Published online Sep 15, 2023. doi: 10.4251/wjgo.v15.i9.1595
Peer-review started: June 1, 2023
First decision: July 17, 2023
Revised: July 24, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 15, 2023
Processing time: 104 Days and 2 Hours
Hepatic arterioportal fistulas (APFs) are common in hepatocellular carcinoma (HCC). Moreover, correlated with poor prognosis, APFs often complicate anti-tumor treatments, including transarterial chemoembolization (TACE).
To compare the efficacy of ethanol-soaked gelatin sponges (ESG) and micro
Data from patients diagnosed with HCC or hepatic APFs between June 2016 and December 2019 were retrospectively analyzed. Furthermore, APFs were embolized with ESG (group E) or microspheres (group M) during TACE. The primary outcomes were disease control rate (DCR) and objective response rate (ORR). The secondary outcomes included immediate and first follow-up APF improvement, overall survival (OS), and progression-free survival (PFS).
Altogether, 91 participants were enrolled in the study, comprising 46 in group E and 45 in group M. The DCR was 93.5% and 91.1% in groups E and M, respectively (P = 0.714). The ORRs were 91.3% and 66.7% in groups E and M, respectively (P = 0.004). The APFs improved immediately after the procedure in 43 (93.5%) patients in group E and 40 (88.9%) patients in group M (P = 0.485). After 2 mo, APF improvement was achieved in 37 (80.4%) and 33 (73.3%) participants in groups E and M, respectively (P = 0.421). The OS was 26.2 ± 1.4 and 20.6 ± 1.1 mo in groups E and M, respectively (P = 0.004), whereas the PFS was 16.6 ± 1.0 and 13.8 ± 0.7 mo in groups E and M, respectively (P = 0.012).
Compared with microspheres, ESG embolization demonstrated a higher ORR and longer OS and PFS in patients of HCC with hepatic APFs.
Core Tip: Hepatocellular carcinoma (HCC) was considered the seventh most common cancer and the second leading cause of cancer-related deaths worldwide in 2020. Hepatic arterioportal fistulas (APFs) are common in HCC and often complicate anti-tumor treatments, including transarterial chemoembolization. The ethanol-soaked gelatin sponge combined the advantages of alcohol and gelatin sponges, contributed to better local control of hepatic APFs, and improved the survival of patients with HCC.
- Citation: Yuan GS, Zhang LL, Chen ZT, Zhang CJ, Tian SH, Gong MX, Wang P, Guo L, Shao N, Liu B. Comparison of ethanol-soaked gelatin sponge and microspheres for hepatic arterioportal fistulas embolization in hepatic cellular carcinoma. World J Gastrointest Oncol 2023; 15(9): 1595-1604
- URL: https://www.wjgnet.com/1948-5204/full/v15/i9/1595.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i9.1595
Hepatocellular carcinoma (HCC) was the seventh most common cancer and the second leading cause of cancer-related deaths worldwide in 2020, with 905677 new cases and 830180 deaths recorded annually[1]. Hepatic arterioportal fistulas (APFs), defined as fistulas between the hepatic artery and the neighboring portal vein[2,3], are common in HCC owing to tumor infiltration, vascular damage[4], or remodeling of the cirrhotic parenchyma.
Hepatic APFs may cause portal hypertension, ascites, and varices[5], which are strongly associated with poor prognosis[6]. The presence of hepatic APFs often complicates anti-tumor treatments, including transarterial chemoembolization (TACE). Chemotherapeutic agents and embolic materials run off through the fistulas, and tumor cells may detach from the hepatic artery, resulting in portal vein thrombosis[7].
Many materials have been used to treat hepatic APFs, including gelatin sponges[8], microspheres[9], coils[10], histoacryl[10], absolute ethanol[10], polyvinyl alcohol particles[10], and ethanol-soaked gelatin sponges (ESG)[11,12]. Additionally, ESG combines the advantages of alcohol and gelatin sponges and provides convincing results at different APF stages[12]. However, to the best of our knowledge, no study has compared the efficacies of ESG and microspheres. We conducted a retrospective study to evaluate the efficacy of ESGs and microspheres for the treatment of HCC with hepatic APF.
Patients with HCC and hepatic APF treated with TACE and ESG (group E) or microspheres (group M) were enrolled between June 2016 and December 2019. The study protocol was approved by the ethics committee of the leading center. The requirement for written informed consent was waived owing to the retrospective nature of the study. All the experiments were performed in compliance with the Ethical Principles for Medical Research Involving Human Subjects outlined in the 1975 Declaration of Helsinki (revised in 2000).
The inclusion criteria were as follows: (1) Confirmed diagnosis of HCC based on the American Association for the Study of Liver Diseases practice guidelines[13]; (2) Hypervascular tumor with Barcelona Clinic Liver Cancer (BCLC) Staging A-C; (3) Hepatic APF confirmed by angiography; (4) Predicted life span ≥ 1 year; and (5) Karnofsky score > 70.
The exclusion criteria were as follows: (1) Other malignancies within 5 years; (2) Child-Pugh score ≤ 10; and (3) Severe coagulopathy (prothrombin time > 17 s and/or platelet count ≤ 60 × 109/L).
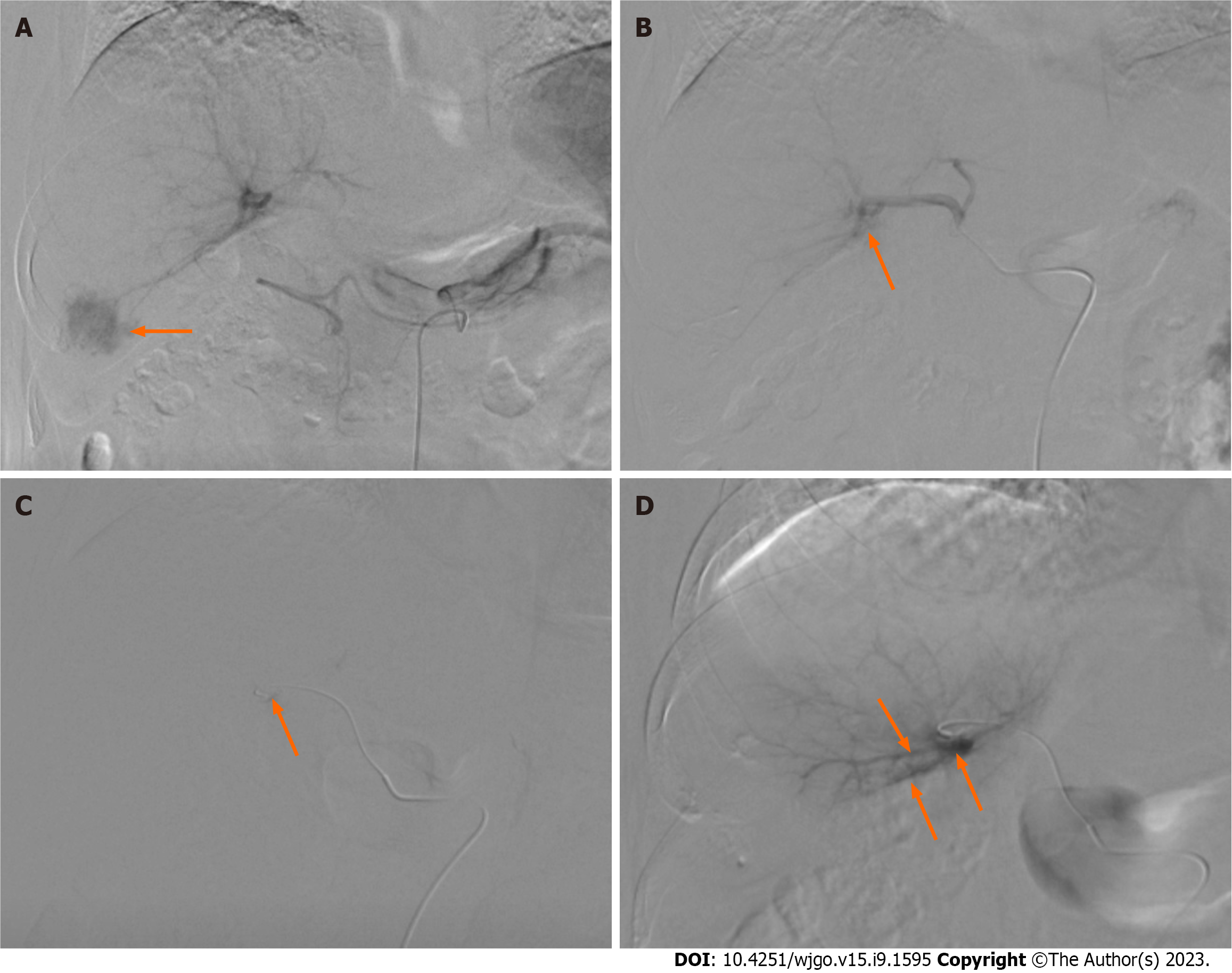
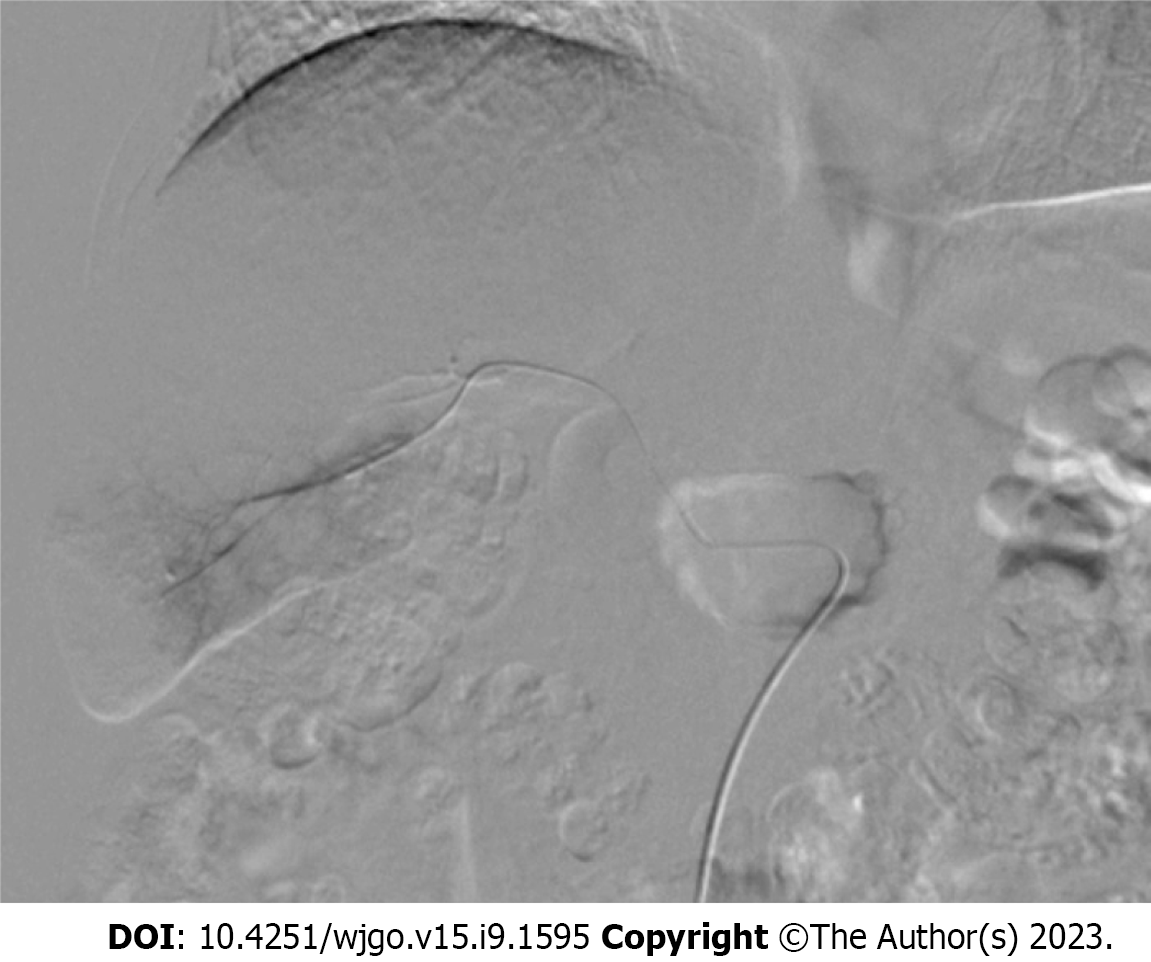
For group E, an appropriate-sized gelatin sponge (Alicon Inc., Hangzhou, China) was mixed with 10 mL of iodixanol (Hengrui Co. Ltd, Lianyungang, China) and 10 mL of ethanol (Lingfeng Inc, Shanghai, China). For group M, appropriate-sized microspheres (Embosphere, Merit Medical, UT, United States) were mixed with 10 mL of iodixanol. Digital subtraction angiography (DSA) was performed after catheterization of the celiac or superior mesenteric artery to validate the location and size of the hepatic APFs (Figure 1). APFs were classified according to a previous study by Zhou et al[12] (Table 1). Each APF feeding artery was superselected using a 2.7-F microcatheter. ESG or the microspheres were injected under fluoroscopic guidance until the fistula was blocked. Coils were used if the fistula was not completely blocked. DSA was repeated to confirm the complete embolization of the APFs (Figure 2).
| Grade | Definition | Class |
| 0 | APFs were not observed | - |
| 1 | APFs flow to the subsegmental portal branch | Mild |
| 2 | APFs flow to the segmental portal branch | Moderate |
| 3 | APFs flow into the main portal branch of the ipsilateral lobe | Moderate |
| 4 | APFs flow into the main portal branch of the contralateral lobe and/or the main portal vein | Severe |
| 5 | APFs flow into the main portal vein presenting with hepatofugal portal venous flow | Severe |
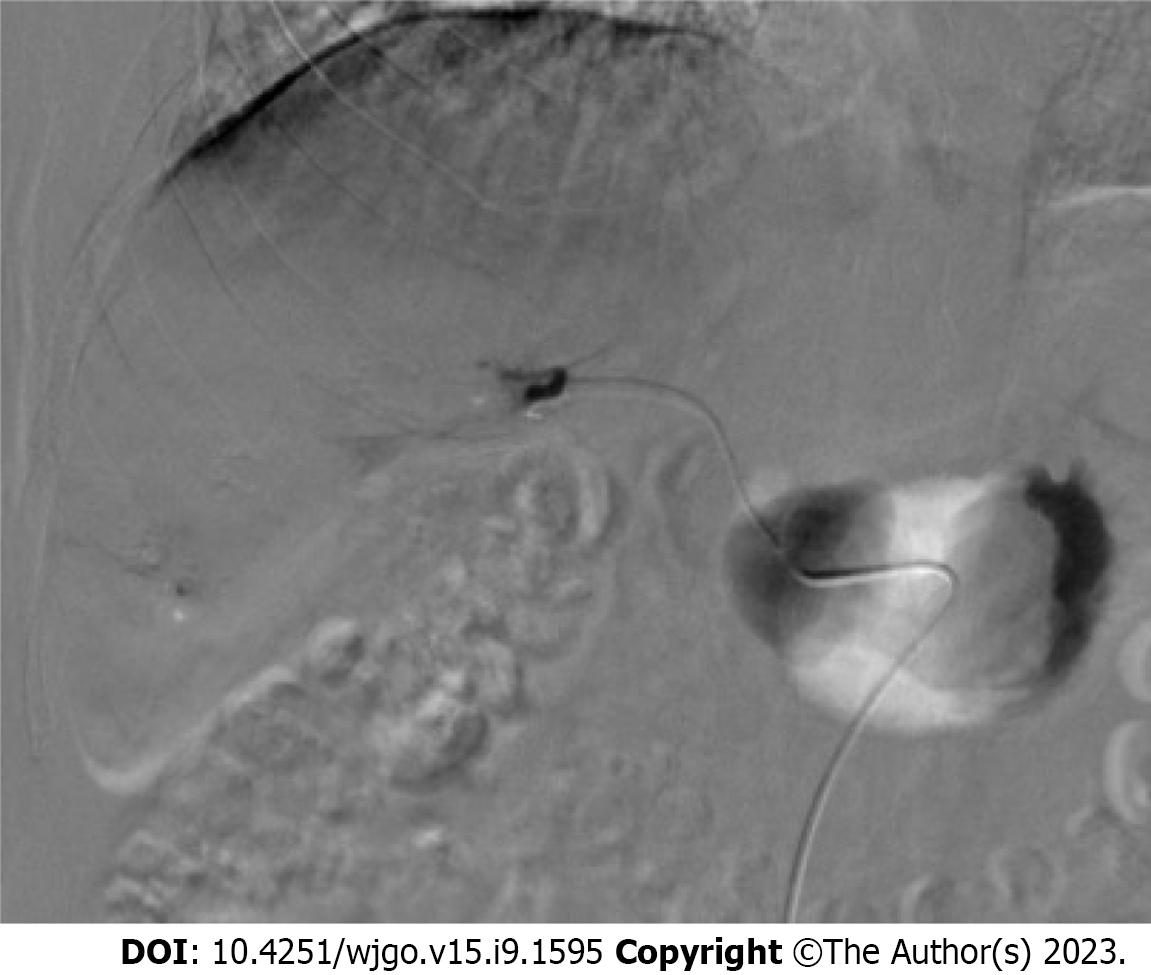
After APF embolization, a microcatheter was advanced into each feeding artery of the HCC. An emulsion of poppy Lipiodol (Hengrui Co. Ltd., Lianyungang, China) and epirubicin (Qilu Co. Ltd., Jinan, China) was injected via a microcatheter until complete embolization of the tumor was achieved (Figure 3)[14].
Follow-up was conducted every 2 mo and included standard blood count, liver functional tests, alpha-fetoprotein (AFP), and abdominal contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI). The images were interpreted based on the consensus of three skilled interventional radiologists.
In case the tumor recurrence was detected on CECT or MRI, TACE was repeated. If APF recurrence with a grade ≥ 2 was observed, ESG or microsphere APF embolization was repeated; however, if APFs did not recur, TACE was the only procedure performed. Follow-up intervention was determined based on the tumor condition and general status.
The modified Response Evaluation Criteria in Solid Tumors for HCC[15] were applied to assess tumor response after 4 mo. The primary outcomes were disease control rate (DCR) and objective response rate (ORR), and the secondary outcomes included immediate and first-time follow-up of APF improvement, overall survival (OS), and progression-free survival (PFS).
Immediate APF improvement was defined as a decrease in grade to 1 or 0. First-time follow-up APF improvement was defined as a decrease in at least two grades confirmed by angiography in the second session, whereas APF progression was defined as an increased grade on the first-time follow-up angiography. If the grade remained the same or decreased by one, the APFs were not considered to improve. Moreover, OS was defined as the time interval between the initial TACE and death or the last follow-up. Furthermore, PFS was defined as the time interval between initial TACE and disease progression or death.
Continuous variables were analyzed using Student’s t-test to determine whether the variables were normally distributed; otherwise, the Mann–Whitney U test was used. Categorical variables were analyzed using the χ2 or Fisher’s exact tests.
Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Statistical significance was defined as a two-tailed P < 0.05. All statistical analyses were conducted using the SPSS software (version 24.0; IBM Inc., Armonk, NY, United States).
A consecutive series of 91 patients were enrolled in the study. During TACE, APFs were embolized using ESG in 46 participants and microspheres in 45 participants. The ratios of men to women were 33/13 in group E and 33/12 in group M (χ2 = 0.029, P = 0.865), with a mean age of 63.4 ± 8.5 and 58.4 ± 10.1 years (P = 0.092), respectively. The etiologies included hepatitis B virus (HBV) (38/46, 82.6% in group E; 39/45, 86.7% in group M), hepatitis C (4/46, 8.7% in group E; and 2/45, 4.4% in group M), HBV + hepatitis C virus (2/46, 4.3% in group E; 2/45, 4.4% in group M), and alcohol consumption (2/46, 4.3% in group E; 2/45, 4.4% in group M) (P = 0.952). No significant differences in the Child-Pugh stage, BCLC stage, or tumor location were observed between the two groups. The mean tumor diameters were 6.8 ± 2.9 mm and 7.1 ± 1.6 mm in groups E and M (P = 0.765), respectively. Portal vein thrombi were identified in 24 participants (24/46, 52.2%) in group E and 22 participants (22/45, 48.9%) in group M (χ2 = 0.098, P = 0.754), respectively. The treatments administered before TACE included surgery, microwave ablation (MWA), radiofrequency ablation (RFA), TACE, radiation, and TACE + MWA/RFA. We observed no significant differences in previous treatments between the two groups (P = 0.925). The median levels of AFP were 137 [interquartile range (IQR): 9.8, 970.1] and 114.9 (IQR: 3.7, 725.7) ng/mL in groups E and M, respectively (P = 0.734). APF grades 1, 2, 3, 4, and 5 were recorded in 5 (5/46, 10.9%) and 6 (6/45, 13.3%); 15 (15/46, 32.6%) and 16 (16/45, 35.6%); 11 (11/46, 23.9%) and 14 (14/45, 31.1%); 9 (9/46, 19.6%), and 7 (7/45, 15.6%); and 6 (6/46, 13%) and 2 (2/45, 4.4%) participants in groups E and M, respectively (P = 0.636) (Table 2).
| Characteristics | E group (n = 46) | M group (n = 45) | χ2 | P value |
| Sex, n (%) | 0.029 | 0.865 | ||
| Male | 33 (71.7) | 33 (73.3) | ||
| Female | 13 (28.3) | 12 (26.7) | ||
| Age (yr) | 63.4 ± 8.5 | 58.4 ± 10.1 | - | 0.092 |
| Etiology, n (%) | 0.909 | 0.952 | ||
| HBV | 38 (82.7) | 39 (86.8) | ||
| HCV | 4 (8.7) | 2 (4.4) | ||
| HBV + HCV | 2 (4.3) | 2 (4.4) | ||
| Alcohol | 2 (4.3) | 2 (4.4) | ||
| Child-Pugh stage, n (%) | 0.297 | 0.586 | ||
| A | 25 (54.3) | 27 (60) | ||
| B | 21 (45.7) | 18 (40) | ||
| BCLC stage, n (%) | 0.271 | 0.873 | ||
| A | 7 (15.2) | 6 (13.3) | ||
| B | 19 (41.3) | 21 (45.7) | ||
| C | 20 (43.5) | 18 (40) | ||
| Tumor location | 0.837 | 0.658 | ||
| Right lobe | 30 (65.2) | 28 (62.2) | ||
| Left lobe | 9 (19.6) | 10 (22.2) | ||
| Right and left lobes | 7 (15.2) | 7.1 ± 1.6 | ||
| Mean tumor diameter (cm) | 6.8 ± 2.9 | - | 0.765 | |
| Portal vein thrombus | 0.098 | 0.754 | ||
| Present | 24 (52.2) | 22 (48.9) | ||
| Absent | 22 (47.8) | 23 (51.1) | ||
| Previous treatment | 1.639 | 0.925 | ||
| Surgery | 7 (15.2) | 6 (13.3) | ||
| MWA/RFA | 9 (19.6) | 7 (15.6) | ||
| TACE | 4 (8.7) | 5 (11.1) | ||
| Radiation | 4 (8.7) | 6 (13.3) | ||
| TACE + MWA/RFA | 2 (4.3) | 3 (6.7) | ||
| None | 20 (43.5) | 18 (40) | ||
| AFP [ng/mL, median (IQR)] | 137 (9.8, 970.1) | 114.9 (3.7, 725.7) | - | 0.734 |
| APF grade, n (%) | 2.689 | 0.636 | ||
| 1 | 5 (10.9) | 6 (13.3) | ||
| 2 | 15 (32.6) | 16 (35.6) | ||
| 3 | 11 (23.9) | 14 (31.1) | ||
| 4 | 9 (19.6) | 7 (15.6) | ||
| 5 | 6 (13) | 2 (4.4) |
The mean follow-up period was 35.3 ± 2.7 mo in group E and 30.9 ± 3.8 mo in group M (P = 0.195). After 4 mo, complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were achieved in 18 (18/46, 39.1%) and 8 (8/45, 17.8%) patients; 21 (21/46, 45.7%) and 18 (18/45, 40%) patients; 4 (4/46, 8.7%) and 15 (15/45, 33.3%) patients; and 3 (3/46, 6.5%) and 4 (4/45, 8.9%) participants in groups E and M, respectively (P = 0.014). The DCR was 93.5% (43/46) in group E and 91.1% (41/45) in group M (P = 0.714). The ORRs were 91.3% (42/46) and 66.7% (30/45) in groups E and M, respectively (P = 0.004).
The APFs immediately improved after the procedure in 43 (43/46, 93.5%) and 40 (40/45, 88.9%) participants in groups E and M, respectively (P = 0.485). After 2 mo, APF improvement was achieved in 37 (37/46, 80.4%) and 33 (33/45, 73.3%) participants in groups E and M, respectively (P = 0.421). The median AFP levels at 4 mo after the procedure were 28.48 (IQR: 4, 257.9) and 45.25 (IQR: 4.43, 359.5) ng/mL in groups E and M, respectively (P = 0.045). After 4 mo, the difference in Child-Pugh class distribution between the two groups was not significant (P = 0.083) (Table 3).
| Characteristics | E group (n = 46) | M group (n = 45) | χ2 | P value |
| Tumor response after four months (%) | 10.578 | 0.014 | ||
| CR | 18 (39.1) | 8 (17.8) | ||
| PR | 21 (45.7) | 18 (40) | ||
| SD | 4 (8.7) | 15 (33.3) | ||
| PD | 3 (6.5) | 4 (8.9) | ||
| DCR | 43 (93.5) | 41 (91.1) | 0.714 | |
| ORR | 42 (91.3) | 30 (66.7) | 8.358 | 0.004 |
| Immediate improvement of APF (%) | - | 0.485 | ||
| Yes | 43 (93.5) | 40 (88.9) | - | |
| No | 3 (6.5) | 5 (11.1) | ||
| First-time follow-up APF improvement (%) | 0.646 | 0.421 | ||
| Improved | 37 (80.4) | 33 (73.3) | ||
| Not improved | 9 (19.6) | 12 (26.7) | ||
| AFP after 4 mo [ng/mL, median (IQR)] | 28.48 (4, 257.9) | 45.25 (4.43, 359.5) | 0.045 | |
| Child-Pugh score after 4 mo (%) | 5.321 | 0.083 | ||
| A | 33 (71.7) | 23 (51.1) | ||
| B | 10 (21.7) | 20 (44.4) | ||
| C | 3 (6.6) | 2 (4.5) | ||
| OS, months (mean ± SD) | 26.2 ± 1.4 | 20.6 ± 1.1 | 10.3 | 0.004 |
| PFS, months (mean ± SD) | 16.6 ± 1.0 | 13.8 ± 0.7 | 6.3 | 0.012 |
The OS was 26.2 ± 1.4 and 20.6 ± 1.1 mo in groups E and M, respectively (χ2 = 10.3, P = 0.004; Figure 4A) (Table 3). The PFS was 16.6 ± 1.0 and 13.8 ± 0.7 mo in groups E and M, respectively (P = 0.012; Figure 4B) (Table 3).
According to the updated BCLC prognosis and treatment strategy[16], TACE is recommended for intermediate-stage B HCC. With its tendency to infiltrate the portal and hepatic venous structures, HCC is often accompanied by APFs, which may reduce the therapeutic benefits of TACE[7]. Our study focused on comparing ESG and microspheres for the treatment of hepatic APFs. The DCRs were 93.5% (43/46) in group E and 91.1% (41/45) in group M (P = 0.714). The ORRs were 91.3% and 66.7% in groups E and M, respectively (P = 0.004). The OS was 26.2 ± 1.4 and 20.6 ± 1.1 mo in groups E and M, respectively (P = 0.004). The PFS was 16.6 ± 1.0 and 13.8 ± 0.7 mo in groups E and M, respectively (P = 0.012; Figure 4B) (Table 3).
Gelatin sponges and microspheres have several disadvantages in the treatment of hepatic APF. Gelatin sponges are absorbed 2-3 wk after the procedure, and APFs can be recanalized. Microspheres exerted a physical embolic effect without causing protein degradation in the vascular wall. Ethanol has been widely used in the embolization of arteriovenous malformations[17], which can denature blood proteins, dehydrate vascular endothelial cells, and cause segment fractures in the vascular wall[18-20]. Compared to gelatin sponges alone, ethanol demonstrated an improved long-term effect on hepatic APFs[21]. However, because of its liquid properties, ethanol alone is not suitable for shunts with high blood flow. ESG combines the advantages of ethanol and gelatin sponges, promoting local control of hepatic APFs and liver tumors[12].
In our study, the immediate improvement and first-time follow-up rates of APFs in group E were not significantly higher than those in group M (93.5% and 88.9%, P = 0.485, 80.4% and 73.3%, P = 0.421, respectively). Thus, ESG and microspheres may have similar short-term effects on the treatment of hepatic APFs. The immediate improvement rate in group E was comparable to the 97% reported by Zhou et al[12], whereas the first follow-up APF improvement rate was higher in both groups than that reported by Zhou et al[12] (54%). This discrepancy may be attributed to the higher proportion of patients with grades 1–3 APFs in our study.
Our study investigated tumor response 4 mo after the procedure and revealed that the CR, PR, SD, and PD rates were 39.1% and 17.8%, 45.7% and 40%, 8.7% and 33.3%, and 6.5% and 8.9% in groups E and M, respectively (P = 0.014). Moreover, the ORR was 84.8% and 57.8% in groups E and M, respectively (P = 0.004). Compared with microspheres, ESG led to complete long-term control of hepatic APF, including physical blockade and chemical destruction and yielded a significantly better local tumor response. Both the DCRs (93.5%) and ORRs (84.8%) in group E patients were higher than those reported in Zhou et al’s study (81.9% and 42.6%, respectively)[12]. This has three possible reasons. First, the tumor response in our study was evaluated 4 mo after the procedure, which provided an additional opportunity for tumor control. Second, the percentage of participants with portal vein thrombus (52.5%) was lower than that reported by Zhou et al’s study[12]. Third, the proportion of grade 1-3 APFs in our study was higher, resulting in a better embolic response.
The OS, PFS, and median AFP levels at 4 mo after the procedure in group E were significantly better than those in group M. The aforementioned outcome may be attributed to the complete blockage of hepatic APFs and well-controlled tumors. Compared with microspheres, ESG embolization demonstrated complete long-term blockade of hepatic APFs and therefore improved the local control of HCC and survival of patients with HCC.
Nevertheless, the study had some limitations. As this was a retrospective study, selection bias may have reduced the value of the results. However, further prospective studies are required to validate the findings.
Compared to microsphere embolization, ESG embolization resulted in a higher ORR and longer OS and PFS. The findings may contribute to the selection of embolic agents for treating hepatic APFs in patients with HCC.
Hepatic arterioportal fistulas (APFs) are common in hepatocellular carcinoma (HCC) because of tumor infiltration, vascular damage, and remodeling of the cirrhotic parenchyma. The presence of hepatic APFs often complicates anti-tumor treatments, including transarterial chemoembolization (TACE).
Ethanol-soaked gelatin sponges (ESG) combine the advantages of alcohol and gelatin sponges, demonstrating a convincing effect at different stages of hepatic APFs. However, to date, no study has compared the efficacy of ESG and microspheres.
This retrospective study aimed to compare the efficacy of ESG and microspheres in the management of APFs, and their impact on the prognosis of HCC.
The APFs were embolized using ESG (group E) or microspheres (group M) during TACE. The disease control rate (DCR) and objective response rate (ORR) were considered the primary outcomes. The secondary outcomes included immediate and first follow-up APF improvement, overall survival (OS), and progression-free survival (PFS).
The DCR was 93.5% and 91.1% in groups E and M, respectively (P = 0.714). The ORRs were 91.3% and 66.7% in groups E and M, respectively (P = 0.004). In 43 (93.5%) patients in group E and 40 (88.9%) patients in group M. the APFs improved immediately after the procedure (P = 0.485). After 2 mo, APF improvement was achieved in 37 (80.4%) and 33 (73.3%) participants in groups E and M, respectively (P = 0.421). The OS was 26.2 ± 1.4 and 20.6 ± 1.1 mo in groups E and M, respectively (P = 0.004). The PFS was 16.6 ± 1.0 and 13.8 ± 0.7 mo in groups E and M, respectively (P = 0.012).
Compared with microspheres, ESG embolization demonstrated a higher ORR and longer OS and PFS in patients with HCC with hepatic APFs.
The findings may aid the selection of embolic agents for the treatment of hepatic APFs in patients with HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single-blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lykoudis PM, United Kingdom; Ueda H, Japan S-Editor: Fan JR L-Editor: A P-Editor: Zhao S
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63311] [Article Influence: 15827.8] [Reference Citation Analysis (174)] |
| 2. | Gülberg V, Haag K, Rössle M, Gerbes AL. Hepatic arterial buffer response in patients with advanced cirrhosis. Hepatology. 2002;35:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 3. | Richter S, Mücke I, Menger MD, Vollmar B. Impact of intrinsic blood flow regulation in cirrhosis: maintenance of hepatic arterial buffer response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G454-G462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002;3:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Kakati BR, Pedersen MR, Chen SY, Hirsch KS, Berggreen PJ, Seetharam AB. Hepatic arterioportal fistula presenting as gastric variceal hemorrhage. J Gastrointestin Liver Dis. 2014;23:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Wakamatsu T, Ogasawara S, Chiba T, Yokoyama M, Inoue M, Kanogawa N, Saito T, Suzuki E, Ooka Y, Tawada A, Yokosuka O. Impact of Radiofrequency Ablation-Induced Glisson's Capsule-Associated Complications in Patients with Hepatocellular Carcinoma. PLoS One. 2017;12:e0170153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6552] [Article Influence: 468.0] [Reference Citation Analysis (1)] |
| 8. | Kanogawa N, Chiba T, Ogasawara S, Ooka Y, Suzuki E, Motoyama T, Saito T, Sekimoto T, Tawada A, Maruyama H, Yoshikawa M, Yokosuka O. Successful interventional treatment for arterioportal fistula caused by radiofrequency ablation for hepatocellular carcinoma. Case Rep Oncol. 2014;7:833-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Cai L, Li H, Guo J, Zhao W, Duan Y, Hou X, Cheng L, Du H, Shao X, Diao Z, Hao Y, Zheng X, Li C, Li W. Treatment efficacy and safety of drug-eluting beads transarterial chemoembolization versus conventional transarterial chemoembolization in hepatocellular carcinoma patients with arterioportal fistula. Cancer Biol Ther. 2022;23:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Chan WS, Poon WL, Cho DH, Chiu SS, Luk SH. Transcatheter embolisation of intrahepatic arteriovenous shunts in patients with hepatocellular carcinoma. Hong Kong Med J. 2010;16:48-55. [PubMed] |
| 11. | Li J, Kang X, Guo L, Xiao J, Cheng J. Embolization of hepatic arterioportal shunt with ethanol-soaked gelatin sponge. J Cancer Res Ther. 2019;15:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Zhou WZ, Shi HB, Liu S, Yang ZQ, Zhou CG, Xia JG, Zhao LB, Li LS. Arterioportal shunts in patients with hepatocellular carcinoma treated using ethanol-soaked gelatin sponge: therapeutic effects and prognostic factors. J Vasc Interv Radiol. 2015;26:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 2987] [Article Influence: 426.7] [Reference Citation Analysis (3)] |
| 14. | Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 3270] [Article Influence: 218.0] [Reference Citation Analysis (36)] |
| 16. | Reig M, Forner A, Rimola J, Ferrer-Fábrega J, Burrel M, Garcia-Criado A, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J Hepatol. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2488] [Article Influence: 829.3] [Reference Citation Analysis (59)] |
| 17. | Do YS, Yakes WF, Shin SW, Lee BB, Kim DI, Liu WC, Shin BS, Kim DK, Choo SW, Choo IW. Ethanol embolization of arteriovenous malformations: interim results. Radiology. 2005;235:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Buchta K, Sands J, Rosenkrantz H, Roche WD. Early mechanism of action of arterially infused alcohol U.S.P. in renal devitalization. Radiology. 1982;145:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Hammer FD, Boon LM, Mathurin P, Vanwijck RR. Ethanol sclerotherapy of venous malformations: evaluation of systemic ethanol contamination. J Vasc Interv Radiol. 2001;12:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Takebayashi S, Hosaka M, Kubota Y, Ishizuka E, Iwasaki A, Matsubara S. Transarterial embolization and ablation of renal arteriovenous malformations: efficacy and damages in 30 patients with long-term followup. J Urol. 1998;159:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Huang MS, Lin Q, Jiang ZB, Zhu KS, Guan SH, Li ZR, Shan H. Comparison of long-term effects between intra-arterially delivered ethanol and Gelfoam for the treatment of severe arterioportal shunt in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:825-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |