Published online Aug 15, 2023. doi: 10.4251/wjgo.v15.i8.1497
Peer-review started: April 8, 2023
First decision: May 27, 2023
Revised: June 7, 2023
Accepted: July 18, 2023
Article in press: July 18, 2023
Published online: August 15, 2023
Processing time: 123 Days and 21 Hours
The molecular changes present in gastric neuroendocrine tumors (NETs) include a loss of heterozygosity or mutation of MEN1, CDKN1B gene mutation, P27 heterozygous mutation, and ATP4A gene missense mutation. We identified and are the first to report a case of type 1 histamine-producing enterochromaffin-like cell NETs (ECL-cell NETs) with a BRCA2 gene germline mutation.
The patient had a history of iron-deficient anemia for 5 years, and gastroscopic examination indicated multiple gastric tumors. Then, the patient underwent distal gastrectomy. Microscopically, multifocal tumor cells were found in the mucosa and submucosa; tumor cells were organoid and arranged in nests and cords, and the stroma was rich in sinusoids. The surrounding gastric mucosa showed atrophy with mild intestinal metaplasia or pseudopyloric gland metaplasia. Neuroendocrine cells could be seen with diffuse linear, nodular, and adeno
This is the first report of a case of type 1 gastric ECL-cell NETs with a pathogenic germline mutation of the BRCA2 gene. The findings of this report will expand the germline mutation spectrum of gastric NETs and increase the understanding of the molecular changes present in these tumors for their improved diagnosis in the future.
Core Tip: Type 1 enterochromaffin-like neuroendocrine tumors (ECL-cell NETs) occur most frequently and are associated with autoimmune gastritis. In gastric neuroendocrine tumors, molecular changes occur in genes including MEN1, CDKN1B, P27, and ATP4A. This is the first report of type 1 ECL-cell NETs with a pathogenic germline mutation of the BRCA2 gene.
- Citation: Zhang HF, Zheng Y, Wen X, Zhao J, Li J. Gastric neuroendocrine tumors in a BRCA2 germline mutation carrier: A case report. World J Gastrointest Oncol 2023; 15(8): 1497-1504
- URL: https://www.wjgnet.com/1948-5204/full/v15/i8/1497.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i8.1497
Among the digestive system tumors of the World Health Organization tumor classification series, gastric neuroendocrine tumors (NETs) include histamine-producing enterochromaffin-like NETs (ECL-cell NETs), somatostatin-producing D-cell NETs, gastrin-producing G-cell NETs, and serotonin-producing enterochromaffin-cell NETs (EC-cell NETs). ECL-cell NETs are divided into type 1, type 2, and type 3 according to their clinicopathological characteristics. Type 1 ECL-cell NETs account for the highest percentage of gastric NETs (approximately 80-90%), and are associated with autoimmune gastritis (AIG), anti-parietal cell antibodies (PCAb), and/or anti-intrinsic factor antibodies (IFAb)[1].
The molecular changes present in gastric NETs include a loss of heterozygosity or mutation of MEN1, CDKN1B gene mutation, P27 heterozygous mutation, and ATP4A gene missense mutation[2-5]. At present, further research on the molecular mechanisms of gastric NETs is still being conducted.
In this research, we identified and are the first to report a case of type 1 ECL-cell NETs with a BRCA2 gene germline mutation. In addition, we performed a review of the relevant literature to expand the understanding of the molecular changes present in gastric NETs.
A young woman was admitted to our hospital because of recurrent abdominal discomfort.
The patient's symptoms had lasted for 2 mo.
The patient had a history of iron-deficient anemia for 5 years, which was treated with oral iron. The patient had no history of prior surgeries.
The patient did not disclose any family genetic or aggregation diseases. Other family members had no clear history of cancers.
The physical examination of the patient showed no abnormalities, and there were no obvious signs or symptoms of anemia, such as pale oral mucosa.
Routine blood test results showed that the patient’s hemoglobin level was 106 g/L (normal range: 113-151 g/L). Biochemical indices were all normal. The levels of tumor markers, such as alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 125, and carbohydrate antigen 19-9, were all normal. Serum ferritin was markedly lower than normal at 1.8 U/mL (normal range: 7.0-323.0 U/mL).
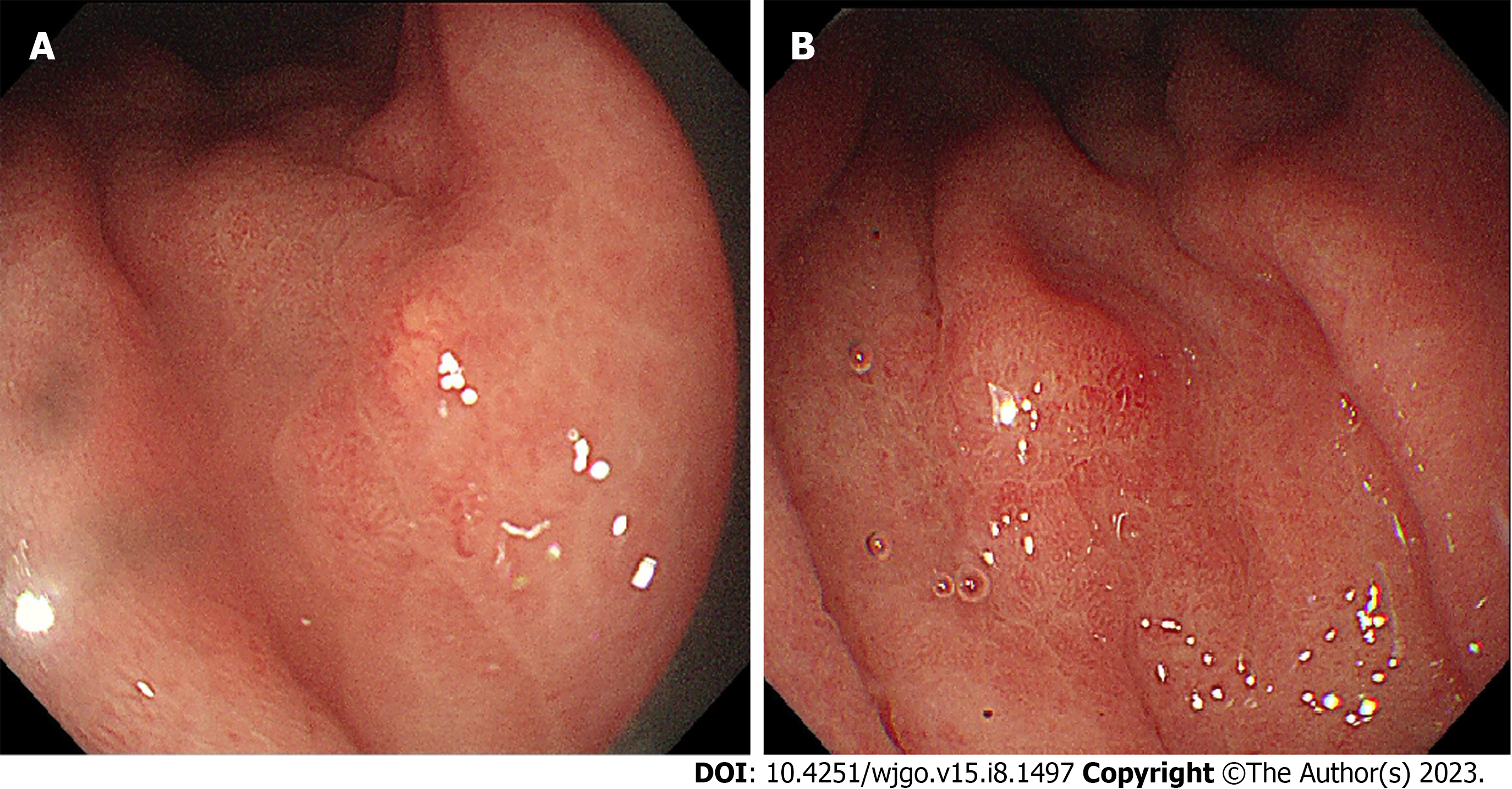
The patient next underwent gastroscopy and abdominal ultrasound examination. Gastroscopy showed that there were multiple grain-like protrusions in the great curvature of the stomach, with a hyperemic erosive focus found in the middle of the great curvature of the stomach (Figure 1). Abdominal ultrasound examination showed no abnormalities.
The patient underwent a biopsy after gastroscopy for pathological examination. Tumor cells could be seen in the lamina propria of the gastric mucosa in the pathological analysis of the biopsy sample. Immunohistochemically, the tumor cells diffusely expressed cytokeratin (CK), chromogranin A (CGA), synaptophysin (Syn), and CD56, indicating that the tumors were gastric NETs.
Because gastroscopy revealed multiple lesions in the stomach, the patient underwent distal gastrectomy surgery, and the excised distal gastric tissue was sent for pathological examination.
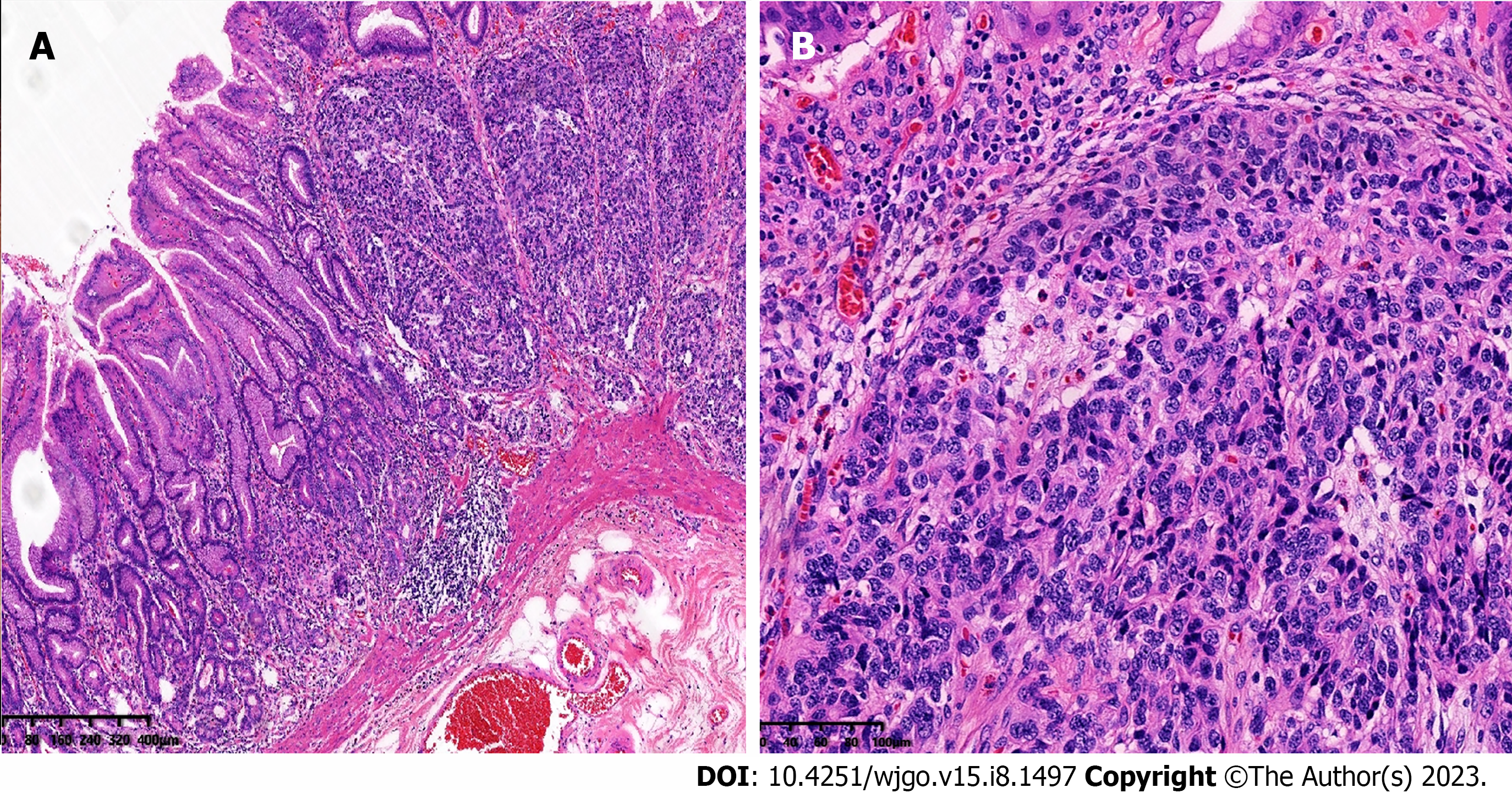
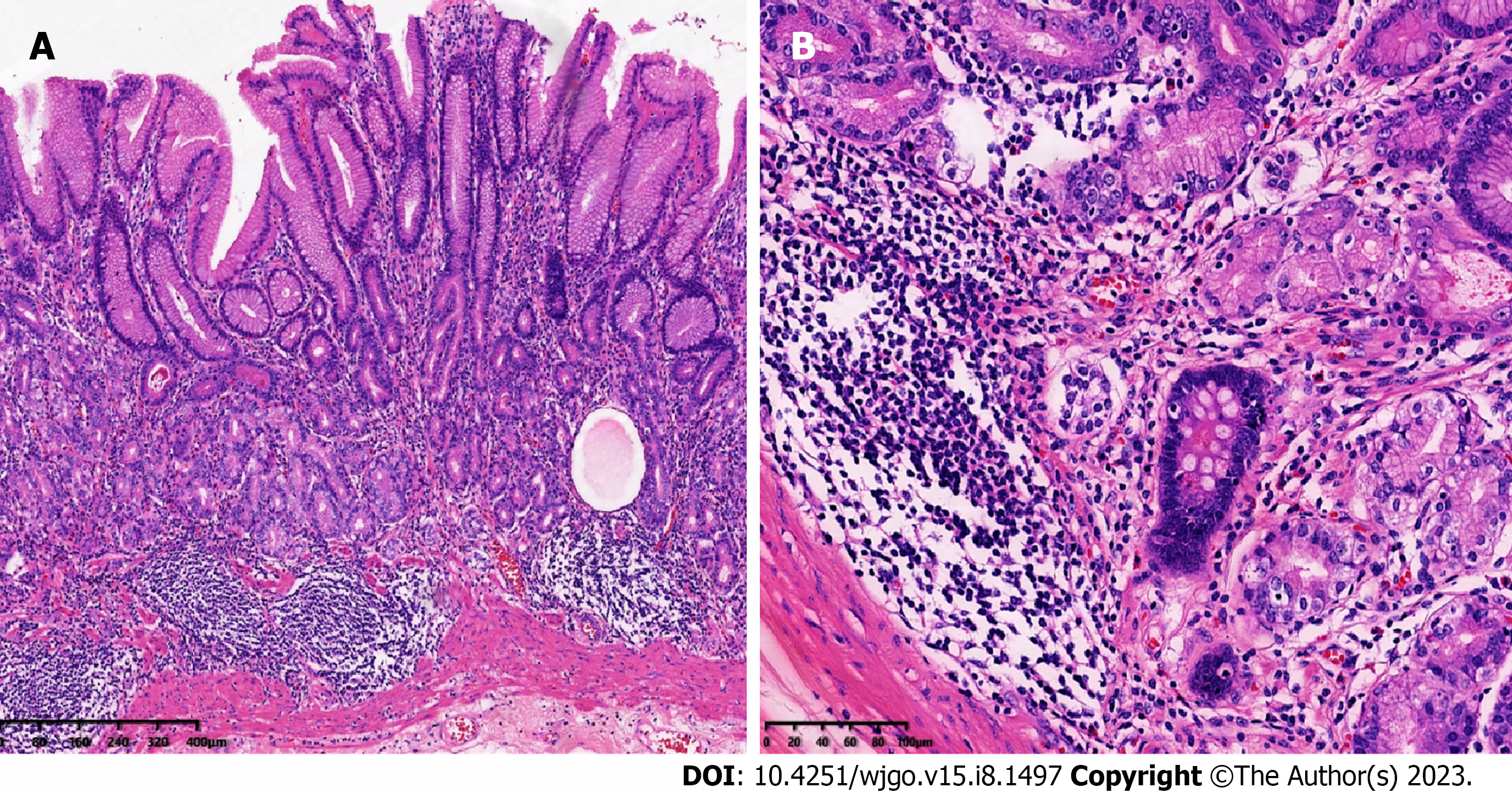
Grossly, in distal gastrectomy specimens, several polyps, 4-5 mm in diameter, were found in the antrum. Microscopically, multiple foci of tumor cells were found in the mucosa and submucosa, with tumor cells being organoid and arranged in nests and cords, with mild atypia; mitotic figures were not easily visible, and the stroma was rich in sinusoids (Figure 2). The surrounding gastric mucosa showed atrophy with mild intestinal metaplasia or pseudopyloric gland metaplasia (Figure 3). Neuroendocrine cells could be seen with diffuse linear, nodular, and adenomatous hyperplasia. Nests of neuroendocrine cells were observed at the upper resection margin but not at the lower margin. No tumor metastasis was observed in the surrounding lymph nodes.
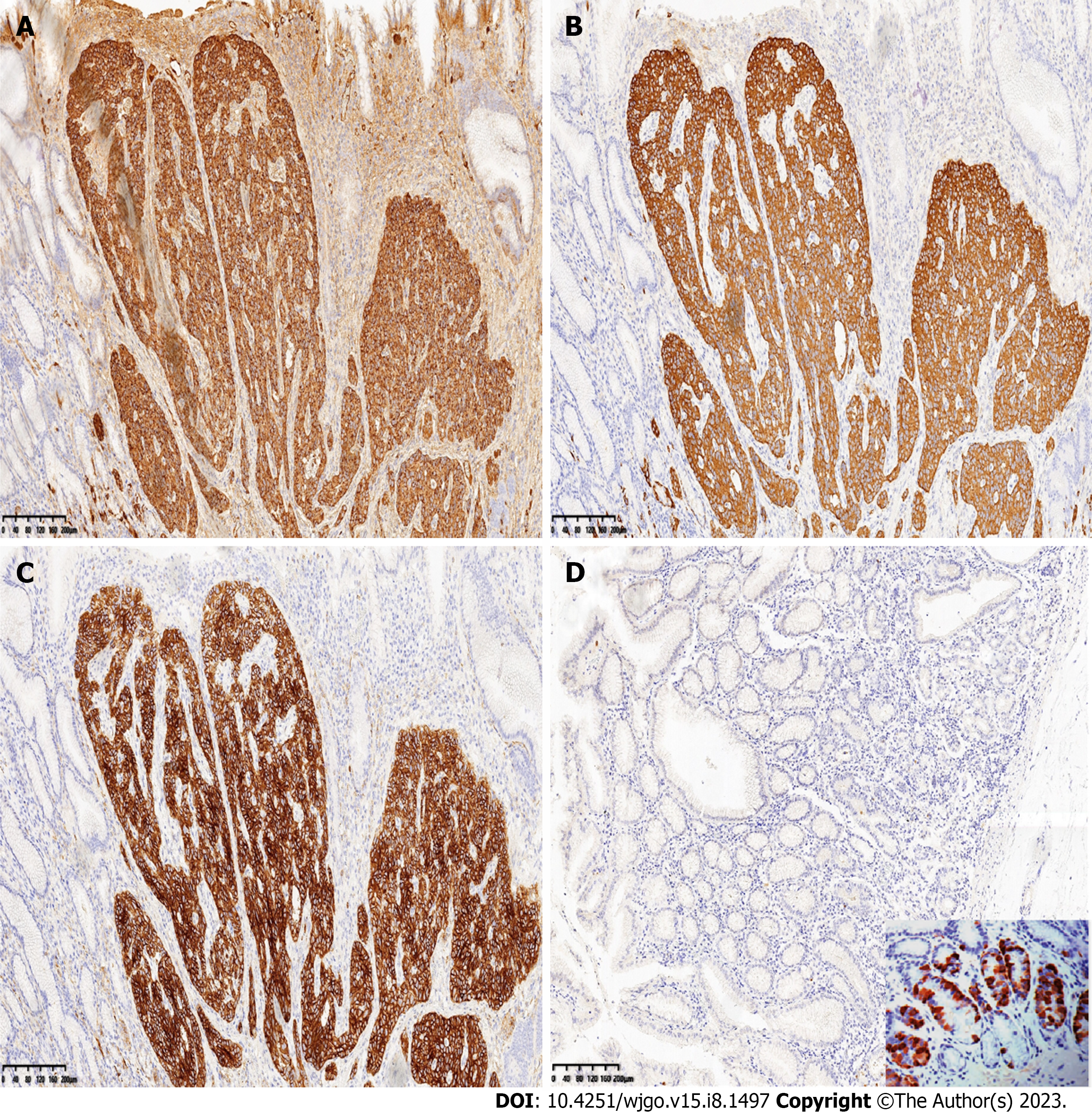
Immunohistochemically, the tumor cells diffusely expressed CK, CGA, Syn, and CD56. MLH1, PMS2, MSH2, and MSH6 were positive. CK20 and CDX2 were negative (Figure 4). Staining for gastrin in the surrounding gastric mucosa was negative or focally positive (Figure 4).
Tumor cells were positive for neuroendocrine markers, with 1 mitotic cell/2 mm2 at high magnification, and the Ki-67 index was 1%. The diagnosis was gastric NETs (G1).
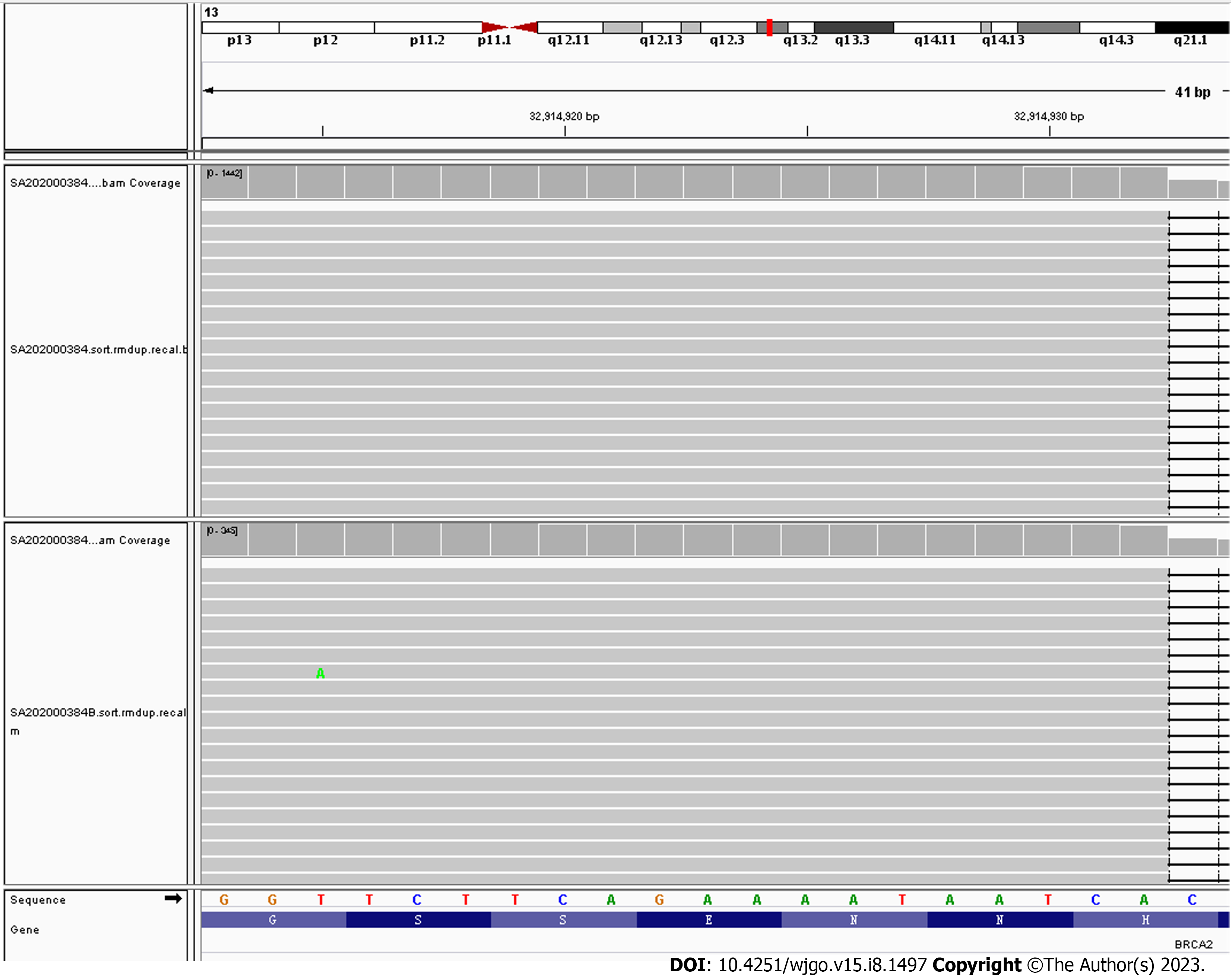
Combined with the patient's history and microscopic histomorphological changes, it was recommended that the patient undergo a test for anti-parietal cell antibodies and/or anti-intrinsic factor antibodies, and the serum results for anti-parietal cell antibodies were positive. The patient subsequently underwent whole-genome high-throughput molecular sequencing, which revealed a pathogenic germline mutation in the BRCA2 gene, a heterozygous germline frameshift mutation in exon 11, c.6443_6444del (p.S2148Yfs*2) (Figure 5).
The patient has been followed up to date, with regular routine blood examinations and semiannual gastroscopies performed. The patient still has anemia at present, and gastroscopies have shown no abnormalities. The latest routine blood test results showed a hemoglobin level of 72 g/L (normal range: 113-151 g/L). However, ultrasound examination revealed a cyst in the left ovary, with a diameter of < 2 cm, and the cyst was only regularly followed up without further treatment.
AIG is a progressive form of chronic gastritis. The histopathological changes that occur in AIG are atrophy of the secretory glands in the gastric body and fundus with intestinal metaplasia or pseudopyloric metaplasia, but changes in the gastric antrum mucosa are not obvious. Serological examinations show positivity for parietal cell antibodies and/or intrinsic factor antibodies. AIG has no characteristic symptoms in the early stage. Most patients experience dyspepsia or anemia as the first symptoms. Some cases of AIG can evolve to gastric adenocarcinoma or gastric NETs. In one study of 245 AIG patients with pernicious anemia, 28 patients (11.4%) developed type 1 NETs, 24 (9.8%) developed adenocarcinoma, and 52 (21.1%) developed hyperplastic polyps[6]. The patient in this report had iron-deficiency anemia for 5 years and developed type 1 gastric NETs.
Gastric anacidity in AIG stimulates the continuous secretion of gastrin by gastric antrum G cells, and hypergastrinemia promotes the proliferation of ECL cells. Histopathological analysis of early AIG shows a linear proliferation of ECL cells, which is manifested by the proliferation of five adjacent ECL cells in the glandular neck region and the expression of CGA as detected by immunohistochemistry. With the continuous progression of the disease, ECL cells may proliferate and develop into NETs[7]. In the patient whose case is presented here, a series of changes, such as linear hyperplasia of neuroendocrine cells and micronodular hyperplasia, could be seen in the glands of the gastric mucosa around the tumor.
The BRCA2 gene is located on the long arm of chromosome 13 and is normally expressed in breast cells. The BRCA2 gene is involved in DNA damage repair. Germline mutation of the BRCA2 gene can lead to tumors. At present, BRCA2 gene germline mutations have been reported in prostate neuroendocrine carcinoma, gallbladder neuroendocrine carcinoma, and ovarian non-small cell neuroendocrine carcinoma[8-10]. BRCA2 gene germline mutations can also be seen in hereditary diffuse gastric cancer syndrome[11,12]. The finding of BRCA2 gene germline mutations in gastric NETs has not previously been reported. Our patient was the first case of type I gastric NETs with a pathogenic germline mutation in the BRCA2 gene. A review of the literature shows that the homologous recombination pathway (HRD) involved in DNA repair pathways leads to tumorigenesis in pancreatic NETs with BRCA2 germline mutations[13]. However, more research is needed on the exact role of BRCA2 germline mutations in the pathogenesis of gastric NETs.
Some studies have shown that the incidence of type 1 ECL-cell NETs is low (approximately 4.37-11.4%)[6,14,15]; these NETs are usually small (< 1 cm) and have a median diameter of 5 mm, but they are prone to recurrence and can be complicated by gastric adenocarcinoma[16]. Metastasis can occur when the tumor diameter is greater than 1 cm. Type 1 ECL-cell NETs are gastrin dependent and are treated by controlling hypergastrinemia. A clinical trial by Lloyd et al[17] found that the application of netazepide (YF476), a gastrin/CCK-2 receptor antagonist, could eradicate some type 1 ECL-cell NETs after one year of treatment[17]. Somatostatin analogs (SSAs) can inhibit gastrin secretion and the proliferation of ECL cells to shrink the tumor and reduce recurrence[18]. SSAs have been reported to selectively treat multiple, unresectable, relapse-prone type I gastric NETs[19-21]. Studies have shown that tumors with BRCA2 gene germline mutations are sensitive to PARP inhibitors[22]. In patients with type 1 ECL-cell NETs with a BRCA2 gene germline mutation, further studies are needed to determine whether a benefit could be achieved with PARP inhibitor treatment.
The following appears to be steps for the differential diagnosis of type 1 ECL-cell NETs. First, it is necessary to differentiate gastric adenocarcinoma from type 1 ECL-cell NETs associated with AIG. Immunohistochemical analyses of type 1 ECL-cell NETs show the expression of the neuroendocrine markers CgA, Syn, and CD56, which are not expressed in gastric adenocarcinoma. In addition, in type 1 ECL-cell NETs, the tumor cell heterogeneity and mitotic index are lower than those of gastric adenocarcinoma. Second, type 1 ECL-cell NETs in the stomach need to be differentiated from type 2 ECL-cell NETs and type 3 ECL-cell NETs. Type 1 ECL-cell NETs are highly correlated with AIG and have unique clinical and pathological characteristics, such as changes including atrophic gastritis seen under gastroscopy, anti-intrinsic factor antibody and/or anti-parietal cell antibody positivity, changes in the fundus of the stomach, a decrease in the number of glands in the mucosa of the gastric fundus, and pyloric gland or intestinal metaplasia.
This is the first case report of gastric NETs (type 1 ECL-cell NETs) with a pathogenic germline mutation of the BRCA2 gene. The findings presented in this report will expand the germline mutation spectrum of gastric NETs and increase the understanding of the molecular changes present in gastric NETs for the improved diagnosis of gastric NETs in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Massironi S, Italy S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Cai YX
| 1. | La Rosa S, Inzani F, Vanoli A, Klersy C, Dainese L, Rindi G, Capella C, Bordi C, Solcia E. Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum Pathol. 2011;42:1373-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 2. | Bordi C. Neuroendocrine pathology of the stomach: the Parma contribution. Endocr Pathol. 2014;25:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Malanga D, De Gisi S, Riccardi M, Scrima M, De Marco C, Robledo M, Viglietto G. Functional characterization of a rare germline mutation in the gene encoding the cyclin-dependent kinase inhibitor p27Kip1 (CDKN1B) in a Spanish patient with multiple endocrine neoplasia-like phenotype. Eur J Endocrinol. 2012;166:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Lee M, Pellegata NS. Multiple endocrine neoplasia syndromes associated with mutation of p27. J Endocrinol Invest. 2013;36:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 5. | Fossmark R, Calvete O, Mjønes P, Benitez J, Waldum HL. ECL-cell carcinoids and carcinoma in patients homozygous for an inactivating mutation in the gastric H(+) K(+) ATPase alpha subunit. APMIS. 2016;124:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Terao S, Suzuki S, Yaita H, Kurahara K, Shunto J, Furuta T, Maruyama Y, Ito M, Kamada T, Aoki R, Inoue K, Manabe N, Haruma K. Multicenter study of autoimmune gastritis in Japan: Clinical and endoscopic characteristics. Dig Endosc. 2020;32:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (2)] |
| 7. | Cockburn AN, Morgan CJ, Genta RM. Neuroendocrine proliferations of the stomach: a pragmatic approach for the perplexed pathologist. Adv Anat Pathol. 2013;20:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Symonds L, Konnick E, Vakar-Lopez F, Cheng HH, Schweizer MT, Nelson PS, Pritchard CC, Montgomery B. BRCA2 Alterations in Neuroendocrine/Small-Cell Carcinoma Prostate Cancer: A Case Series. JCO Precis Oncol. 2022;6:e2200091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Liu F, Li Y, Ying D, Qiu S, He Y, Li M, Liu Y, Zhang Y, Zhu Q, Hu Y, Liu L, Li G, Pan W, Jin W, Mu J, Cao Y. Whole-exome mutational landscape of neuroendocrine carcinomas of the gallbladder. Signal Transduct Target Ther. 2021;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 10. | Herold N, Wappenschmidt B, Markiefka B, Keupp K, Kröber S, Hahnen E, Schmutzler R, Rhiem K. Non-small cell neuroendocrine carcinoma of the ovary in a BRCA2-germline mutation carrier: A case report and brief review of the literature. Oncol Lett. 2018;15:4093-4096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, Santos TA, Claro I, Carvalho J, Nielsen C, Padilla S, Lum A, Talhouk A, Baker-Lange K, Richardson S, Lewis I, Lindor NM, Pennell E, MacMillan A, Fernandez B, Keller G, Lynch H, Shah SP, Guilford P, Gallinger S, Corso G, Roviello F, Caldas C, Oliveira C, Pharoah PD, Huntsman DG. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 12. | Dulskas A, Al Bandar M, Choi YY, Shin SJ, Beom SH, Son T, Kim HI, Cheong JH, Hyung WJ, Noh SH. A case of gastric cancer metastasis to the breast in a female with BRCA2 germline mutation and literature review. Acta Chir Belg. 2019;119:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Szybowska M, Mete O, Weber E, Silver J, Kim RH. Neuroendocrine Neoplasms Associated with Germline Pathogenic Variants in the Homologous Recombination Pathway. Endocr Pathol. 2019;30:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Park JY, Cornish TC, Lam-Himlin D, Shi C, Montgomery E. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am J Surg Pathol. 2010;34:1591-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Zhang H, Jin Z, Cui R, Ding S, Huang Y, Zhou L. Autoimmune metaplastic atrophic gastritis in chinese: a study of 320 patients at a large tertiary medical center. Scand J Gastroenterol. 2017;52:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Lahner E, Esposito G, Pilozzi E, Galli G, Corleto VD, Di Giulio E, Annibale B. Gastric cancer in patients with type I gastric carcinoids. Gastric Cancer. 2015;18:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Lloyd KA, Parsons BN, Burkitt MD, Moore AR, Papoutsopoulou S, Boyce M, Duckworth CA, Exarchou K, Howes N, Rainbow L, Fang Y, Oxvig C, Dodd S, Varro A, Hall N, Pritchard DM. Netazepide Inhibits Expression of Pappalysin 2 in Type 1 Gastric Neuroendocrine Tumors. Cell Mol Gastroenterol Hepatol. 2020;10:113-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Roberto GA, Rodrigues CMB, Peixoto RD, Younes RN. Gastric neuroendocrine tumor: A practical literature review. World J Gastrointest Oncol. 2020;12:850-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (8)] |
| 19. | Massironi S, Zilli A, Fanetti I, Ciafardini C, Conte D, Peracchi M. Intermittent treatment of recurrent type-1 gastric carcinoids with somatostatin analogues in patients with chronic autoimmune atrophic gastritis. Dig Liver Dis. 2015;47:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 20. | Rossi RE, Invernizzi P, Mazzaferro V, Massironi S. Response and relapse rates after treatment with long-acting somatostatin analogs in multifocal or recurrent type-1 gastric carcinoids: A systematic review and meta-analysis. United European Gastroenterol J. 2020;8:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Massironi S, Zilli A, Conte D. Somatostatin analogs for gastric carcinoids: For many, but not all. World J Gastroenterol. 2015;21:6785-6793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1709] [Cited by in RCA: 1987] [Article Influence: 248.4] [Reference Citation Analysis (0)] |