Published online Sep 15, 2023. doi: 10.4251/wjgo.v15.i9.1531
- This article has been retracted.
- Retraction in: World J Gastrointest Oncol. Jun 15, 2024; 16(6): 2865-2866 See also: Errata, Retraction, Duplicate Publication and Comment Policy
Peer-review started: April 7, 2023
First decision: July 19, 2023
Revised: July 31, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 15, 2023
Processing time: 159 Days and 0.9 Hours
Extensive evidence has illustrated the promotive role of integrin binding sialoprotein (IBSP) in the progression of multiple cancers. However, little is known about the functions of IBSP in gastric cancer (GC) progression.
To investigate the mechanism underlying the regulatory effects of IBSP in GC progression, and the relationship between IBSP and cleavage and polyadenylation factor 6 (CPSF6) in this process.
The mRNA and protein expression of relevant genes were assessed through real-time quantitative polymerase chain reaction and Western blot, respectively. Cell viability was evaluated by Cell Counting Kit-8 assay. Cell invasion and migration were evaluated by Transwell assay. Pyroptosis was measured by flow cytometry. The binding between CPSF6 and IBSP was confirmed by luciferase reporter and RNA immunoprecipitation (RIP) assays.
IBSP exhibited higher expression in GC tissues and cell lines than in normal tissues and cell lines. IBSP knockdown suppressed cell proliferation, migration, and invasion but facilitated pyroptosis. In the exploration of the regulatory mechanism of IBSP, potential RNA binding proteins for IBSP were screened with catRAPID omics v2.0. The RNA-binding protein CPSF6 was selected due to its higher expression in stomach adenocarcinoma. Luciferase reporter and RIP assays revealed that CPSF6 binds to the 3’-untranslated region of IBSP and regulates its expression. Knockdown of CPSF6 inhibited cell proliferation, migration, and invasion but boosted pyroptosis. Through rescue assays, it was uncovered that the retarded GC progression mediated by CPSF6 knockdown was reversed by IBSP overexpression.
Our study highlighted the vital role of the CPSF6/IBSP axis in GC, suggesting that IBSP might be an effective bio-target for GC treatment.
Core Tip: This study, for the first time, revealed the crucial role of the cleavage and polyadenylation factor 6 (CPSF6)/integrin binding sialoprotein (IBSP) axis in gastric cancer (GC). This discovery might shed light on GC treatment. However, although this study explored this regulatory axis on cell proliferation, metastasis, and pyroptosis in GC, its data regarding the regulatory effects of CPSF6/IBSP on GC progression are limited. In the future, the regulatory effects of the CPSF6/IBSP axis on stemness, autophagy, and inflammation should be investigated through more experiments.
- Citation: Wang XJ, Liu Y, Ke B, Zhang L, Liang H. RNA-binding protein CPSF6 regulates IBSP to affect pyroptosis in gastric cancer. World J Gastrointest Oncol 2023; 15(9): 1531-1543
- URL: https://www.wjgnet.com/1948-5204/full/v15/i9/1531.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i9.1531
Similar to other malignant tumors, gastric cancer (GC) is featured by immoderate cell proliferation and delayed cell apoptosis[1,2]. The activation of oncogenes and the inactivation of tumor suppressor genes are the main inducements for tumors[3]. At present, treatments for GC are mainly surgery, chemotherapy, and radiotherapy[4,5]. However, there are no ideal treatment strategies. GC cells are often resistant to chemotherapy or radiotherapy, which is the main reason for tumor recurrence after treatment[6,7]. In view of the serious threat of GC to patient lives, there is an urgent need to look for effective bio-targets for GC treatment.
Different factors [proteins, long non-coding/circular RNAs (lnc/circRNAs), microRNAs (miRNAs), etc.] play critical roles in the progression of cancers, including GC[8-11]. For example, SRY-box transcription factor 4 accelerates transforming growth factor β-stimulated epithelial-mesenchymal transition and stemness in GC[12]. Tripartite motif containing 58 inactivates β-catenin signaling through ubiquitination to suppress tumor growth in GC[13]. Besides, the lncRNA bladder cancer associated transcript 1/microRNA 361 (miR-361)/ATP binding cassette subfamily B member 1 (ABCB1) competitive exogenous RNA axis contributes to oxaliplatin resistance in GC[14]. Centromere protein U promotes GC cell proliferation and glycolysis by modulating high mobility group box 2[15]. Integrin binding sialoprotein (IBSP) serves as a member of the small integrin-binding ligand, N-linked glycoprotein family, and the gene encoding this protein is located on 4q21.1[16,17]. IBSP has higher expression and important function in various types of cancers. For instance, IBSP modulates the Fyn/β-catenin signaling pathway to aggravate colorectal cancer progression[18]. Exosomal miR-19a interacts with IBSP in estrogen receptor-positive breast cancer to stimulate osteolytic bone metastasis[19]. Besides, overexpression of IBSP results in a poor prognosis in esophageal squamous cell carcinoma patients[20]. However, the functions and related regulatory mechanism of IBSP are still unclear in GC. Some studies have confirmed the oncological function of cleavage and polyadenylation factor 6 (CPSF6) in various kinds of cancers[21-24]. However, the relationship between IBSP and CPSF6 in GC progression remains to be investigated.
This study aimed to investigate the mechanism underlying the regulatory effects of IBSP in GC progression, and the relationship between IBSP and CPSF6 in this process. Our study revealed that CPSF6-mediated IBSP facilitated cell proliferation, invasion, and migration and reduced cell pyroptosis in GC. This discovery is of great clinical significance for identifying promising bio-targets for GC treatment.
Thirty paired GC tissues and adjacent non-cancer tissues were obtained from January 2020 to March 2023 from patients who had undergone surgery at Tianjin Medical University Cancer Institute and Hospital, Tianjin, China. All patients were histologically or pathologically verified as having GC by two independent pathologists and did no receive prior anti-cancer treatments. The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (E2020216) and written informed consent was obtained from all patients. Resected tissues were immediately frozen in liquid nitrogen and then stored at -80 °C.
GC cell lines (HGC-27, MKN45, SGC7901, and BGC823) and the human normal gastric mucosal cell line (GES-1) were obtained from American Type Culture Collection (ATCC, Manassas, VA, United States). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma, St. Louis, MO, United States) with 10% fetal bovine serum (FBS; Gibco, Waltham, MA, United States) and 1% penicillin-streptomycin at 37 °C in a humidified incubator with 5% CO2.
Small interfering RNAs (siRNAs) against IBSP (si-IBSP#1 and si-IBSP#2) and siRNAs against CPSF6 (si-CPSF6#1 and si-CPSF6#2) were designed for silencing IBSP and CPSF6, respectively. A negative control siRNA (si-NC) was also used. To overexpress IBSP, pcDNA3.1/IBSP (OV-IBSP) plasmid was constructed, and the empty vector pcDNA3.1 was used as the negative control. These vectors were obtained from Genepharma (Shanghai, China) and transfected into GC cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States).
TRIzol reagent (Invitrogen, Carlsbad, CA, United States) was utilized to isolate total RNA from GC tissues or cells. The ReverTra Ace quantitative polymerase chain reaction (RT-qPCR) RT Kit (Takara, Beijing, China) was employed to synthesize cDNA from RNA. The SYBR Green Real-time PCR Master Mix (Takara, Beijing, China) was used for qPCR amplification on the ABI 7500 real-time PCR system (Applied Biosystems, Bedford, MA, United States). β-actin was used as the internal reference. The 2-ΔΔCt method was used for calculating gene expression.
Cell Counting Kit-8 (CCK-8) assay was performed to examine the viability of GC cells as described previously[25,26]. In brief, GC cells (1 × 104 cells/well) were plated into 96-well plates and then incubated for 0 h, 24 h, 48 h, and 72 h. The CCK8 solution (10 μL, Dojindo, Japan) was added into each well and incubated for 2 h, and the absorbance (450 nm) was then measured with a microplate reader.
GC cells (1 × 104 cells/well) were seeded into the upper chamber (8 µm pore size; Millipore, Billerica, MA, United States) coated with (for invasion assay) or without Matrigel (for migration assay). The culture medium with 10% FBS was added into the lower chamber. The invading and migrating cells were fixed with methanol and dyed with crystal violet. Subsequently, a microscope (Olympus, Tokyo, Japan) was employed to count these cells.
Cell apoptosis was assessed by flow cytometry with the propidium iodide (PI) and FITC Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, United States) as described previously[27]. GC cells were cultured for 72 h, followed by washing with cold phosphate-buffered saline and resuspending in 1 × binding buffer. Annexin V-FITC (5 µL) was utilized for dyeing the cells, followed by mixing with PI (5 µL) in the darkness. FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, United States) was employed for evaluating cell apoptosis.
Luciferase reporter assay was performed as described in previous studies[28,29]. The wild-type (wt) and mutant-type (mut) 3’-untranslated region (3’-UTR) sequences of IBSP (IBSP 3’-UTR-wt/mut) were inserted into psiCHECK2 dual-luciferase vector (Promega, Madison, United States) to generate reporter vectors. Then, IBSP 3’-UTR-wt or mut reporters were separately transfected with pcDNA3.1 or pcDNA3.1-CPSF6 into GC cells. After 48 h, the luciferase reporter assay system (Promega, Madison, Wisconsin, United States) was applied to measure the luciferase activity.
RNA immunoprecipitation (RIP) assay was performed following previous studies[30,31]. GC cells were lysed with the lysis buffer. Cell lysate was mixed with anti-CPSF6 or anti-immunoglobulin G (IgG) antibodies, and then magnetic beads were added to immunoprecipitate the RNA-protein immunocomplexes. After washing, IBSP expression was assessed by RT-qPCR.
GC cells were lysed with RIPA lysis buffer. Then, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride or polyvinylidene difluoride membranes (Amersham, United States). After blocking with non-fat milk, the membranes were incubated with primary antibodies, including those against IBSP, CPSF6, NLR family pyrin domain containing 3 (NLRP3), cleaved caspase-1, interleukin (IL)-18, IL-1β, and β-actin, at 4 °C overnight. Subsequently, the secondary antibody was added and incubated for 2 h. All antibodies were bought from Abcam (Shanghai, China). After washing, the ECL detection (ThermoScientific, Waltham, MA, United States) was utilized to visualize protein bands.
Male BALB/c nude mice (4-wk-old, n = 15) were purchased from Vital River (Beijing, China). Mice were randomly divided into three groups (n = 5 for each group; si-NC, si-CPSF6, and si-CPSF6 + OV-IBSP groups). The transfected GC cells were injected into the right flanks of mice. After 4 wk, tumor size, volume, and weight were assessed. This work was approved by the Animal Care and Use Committee of Beijing Viewsolid Biotechnology Co. LTD (VS212601449).
Data are shown as the mean ± SD. Statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, United States). The correlation between IBSP and CPSF6 expression was assessed by Pearson correlation analysis. The comparison between two groups or among multiple groups was done by Student’s t test and one-way analysis of variance, respectively. P < 0.05 was considered statistically significant.
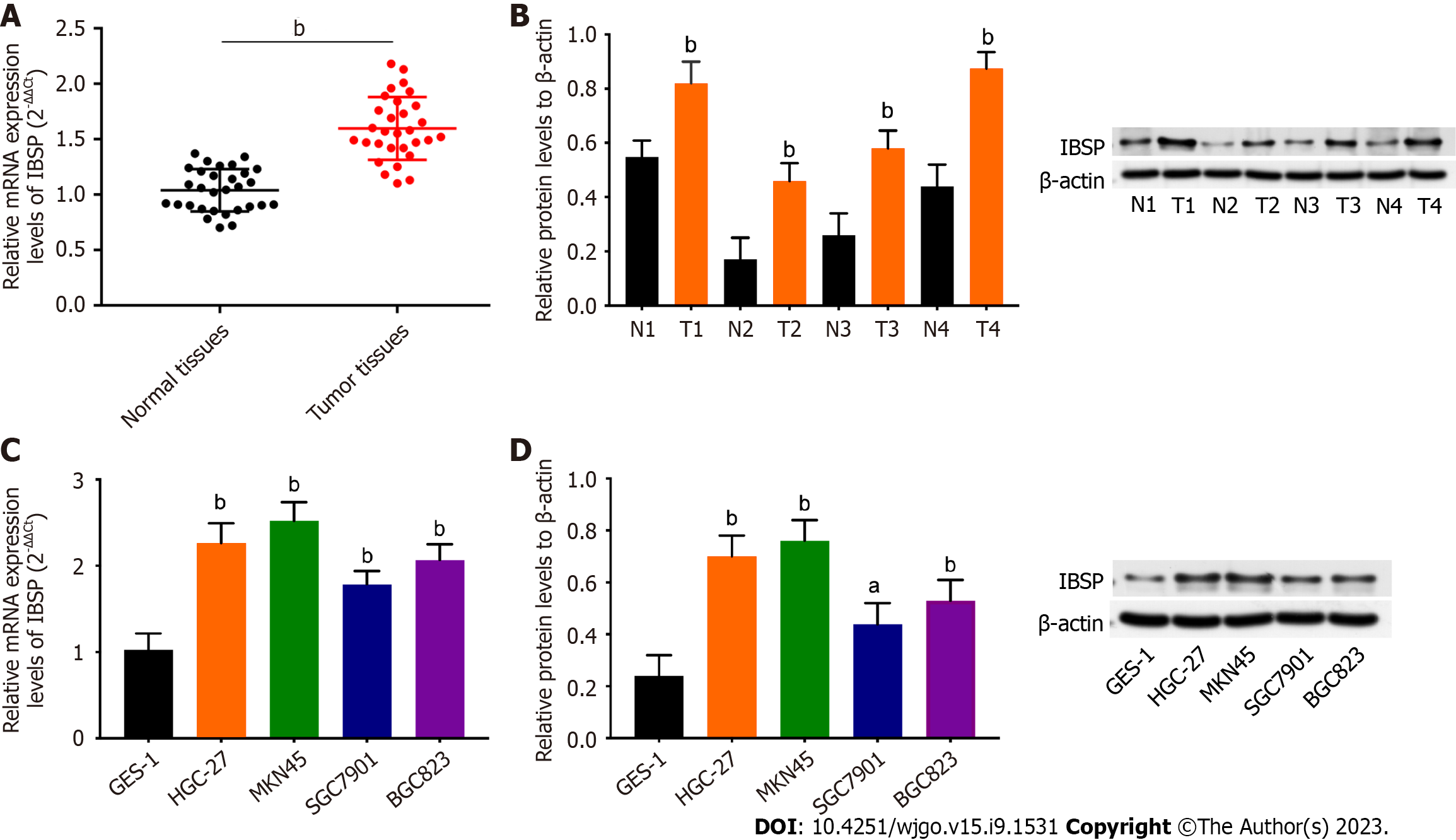
As shown in Figure 1A and B, the mRNA and protein expression levels of IBSP were higher in the GC tissues than in the normal tissues. The correlation between GC patients’ clinicopathological features and IBSP expression is shown in Table 1. IBSP expression was not significantly correlated with age, gender, or distant metastasis but was significantly related with tumor diameter and TNM stage (P < 0.05). The mRNA and protein expression levels of IBSP were up-regulated in GC cell lines (HGC-27, MKN45, SGC-7901, and BGC823) compared with the human normal gastric mucosal cell line GES-1 (Figure 1C and D). Additionally, the prognosis of GC patients with high IBSP expression was poor (Supplementary Figure 1A). Taken together, IBSP shows higher expression in GC tissues and cell lines.
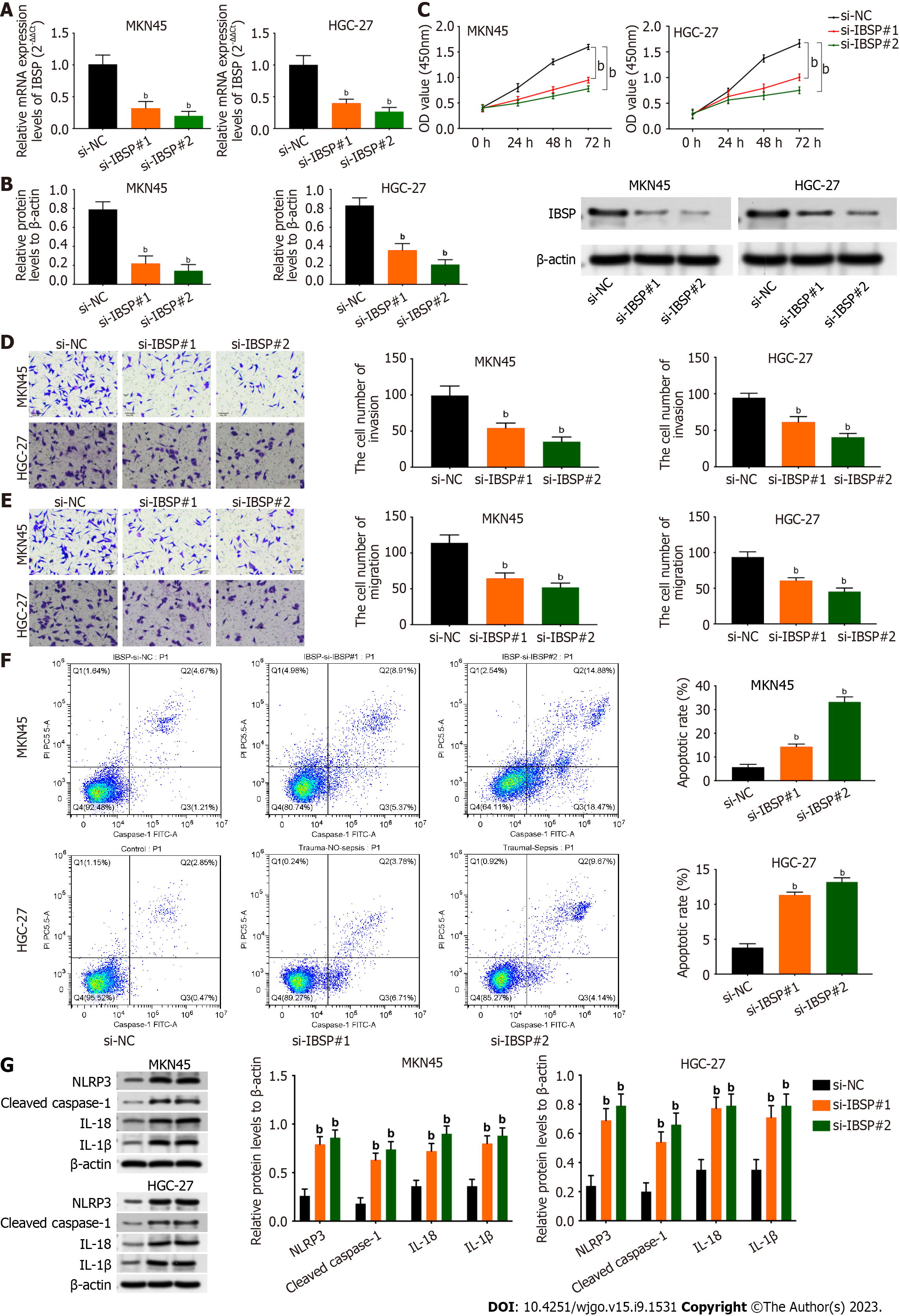
The efficiency of IBSP knockdown was verified by the decreased mRNA and protein expression levels of IBSP after IBSP silencing (Figure 2A and B). Cell viability was attenuated after suppressing IBSP in MKN45 and HGC-27 cells (Figure 2C). Furthermore, the invasion and migration of MKN45 and HGC-27 cells were weakened after IBSP inhibition (Figure 2D and E). The cell apoptosis rate was increased after IBSP knockdown in MKN45 and HGC-27 cells (Figure 2F). In addition, the protein levels of NLRP3, cleaved caspase-1, IL-18, and IL-1β were all upregulated after inhibiting IBSP in MKN45 and HGC-27 cells (Figure 2G). Thus, MKN45 cells were used for further experiments. These findings demonstrate that IBSP downregulation suppresses cell proliferation, migration, and invasion and facilitates pyroptosis.
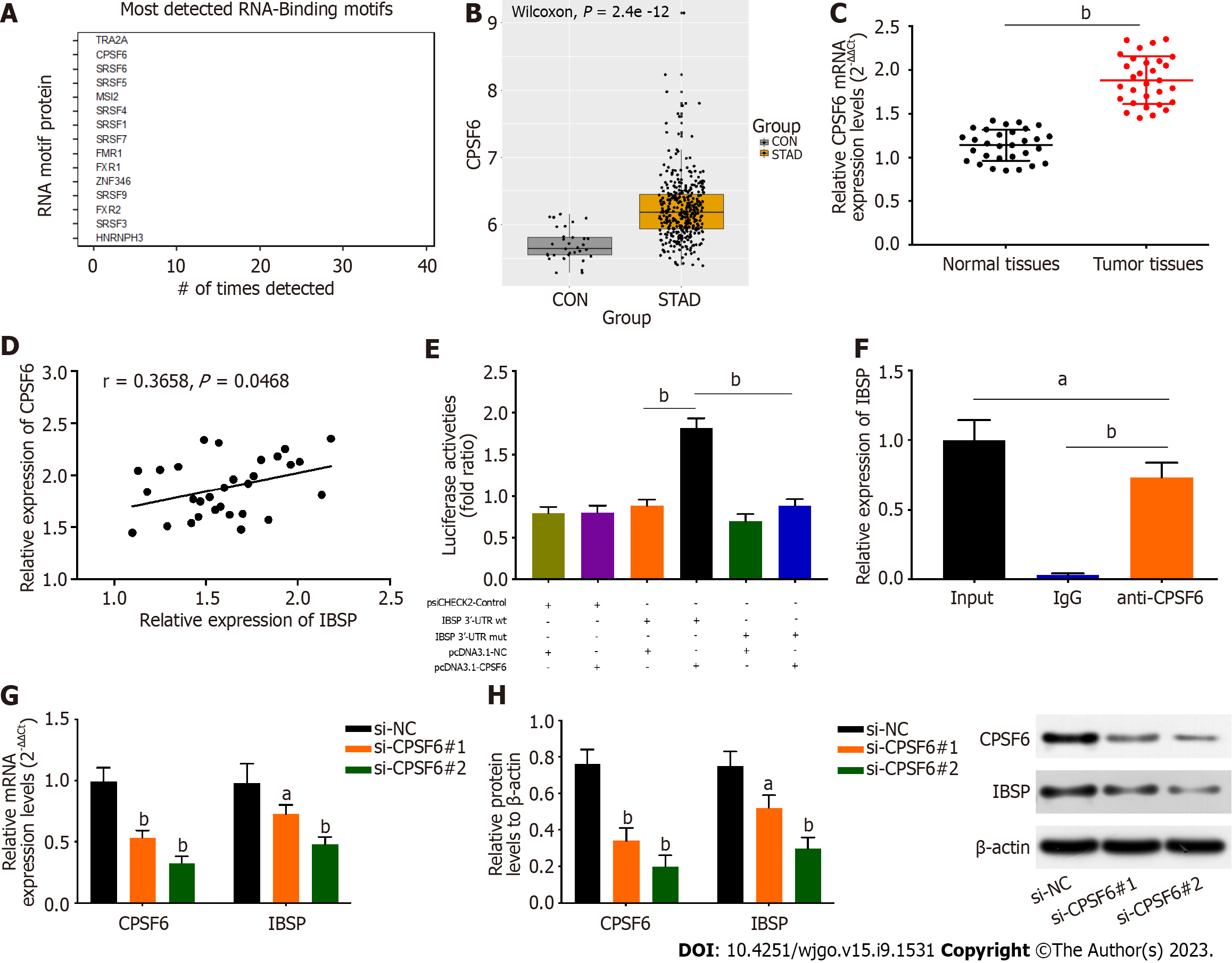
catRAPID omics v2.0 was used to predict and screen potential RNA binding proteins for IBSP (Figure 3A). CPSF6 ranked second in the binding ability to IBSP and was differentially expressed in GC. The transformer 2 alpha homolog, which ranked first in binding ability, was not differentially expressed in GC. Thus, CPSF6 was selected for the subsequent study. The expression of CPSF6 was upregulated in stomach adenocarcinoma tissues (Figure 3B). Similarly, CPSF6 expression was higher in GC tissues and was positively correlated with IBSP expression (Figure 3C and D). Moreover, the prognosis of GC patients with high CPSF6 expression was poor (Supplementary Figure 1B). The luciferase activity of IBSP-wt reporters was increased after overexpressing CPSF6, but that of IBSP-mut reporters had no noticeable change (Figure 3E). RIP assay revealed that CPSF6 binds to IBSP (Figure 3F). The mRNA and protein levels of CPSF6 and IBSP were reduced after silencing CPSF6 (Figure 3G and H). Thus, CPSF6 binds to the 3’-UTR of IBSP and regulates its expression.
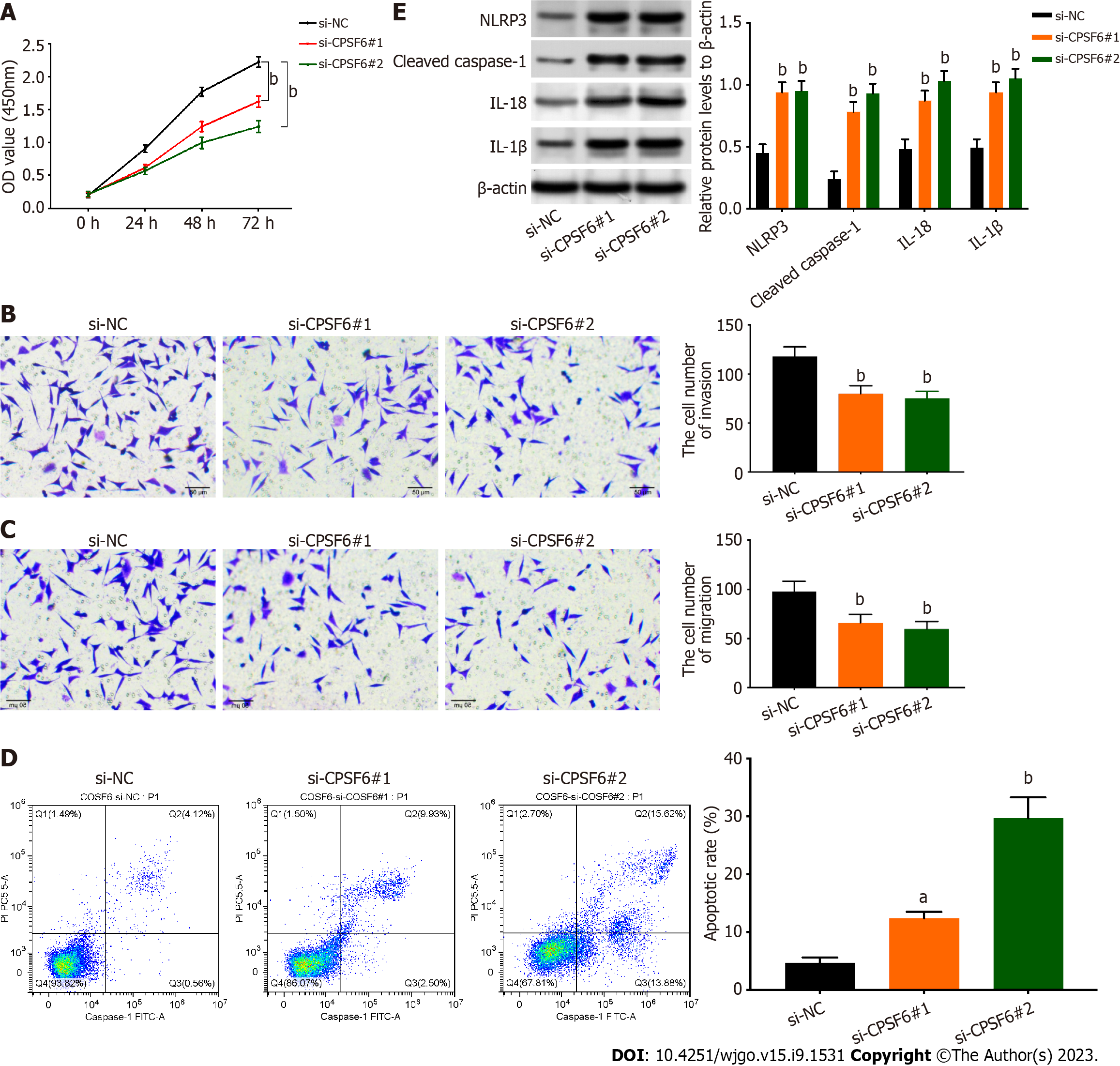
The proliferation of GC cells was weakened after repressing CPSF6 (Figure 4A). In addition, cell invasion and migration were reduced after silencing CPSF6 (Figure 4B and C). In contrast, cell apoptosis was strengthened after CPSF6 suppression (Figure 4D). The protein levels of NLRP3, cleaved caspase-1, IL-18, and IL-1β were increased after CPSF6 knockdown (Figure 4E). Collectively, knockdown of CPSF6 represses cell proliferation, migration, and invasion but boosts pyroptosis.
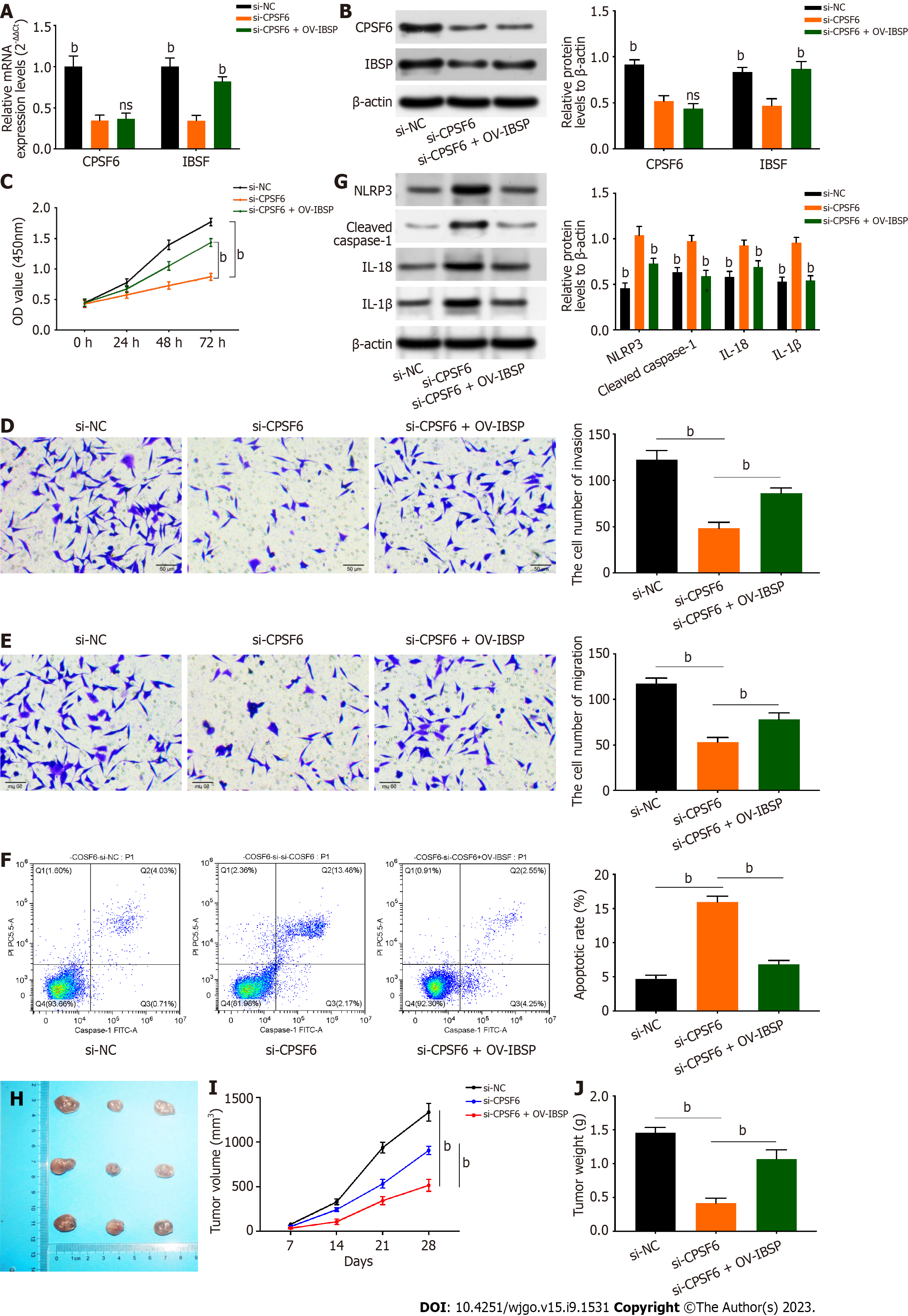
Rescue assays were conducted to verify the interaction between CPSF6 and IBSP. IBSP expression was decreased after CPSF6 knockdown, but this effect could be reversed by IBSP overexpression (Figure 5A and B). The reduced cell viability mediated by CPSF6 inhibition was rescued by IBSP overexpression (Figure 5C). Additionally, the weakened cell invasion and migration induced by CPSF6 suppression were counteracted by IBSP upregulation (Figure 5D and E). Cell apoptosis was reduced after repressing CPSF6, but this effect was offset by overexpressing IBSP (Figure 5F). Besides, the protein levels of NLRP3, cleaved caspase-1, IL-18, and IL-1β were upregulated after CPSF6 knockdown, but these changes were neutralized by IBSP overexpression (Figure 5G). Tumor size, volume, and weight were decreased after CPSF6 inhibition, but these effects were rescued by IBSP upregulation (Figure 5H-J).
GC is one of the most common cancers[32]. Most GC patients are diagnosed at the advanced stage, and the 5-year survival rate of advanced GC patients is less than 15%[33,34]. The emergence of novel bio-targets can improve the early diagnosis and treatment of GC. IBSP has been discovered to exhibit higher expression and important regulatory function in various types of cancers[18-20]. However, the regulatory functions of IBSP in GC progression remain unclear. Similar to the above studies, our study demonstrated that IBSP showed higher expression in GC tissues and cell lines. In addition, IBSP knockdown suppressed cell proliferation, migration, and invasion and facilitated cell apoptosis. In addition, IBSP knockdown strengthened pyroptosis.
RNA-binding protein could bind to the 3′-UTR of mRNAs to regulate their expression levels in various cancers. For instance, the RNA-binding protein NONO post-transcriptionally regulates S-phase kinase associated protein 2 and E2F transcription factor 8 to boost breast cancer tumorigenesis[35]. Additionally, the RNA-binding protein sorbin and SH3 domain containing 2 (SORBS2) stabilizes RAR related orphan receptor A (RORA) mRNA to repress tumor growth and metastasis in hepatocellular carcinoma[36]. The RNA-binding protein DAZ-associated protein 1 interacts with solute carrier family 7 member 11 (SLC7A11) mRNA to aggravate hepatocellular carcinoma progression and modulate ferroptosis[37]. The RNA-binding protein SORBS2 strengthens microtubule associated scaffold protein 1 (MTUS1) mRNA stability to inhibit metastasis in clear cell renal cell carcinoma[38]. Previous studies also verified the oncological function of CPSF6 in acute myeloid leukemia and breast cancer[21,24]. Inhibition of CPSF6 enhances apoptosis by shortening human von Hippel-Lindau (VHL) 3’-UTR in GC[22]. Also, nudix hydrolase 21 (NUDT21) regulates CPSF6 to inhibit tumorigenesis in breast cancer[23]. Similar to these previous reports, this study also revealed that CPSF6 expression was upregulated in GC tissues.
Similar regulatory mechanism (RNA-binding protein-mRNA) also exists in GC. For example, the RNA-binding protein RNPC1 stabilizes aurora kinase B (AURKB) mRNA to enhance GC progression[39]. The RNA binding protein Lin28B interacts with neuropilin-1 to affect stemness in GC[40]. LINC00668 interacts with human antigen R (HuR) to upregulate protein kinase N2 (PKN2) and facilitate GC metastasis[41]. The lncRNA small nucleolar RNA host gene 12 (SNHG12) aggravates cisplatin resistance by regulating the HuR/X-linked inhibitor of apoptosis protein axis in non-small cell lung cancer[42]. In this work, potential RNA binding proteins that can bind to IBSP were predicted and screened using catRAPID omics v2.0. The RNA-binding protein CPSF6 was selected due to its higher expression in GC. However, the relationship between IBSP and CPSF6 has not been studied in GC progression. CPSF6 expression was positively correlated with IBSP expression. Furthermore, through luciferase reporter and RIP assays, it was showed that CPSF6 binds to the 3’-UTR of IBSP and positively regulates IBSP expression. Knockdown of CPSF6 inhibited cell proliferation, migration, and invasion but boosted pyroptosis. Rescue assays revealed that the retarded GC progression mediated by CPSF6 knockdown was reversed by IBSP overexpression.
This study, for the first time, revealed the crucial role of the CPSF6/IBSP axis in GC progression, shedding light on GC treatment. The main findings in previous studies and our findings in this study are shown in Table 2. However, some limitations exist in this study: The luciferase reporter assay is unable to determine whether the protein directly interacts with DNA itself; the RIP assay used native immunoprecipitation without any form of cross-linking; the number of human samples and animal samples was not large; and other phenotypes (such as stemness, exosome, autophagy, and glycolysis) were not assessed. In the future, the regulatory effects of the CPSF6/IBSP axis on these phenotypes will be investigated through more experiments.
| No. | Findings in previous studies | Findings in this work |
| 1 | IBSP has been discovered to exhibit higher expression and important regulatory function in colorectal cancer, breast cancer, and esophageal squamous cell carcinoma. However, the regulatory functions of IBSP in GC progression remain unclear | IBSP exhibits higher expression in GC tissues and cell lines. IBSP facilitates GC cell proliferation, migration, and invasion but suppresses pyroptosis |
| 2 | Previous studies have verified the oncological function of the RNA-binding protein CPSF6 in acute myeloid leukemia, breast cancer, and GC. However, no reports have focused on the regulatory effects of CPSF6 on metastasis and pyroptosis | CPSF6 promotes cell proliferation, migration, and invasion but boosts pyroptosis |
| 3 | The RNA-binding protein CPSF6 binds to the 3’-UTR of genes to participate in the progression of hepatocellular carcinoma, lung adenocarcinoma, and GC. But, the relationship between IBSP and CPSF6 has not been studied in GC progression | CPSF6 binds to the 3’-UTR of IBSP and positively regulates IBSP expression |
| 4 | The regulatory mechanism (RNA binding protein-mRNA 3’-UTR) exists in GC progression. However, the regulatory effects of CPSF6/IBSP remain unclear | The retarded GC progression mediated by CPSF6 knockdown is reversed by IBSP overexpression |
Previous studies have illustrated that integrin binding sialoprotein (IBSP) exhibits a promotive role in the progression of cancers. However, the regulatory functions of IBSP in gastric cancer (GC) progression remain unclear.
To find effective bio-targets for GC prognosis and treatment.
To probe the regulatory effects and underlying molecular mechanism of IBSP in GC progression.
Real-time quantitative polymerase chain reaction and Western blot were used to detect the mRNA and protein expression of IBSP, respectively. The prognosis of GC patients with high or low IBSP expression was evaluated. The regulatory effects of IBSP in GC progression was assessed via in vitro and in vivo experiments. The molecular mechanism of the IBSP/cleavage and polyadenylation factor 6 (CPSF6) axis was validated.
IBSP exhibited higher expression in GC, and IBSP knockdown suppressed cell proliferation, migration, and invasion but facilitated pyroptosis. Moreover, the results revealed that CPSF6 binds to the 3’-untranslated region of IBSP and positively regulates IBSP expression in GC.
Other regulatory functions and related mechanisms of ISBP in GC may be investigated in the future, and its application in GC treatment will be explored.
IBSP expression is upregulated in GC tissues and cells, which results in a poor prognosis in GC. CPSF6 positively regulates IBSP to affect pyroptosis and aggravate tumor growth in GC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baryshnikova NV, Russia; Cabezuelo AS, Spain; Osera S, Japan; Yakar M, Turkey S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 377] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2792] [Article Influence: 558.4] [Reference Citation Analysis (5)] |
| 3. | Baylin SB, Jones PA. Epigenetic Determinants of Cancer. Cold Spring Harb Perspect Biol. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 830] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 4. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 834] [Article Influence: 166.8] [Reference Citation Analysis (0)] |
| 5. | Ilson DH. Advances in the treatment of gastric cancer: 2019. Curr Opin Gastroenterol. 2019;35:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Chen Z, Li Y, Tan B, Zhao Q, Fan L, Li F, Zhao X. Progress and current status of molecule-targeted therapy and drug resistance in gastric cancer. Drugs Today (Barc). 2020;56:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Huang T, Song C, Zheng L, Xia L, Li Y, Zhou Y. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer. 2019;18:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Li D, Wang J, Zhang M, Hu X, She J, Qiu X, Zhang X, Xu L, Liu Y, Qin S. LncRNA MAGI2-AS3 Is Regulated by BRD4 and Promotes Gastric Cancer Progression via Maintaining ZEB1 Overexpression by Sponging miR-141/200a. Mol Ther Nucleic Acids. 2020;19:109-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 9. | Li D, She J, Hu X, Zhang M, Sun R, Qin S. The ELF3-regulated lncRNA UBE2CP3 is over-stabilized by RNA-RNA interactions and drives gastric cancer metastasis via miR-138-5p/ITGA2 axis. Oncogene. 2021;40:5403-5415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Wang J, Zhang M, Hu X, She J, Sun R, Qin S, Li D. miRNA-194 predicts favorable prognosis in gastric cancer and inhibits gastric cancer cell growth by targeting CCND1. FEBS Open Bio. 2021;11:1814-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Li D, Xu M, Wang Z, Huang P, Huang C, Chen Z, Tang G, Zhu X, Cai M, Qin S. The EMT-induced lncRNA NR2F1-AS1 positively modulates NR2F1 expression and drives gastric cancer via miR-29a-3p/VAMP7 axis. Cell Death Dis. 2022;13:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 12. | Peng X, Liu G, Peng H, Chen A, Zha L, Wang Z. SOX4 contributes to TGF-β-induced epithelial-mesenchymal transition and stem cell characteristics of gastric cancer cells. Genes Dis. 2018;5:49-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Liu X, Long Z, Cai H, Yu S, Wu J. TRIM58 suppresses the tumor growth in gastric cancer by inactivation of β-catenin signaling via ubiquitination. Cancer Biol Ther. 2020;21:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Wu X, Zheng Y, Han B, Dong X. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomed Pharmacother. 2018;99:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Deng T, Jiang X, He Z, Cai M, Chen C, Xu Z. Centromere protein U (CENPU) promotes gastric cancer cell proliferation and glycolysis by regulating high mobility group box 2 (HMGB2). Bioengineered. 2021;12:10194-10202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Malaval L, Aubin JE, Vico L. Role of the small integrin-binding ligand N-linked glycoprotein (SIBLING), bone sialoprotein (BSP) in bone development and remodeling. Osteoporos Int. 2009;20:1077-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501-8511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Qin Y, Dai M, Liu L, Ni Y, Sun Q, Li L, Zhou Y, Qiu C, Jiang Y. IBSP, a potential recurrence biomarker, promotes the progression of colorectal cancer via Fyn/β-catenin signaling pathway. Cancer Med. 2021;10:4030-4045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Wu K, Feng J, Lyu F, Xing F, Sharma S, Liu Y, Wu SY, Zhao D, Tyagi A, Deshpande RP, Pei X, Ruiz MG, Takahashi H, Tsuzuki S, Kimura T, Mo YY, Shiozawa Y, Singh R, Watabe K. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat Commun. 2021;12:5196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Wang M, Liu B, Li D, Wu Y, Wu X, Jiao S, Xu C, Yu S, Wang S, Yang J, Li Y, Wang Q, Luo S, Tang H. Upregulation of IBSP Expression Predicts Poor Prognosis in Patients With Esophageal Squamous Cell Carcinoma. Front Oncol. 2019;9:1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Binothman N, Hachim IY, Lebrun JJ, Ali S. CPSF6 is a Clinically Relevant Breast Cancer Vulnerability Target: Role of CPSF6 in Breast Cancer. EBioMedicine. 2017;21:65-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Shi X, Ding K, Zhao Q, Li P, Kang Y, Tan S, Sun J. Suppression of CPSF6 Enhances Apoptosis Through Alternative Polyadenylation-Mediated Shortening of the VHL 3'UTR in Gastric Cancer Cells. Front Genet. 2021;12:707644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Wang BJ, Liu DC, Guo QY, Han XW, Bi XM, Wang H, Wu ZS, Wu WY. NUDT21 Suppresses Breast Cancer Tumorigenesis Through Regulating CPSF6 Expression. Cancer Manag Res. 2020;12:3069-3078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Zhang Z, Jiang M, Borthakur G, Luan S, Huang X, Tang G, Xu Q, Ji D, Boyer AD, Li F, Huang R, You MJ. Acute myeloid leukemia with a novel CPSF6-RARG variant is sensitive to homoharringtonine and cytarabine chemotherapy. Am J Hematol. 2020;95:E48-E51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Chen MS, Lo YH, Chen X, Williams CS, Donnelly JM, Criss ZK 2nd, Patel S, Butkus JM, Dubrulle J, Finegold MJ, Shroyer NF. Growth Factor-Independent 1 Is a Tumor Suppressor Gene in Colorectal Cancer. Mol Cancer Res. 2019;17:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Wang H, Wu M, Lu Y, He K, Cai X, Yu X, Lu J, Teng L. LncRNA MIR4435-2HG targets desmoplakin and promotes growth and metastasis of gastric cancer by activating Wnt/β-catenin signaling. Aging (Albany NY). 2019;11:6657-6673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Zhao Z, Xiao S, Yuan X, Yuan J, Zhang C, Li H, Su J, Wang X, Liu Q. AHNAK as a Prognosis Factor Suppresses the Tumor Progression in Glioma. J Cancer. 2017;8:2924-2932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Shan Z, An N, Qin J, Yang J, Sun H, Yang W. Long non-coding RNA Linc00675 suppresses cell proliferation and metastasis in colorectal cancer via acting on miR-942 and Wnt/β-catenin signaling. Biomed Pharmacother. 2018;101:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Shu D, Xu Y, Chen W. Knockdown of lncRNA BLACAT1 reverses the resistance of afatinib to non-small cell lung cancer via modulating STAT3 signalling. J Drug Target. 2020;28:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Guo M, Zhang X. LncRNA MSTO2P promotes colorectal cancer progression through epigenetically silencing CDKN1A mediated by EZH2. World J Surg Oncol. 2022;20:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Hu S, Song Y, Zhou Y, Jiao Y, Li G. METTL3 Accelerates Breast Cancer Progression via Regulating EZH2 m(6)A Modification. J Healthc Eng. 2022;2022:5794422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1193] [Cited by in RCA: 1252] [Article Influence: 65.9] [Reference Citation Analysis (8)] |
| 33. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 639] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 34. | Tan Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit. 2019;25:3537-3541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 305] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 35. | Iino K, Mitobe Y, Ikeda K, Takayama KI, Suzuki T, Kawabata H, Suzuki Y, Horie-Inoue K, Inoue S. RNA-binding protein NONO promotes breast cancer proliferation by post-transcriptional regulation of SKP2 and E2F8. Cancer Sci. 2020;111:148-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Han L, Huang C, Zhang S. The RNA-binding protein SORBS2 suppresses hepatocellular carcinoma tumourigenesis and metastasis by stabilizing RORA mRNA. Liver Int. 2019;39:2190-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Wang Q, Guo Y, Wang W, Liu B, Yang G, Xu Z, Li J, Liu Z. RNA binding protein DAZAP1 promotes HCC progression and regulates ferroptosis by interacting with SLC7A11 mRNA. Exp Cell Res. 2021;399:112453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 38. | Lv Q, Dong F, Zhou Y, Cai Z, Wang G. RNA-binding protein SORBS2 suppresses clear cell renal cell carcinoma metastasis by enhancing MTUS1 mRNA stability. Cell Death Dis. 2020;11:1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Ji CM, Zhang X, Fang W, Meng L, Wei X, Lu C. RNA-binding protein RNPC1 acts as an oncogene in gastric cancer by stabilizing aurora kinase B mRNA. Exp Cell Res. 2021;406:112741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Wang X, Hu H, Liu H. RNA binding protein Lin28B confers gastric cancer cells stemness via directly binding to NRP-1. Biomed Pharmacother. 2018;104:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Li J, Dong W, Jiang Q, Zhang F, Dong H. LINC00668 cooperated with HuR dependent upregulation of PKN2 to facilitate gastric cancer metastasis. Cancer Biol Ther. 2021;22:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Tan D, Li G, Zhang P, Peng C, He B. LncRNA SNHG12 in extracellular vesicles derived from carcinoma-associated fibroblasts promotes cisplatin resistance in non-small cell lung cancer cells. Bioengineered. 2022;13:1838-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |