FAR and prognosis and survival in patients with HCC.
A total of 366 histologically confirmed HCC patients diagnosed between 2013 and 2018 in a provincial cancer hospital in southwestern China were retrospectively selected. Relevant data were extracted from the hospital information system. The optimal cutoff for baseline serum FAR measured upon disease diagnosis was established using the receiver operating characteristic (ROC) curve. Univariate and multivariate Cox proportional hazards models were used to determine the crude and adjusted associations between FAR and the overall survival (OS) of the HCC patients while controlling for various covariates. The restricted cubic spline (RCS) was applied to estimate the dose-response trend in the FAR-OS association.
The optimal cutoff value for baseline FAR determined by the ROC was 0.081. Multivariate Cox proportional hazards model revealed that a lower baseline serum FAR level was associated with an adjusted hazard ratio of 2.43 (95% confidence interval: 1.87–3.15) in the OS of HCC patients, with identifiable dose-response trend in the RCS. Subgroup analysis showed that this FAR-OS association was more prominent in HCC patients with a lower baseline serum aspartate aminotransferase or carbohydrate antigen 125 level.
Serum FAR is a prominent prognostic indicator for HCC. Intervention measures aimed at reducing FAR might result in survival benefit for HCC patients.
Core Tip: It is important to explore the affecting factors of survival for hepatocellular carcinoma (HCC) patients. A receiver operating characteristic curve was used to establish the optimal cutoff value for baseline serum fibrinogen-to-albumin ratio (FAR) in disease diagnosis. Univariate and multivariate Cox proportional risk models were employed to determine the correlation between FAR and overall survival (OS) in HCC patients. Restricted cubic spline was used to estimate dose-response trends in FAR-OS associations. Serum FAR is an important prognostic index of HCC. Effective FAR reduction may benefit HCC patient survival.
- Citation: Sun H, Ma J, Lu J, Yao ZH, Ran HL, Zhou H, Yuan ZQ, Huang YC, Xiao YY. Fibrinogen-to-albumin ratio predicts overall survival of hepatocellular carcinoma. World J Gastrointest Oncol 2023; 15(9): 1662-1672
- URL: https://www.wjgnet.com/1948-5204/full/v15/i9/1662.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i9.1662
As one of the most common malignant tumors of the digestive system, liver cancer is a burden on human health and social economy due to its insidious onset and poor prognosis. The latest global cancer data released by the International Agency for Research on Cancer in 2020 showed that liver cancer accounted for 4.7% of all new cancer cases and 8.3% of all cancer-related deaths worldwide[1]. In China, liver cancer accounted for 9.0% and 13.0% of new cancer cases and cancer deaths in 2020, respectively[1]. Early diagnosis and treatment of cancer patients are critical. However, since patients with liver cancer only experience minor clinical symptoms in the early stages of the disease, only a minority of them can achieve early diagnosis. A 2018 study noted that the one, three, and five-year survival rates of liver cancer patients in Asian countries were 34.8%, 19%, and 18.1%, respectively, which were comparatively lower than those in the European and North American countries[2].
Considering the less optimistic survival rates of liver cancer patients, it is imperative to explore its potential prognostic factors, especially those that are easy to monitor and allow for a possible intervention. Hepatocellular carcinoma (HCC) is the predominant histological type of liver cancer and accounts for 75%–85% of all liver cancer cases[3,4]. Major risk factors for HCC include viral hepatitis B or C, consumption of aflatoxin-contaminated food, alcohol abuse, and metabolic and endocrine diseases[5,6]. HCC prognosis is related to various factors, such as clinical stage, tumor size, portal vein invasion, and non-alcoholic fatty liver disease[7-9].
In recent years, serum markers and their significance in cancer survival have gradually attracted considerable research interest in the field of cancer epidemiology, probably due to their easy accessibility and low cost. Many serum indicators had been found to be associated with HCC prognosis, such as blood profiling indexes [total bilirubin (TBIL), platelets, and albumin][10], serum enzymes [alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl aminotransferase, and alkalinephosphatase][11], systemic inflammatory indicators (neutrophil-to-lymphocyte ratio, systemic immune-inflammatory index)[12], and tumor biomarkers [carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), and carbohydrate antigen 19-9 (CA19-9)][10,13-15].
Fibrinogen is a glycoprotein synthesized by hepatic epithelial cells. Elevated plasma fibrinogen level has been shown to be associated with poorer survival in various malignancies[16]. Albumin is also produced by the liver and is generally used to assess nutritional status. In the advanced stage of cancer, malnutrition and inflammation jointly inhibit albumin synthesis[17]. Therefore, a significant decrease in serum albumin level can predict compromised cancer prognosis[18]. It has been reported that the composite index of fibrinogen and albumin, fibrinogen-to-albumin ratio (FAR), is an independent prognostic factor for progression-free-survival and overall survival (OS) in patients with head and neck squamous cell carcinoma[19]. In another newly published study, Yang et al[20] have found that FAR was also related to disease-free survival (DFS) and OS in triple-negative breast cancer patients, with longer median DFS and OS observed in patients with a lower FAR.
In two previously published studies, scholars have evaluated the association between FAR and survival outcomes in early-stage HCC patients who underwent curative resection and found that an elevated FAR was significantly associated with poorer survival and higher risk of recurrence[21]. Nevertheless, the general prognostic significance of serum FAR measured upon disease diagnosis in HCC patients remains unknown. The present study explores this issue in a large sample of HCC patients. The independent association between serum FAR and OS in HCC patients was analyzed and its dose-response trend was estimated.
Patients with histologically confirmed HCC between January 1, 2013 and December 31, 2018 at the Third Affiliated Hospital of Kunming Medical University (Yunnan Provincial Cancer Hospital) were retrospectively identified. The hospital had a well-established hospital information system, including a digital clinical information system and a computer-assisted telephone interview follow-up system. In the digital clinical information system, all data related to the clinical practice of inpatients and outpatients are recorded and updated on a daily basis. The following information was extracted from the clinical information system in the present study: Gender, age at diagnosis, ethnicity, cigarette smoking status, alcohol drinking status, body mass index (BMI), clinical stage, and serum indicator levels measured at diagnosis (fibrinogen, albumin, AST, ALT, alpha-fetoprotein (AFP), TBIL, neutrophil-to-lymphocyte ratio (NLR), CEA, CA125, and CA19-9). Information about patient death, such as date and cause, was obtained from the follow-up system. HCC patients with complete required information were included in the final study analysis. The study protocol was approved by the Ethics Review Committee of Kunming Medical University. The requirement for informed consent was waived by the committee due to the retrospective study design.
The OS served as the study outcome. Survival interval was defined as the time from the histological diagnosis to the date of death from any cause or the end of follow-up (December 31, 2018), whichever came first. Baseline serum FAR was calculated as serum fibrinogen level divided by serum albumin level measured around the date of disease diagnosis (within seven days prior to or post diagnosis). Because no commonly used cutoffs have been proposed for serum FAR, the receiver operating characteristic (ROC) curve was used to establish the best cutoff value. Other baseline blood indicators to be controlled for, including AST, ALT, AFP, TBIL, NLR, CEA, CA125, and CA19-9, were also extracted from the system using the same time requirements as those for serum FAR. The most commonly adopted cutoffs were employed to dichotomize these blood indicators as follows: 40 U/L for AST and ALT, 17.1 μmol/L for TBIL, 400 ng/mL for AFP[22,23], 5 U/L for CEA, 35 U/L for CA125, and 37 U/L for CA19-9. BMI and serum NLR were dichotomized using their medians.
Descriptive statistics were used to illustrate and compare the general characteristics of the participants. The Kaplan-Meier survival curves were plotted for HCC patients based on different baseline FAR levels and compared using the log-rank test. Univariate and multivariate Cox proportional hazards models were employed to evaluate the crude and adjusted associations between baseline serum FAR and the OS of HCC patients. Variables that achieved a less strict significance level (P < 0.10) in univariate analyses were incorporated into the subsequent multivariate model. Considering the quantitative nature of FAR, the dose-response trend in its association with the OS of HCC patients was subsequently estimated using the restricted cubic spline (RCS). A two-tailed probability of < 0.05 was deemed statistically significant. Subgroup analyses based on clinical stage, AST level, and CA125 level were further performed. All statistical analyses were carried out in R software (version 4.2.2).
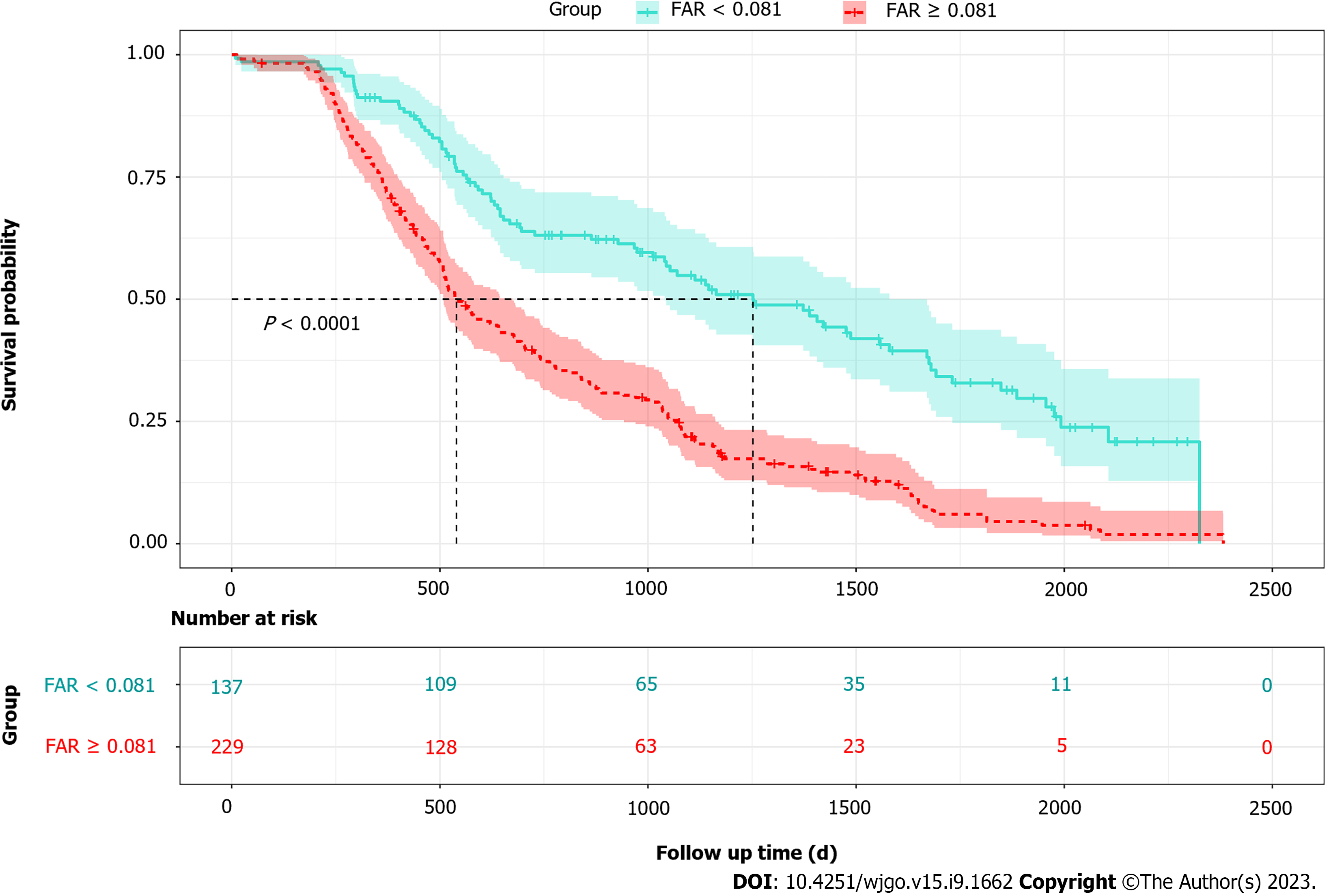
A total of 366 histologically confirmed HCC patients were retrospectively identified between 2013 and 2018. The ROC curve determined an optimal cutoff of 0.081 for baseline FAR (Supplementary Figure 1). Therefore, the patients were dichotomized into higher (FAR ≥ 0.081) and lower (FAR < 0.081) FAR groups. General characteristics of the HCC patients are represented and compared in Table 1. Except for age at diagnosis, alcohol drinking status, BMI, and baseline CEA, all other characteristics were statistically different in HCC patients with different baseline serum FAR levels. The median survival time for all patients was 645.00 d (interquartile range: 757.25 d). Compared to patients with a lower baseline FAR level, HCC patients with higher baseline FAR levels (FAR ≥ 0.081) had a significantly shorter median survival length (947.50 vs. 530.50 d).
| Characteristics | All patients (n = 366) | The lower group (FAR < 0.081, n = 138) | The higher group (FAR 0.081, n = 228) | P value |
| Gender | ||||
| Male | 312 (85.24)c | 113 (81.88)c | 199 (87.28)c | 0.16 |
| Female | 54 (14.76)c | 25 (18.12)c | 29 (12.72)c | |
| Age at diagnosis | 54.59 (10.60)a | 52.98 (10.83)a | 55.56 (10.36)a | 0.02 |
| Cigarette smoking | ||||
| No | 129 (35.24)c | 54 (39.19)c | 75 (32.89)c | 0.23 |
| Yes | 237 (64.76)c | 84 (60.81)c | 153 (67.11)c | |
| Alcohol drinking | ||||
| No | 161 (43.99)c | 64 (46.38)c | 97 (42.54)c | 0.47 |
| Yes | 205 (56.01)c | 74 (53.62)c | 131 (57.46)c | |
| BMI (kg/m2) | 21.55 (2.71)b | 21.93 (2.70)b | 21.32 (2.70)b | 0.04 |
| Stage | < 0.01 | |||
| I-II | 68 (18.58)c | 53 (38.41)c | 15 (6.58)c | |
| III | 166 (45.35)c | 52 (37.68)c | 114 (50.00)c | |
| IV | 132 (36.07)c | 33 (23.91)c | 99 (43.42)c | |
| Survival length (d) | 645.00 (757.25)b | 947.50 (1002.25)b | 530.50 (683.50)b | < 0.01 |
| AST (U/L) | 66.35 (75.22)b | 46.50 (48.08)b | 80.15 (81.30)b | < 0.01 |
| ALT (U/L) | 43.80 (38.68)b | 38.95 (28.10)b | 50.05 (40.93)b | 0.01 |
| AFP (ng/mL) | 593.35 (9018.82)b | 315.20 (483.18)b | 743.25 (10910.73)b | 0.53 |
| TBIL (μmol/L) | 17.40 (14.00)b | 16.35 (13.55)b | 19.45 (14.13)b | 0.81 |
| NLR (unit free) | 2.75 (2.09)b | 1.93 (1.29)b | 3.30 (2.18)b | < 0.01 |
| CEA (U/L) | 2.80 (3.10)b | 2.90 (3.11)b | 2.73 (3.14)b | 0.56 |
| CA125 (U/L) | 34.77 (83.51)b | 19.63 (30.11)b | 55.96 (104.58)b | 0.01 |
| CA19-9 (U/L) | 29.50 (67.38)b | 21.56 (36.71)b | 36.56 (81.11)b | 0.09 |
| FAR (unit free) | 0.09 (0.05)b | - |
Figure 1 represents an overview of the OS for HCC patients with higher (≥ 0.081) and lower (< 0.081) baseline FAR levels. Patients with a lower baseline FAR had a superior OS (log-rank = 47.8, P < 0.01). The results of univariate and multivariate Cox proportional hazard model fitting results are shown in Table 2. Baseline FAR remained a significant prognostic factor after adjusting for possible covariates, while HCC patients with higher FAR levels showed a hazard ratio (HR) of 2.43 [95% confidence interval (95%CI): 1.87–3.15].
| Covariates | Univariate Cox model | Multivariate Cox model | ||
| Crude HR (90%CI) | P value | Adjusted HR (95%CI) | P value | |
| Sex: Male | 1.74 (1.30, 2.35) | < 0.01 | ||
| Age at diagnosis: + 5 yr | 1.01 (0.96, 1.06) | 0.72 | ||
| Cigarette smoking: Yes | 1.51 (1.23, 1.87) | < 0.01 | ||
| Alcohol drinking: Yes | 1.23 (1.01, 1.50) | 0.08 | ||
| BMI: + 1 kg/m2 | 0.97 (0.93, 1.00) | 0.14 | ||
| Stage (Ref: I-II) | ||||
| III | 3.49 (2.46, 4.96) | < 0.01 | 2.14 (1.37, 3.35) | < 0.01 |
| IV | 4.86 (3.40, 6.95) | < 0.01 | 2.33 (1.45, 3.74) | < 0.01 |
| AST: 40 U/L | 2.74 (2.12, 3.53) | < 0.01 | 1.50 (1.01, 2.22) | 0.04 |
| ALT: 40 U/L | 1.72 (1.41, 2.10) | < 0.01 | ||
| AFP: 400 ng/mL | 1.93 (1.58, 2.36) | < 0.01 | 1.53 (1.19, 1.96) | < 0.01 |
| TBIL: 17.1 μmo/L | 1.81 (1.49, 2.20) | < 0.01 | ||
| NLR: + 5 | 1.72 (1.45, 2.05) | < 0.01 | ||
| CEA: 5 U/L | 1.26 (1.01, 1.57) | < 0.01 | ||
| CA125: 35 U/L | 2.89 (2.36, 3.55) | < 0.01 | 1.72 (1.30, 2.27) | < 0.01 |
| CA19-9: > 37 U/L | 1.95 (1.60, 2.37) | < 0.01 | ||
| FAR: 0.081 | 2.43 (1.95, 3.02) | < 0.01 | 1.39 (1.05, 1.83) | < 0.01 |
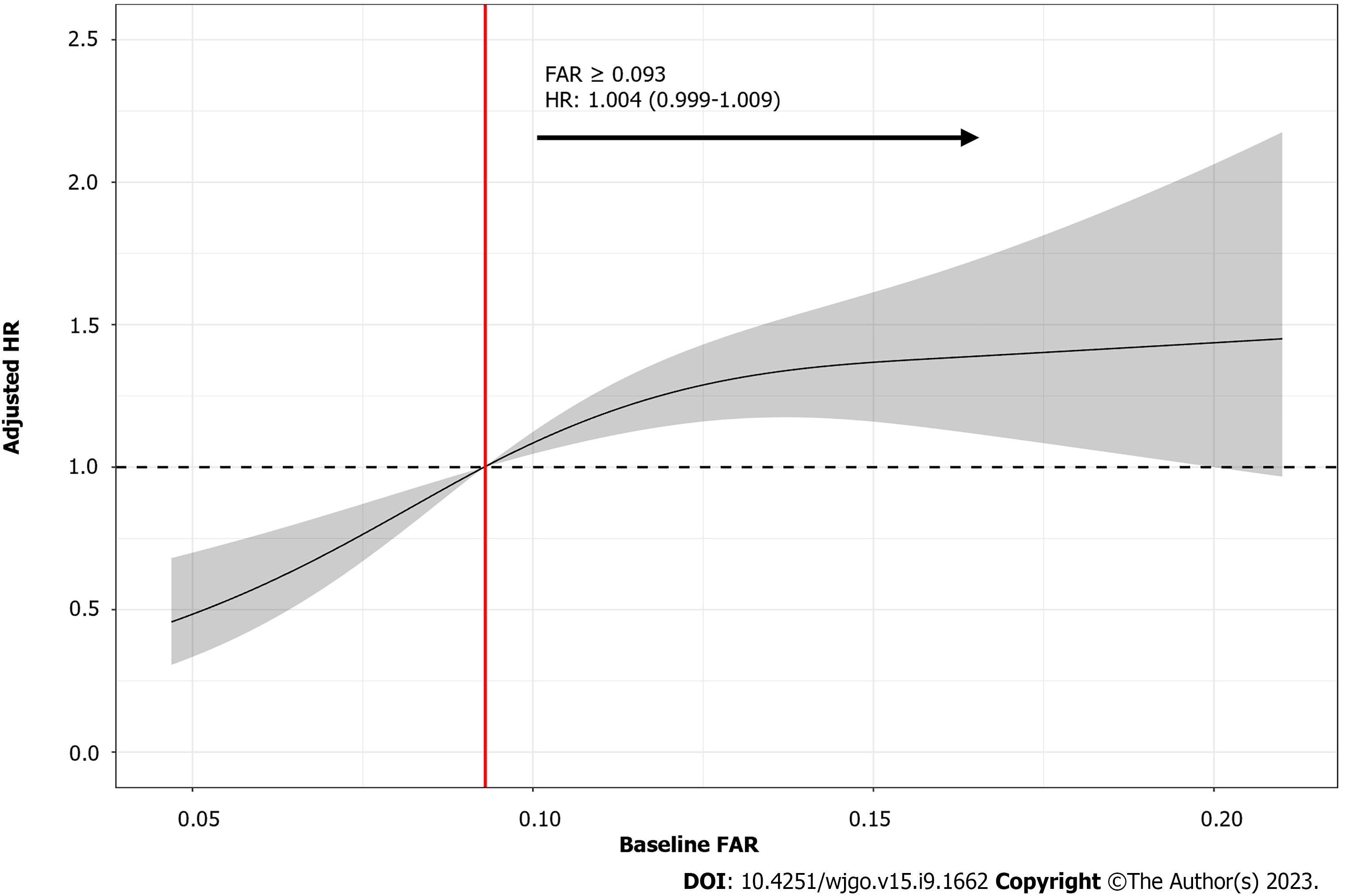
To verify the reliability and determine the trend of this association, HCC patients were further divided into four groups based on their baseline serum FAR quartiles as follows: Group 1 (FAR < 0.071), group 2 (0.071 ≤ FAR < 0.093), group 3 (0.093 ≤ FAR < 0.131), and group 4 (FAR ≥ 0.131). After adjustment for potential covariates identified in the previous univariate model, the adjusted HRs for groups 2 to 4 compared to group 1 were 1.07 (95%CI: 0.73–1.57), 1.62 (95%CI: 1.10–2.39), and 1.37 (95%CI: 0.92–2.04), respectively (Figure 2). The RCS fitting results showed that after controlling for the same potential confounders as those in the multivariate Cox proportional hazards model, a nonlinear relationship between baseline FAR levels and HR was observed, with the overall risk of death in HCC patients arising with an increasing baseline FAR. Furthermore, the risk of death was significantly higher when the baseline FAR exceeded 0.093 (Figure 3).
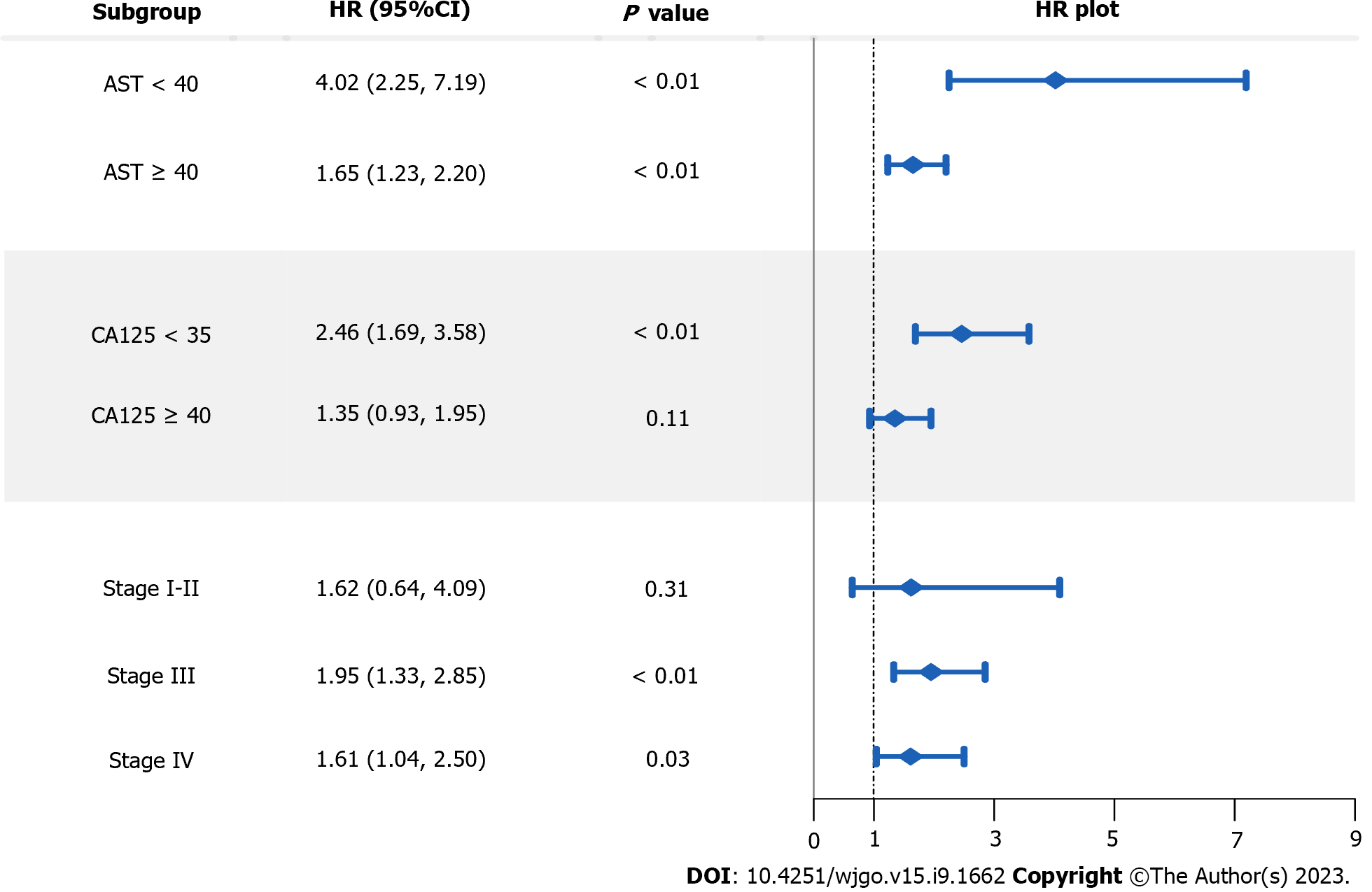
A series of subgroup analyses were performed separately using three important characteristics of the HCC patients, including baseline serum AST, CA125, and clinical stage (Figure 4). Among the three stratified factors, baseline serum AST and CA125 levels presented as noticeable effect modifiers. Specifically, the FAR-OS association tended to be much stronger for HCC patients with lower baseline serum AST or CA125 levels.
The present retrospective study examined the prognostic role of serum FAR measured upon diagnosis for the OS in 366 HCC patients. As a result, a higher baseline FAR was related to significantly inferior OS in HCC. Further analysis using either FAR quartiles or the RCS revealed a prominent dose-response trend in this association. Subgroup analysis revealed that the FAR-OS association was more significant in patients with lower baseline serum AST or CA125 Levels. These findings may have potential clinical significance in guiding the HCC treatment.
FAR is a composite index. It increases along with an increase in serum fibrinogen level or a decrease in serum albumin level. Elevated serum fibrinogen level may be associated with increased fibrinogen deposition in tumor tissue[24]. It has been found that fibrinogen has strong adhesion to tumor cells. With the help of thrombin, fibrinogen is converted into fibrin, which forms a physical barrier around tumor cells, helps them survive, and plays an important role in cancer progression[25]. Serum albumin has been recognized as not only an important indicator of nutritional status in cancer patients[26,27], but also a tumor suppressor that inhibits matrix metalloproteinase (MMP)-induced invasion and metastasis of HCC by modulating urokinase plasminogen activator surface receptor signaling[28]. In the advanced stages of cancer, albumin level also decreases with the increase in concentrations of other acute phase proteins[29]. These mechanisms can justify the positive connection between elevated serum FAR and compromised OS in HCC patients identified in the present study.
Subgroup analysis suggested that the FAR-OS association appeared to be stronger in patients with lower serum AST or CA125 Levels. AST is an enzyme that reflects liver damage and is often used to evaluate the progression of liver-related diseases. The clearance of serum AST decreases as liver function declines, causing elevated serum AST levels[30]. Elevated serum AST levels have been found to be independently related to inferior survival rates of HCC patients[31]. CA125 is a macromolecular glycoprotein synthesized and stored in the somatic cavity of epithelial cells and is not normally accessible to the circulation. Elevation in this indicator reflects various tumor behaviors that may be associated with severe cell damage, angiogenesis, vascular invasion, and destruction[32]. In some previously published studies, a higher serum CA125 level has been demonstrated to be prominently related to compromised prognosis of liver cancer patients[33]. These findings suggest that even if the reduction in serum FAR can be manipulated, little survival benefit can be achieved in HCC patients with higher AST or CA125 levels.
The present study’s major findings highlight the prognostic significance of serum FAR in HCC patients. The decrease in fibrinogen level or increase in albumin level, which result in reduced serum FAR, might be related to improved survival. Many drugs that lower serum fibrinogen level are currently available. For instance, fibrates directly reduce fibrinogen mRNA transcription in vitro and in vivo through their effect on peroxisome proliferator-activated receptor alpha[34]. It has been observed that benzofibrate significantly reduces serum fibrinogen level, which, in turn, is associated with the anti-tumor effect of benzofibrate as a biomarker or potential mediator[35]. In addition, MMPs and serine proteases contained in snake venom drugs also have a good fibrinogen-lowering effect[36]. Moreover, it has been reported that the median survival of HCC patients can be extended by taking low doses of aspirin[37]. Branched-chain amino acid (BCAA) supplementation is commonly used for correcting malnutrition issues in cancer patients, and it significantly increases serum albumin concentration[38]. It has been reported that BCAA supplementation can also alleviate the impairment of liver function in HCC patients after transarterial chemoembolization[39]. Some literature has reported that the administration of n-3 polyunsaturated fatty acids, especially eicosa-pentaenoic, can also help to maintain serum albumin level in cancer patients[40]. Nonetheless, the effect of either fibrinogen reduction or albumin supplementation aimed at improving the survival outcomes in HCC patients should be further validated by clinical experimental studies.
The main strengths of the present study include its comparatively large sample size. In addition, many potential confounders were simultaneously controlled for when analyzing the FAR-OS association. Furthermore, a dose-response trend revealed by the RCS further corroborated the stability of this FAR-OS association. However, some study limitations were present. First, analytical data were collected retrospectively, introducing a risk of information bias. Second, all HCC patients were from a single institution, which limits the ability to generalize the study results. Multi-centered longitudinal studies are needed to further validate the present study’s major findings.
In this retrospective study of 366 histologically confirmed HCC patients, serum FAR measured at disease diagnosis was found to be significantly associated with the OS. Patients with a higher baseline FAR had an increased death hazard with a prominent dose-response trend. This FAR-OS association was more prominent in HCC patients with lower serum AST or CA125 levels. The study findings have important implications for clinical treatment of HCC, suggesting that intervention measures aiming at reducing FAR might provide a survival benefit for this group of patients. Prospective studies with more representative samples should be carried out.
Fibrinogen-to-albumin ratio (FAR) has been found significantly associated with survival of some types of cancer. Less is known regarding to its association with prognosis for hepatocellular carcinoma (HCC) patients.
We intend to thoroughly discuss the association between baseline serum FAR and the overall survival (OS) for HCC patients.
To provide estimation for the association between baseline FAR and the OS of HCC patients, and to discuss potential effect modification by some important characteristics of the patients.
Retrospective study design was used to identify qualified HCC patients from a provincial cancer hospital in China. Relevant information was extracted from the Hospital Information System. Kaplan-Meier survival curves were plotted to compare the OS of HCC patients with different baseline serum FAR levels. Cox proportional hazards models were applied to estimate the adjusted association between FAR and the OS of HCC patients. The Restricted Cubic Spline was used to further delineate the dose-response association.
A lower baseline serum FAR level was associated with an adjusted hazard ratio of 2.43 (95% confidence interval: 1.87–3.15) in the OS of HCC patients, with identifiable dose-response trend. The FAR-OS association was more prominent in HCC patients with a lower baseline serum aspartate aminotransferase or carbohydrate antigen 125 level.
Serum FAR is a prominent prognostic indicator for HCC.
Intervention measures which aiming at regulating serum FAR might of clinical interest for treating HCC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding H, China; Ker CG, Taiwan S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63989] [Article Influence: 15997.3] [Reference Citation Analysis (174)] |
| 2. | Hassanipour S, Vali M, Gaffari-Fam S, Nikbakht HA, Abdzadeh E, Joukar F, Pourshams A, Shafaghi A, Malakoutikhah M, Arab-Zozani M, Salehiniya H, Mansour-Ghanaei F. The survival rate of hepatocellular carcinoma in Asian countries: a systematic review and meta-analysis. EXCLI J. 2020;19:108-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 3. | Mohammadian M, Mahdavifar N, Mohammadian-Hafshejani A, Salehiniya H. Liver cancer in the world: epidemiology, incidence, mortality and risk factors. World Cancer Res J. 2018;5:e1082. |
| 4. | Karaoğullarından Ü, Üsküdar O, Odabaş E, Ak N, Kuran S. Hepatocellular Carcinoma in Cirrhotic Versus Noncirrhotic Livers: Clinicomorphologic Findings and Prognostic Factors. Turk J Gastroenterol. 2023;34:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 186] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 6. | Volc-Platzer B, Hanak H, Weiss W, Denk H. [Immunohistochemical studies on the association between hepatitis B surface antigen and primary hepatocellular carcinoma (author's transl)]. Wien Klin Wochenschr. 1982;94:108-114. [PubMed] |
| 7. | Petrelli F, Manara M, Colombo S, De Santi G, Ghidini M, Mariani M, Iaculli A, Rausa E, Rampulla V, Arru M, Viti M, Lonati V, Ghidini A, Luciani A, Facciorusso A. Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis: HCC and Steatosis or Steatohepatitis. Neoplasia. 2022;30:100809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Kim TH, Kim BH, Park JW, Cho YR, Koh YH, Chun JW, Oh ES, Lee DY, Lee SU, Suh YG, Woo SM, Moon SH, Kim SS, Lee WJ. Proton Beam Therapy for Treatment-Naïve Hepatocellular Carcinoma and Prognostic Significance of Albumin-Bilirubin (ALBI) Grade. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Qiu Y, Yang Y, Wang T, Shen S, Wang W. Efficacy of Postoperative Adjuvant Transcatheter Arterial Chemoembolization in Hepatocellular Carcinoma Patients With Microscopic Portal Vein Invasion. Front Oncol. 2022;12:831614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Wu B, Wu Y, Guo X, Liu Y, Yue Y, Zhao W, Liu J, Wu X, Shen A, Zhang S. Prognostic Significance of Preoperative Integrated Liver Inflammatory Score in Patients with Hepatocellular Carcinoma. Med Sci Monit. 2022;28:e937005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Chen Y, Liu H, Zhang J, Wu Y, Zhou W, Cheng Z, Lou J, Zheng S, Bi X, Wang J, Guo W, Li F, Zheng Y, Li J, Cheng S, Zeng Y, Liu J. Prognostic value and predication model of microvascular invasion in patients with intrahepatic cholangiocarcinoma: a multicenter study from China. BMC Cancer. 2021;21:1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Li YN, Qin J, Li WW. Systemic immune-inflammation index in hepatocellular carcinoma patients survival outcomes: a meta-analysis. Acta Medica Mediterr. 2021;37:129-134. [DOI] [Full Text] |
| 13. | Huang Y, Zeng J, Liu T, Lin X, Guo P, Zhou W, Liu J. Prognostic Significance of Elevated Preoperative Serum CA125 Levels After Curative Hepatectomy for Hepatocellular Carcinoma. Onco Targets Ther. 2020;13:4559-4567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Lee CW, Lin SE, Yu MC, Kou HW, Lee CH, Kuo T, Lee KC, Tsai HI. Does Neutrophil to Lymphocyte Ratio Have a Role in Identifying Cytokeratin 19-Expressing Hepatocellular Carcinoma? J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Li H, Liu R, Qiu H, Huang Y, Liu W, Li J, Wu H, Wang G, Li D. Tumor Burden Score Stratifies Prognosis of Patients With Intrahepatic Cholangiocarcinoma After Hepatic Resection: A Retrospective, Multi-Institutional Study. Front Oncol. 2022;12:829407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Zhao G. Albumin/fibrinogen ratio, a predictor of chemotherapy resistance and prognostic factor for advanced gastric cancer patients following radical gastrectomy. BMC Surg. 2022;22:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wu M, Pan Y, Jia Z, Wang Y, Yang N, Mu J, Zhou T, Guo Y, Jiang J, Cao X. Preoperative Plasma Fibrinogen and Serum Albumin Score Is an Independent Prognostic Factor for Resectable Stage II-III Gastric Cancer. Dis Markers. 2019;2019:9060845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Gan W, Yi Y, Fu Y, Huang J, Lu Z, Jing C, Fan J, Zhou J, Qiu S. Fibrinogen and C-reactive protein score is a prognostic index for patients with hepatocellular carcinoma undergoing curative resection: a prognostic nomogram study. J Cancer. 2018;9:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Wang S, Feng Y, Xie Y, Zhao X, Ma J, Liu X, Hu C, Hou T. High fibrinogen-albumin ratio index (FARI) predicts poor survival in head and neck squamous cell carcinoma patients treated with surgical resection. Eur Arch Otorhinolaryngol. 2022;279:4541-4548. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Yang Q, Liang D, Yu Y, Lv F. The Prognostic Significance of the Fibrinogen-to-Albumin Ratio in Patients With Triple-Negative Breast Cancer: A Retrospective Study. Front Surg. 2022;9:916298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, Zhou Z, Chen Z, Zheng S, Liang J, Lin Z, Wang J, Yan J, Xiao Z. A Novel Inflammation-Based Prognostic Score: The Fibrinogen/Albumin Ratio Predicts Prognoses of Patients after Curative Resection for Hepatocellular Carcinoma. J Immunol Res. 2018;2018:4925498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Liang L, Wang MD, Zhang YM, Zhang WG, Zhang CW, Lau WY, Shen F, Pawlik TM, Huang DS, Yang T. Association of Postoperative Biomarker Response with Recurrence and Survival in Patients with Hepatocellular Carcinoma and High Alpha-Fetoprotein Expressions (>400 ng/ml). J Hepatocell Carcinoma. 2021;8:103-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Zhang J, Qin SD, Li Y, Lu F, Gong WF, Zhong JH, Ma L, Zhao JF, Zhan GH, Li PZ, Song B, De Xiang B. Prognostic significance of combined α-fetoprotein and CA19-9 for hepatocellular carcinoma after hepatectomy. World J Surg Oncol. 2022;20:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, Aquino JB, Fiore E, Rizzo MM, Rodriguez A, Lorenti A, Andriani O, Podhajcer O, Mazzolini G. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm. 2011;8:1538-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 25. | Sharma BK, Mureb D, Murab S, Rosenfeldt L, Francisco B, Cantrell R, Karns R, Romick-Rosendale L, Watanabe-Chailland M, Mast J, Flick MJ, Whitlock PW, Palumbo JS. Fibrinogen activates focal adhesion kinase (FAK) promoting colorectal adenocarcinoma growth. J Thromb Haemost. 2021;19:2480-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Yang M, Liu Z, Li G, Li B, Li C, Xiao L, Zhou J. Geriatric Nutritional Risk Index as a Prognostic Factor of Patients with Non-Small Cell Lung Cancer: A Meta-Analysis. Horm Metab Res. 2022;54:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Harimoto N, Muranushi R, Hoshino K, Yamanaka T, Hagiwara K, Ishii N, Tsukagoshi M, Igarashi T, Watanabe A, Kubo N, Araki K, Shirabe K. Albumin-Indocyanine Green Evaluation (ALICE) grade predicts bile leakage after hepatic resection. Surg Today. 2020;50:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Fu X, Yang Y, Zhang D. Molecular mechanism of albumin in suppressing invasion and metastasis of hepatocellular carcinoma. Liver Int. 2022;42:696-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Li M, Kong YD, Zou JX, Wu XQ, Yin Z, Niu XT, Wang GQ. Transcriptome and physiological analyses reveal the mechanism of the liver injury and pathological alterations in northern snakehead (Channa argus) after dietary exposure to aflatoxin B1. Aquaculture. 2022;561:e738727. [DOI] [Full Text] |
| 31. | Zhang LX, Lv Y, Xu AM, Wang HZ. The prognostic significance of serum gamma-glutamyltransferase levels and AST/ALT in primary hepatic carcinoma. BMC Cancer. 2019;19:841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Zhang M, Zhang Y, Fu J, Zhang L. Serum CA125 levels are decreased in rectal cancer but increased in fibrosis-associated diseases and in most types of cancers. Prog Mol Biol Transl Sci. 2019;162:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Meng Q, Shi S, Liang C, Xiang J, Liang D, Zhang B, Qin Y, Ji S, Xu W, Xu J, Ni Q, Yu X. Diagnostic Accuracy of a CA125-Based Biomarker Panel in Patients with Pancreatic Cancer: A Systematic Review and Meta-Analysis. J Cancer. 2017;8:3615-3622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Bordbar M, de Mutsert R, Cevval M, Rosendaal FR, Jukema JW, Lijfering WM. Differential effect of statin use on coagulation markers: an active comparative analysis in the NEO study. Thromb J. 2021;19:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Iakobishvili Z, Hasin T, Klempfner R, Shlomo N, Goldenberg I, Brenner R, Kornowski R, Gerber Y. Association of Bezafibrate Treatment With Reduced Risk of Cancer in Patients With Coronary Artery Disease. Mayo Clin Proc. 2019;94:1171-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Op den Brouw B, Ghezellou P, Casewell NR, Ali SA, Fathinia B, Fry BG, Bos MHA, Ikonomopoulou MP. Pharmacological Characterisation of Pseudocerastes and Eristicophis Viper Venoms Reveal Anticancer (Melanoma) Properties and a Potentially Novel Mode of Fibrinogenolysis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Sitia G, Iannacone M, Guidotti LG. Anti-platelet therapy in the prevention of hepatitis B virus-associated hepatocellular carcinoma. J Hepatol. 2013;59:1135-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Zeid SA, Rabiee A, El Refaey FA, Sherif N. Effect of branched chain amino acid supplementation on dialysis adequacy and nutritional parameters in hemodialysis patients. Saudi J Kidney Dis Transpl. 2020;31:1361-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Shiozawa S, Usui T, Kuhara K, Tsuchiya A, Miyauchi T, Kono T, Asaka S, Yamaguchi K, Yokomizo H, Shimakawa T, Yoshimatsu K, Katsube T, Naritaka Y. Impact of Branched-Chain Amino Acid-Enriched Nutrient on liver Cirrhosis with Hepatocellular Carcinoma Undergoing Transcatheter Arterial Chemoembolization in Barcelona Clinic Liver Cancer Stage B: A Prospective Study. J Nippon Med Sch. 2016;83:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Mocellin MC, Fernandes R, Chagas TR, Trindade EBSM. A meta-analysis of n-3 polyunsaturated fatty acids effects on circulating acute-phase protein and cytokines in gastric cancer. Clin Nutr. 2018;37:840-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |