Published online Sep 15, 2023. doi: 10.4251/wjgo.v15.i9.1544
Peer-review started: April 11, 2023
First decision: July 9, 2023
Revised: July 14, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: September 15, 2023
Processing time: 154 Days and 19.9 Hours
Gastric cancer (GC) is one of the most common malignant tumors. Osteopontin (OPN) is thought to be closely related to the occurrence, metastasis and prognosis of many types of tumors.
To investigate the effects of OPN on the proliferation, invasion and migration of GC cells and its possible mechanism.
The mRNA and protein expression of OPN in the GC cells were analyzed by real-time quantitative-reverse transcription polymerase chain reaction and western blotting, and observe the effect of varying degree expression OPN on the proliferation and other behaviors of GC. Next, the effects of OPN knockdown on GC cells migration and invasion were examined. The short hairpin RNA (shRNA) and negative control shRNA targeting OPN-shRNA were transfected into the cells according to the manufacturer’s instructions. Non transfected cells were classified as control in the identical transfecting process. 24 h after RNA transfection cell proliferation activity was detected by 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide assay, and cell invasiveness and migration were detected by Trans well assay. Meanwhile, the expression of protein kinase B (AKT), matrix metalloproteinase 2 (MMP-2) and vascular endothelial growth factor (VEGF) in the human GC cell lines was detected by reverse transcription polymerase chain reaction and western blotting.
The results of this study revealed that OPN mRNA and protein expression levels were highly expressed in SGC-7901 cells. OPN knockdown by specific shRNA noticeably reduced the capabilities of proliferation, invasion and migration of SGC-7901 cells. Moreover, in the experiments of investigating the underlying mechanism, results showed that OPN knockdown could down-regulated the expression of MMP-2 and VEGF, it also decreased the phosphorylation of AKT. Meanwhile, the protein expression levels of MMP-2, VEGF and phosphorylated AKT was noticeable lower than that in control group in the GC cells after they were added to phosphatidylinositol-3-kinase (PI3K) inhibitor (LY294002).
These results suggested that OPN though PI3K/AKT/mammalian target of rapamycin signal pathway to up-regulate MMP-2 and VEGF expression, which contribute SGC-7901 cells to proliferation, invasion and migration. Thus, our results demonstrate that OPN may serve as a novel prognostic biomarkers as well as a potential therapeutic targets for GC.
Core Tip: We investigated the effects of osteopontin (OPN) on the proliferation, invasion and migration of gastric cancer (GC) cells and its possible mechanism. The results of this study revealed that OPN mRNA and protein expression levels were highly expressed in SGC-7901 cells. OPN knockdown by specific short hairpin RNA noticeably reduced the capabilities of proliferation, invasion and migration of SGC-7901 cells. Moreover, our results showed that OPN though phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway signal pathway to up-regulate matrix metalloproteinase 2 and vascular endothelial growth factor expression, which contribute SGC-7901 cells to proliferation, invasion and migration. These results demonstrate that OPN may serve as a novel prognostic biomarkers as well as a potential therapeutic targets for GC.
- Citation: Qin YC, Yan X, Yuan XL, Yu WW, Qu FJ. Osteopontin promotes gastric cancer progression via phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. World J Gastrointest Oncol 2023; 15(9): 1544-1555
- URL: https://www.wjgnet.com/1948-5204/full/v15/i9/1544.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i9.1544
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide[1]. At present, the development of comprehensive treatment strategies has greatly improved the therapeutic effect of GC patients. Because the accurate diagnosis of early GC is difficult[2], the prognosis of most GC patients is still poor, and the 5-year survival rate of patients with advanced GC is approximately 25%[3]. Therefore, more accurate identification of prognostic biomarkers and molecular basis of GC invasion and metastasis has important clinical value for understanding GC and developing new effective treatment strategies.
Osteopontin (OPN), an extracellular matrix (ECM) phosphoglycoprotein, is expressed at elevated levels in a variety of malignant tumors (such as breast cancer, lung cancer, urogenital tumors, head and neck cancer, osteosarcoma, etc.) and is involved in many pathophysiological processes including tumorigenesis, leading to poor prognosis. Since it is involved in promoting aggressive and metastatic progression of many cancers, it is considered as a potential important biomarker for monitoring cancer progression[4-13]. In addition, the up-regulation of OPN expression is also closely related to the occurrence, metastasis and prognosis of tumors in the digestive system, and even the size and grade of tumors[14-18].
With the ongoing study of OPN, OPN is also being explored as a potential therapeutic target. For example, reducing OPN expression could provide novel strategies for the treatment of patients with various types of metastatic cancer[15,19-22].
A number of studies have reported that the expression of OPN in GC tissues is significantly higher than that in non-tumor tissues, and is closely related to the invasion, metastasis and prognosis of GC[15,23-28]. But there are conflicting stories. Tang et al[29] concluded that the expression of OPN in GC tissues was not related to prognosis.
Previous studies of our research group have found[30] that OPN is significantly up-regulated in GC tissues, and its expression level is closely related to clinicopathological parameters, overall survival (OS) and disease-free survival of patients, suggesting that OPN is closely related to poor prognosis of GC. The results are consistent with those of Sun et al[31]. Final results in a meta-analysis showed that high OPN expression was associated with poor OS, suggesting that OPN is a promising prognostic biomarker for GC[32].
These data indicated that OPN may play a crucial role in the carcinogenesis of GC. Despite increasing insights into the function of OPN-promoted progression of GC, the exact mechanism of OPN-promoted invasion and progression in GC remains unclear.
Phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) signaling pathway is one of the most widely studied signaling pathways. PI3K/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling pathway is involved in cell proliferation, invasion and metastasis after abnormal activation of malignant tumors[33]. PI3K/AKT/mTOR signaling pathway is considered to be one of the most common regulatory pathways in GC molecular mechanism studies[33-35].
However, whether OPN can regulate the PI3K-AKT-mTOR signaling pathway in GC cells has not been reported in the literature. Therefore, our study comprehensively analyzed whether OPN in GC cells regulates the expression of PI3K and phosphorylation of its downstream signal transduction pathway protein by exerting its kinase activity, thus promoting the proliferation, migration and invasion of GC cells. This study aims to explore the mechanism of the regulation of PI3K-AKT-mTOR signal transduction pathway by OPN in GC cells, so as to provide a new theoretical basis for elucidate the metastasis and invasion mechanism of GC and further develop the targeted treatment of GC with protein kinase inhibitors.
The GC cell lines (SGC-7901, HGC-27, and AGS) and normal gastric mucosa epithelial cell line (GES-1) were provided by China Center for Type Culture Collection (China); roswell park memorial institute (RPMI) 1640 cell culture medium, Ham’s F 12 nutrient medium (F12), and 0.25% trypsin-ethylenediaminetetraacetic acid were provided by HYclone (United States); fetal bovine serum (FBS) was offered by Haoyang (China); OPN-short hairpin RNA (shRNA) interference vector and negative control shRNA (NC-shRNA) interference vector were designed and developed by Sangon (China); RNAiso Plus TB Green™ Premix Ex Taq™ II PrimeScript™ RT reagent kit with gDNA Eraser were provided by Takara (Japan); The primer sequence of OPN, matrix metalloproteinase 2 (MMP-2), vascular endothelial growth factor (VEGF) and β-actin were designed and fabricated by Sangon (China); primary monoclonal antibodies for mTOR (Abp54398), OPN (Abp52084), AKT (Abp50636), phosphorylated AKT (p-AKT) (phosphorySer473), β-actin (A01010) and horseradish peroxidase (HRP) conjugated secondary antibodies (A21010, A21020) were offered by Abbkine (United States); primary monoclonal antibodies for MMP-2 (BS-0412R) and VEGF (BS-0279R) were provided by Bioss (China); LY294002 inhibitor was purchased from Meilun (China).
Human GC cell lines were cultured in RPMI-1640 medium supplemented with 10% FBS and antibiotics (100 U/mL streptomycin and 100 μg/mL penicillin); the AGS cells underwent culture process in F-12 medium supplemented with 10% FBS and antibiotics (100 U/mL streptomycin and 100 μg/mL penicillin). All of the cells were grown in a humid incubator with 5% CO2 at 37 ℃, besides, the adherent cells were cleaned twice consecutively with phosphate-buffered saline. Cells were harvested with 0.25% trypsin and passaged at a ratio of 1: 3 every three days.
The shRNA targeting OPN-shRNA and the NC-shRNA were transiently transfected cells with Ultra Fectin according to the instruction of the manufacturer. Non transfected cells were classified as control in the identical transfecting process. TTCAAGAA was taken as the loop structure of shRNA template to avoid the formation of termination signal, and T6 structure acted as the transcription termination sequence of shRNA. ShRNA expression vector covered the expression framework of green fluorescent protein, which can be expressed after being transferred into cells. The transfection efficiency can be easily determined under a fluorescence microscopy or by flow cytometry. Cells were cultured for 24 h or 48 h and subsequently harvested for further experiments.
The sequences of the shRNAs include: OPN-shRNA1: Sense: 5'-CACCGAGGAGTTGAATGGTGCATACTTCAAGAGAGTATGCACCATTCAACTCCTCTTTTTTG-3', Anti-sense: 5'-AGCTCAAAAAAGAGGAGTTGAATGGTGCATACTCTCTTGAAGTATGCACCATTCAACTCCTC-3'; OPN-shRNA2: Sense: 5'-CACCGTAAGGAAGAAGATAAACACCTTCAAGAGAGGTGTTTATCTTCTTCCTTACTTTTTTG-3', Anti-sense: 5'-AGCTCAAAAAAGTAAGGAAGAAGATAAACACCTCTCTTGAAGGTGTTTATCTTCTTCCTTAC-3'; OPN-shRNA3: Sense: 5'-CACCGTGCATCTTCTGAGGTCAATTTTCAAGAGAAGACCTCAGAAGATGCACTTTTTTG-3', Anti-sense: 5'-AGCTCAAAAAAGTGCATCTTCTGAGGTCAATTTCTCTTGAAAATTGACCTCAGAAGATGCAC-3'; NC-shRNA: Sense: 5'-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3', Anti-sense: 5'-AGCTCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3’.
24 h after RNA transfection, human GC cell line (SGC-7901) was seeded in 96-plates (4 × 103 cells/well). For this experiment, 1 h post-cell seeding was defined as the 0 h time point. After 0, 24, 48, 72, 96 h, the cells were incubated with MTT solution (5 mg/mL) in an incubator for 4 h, respectively. The formed formazan crystals were dissolved with 200 µL of dimethyl sulfoxide and then mixed well. The optical density of each sample was determined at 490 nm with Epoch™ Microplate Spectrophotometer (United States).
The transwell chamber was placed into a 24-well plates. In the upper chamber coated with matrigel (for invasion assay), SGC-7901 (4 × 104) cells in 200 μL serum-free XGI-1640 medium were added. In the upper chamber without being coated with matrigel (for migration assay), SGC-7901 (2 × 104) cells in 200 μL serum-free XGI-1640 were added. The lower chamber was filled with 600 μL conditioned media. After incubated for 48 h (for invasion) or 24 h (for migration), the cells were incubated with formaldehyde 4% for 20 min at ambient temperature. Subsequently, cells were stained with 1% crystal violet for 30 min at ambient temperature. Next, the images of various fields (n = 3) at 100 × magnification for each insert were counted.
Total RNA was extracted from human gastric cell lines using RNAiso Plus and then quantified spectrophotometrically by its absorbance at 260 nm. Forward primer 5'-AGCGAGGAGTTGAATGGTGCATAC-3' reverse primer 5'-AATCTGGACTGCTTGTGGCTGTG-3'; MMP-2, forward primer 5'-GGCGGTCACAGCTACTTCTTCAAG-3', reverseprimer 5'-ATCGAAGGCAGTGGAGAGGAAGG-3'; VEGF, forwardprimer 5'-CCTTCGCTTACTCTCACCTGCTTC-3', reverse primer 5'-GGCTGCTTCTTCCAACAATGTGTC-3'; β-actin, human beta-actin Endogenous Reference Genes Primers, 10μM (B661102-0001, Sangon Biotech). Reverse transcription was performed with PrimeScript™ RT reagent Kit with gDNA Eraser (perfect real time) following the directives of the manufacturer. RT-PCR was performed with the following protocol: An initial pre-denaturation step at 95 ℃ for 30 s, 40 cycles of denaturation at 95 ℃ for 5 s, and then annealing process at 60 ℃ for 30 s. Meantime, the β-actin RNA was amplified and acted as an internal control. The cycle threshold values for β-actin RNA the samples were calculated by computer software.
Human GC cell lines and treated SGC-7901 cells were harvested and lysed with ice-cold radio immunoprecipitation assay solution, and then added to phenylmethanesulfonyl fluoride (99: 1) as well as phosphatase inhibitors (99: 1), Then, the resulting homogenate was centrifuged for 5 min at 12000 rmp and 4 ℃. Subsequently, the total protein in the supernatant was quantified with a protein quantification kit protein assay kit, resolved by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane. Next, the protein was blocked by 5% BSA blocking buffer or evaporated milk at ambient temperature for 1-2 h, incubated overnight with polyclonal antibody at 4 ℃ under gentle agitation, sub
Each experiment was performed at least three times. Data (mean ± SE) were studied by one-way ANOVA or independent t-test. All of the calculations were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, United States). The level of significance was set at aP < 0.05; bP < 0.01; cP < 0.001.
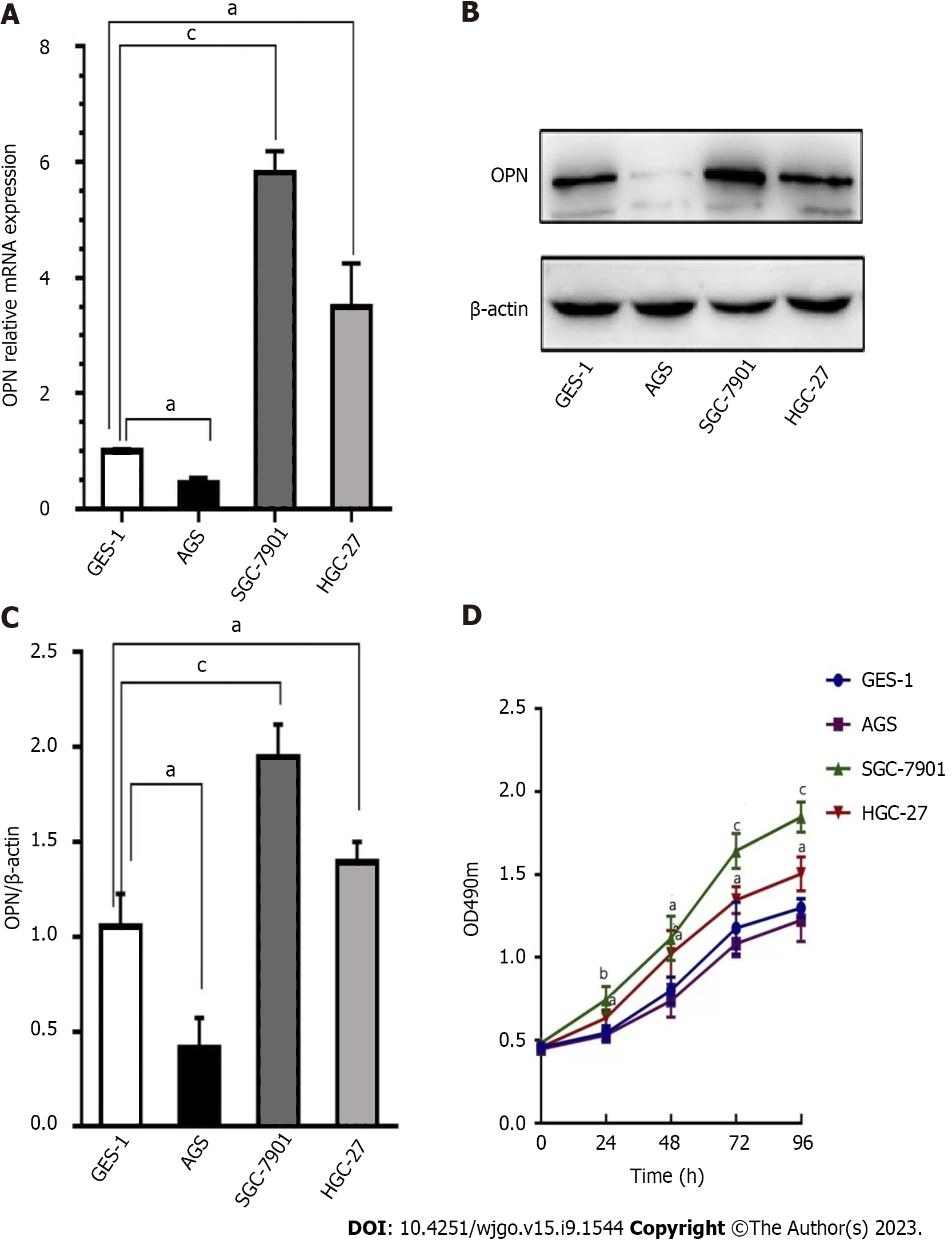
The mRNA and protein expression of OPN in the GC cells and GES-1 cells were analyzed by real-time quantitative-reverse transcription and western blotting, respectively. Results revealed that GC cell lines (AGS, SGC-7901 and HGC-27) and normal cell line GES-1 expressed OPN mRNA and protein to varying degree. The mRNA and protein expression levels of OPN in the GC cell lines (SGC-7901, HGC-27) were markedly higher than those in GES-1 cells (Figure 1A-C). Moreover, we examined the effects of OPN expression on GC cell proliferation by MTT assay; results revealed the capacity of proliferation related with the OPN expression levels; SGC-7901 cells with high level expressed OPN possess strong capacity of proliferation (Figure 1D).
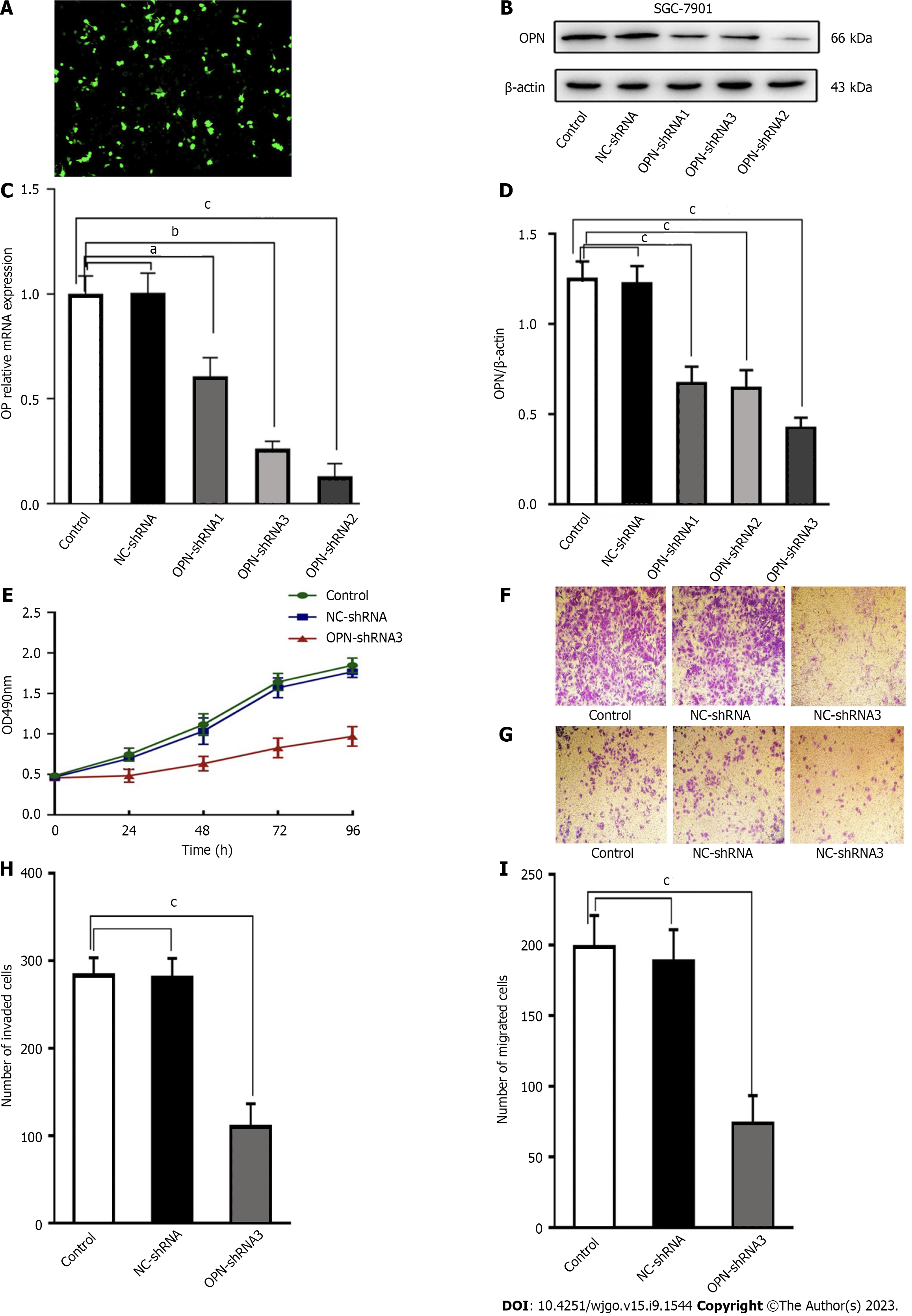
To explore the effects of OPN on GC cells, the proliferation, invasion and metastasis capacities of high level OPN expression SGC-7901 cells and low level OPN expression SGC-7901 cells were examined. In order to create low level OPN expression SGC-7901 cells, three OPN-shRNA transfect vector were built. The transfection efficiency can be easily determined under a fluorescence microscopy (Figure 2A). Results reveal that the expression of OPN of all three sequences of OPN-shRNA-transfected SGC-7901 cells were significantly lower than that of control (blank control) cells( Figure 2B-D). And the OPN-shRNA3 exhibited the optimal interference efficiency of OPN, revealing that it acts as a right model for ascertaining the effects of OPN knockdown (Figure 2B-D). Meanwhile, there were no noticeable differences in the expression between control and NC-shRNA-transfected SGC-7901 cells ( Figure 2B-D).
The MTT assay and transwell assay revealed that capacities of proliferation, invasion and metastasis of OPN-shRNA3-transfected group were significantly lower than that of control group.
Meanwhile, there were no differences between in the control group and NC-shRNA-transfected group (Figure 2E-I). These results demonstrated that OPN play a key role in promoting SGC-7901 cells proliferation, invasion and migration.
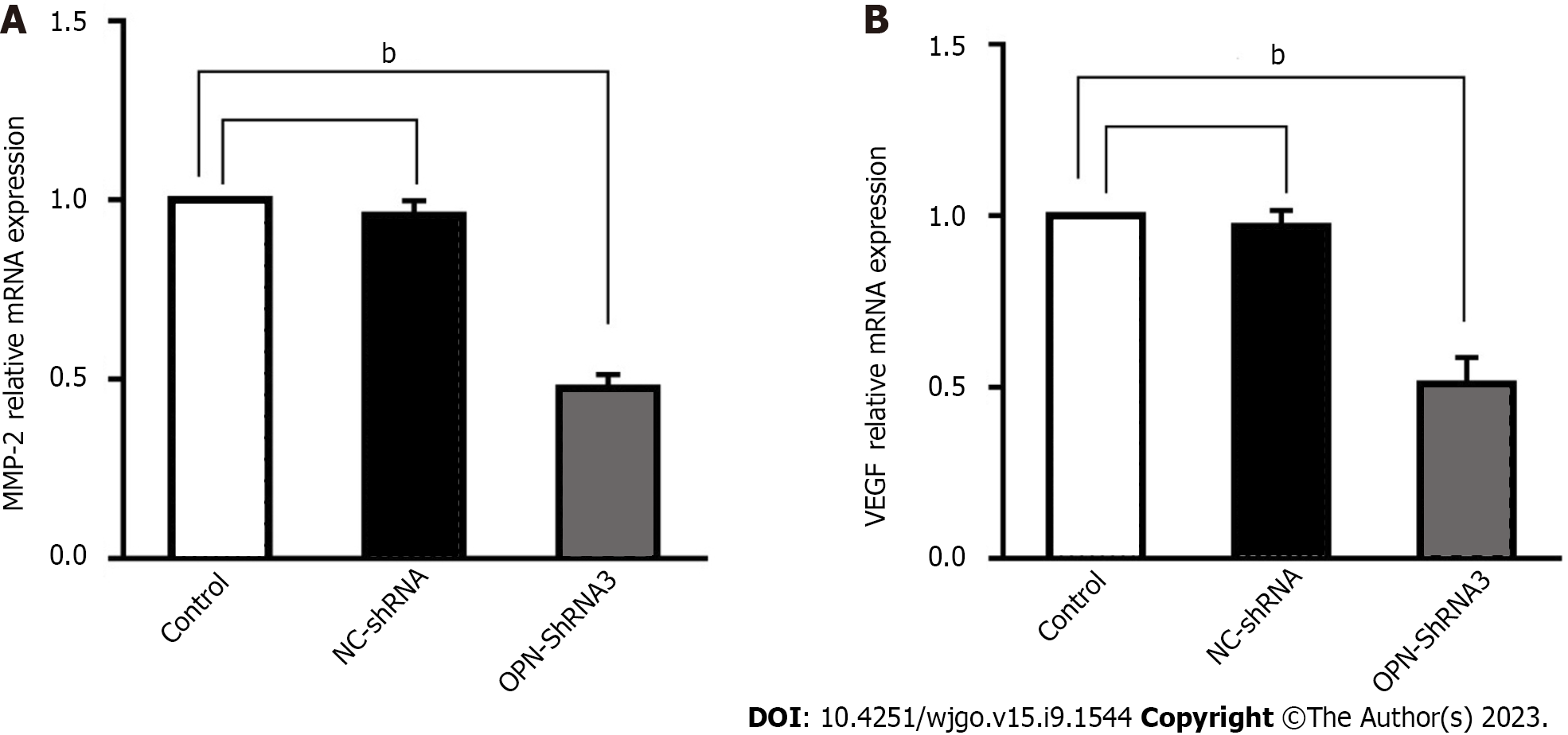
In order to observe the relevance between OPN and the expression MMP-2 and VEGF, the effect of OPN on regulating MMP-2 and VEGF expression was studied. Results show that the MMP-2 and VEGF mRNA in OPN-shRNA3 group were significantly down-regulated by 52.6% and 49.0% compared with control group, respectively (Figure 3), the result revealed that OPN knockdown could down-regulate the expressions of MMP-2 and VEGF.
To further investigate the underlying mechanism of OPN in proliferation, invasion and migration of GC cells. We analyzed the protein expression levels of mTOR, AKT, p-AKT, MMP-2, VEGF in the SGC-7901 after they were OPN knockdown or added to PI3K inhibitor (LY294002).
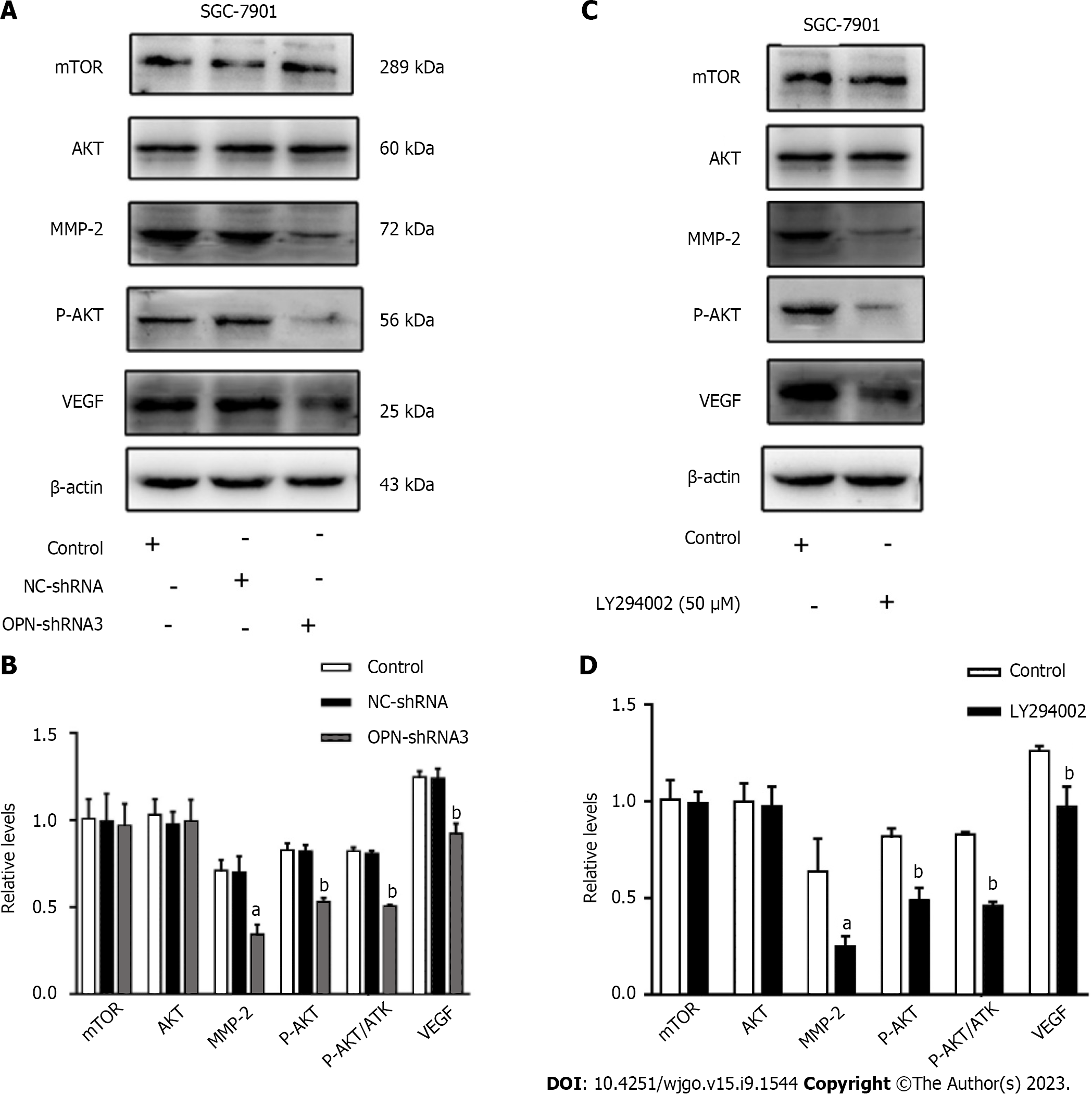
The western blotting analysis revealed that protein expression levels of total mTOR and AKT among control, NC-shRNA and OPN-shRNA3 group remained constant, while that of p-AKT in OPN-shRNA3 group was noticeably lower than that in control group (Figure 4A and B). Meanwhile, MMP-2 and VEGF expression in OPN-shRNA3 were lower than the groups of control (Figure 4A and B).
In order to further study the relationship between OPN and PI3K/AKT/mTOR pathway, we administrated SGC-7901 cells with PI3K inhibitor (LY294002). As shown in Figure 4C and D, the protein expression levels of total mTOR and AKT in control and LY294002 group remained constant, while the protein expression levels of p-AKT, MMP-2 and VEGF in LY294002 group significantly decreased as compared with control group. These results suggested that OPN up-regulate the expressions of MMP-2 and VEGF via PI3K/AKT/mTOR signaling pathway, thus promote the proliferation, invasion and migration of GC SGC-7901 cells.
It is well known that patients with metastatic GC have a poor prognosis[3], so it is of great clinical value to search for more accurate prognostic markers to better understand the molecular mechanism of the occurrence and development of GC and develop new therapeutic strategies. In this study, the effects of OPN on proliferation, invasion and migration of GC SGC-7901 cells were investigated through a number of in vitro experiments, and the mechanism of the regulation of PI3K/AKT/mTOR signaling pathway by OPN in GC cells was also explored, providing a new theoretical basis for clarifying the metastasis and invasion mechanism of GC and further developing the targeted treatment of GC with protein kinase inhibitors.
In recent years, numerous studies demonstrated that OPN overexpressed and promoted the cancer progression in various cancers via various signaling pathways[4-18]. With the ongoing study of OPN, OPN is also being explored as a potential therapeutic target. For example, reducing OPN expression could provide novel strategies for the treatment of patients with various types of metastatic cancer[19-22]. Wang et al[36] reported that silencing the expression of OPN in GC cell line SGC7901 inhibited the growth and metastasis of GC. Park et al[37] also reported that the migration ability of GC cells with OPN knockdown was reduced.
We found in our study that the mRNA and protein expression levels of OPN were highly expressed in GC SGC-7901 cells (Figure 1), and OPN knockdown inhibits cell proliferation, invasion and migration in SGC-7901 cells (Figure 2). Our results indicated that OPN as an inducer of cell proliferation, invasion and migration.
Another important finding of our study was that overactivation of the PI3K/AKT signaling pathway was associated with OPN-induced progression of GC cells. Several studies have demonstrated that OPN promotes tumor invasion and metastasis by inducing activation of signaling pathways that regulate cell migration and tumor progression, such as mitogen-activated protein kinase and PI3K/AKT[38].
PI3K/AKT signaling pathway is one of the most widely studied signaling pathways. Some studies have shown that the PI3K/AKT/mTOR signaling pathway is involved in the proliferation, invasion and metastasis of GC cells[34,35]. In an in vitro study of breast cancer, OPN expression was found to increase with the aggressiveness of the breast cancer phenotype. Knockdown of OPN may reduce breast cancer metastasis by regulating αv and β3 integrin expression and inhibiting PI3K/AKT/mTOR signaling pathway[39].
PKB/AKT, a serine/threonine (Ser/Thr) protein kinase, is the main effector downstream of PI3K and its activity is regulated by phosphorylation. P-AKT, the active form of AKT, affects a variety of cellular functions. Abnormal activation of AKT has been detected in a variety of malignant tumor cells[40].
In our study, western blot results showed that phosphorylation of AKT in SGC-7901 cells decreased after OPN silencing (Figure 4). This further supports the idea. Similarly, GC cell lines with stable overexpression of OPN were incubated with the PI3K inhibitor LY294002, and phosphorylation of AKT was significantly reduced (Figure 4). In conclusion, OPN may promote GC invasion and migration by activating PI3K/AKT/mTOR signaling pathway. To our knowledge, this study is the first to demonstrate a relevance between OPN and the PI3K/AKT signaling pathway in human GC cell lines.
ECM degradation is a key step in tumor invasion and migration and ECM degradation mainly depends on MMPs (such as MMP-2 and MMP-9), which bind to adhesion molecules and digest ECM-related components during cell migration, thus facilitating the movement of cancer cells[41]. MMP-2 is a proteolytic enzyme that mainly degrades type IV collagen, leading to destruction of basement membrane, infiltration of tumor cells into connective tissue matrix, infiltration of small blood vessels and lymphatic vessels, and thus metastasis[42]. MMPs are highly regulated by growth factors, cytokines and ECM proteins. OPN, as an ECM protein, can induce the production and activation of MMP-2 in cells, and the increased expression of MMP-2 further enhances the ability of tumor cells to digest ECM-related components, and ultimately leads to the promotion of tumor cell invasion and metastasis[43,25].
Tumor growth and metastasis depend on angiogenesis, and VEGF can induce angiogenesis[44,45]. It has been reported that increased VEGF expression can promote angiogenesis, thereby enhancing the ability of tumor cells to enter circulation and ultimately promote tumor metastasis to other organs[45]. Tang et al[29] found that OPN and VEGF were co-expressed in GC tissues, and their expression levels were significantly correlated with tumor node metastasis staging, lymph node metastasis and distant metastasis (P < 0.05). Xu et al[46] also studied the effect of OPN on VEGF expression in articular cartilage and found that OPN may directly up-regulate VEGF expression through PI3K/AKT and mitogen-activated protein kinase 1 pathways.
In this study, we found that mRNA and protein expression levels of MMP-2 and VEGF were inhibited in SGC-7901 cells treated with OPN knockdown and LY294002 inhibitor (Figures 3 and 4). Consequently, we speculate that OPN promoting the invasion and migration of GC SGC-7901 cells, which might be related to the increasing expression of MMP-2 and VEGF. The results of our study are similar to those of previous studies[47].
In our study, we detected a strange phenomenon that OPN was also expressed in normal gastric epithelial GES-1 cells (Figure 1A-C). This phenomenon may be related to the existence of OPN splicing variants (a, b, c). Studies have found that normal gastric GES-1 cells mainly express OPN-a subtype, and GC cell lines mainly express OPN-c subtype[48]. Another study also showed that OPN-c subtypes were overexpressed in GC and correlated with the prognosis of GC, while the other two subtypes were not associated with the progression of GC[31]. These studies may explain why OPN is expressed in GES-1 cells.
In this study, we preliminarily analyzed the effects of OPN on the proliferation, invasion and migration of GC cells, which is similar to previous literature. Our study further found that OPN knockdown and LY294002 inhibitor inhibited the activation of PI3K/AKT/mTOR pathway and down-regulated the mRNA and protein expression of MMP-2 and VEGF in GC-7901 cells, ultimately inhibiting the proliferation, invasion and migration of GC cells.
In conclusion, OPN may promote the progression of GC by activating PI3K/AKT/mTOR signaling pathway and up-regulating the expression of MMP-2 and VEGF. Our findings suggest that OPN is a new prognostic marker and potential therapeutic target for GC.
There are some limitations to our study. Due to the limitation of time and funds, the experimental design was somewhat simple, and only in vitro experiments were designed. In the future, with the support of further research funding, we hope to conduct some in vivo studies to verify this, such as animal trials. Although more remains to be learned about the mechanism, it is clear that OPN is a promising biomarker. OPN targeting therapy may be an effective way to overcome treatment failure and significantly enhance anti-tumor activity. Currently, several OPN inhibitors are under preclinical study for the treatment of solid tumors such as bowel cancer and lung cancer[49,50]. Although initial results from different OPN inhibitors are encouraging, clinical benefits remain to be demonstrated.
Gastric cancer (GC) is one of the most common malignant tumors. Osteopontin (OPN) is thought to be closely related to the occurrence, metastasis and prognosis of many types of tumors.
To search for a potential prognostic biomarker for GC as well as a potential therapeutic target.
The purpose of this study was to investigate the effects of OPN on the proliferation, invasion and migration of GC cells and its possible mechanism.
The mRNA and protein expression of OPN in the GC cells were analyzed by real-time quantitative-reverse transcription and western blotting, and observe the effect of varying degree expression OPN on the proliferation and other behaviors of GC. Next, the effects of OPN knockdown on GC cells migration and invasion were examined. The short hairpin RNA (shRNA) and negative control shRNA targeting OPN-shRNA were transfected into the cells according to the manu
The results of this study revealed that OPN mRNA and protein expression levels were highly expressed in SGC-7901 cells. OPN knockdown by specific shRNA noticeably reduced the capabilities of proliferation, invasion and migration of SGC-7901 cells. Moreover, in the experiments of investigating the underlying mechanism, results showed that OPN knockdown could down- regulated the expression of MMP-2 and VEGF, it also decreased the phosphorylation of AKT. Meanwhile, the protein expression levels of MMP-2, VEGF and phosphorylated AKT was noticeable lower than that in control group in the GC cells after they were added to phosphatidylinositol-3-kinase (PI3K) inhibitor (LY294002).
These results suggested that OPN though PI3K/AKT/mammalian target of rapamycin signal pathway to up-regulate MMP-2 and VEGF expression, which contribute SGC-7901 cells to proliferation, invasion and migration. Thus, our results demonstrate that OPN may serve as a novel prognostic biomarkers as well as a potential therapeutic targets for GC.
The effects of OPN on proliferation, invasion and migration of GC cells were confirmed by preliminary evidence, which may be used as a prognostic biomarker and potential therapeutic target in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baryshnikova NV, Russia; Kukongviriyapan V, Thailand S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Dassen AE, Lemmens VE, van de Poll-Franse LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, Vd Wurff AA, Bosscha K, Coebergh JW. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: a population-based study in the Netherlands. Eur J Cancer. 2010;46:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Psyrri A, Kalogeras KT, Wirtz RM, Kouvatseas G, Karayannopoulou G, Goussia A, Zagouri F, Veltrup E, Timotheadou E, Gogas H, Koutras A, Lazaridis G, Christodoulou C, Pentheroudakis G, Economopoulou P, Laskarakis A, Arapantoni-Dadioti P, Batistatou A, Sotiropoulou M, Aravantinos G, Papakostas P, Kosmidis P, Pectasides D, Fountzilas G. Association of osteopontin with specific prognostic factors and survival in adjuvant breast cancer trials of the Hellenic Cooperative Oncology Group. J Transl Med. 2017;15:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Rizwan A, Paidi SK, Zheng C, Cheng M, Barman I, Glunde K. Mapping the genetic basis of breast microcalcifications and their role in metastasis. Sci Rep. 2018;8:11067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Yan CH, Lv M, Li H, Song X, Yan F, Cao S, Ren X. Osteopontin is a novel prognostic biomarker in early-stage non-small cell lung cancer after surgical resection. J Cancer Res Clin Oncol. 2015;141:1371-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Hao C, Cui Y, Chang S, Huang J, Birkin E, Hu M, Zhi X, Li W, Zhang L, Cheng S, Jiang WG. OPN promotes the aggressiveness of non-small-cell lung cancer cells through the activation of the RON tyrosine kinase. Sci Rep. 2019;9:18101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Xu ST, Guo C, Ding X, Fan WJ, Zhang FH, Xu WL, Ma YC. Role of osteopontin in the regulation of human bladder cancer proliferation and migration in T24 cells. Mol Med Rep. 2015;11:3701-3707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Tilli TM, Bellahcène A, Castronovo V, Gimba ER. Changes in the transcriptional profile in response to overexpression of the osteopontin-c splice isoform in ovarian (OvCar-3) and prostate (PC-3) cancer cell lines. BMC Cancer. 2014;14:433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Song JY, Lee JK, Lee NW, Yeom BW, Kim SH, Lee KW. Osteopontin expression correlates with invasiveness in cervical cancer. Aust N Z J Obstet Gynaecol. 2009;49:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Xu C, Li H, Yin M, Yang T, An L, Yang G. Osteopontin is involved in TLR4 pathway contributing to ovarian cancer cell proliferation and metastasis. Oncotarget. 2017;8:98394-98404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Qin X, Yan M, Wang X, Xu Q, Zhu X, Shi J, Li Z, Zhang J, Chen W. Cancer-associated Fibroblast-derived IL-6 Promotes Head and Neck Cancer Progression via the Osteopontin-NF-kappa B Signaling Pathway. Theranostics. 2018;8:921-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Liang S, Li Y, Wang B. The cancer-related transcription factor Runx2 combined with osteopontin: a novel prognostic biomarker in resected osteosarcoma. Int J Clin Oncol. 2021;26:2347-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Kolb A, Kleeff J, Guweidhi A, Esposito I, Giese NA, Adwan H, Giese T, Büchler MW, Berger MR, Friess H. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol Ther. 2005;4:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E, Lee NP, Wu GH, Luk JM. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol. 2012;18:3923-3930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Ng L, Wan T, Chow A, Iyer D, Man J, Chen G, Yau TC, Lo O, Foo CC, Poon JT, Poon RT, Pang R, Law WL. Osteopontin Overexpression Induced Tumor Progression and Chemoresistance to Oxaliplatin through Induction of Stem-Like Properties in Human Colorectal Cancer. Stem Cells Int. 2015;2015:247892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Lee SH, Park JW, Woo SH, Go DM, Kwon HJ, Jang JJ, Kim DY. Suppression of osteopontin inhibits chemically induced hepatic carcinogenesis by induction of apoptosis in mice. Oncotarget. 2016;7:87219-87231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Loosen SH, Roderburg C, Kauertz KL, Pombeiro I, Leyh C, Benz F, Vucur M, Longerich T, Koch A, Braunschweig T, Ulmer TF, Heidenhain C, Tacke F, Binnebösel M, Schmeding M, Trautwein C, Neumann UP, Luedde T. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J Hepatol. 2017;67:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P, Gorain M, Kale S, Kumar D, Kumar S, Totakura KV, Roy G, Sharma P, Shetti D, Soundararajan G, Thorat D, Tomar D, Nalukurthi R, Raja R, Mishra R, Yadav AS, Kundu GC. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. 2014;18:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Shevde LA, Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014;37:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 21. | Shi L, Wang X. Role of osteopontin in lung cancer evolution and heterogeneity. Semin Cell Dev Biol. 2017;64:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Zhao H, Chen Q, Alam A, Cui J, Suen KC, Soo AP, Eguchi S, Gu J, Ma D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 23. | Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, Chi NH, Chen GH, Lin JT. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Dai N, Bao Q, Lu A, Li J. Protein expression of osteopontin in tumor tissues is an independent prognostic indicator in gastric cancer. Oncology. 2007;72:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Song G, Ouyang G, Mao Y, Ming Y, Bao S, Hu T. Osteopontin promotes gastric cancer metastasis by augmenting cell survival and invasion through Akt-mediated HIF-1alpha up-regulation and MMP9 activation. J Cell Mol Med. 2009;13:1706-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Imano M, Satou T, Itoh T, Sakai K, Ishimaru E, Yasuda A, Peng YF, Shinkai M, Akai F, Yasuda T, Imamoto H, Okuno K, Ito H, Shiozaki H, Ohyanagi H. Immunohistochemical expression of osteopontin in gastric cancer. J Gastrointest Surg. 2009;13:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Di Bartolomeo M, Pietrantonio F, Pellegrinelli A, Martinetti A, Mariani L, Daidone MG, Bajetta E, Pelosi G, de Braud F, Floriani I, Miceli R. Osteopontin, E-cadherin, and β-catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric Cancer. 2016;19:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Yazici O, Dogan M, Ozal G, Aktas SH, Demirkazik A, Utkan G, Senler FC, Icli F, Akbulut H. Osteopontin is a Prognostic Factor in Patients with Advanced Gastric Cancer. Comb Chem High Throughput Screen. 2021;24:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Tang H, Wang J, Bai F, Zhai H, Gao J, Hong L, Xie H, Zhang F, Lan M, Yao W, Liu J, Wu K, Fan D. Positive correlation of osteopontin, cyclooxygenase-2 and vascular endothelial growth factor in gastric cancer. Cancer Invest. 2008;26:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Yu WW, Wang H, Zhang J, Miao JF, Kong Y, Qu FJ. Expression of osteoblastin, matrix metalloproteinase 2 and vascular endothelial growth factor in gastric cancer tissues and its prognostic value. Zhongliu YanjiuYu Linchuang. 2019;031:390-394. [DOI] [Full Text] |
| 31. | Sun X, Wang L, Hou W, Li Y, Liu L, Zuo W, Yu J. [Expression of osteopontin splice variant and its clinical significance in gastric cancer]. Zhonghua Zhong Liu Za Zhi. 2015;37:427-430. [PubMed] |
| 32. | Gu X, Gao XS, Ma M, Qin S, Qi X, Li X, Sun S, Yu H, Wang W, Zhou D. Prognostic significance of osteopontin expression in gastric cancer: a meta-analysis. Oncotarget. 2016;7:69666-69673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Aoki M, Fujishita T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 2017;407:153-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 34. | Singh SS, Yap WN, Arfuso F, Kar S, Wang C, Cai W, Dharmarajan AM, Sethi G, Kumar AP. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J Gastroenterol. 2015;21:12261-12273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Riquelme I, Tapia O, Espinoza JA, Leal P, Buchegger K, Sandoval A, Bizama C, Araya JC, Peek RM, Roa JC. The Gene Expression Status of the PI3K/AKT/mTOR Pathway in Gastric Cancer Tissues and Cell Lines. Pathol Oncol Res. 2016;22:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | Wang ZM, Cui YH, Li W, Chen SY, Liu TS. Lentiviral-mediated siRNA targeted against osteopontin suppresses the growth and metastasis of gastric cancer cells. Oncol Rep. 2011;25:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Park JW, Lee SH, Go du M, Kim HK, Kwon HJ, Kim DY. Osteopontin depletion decreases inflammation and gastric epithelial proliferation during Helicobacter pylori infection in mice. Lab Invest. 2015;95:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 534] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 39. | Zhang H, Guo M, Chen JH, Wang Z, Du XF, Liu PX, Li WH. Osteopontin knockdown inhibits αv,β3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 2014;33:991-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Vivanco I, Sawyers C L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature Reviews Cancer. 2002;2:489-501. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4468] [Cited by in RCA: 4588] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 41. | Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 697] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 42. | Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1911] [Cited by in RCA: 1955] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 43. | Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276:44926-44935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1620] [Cited by in RCA: 1528] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 45. | Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11:1000-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 46. | Xu J, Yi Y, Li L, Zhang W, Wang J. Osteopontin induces vascular endothelial growth factor expression in articular cartilage through PI3K/AKT and ERK1/2 signaling. Mol Med Rep. 2015;12:4708-4712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Liu J, Liu Q, Wan Y, Zhao Z, Yu H, Luo H, Tang Z. Osteopontin promotes the progression of gastric cancer through the NF-κB pathway regulated by the MAPK and PI3K. Int J Oncol. 2014;45:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Tang X, Li J, Yu B, Su L, Yu Y, Yan M, Liu B, Zhu Z. Osteopontin splice variants differentially exert clinicopathological features and biological functions in gastric cancer. Int J Biol Sci. 2013;9:55-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Zagani R, Hamzaoui N, Cacheux W, de Reyniès A, Terris B, Chaussade S, Romagnolo B, Perret C, Lamarque D. Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology. 2009;137:1358-66.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Zhang J, Yamada O, Kida S, Matsushita Y, Murase S, Hattori T, Kubohara Y, Kikuchi H, Oshima Y. Identification of brefelamide as a novel inhibitor of osteopontin that suppresses invasion of A549 lung cancer cells. Oncol Rep. 2016;36:2357-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |