Published online Aug 15, 2023. doi: 10.4251/wjgo.v15.i8.1461
Peer-review started: April 17, 2023
First decision: May 4, 2023
Revised: May 21, 2023
Accepted: June 13, 2023
Article in press: June 13, 2023
Published online: August 15, 2023
Processing time: 115 Days and 0.2 Hours
Mucinous adenocarcinoma (MC) has attracted much attention as a distinct histologic subtype of colorectal cancer in recent years. However, data about its epidemiologic and prognostic characteristics are limited. Therefore, patient data extracted from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program were collected to analyze the epidemiologic and clinicopathological characteristics of MC.
To determine the epidemiologic and clinicopathological characteristics of MC.
The incidence trend of MC was calculated through the Joinpoint Regression Program. Cox regression analyses were performed to identify prognostic factors associated with overall survival (OS). A nomogram was established to predict the survival probability of individual patients with MC.
We found that rates of MC decreased from 4.50/100000 in 2000 to 1.54/100000 in 2018. Rates of MCs in patients aged ≤ 50 years decreased 2.27%/year during 2000-2018. The incidence of appendiceal MCs increased from 0.14/100000 in 2000 to 0.24/100000 in 2018, while the incidence in other anatomic subsites continued to decrease. On multivariable Cox analyses, age, race, tumor site, T stage, N stage, M stage, surgery, and chemotherapy were associated with OS. A nomogram was developed based on these factors, and the area under the curve for 1-year, 3-year, and 5-year OS in the training cohort was 0.778, 0.778, and 0.768, respectively.
Our results demonstrated that MC incidence decreased in almost all anatomic subgroups except for the appendix. A nomogram predicting the survival probability of patients with MCs showed good performance.
Core Tip: When stratified by anatomic location, incidence rates declined in almost all anatomic subgroups except for mucinous adenocarcinoma in the appendix. Clinicians should also pay attention to the increasing incidence of appendiceal mucinous adenocarcinoma. During the survival analysis, we found that age, race, tumor site, surgery, and chemotherapy were significantly associated with survival. A nomogram based on those factors as well as the TNM tumor stages showed good predictive performance, which indicated that in addition to the TNM stages, characteristics such as tumor site, age, and race were also important in developing individualized treatment plans for patients with mucinous colorectal cancer.
- Citation: Jiang J, Tang XW, Huang S, Hu N, Chen Y, Luo B, Ren WS, Peng Y, Yang WX, Lü MH. Epidemiologic characteristics and risk factors associated with overall survival for patients with mucinous colorectal cancer: A population-based study. World J Gastrointest Oncol 2023; 15(8): 1461-1474
- URL: https://www.wjgnet.com/1948-5204/full/v15/i8/1461.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i8.1461
Colorectal cancer (CRC) is the third most frequent cancer and ranks second with respect to cancer-related mortality worldwide[1]. Mucinous adenocarcinoma (MC), defined as having more than 50% of the tumor component as extra
To date, the prognostic significance of MC remains controversial. Few studies have determined the prognostic risk factors that are associated with the overall survival (OS) of mucinous CRC. Currently, the TNM staging system created and updated by the American Joint Committee on Cancer and the International Union Against Cancer serves as the standard to evaluate cancer status and is the main method to assess the prognosis of cancer patients. However, other elements, such as age, sex, race and ethnicity, and primary tumor site, probably play a role in predicting the prognosis of cancer patients[6,7]. Considering the limitations of the TNM staging system, nomograms have emerged and have been widely used as a simpler but more sophisticated method, given that nomograms could integrate diverse prognostic variables into the model from biological to clinicopathological characteristics[8].
Therefore, in the present study, we conducted a population-based analysis of the epidemiologic and clinicopathological characteristics of mucinous CRC on the basis of data extracted from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and developed a nomogram to predict the 1-year, 3-year, and 5-year OS of patients with mucinous CRC.
This is a retrospective cohort study based on data collected from 18 cancer registries in the SEER program, which covers approximately 27.8% of the United States population. This study is reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology statement. Two researchers conducted the data collection process independently to avoid potential selection bias. The review and informed consent were exempted by the institutional review board for the use of a freely available database.
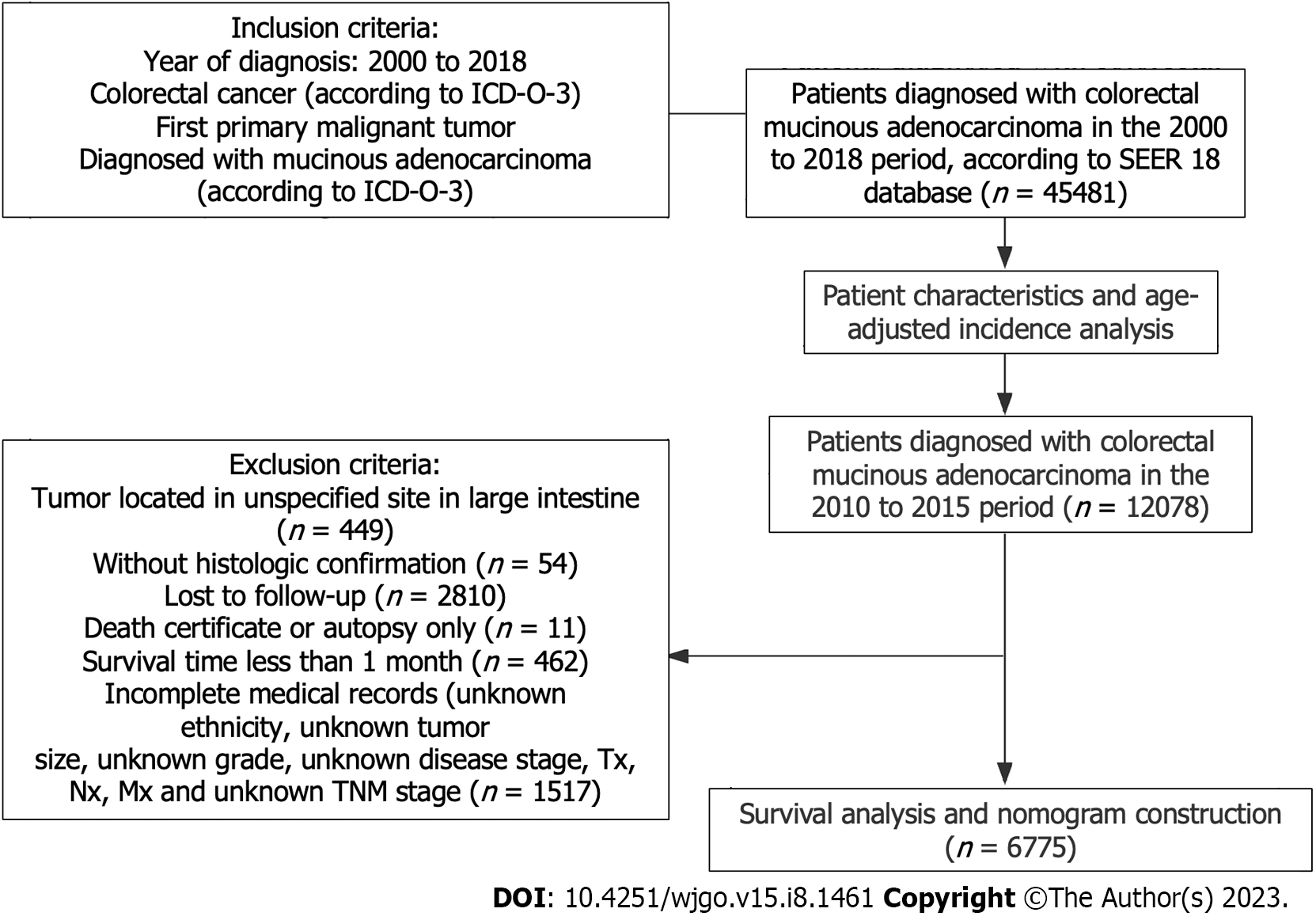
Patients were enrolled with the following criteria: diagnosed with mucinous CRC according to the International Classification of Diseases for Oncology, Third Edition and receiving a diagnosis between 2000 and 2018. Patients with preexisting malignant tumors were excluded. To ensure a follow-up time of at least 3 years, only those patients diagnosed between 2010 and 2015 with complete medical records and sufficient survival data (of complete follow-up and a survival > 1 mo) documented were included in the construction of the nomogram model to predict OS. The study design is presented in Figure 1.
Because of their potential association with OS, the following clinicopathological characteristics were converted into categorical variables and analyzed: Age, sex, race (White, Black, Asian and Pacific Islander, and American Indian and Alaska Native), tumor site, primary tumor size, grade (well differentiated, moderately differentiated, poorly differentiated, and undifferentiated), disease stage (localized, regional, and distant according to SEER staging system), TNM stage derived from American Joint Committee on Cancer staging system 7th edition, surgery, radiation, and chemo
Incidence rates were age-adjusted to the 2000 United States population and acquired by using SEER*Stat software (version 8.4.0.1). Joinpoint Regression Program (version 4.9.1.0) was used to identify yearly trends in annual percent change (APC) in rates. The tool calculated the APC with data fitted to the simplest joinpoint regression model and tested the significance using a Monte Carlo permutation method.
The outcome of interest was OS, defined as the time period that elapsed from diagnosis to death of any cause or the last follow-up. Patients were randomly divided into 7:3 training (n = 4379) and validation (n = 1877) groups using the R program (version 4.2.1, www.r-project.org). Univariate and multivariate Cox proportional hazards regression analyses were performed to identify prognostic factors associated with OS, with hazard ratios (HRs) and 95% confidence intervals (CIs) being calculated. The analyses were conducted with SPSS, version 27 (IBM Corp). A nomogram was constructed to predict 1-year, 3-year, and 5-year OS probability based on those factors identified by multivariable Cox regression analysis.
The concordance index (C-index) and area under the receiver operating characteristic curve (AUC) were calculated to evaluate the predictive accuracy of the nomogram. The calibration curve was plotted to determine how far the predicted survival was from the actual survival. Kaplan–Meier survival curves were used to present OS, and differences were compared using the log-rank test. The “rms,” “Survival,” “survival ROC,” and “foreign” packages of R were used in the construction and verification of the nomogram. Statistical significance was defined as a two-tailed P value less than 0.05.
From 2000 to 2018, a total of 45481 patients with mucinous CRC were identified, with a median age of 68 years at diagnosis (interquartile range, 57-78). Among them, 81.6% were White patients, 11.2% were Black patients, 6.3% were Asian or Pacific Islander patients, and 0.5% were American Indian and Alaska Native patients (Table 1). Females made up a slightly greater proportion of those cancer cases (51.7% compared to 48.3% for males). For primary tumor sites of these mucinous CRC cases, the proximal colon was most commonly involved (45.5%), followed by the distal colon (24.3%), rectum (11.3%), appendix (7.9%), and transverse colon (7.7%). Of 38970 mucinous CRCs with a known grade, 5051 were well differentiated (11.1%), 25253 were moderately differentiated (55.5%), 7703 were poorly differentiated (16.9%), and 963 were undifferentiated (2.1%). Among 44818 mucinous CRCs with a known stage, 11973 were localized (26.3%), 21436 were regional (47.1%), and 11407 were distant (25.1%).
| Variable | n | % among 45481 total |
| Age in yr | ||
| 15-39 | 1865 | 4.1 |
| 40-54 | 7816 | 17.2 |
| 55-69 | 14499 | 31.9 |
| ≥ 70 | 21301 | 46.8 |
| Sex | ||
| Male | 21976 | 48.3 |
| Female | 23505 | 51.7 |
| Race | ||
| White | 37134 | 81.6 |
| Black | 5099 | 11.2 |
| Asian or Pacific Islander | 2865 | 6.3 |
| American Indian and Alaska Native | 249 | 0.5 |
| Unknown | 134 | 0.3 |
| Marital status | ||
| Single | 6476 | 14.2 |
| Married | 24100 | 53.0 |
| Other1 | 14905 | 32.8 |
| Tumor site | ||
| Proximal colon | 20714 | 45.5 |
| Transverse colon | 3510 | 7.7 |
| Distal colon | 11065 | 24.3 |
| Rectum | 5149 | 11.3 |
| Appendix | 3572 | 7.9 |
| Large intestine, unspecified | 1471 | 3.2 |
| Grade2 | ||
| 1 | 5051 | 11.1 |
| 2 | 25253 | 55.5 |
| 3 | 7703 | 16.9 |
| 4 | 963 | 2.1 |
| Unknown | 6511 | 14.3 |
| Disease stage | ||
| In situ | 2 | 0.0 |
| Localized | 11973 | 26.3 |
| Regional | 21436 | 47.1 |
| Distant | 11407 | 25.1 |
| Unknown/unstaged | 663 | 1.5 |
| Surgery | ||
| Yes | 41667 | 91.6 |
| No/Unknown | 3814 | 8.4 |
| Radiation | ||
| Yes | 4865 | 10.7 |
| No/Unknown | 40616 | 89.3 |
| Chemotherapy | ||
| Yes | 19199 | 42.2 |
| No/Unknown | 26282 | 57.8 |
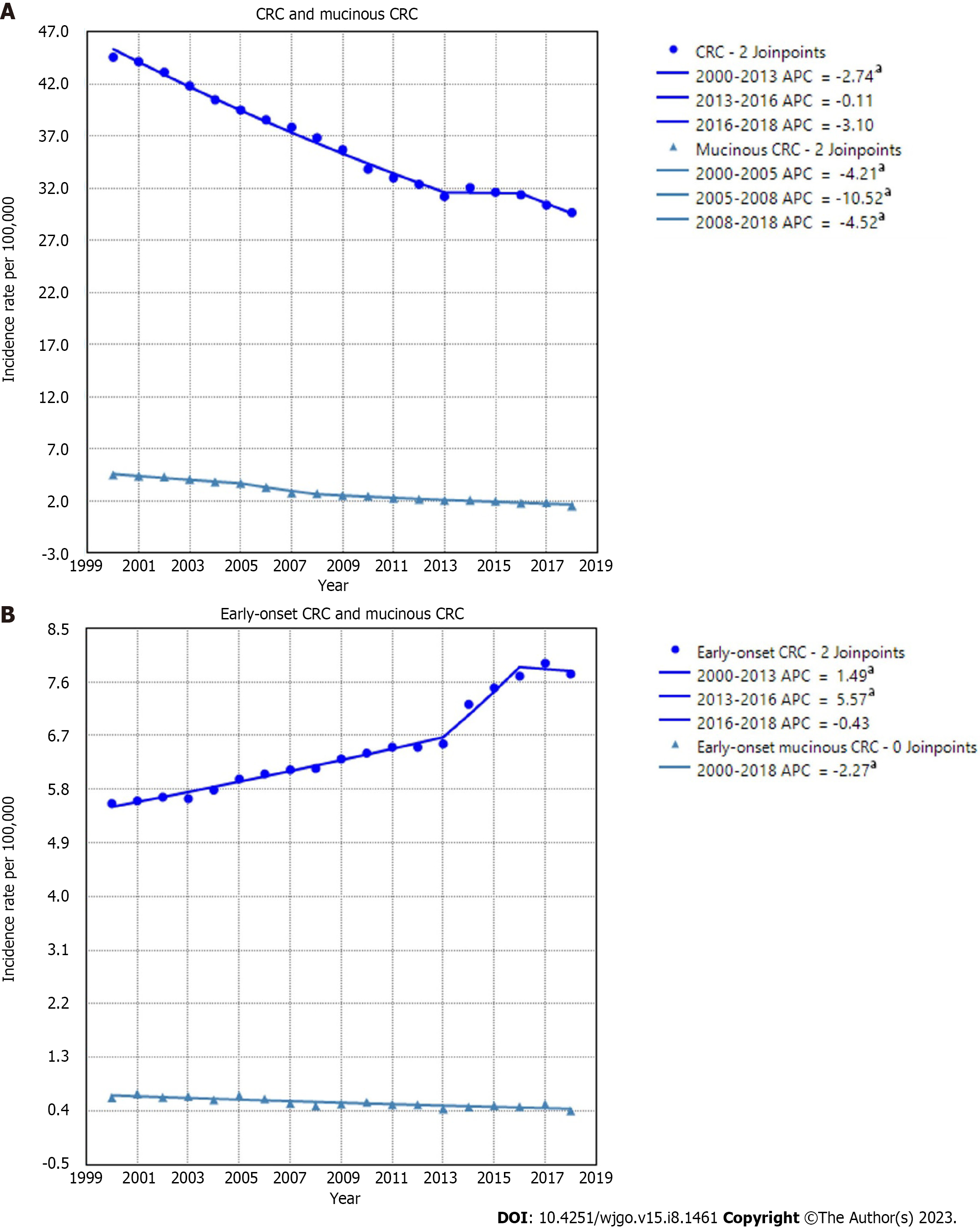
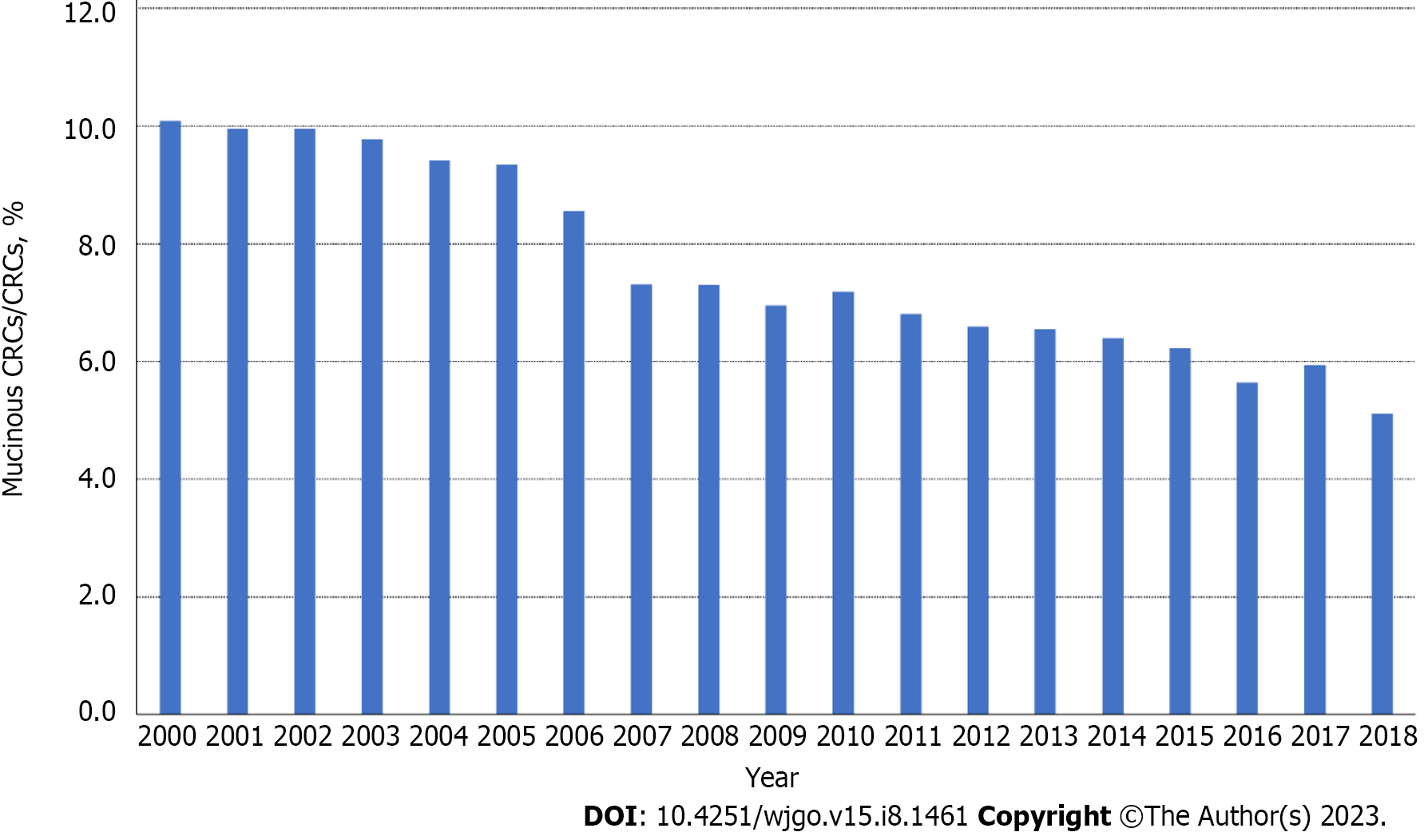
The overall age-adjusted incidence of CRC decreased from 44.56/100000 in 2000 to 29.65/100000 in 2018, with the steepest decline observed between 2016 and 2018 (APC = -3.1, P = 0.06). The rates of mucinous CRC also showed a downward trend over the entire period, decreasing from 4.50/100000 in 2000 to 1.54/100000 in 2018. For CRC patients aged younger than 50 years, i.e. early-onset CRCs, the incidence rate increased 1.49% per year during 2000–2013 and 5.57% per year during 2013–2016 before declining insignificantly during 2016–2018. In contrast to that upward trend, rates of mucinous CRCs in patients younger than 50 years decreased 2.27% per year during 2000–2018 (Figure 2). Of all CRC cases, the proportion of MC decreased similarly during the same period, from 10.1% in 2000 to 5.1% in 2018 (Figure 3).
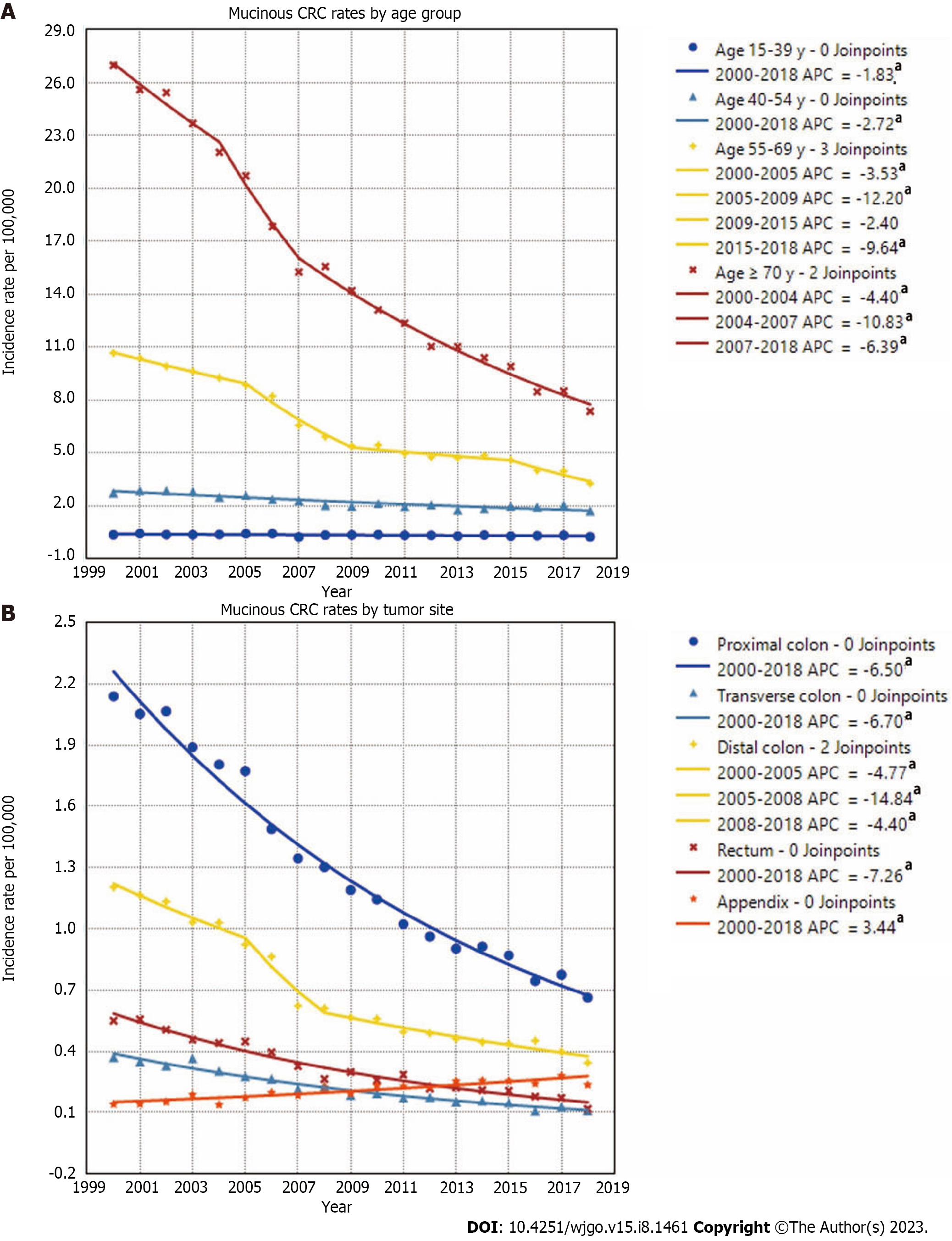
With regard to the sex of patients with mucinous CRC, the incidence rates of male and female patients both decreased significantly over time, with rates decreasing from 4.80/100000 in 2000 to 1.64/100000 in 2018 for males and from 4.22/100000 to 1.43/100000 for females. With respect to the race and ethnicity of patients, the age-adjusted incidence rates of mucinous CRC decreased from 4.60/100000 in 2000 to 1.56/100000 in 2018 among the White population and from 4.88/100000 to 1.68/100000 during the same period among the Black population. The rates for the American Indian and Alaska Native population and the Asian or Pacific Islander population also decreased, with APCs of 6.70 and 5.51, respectively (Supplementary Figure 1).
When stratified by age, declining incidence rates of mucinous CRC were observed among all age groups. The highest mucinous CRC rate was observed in patients aged ≥ 70 years, which fell from 26.96/100000 in 2000 to 7.36/100000 in 2018, with the steepest drop occurring during 2004-2007. In the 55-year-olds to 69-year-olds, mucinous CRC rates decreased from 10.66/100000 to 3.27/100000 during the entire period, with the steepest drop occurring during 2005-2009. Rates decreased 2.72%/year from 2.73/100000 in 2000 to 1.72/100000 in 2018 in the 40-year-olds to 54-year-olds and decreased 1.83%/year from 0.36/100000 to 0.25/100000 in the 15-year-olds to 39-year-olds. With regard to rates of mucinous CRCs in different anatomic subsites, MC was most commonly observed in the proximal colon, followed by the distal colon and rectum. The rates of mucinous CRCs in the proximal colon decreased 6.5%/year from 2.14/100000 in 2000 to 0.66/100000 in 2018. The rates of mucinous CRCs in the distal colon decreased from 1.20/100000 in 2000 to 0.34/100000 in 2018, with the steepest drop occurring during 2005-2008. The incidence of mucinous CRCs located in the transverse colon and rectum also showed a downward trend, presenting a sharp contrast to the increasing incidence of appendiceal MC, which increased from 0.14/100000 in 2000 to 0.24/100000 in 2018 (Figure 4).
A total of 6775 patients were included in this process. Among them, 4742 were randomly allocated to the training cohort, and 2033 were randomly allocated to the validation cohort (Supplementary Table 1). We performed our analysis based on the training cohort. Univariate and multivariate Cox regression models were chosen to explore the independent prognostic risk factors for 1-year, 3-year, and 5-year OS for patients with mucinous CRC during the follow-up. Variables found to be significantly associated with OS were further applied in the multivariate Cox regression analysis. By univariate Cox regression analysis, age, race, tumor site, tumor size, grade, disease stage, T stage, N stage, M stage, surgery, and chemotherapy were identified to be significantly associated with OS. Statistical significance of age, race, tumor site, T stage, N stage, M stage, surgery, and chemotherapy persisted during the multivariate Cox regression analysis. In particular, elderly patients (aged 40 years to 69 years: HR, 1.28; 95%CI: 1.00-1.64; ≥ 70 years: HR, 2.41; 95%CI: 1.88-3.08), Black patients (HR, 1.26; 95%CI: 1.11-1.43), patients with T3 stage (HR, 1.37; 95%CI: 1.03-1.83) or T4 stage (HR, 2.28; 95%CI: 1.68-3.08), patients with lymph node metastasis (N1 stage: HR, 1.72; 95%CI: 1.51-1.97; N2 stage: HR, 2.58; 95%CI: 2.25-2.96), patients with distant metastasis (HR, 2.67; 95%CI: 2.01-3.55), patients without surgery (HR, 4.82; 95%CI: 3.57-6.51), and patients without chemotherapy (HR, 1.58; 95%CI: 1.42-1.76) had poorer OS. Compared with tumors primarily located in the proximal colon, tumors located in the distal colon were associated with poorer OS (HR, 1.15; 95%CI: 1.04-1.28), whereas tumors located in the appendix suggested better survival (HR, 0.58; 95%CI: 0.47-0.72). Details are presented in Table 2.
| Variables | Subgroup | Univariable | Multivariable | ||
| Hazard ratio | P value | Hazard ratio | P value | ||
| Age in yr | < 40 | Reference | < 0.001 | Reference | < 0.001 |
| 40-69 | 1.15 (0.90-1.46) | 1.28 (1.00-1.64) | |||
| ≥ 70 | 1.85 (1.45-2.35) | 2.41 (1.88-3.08) | |||
| Sex | Male | Reference | 0.211 | / | |
| Female | 1.06 (0.97-1.15) | ||||
| Race | White | Reference | 0.035 | Reference | < 0.001 |
| Black | 1.15 (1.02-1.30) | 1.26 (1.11-1.43) | |||
| Other1 | 0.90 (0.75-1.08) | 0.92 (0.77-1.10) | |||
| Tumor site | Proximal colon | Reference | < 0.001 | Reference | < 0.001 |
| Transverse colon | 0.93 (0.79-1.09) | 0.88 (0.75-1.04) | |||
| Distal colon | 1.17 (1.05-1.29) | 1.15 (1.04-1.28) | |||
| Rectum | 0.92 (0.79-1.08) | 1.14 (0.97-1.34) | |||
| Appendix | 0.74 (0.61-0.88) | 0.58 (0.47-0.72) | |||
| Tumor size | ≤ 30 | Reference | < 0.001 | Reference | 0.625 |
| 31-59 | 1.41 (1.24-1.61) | 1.06 (0.92-1.22) | |||
| ≥ 60 | 1.57 (1.38-1.79) | 1.07 (0.93-1.23) | |||
| Grade | I | Reference | < 0.001 | Reference | 0.073 |
| II | 1.20 (1.04-1.39) | 1.11 (0.95-1.30) | |||
| III | 1.71 (1.45-2.02) | 1.23 (1.03-1.46) | |||
| IV | 2.07 (1.67-2.57) | 1.26 (1.01-1.58) | |||
| Disease stage | Localized | Reference | < 0.001 | Reference | 0.105 |
| Regional | 1.49 (1.33-1.67) | 0.90 (0.77-1.06) | |||
| Distant | 4.32 (3.83-4.88) | 1.16 (0.85-1.60) | |||
| T stage | T1 | Reference | < 0.001 | Reference | < 0.001 |
| T2 | 0.86 (0.63-1.16) | 0.99 (0.72-1.35) | |||
| T3 | 1.41 (1.08-1.85) | 1.37 (1.03-1.83) | |||
| T4 | 2.67 (2.03-3.50) | 2.28 (1.68-3.08) | |||
| N stage | N0 | Reference | < 0.001 | Reference | < 0.001 |
| N1 | 1.63 (1.47-1.81) | 1.72 (1.51-1.97) | |||
| N2 | 2.90 (2.62-3.21) | 2.58 (2.25-2.96) | |||
| M stage | M0 | Reference | < 0.001 | Reference | < 0.001 |
| M1 | 3.75 (3.42-4.11) | 2.67 (2.01-3.55) | |||
| Surgery | Yes | Reference | < 0.001 | Reference | < 0.001 |
| No/Unknown | 5.08 (3.83-6.73) | 4.82 (3.57-6.51) | |||
| Radiation | Yes | Reference | 0.764 | / | |
| No/Unknown | 1.02 (0.89-1.17) | ||||
| Chemotherapy | Yes | Reference | < 0.001 | Reference | < 0.001 |
| No/Unknown | 0.87 (0.80-0.95) | 1.58 (1.42-1.76) | |||
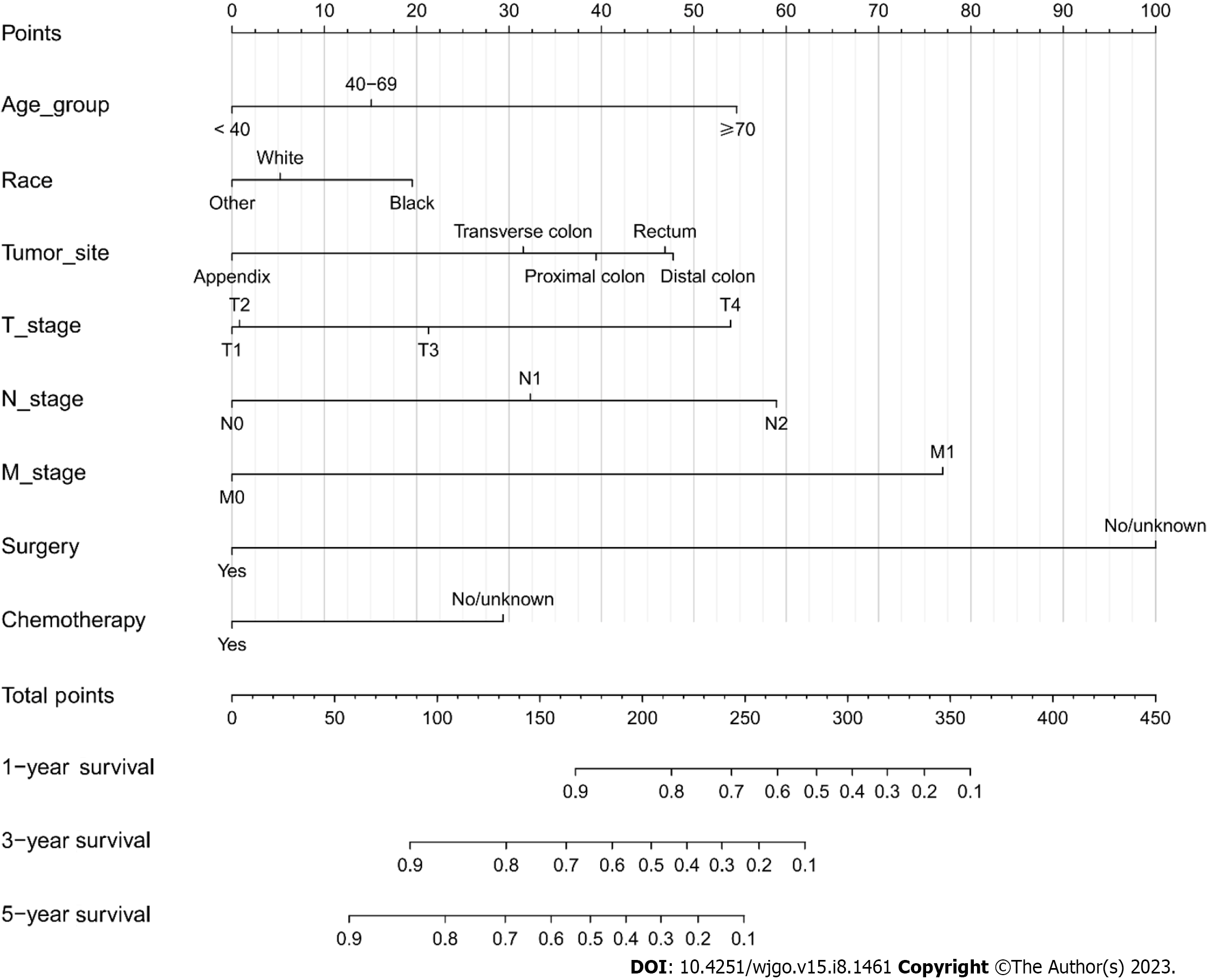
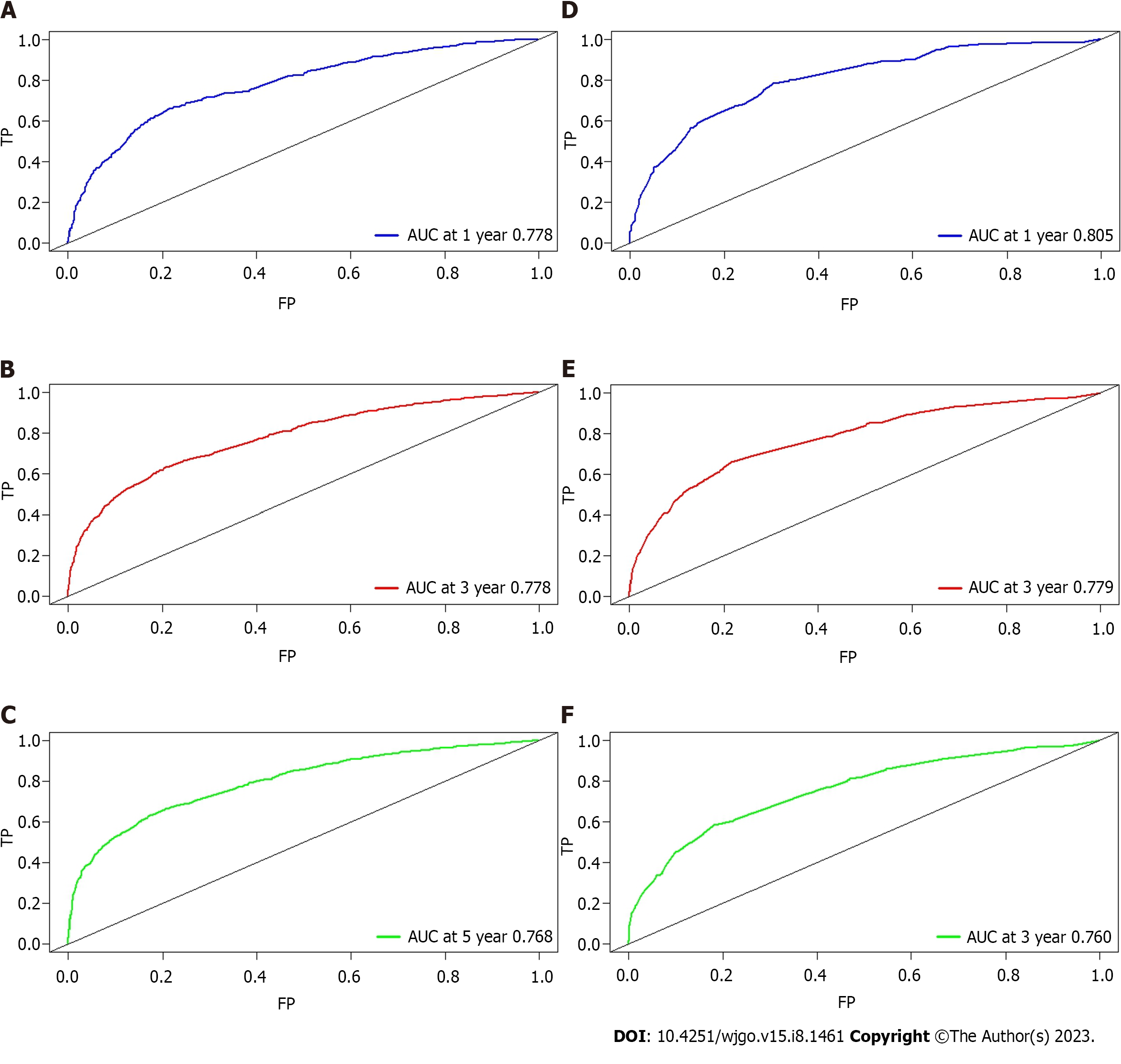
A nomogram model was constructed to predict the 1-year, 3-year, and 5-year OS of patients with mucinous CRC according to multivariate Cox regression analysis (Figure 5). Each vector of these variables was assigned a point value, and the cumulative point score of all variables for a particular individual was matched to the probability of survival. Supplementary Table 2 shows the specific value of each variable. The nomogram had a C-index of 0.730, which was internally validated with a C-index of 0.728 using the validation cohort. The AUCs of the nomogram predicting 1-year, 3-year, and 5-year OS were 0.778, 0.778, and 0.768, respectively. The discrimination performance was validated in the validation cohort with an AUC of 0.805 in predicting the 1-year OS, an AUC of 0.779 in predicting the 3-year OS, and an AUC of 0.760 in predicting the 5-year survival (Figure 6). Additionally, calibration curves were plotted to assess the accuracy of the nomogram model, which presented a good alignment between the nomogram prediction and the actual risk (Supplementary Figure 2).
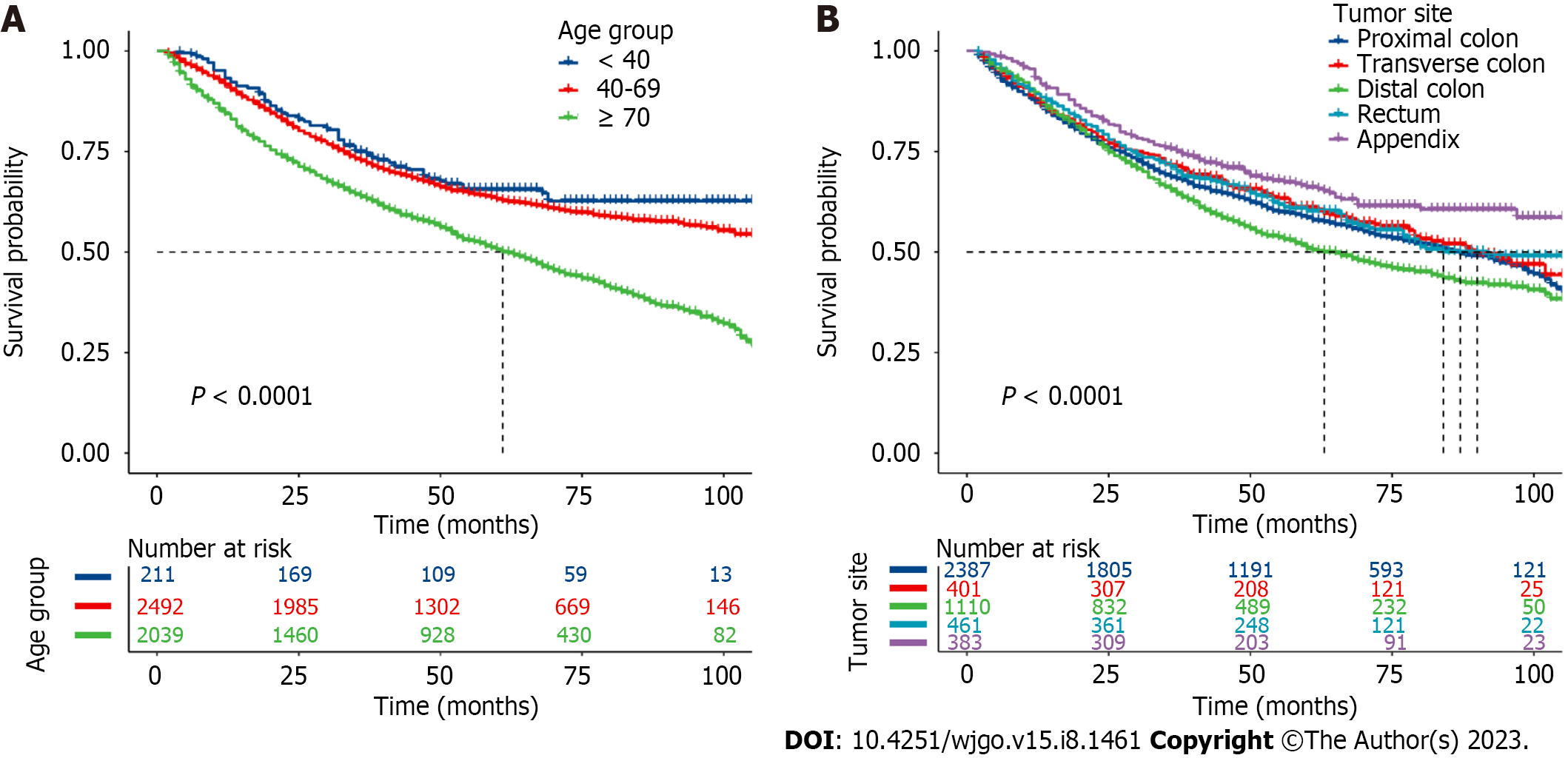
To estimate the survival probability of patients with different levels of those factors, Kaplan-Meier survival curves were plotted. Significant decreases were observed in patients aged ≥ 70 years (1-year, 3-year, and 5-year OS rates: 84.4%, 63.7%, and 50.5%, respectively, Figure 7A), with tumors located in the distal colon (1-year, 3-year, and 5-year OS rates: 89.3%, 65.4%, and 50.8%, respectively, Figure 7B), Black race (1-year, 3-year, and 5-year OS rates: 87.5%, 66.4%, and 54.8%, respectively, Supplementary Figure 3A), and T4 stage (1-year, 3-year, and 5-year OS rates: 81.8%, 51.7%, and 39.8%, respectively, Supplementary Figure 3B). Patients diagnosed with N2 and M1 stage also had a lower survival probability (Supplementary Figure 3C and D ).
By using a national and population-based dataset in this study, we evaluated the incidence trend of mucinous CRC and then constructed a nomogram to predict OS. We found that the age-adjusted incidence rate of mucinous colorectal adenocarcinoma decreased yearly from 2000 to 2018, both in male and female patients and among all age groups. However, a previous study reported that the incidence of mucinous CRC remained stable in earlier time periods, and others reported almost unchanged mucinous CRC rates in males but a slight increase in female incidence[3,9]. The incidence of overall CRC has declined in recent years, mainly because of the enhanced practices of CRC screening and colonoscopic polypectomy[10], which could probably explain the recent decrease in mucinous CRC rates. In addition, the proportion of MCs among all CRC cases also decreased over time from approximately 10% in 2000 to 5% in 2018.
While previous studies reported that MC was more commonly found in younger patients than nonmucinous CRC[9,11], our results indicated that in contrast to the upward trend in the incidence of overall early-onset CRC, the incidence of early-onset CRC with a mucinous histology decreased over the study period. However, a previous study stratifying early-onset CRC incidence by histologic subtype reported that the incidence of adenocarcinoma increased in most age subgroups, with the steepest subsite increase observed in rectal adenocarcinoma, and carcinoid rate increases outpaced adenocarcinoma rates in all age groups. Hence, early-onset CRC rate increases seemed to be driven by other adenocarcinoma subtypes and rectal carcinoid tumors in place of MC[12].
Mucinous CRC rates stratified by anatomic subsites showed a decreasing trend among all subgroups apart from the appendix. In agreement with previous studies, our results demonstrated that the incidence of appendiceal MC in the United States has been increasing over time, partly due to the improved detection and appreciation of the disease entity[13,14].
In the present study, the results demonstrated that MCs were more commonly found in the proximal colon (45.5%). It was reported that MCs were associated with higher mucin 2 expression and high-frequency microsatellite instability, which was partly due to mutations of genes in the RAS/MAPK pathway. Furthermore, mucin 2 and microsatellite instability were associated with proximal location[15,16]. In terms of tumor differentiation and the extent of disease, the largest proportion of MCs were moderately differentiated (55.5%) and were diagnosed at a regional stage (47.1%), similar to previous findings of MCs in the colon and rectum. Nevertheless, MCs in the appendix were more commonly well differentiated but most often diagnosed at a distant stage[17,18].
To date, the prognostic significance of MC remains elusive. Studies comparing the survival and prognosis of mucinous CRC and nonmucinous CRC have shown conflicting results. A few studies found that patients with mucinous CRC had a poorer prognosis than their nonmucinous counterparts[19,20], while others indicated that MC was not an independent prognostic factor[21]. Moreover, some research reported that the survival disadvantage of MC was observed only in specific anatomic subsites[22-24]. Accordingly, it seemed to portend that the prognosis of MC was associated with anatomic location.
During the multivariate Cox regression analysis, we found that the primary tumor site was associated with the OS of patients with mucinous CRC. Compared with MCs in the proximal colon, MCs in the appendix indicated improved survival (HR, 0.58). As a previous study reported, the survival rates of gastrointestinal MC varied by anatomic location, and the best OS was observed in the appendix. When stratified by stage and grade, MCs in the appendix had better OS than those in the colon and rectum, except for grade III/IV appendiceal MC[17]. Appendiceal MC is currently recognized as part of appendiceal mucinous neoplasms (AMNs), which are now divided into low-grade AMNs and high-grade AMNs. AMNs arise most often from low-grade AMNs and are relatively indolent in terms of biological behavior compared with CRC[25,26]. Given that tumor grade is an independent prognostic factor[25], the superior survival of AMNs compared with MCs in the colon and rectum was partly attributed to a greater proportion of well or moderately differentiated tumors.
In addition, our findings demonstrated that compared with MCs in the proximal colon, MCs in the distal colon had poorer prognosis (HR, 1.15), discordant with previous findings that tumor location was not associated with survival differences[20,27], whereas a recent study also found that a tumor site in the left-sided colon was associated with poorer prognosis (HR, 1.16)[28]. CRC is a molecularly and clinically heterogeneous disease, and a large part of its heterogeneity is driven by anatomic location. In particular, molecular and clinical disparities were observed between CRCs in the proximal colon and in the distal colon[29]. Further evaluation of the prognostic significance of tumor location entails studies that focus on the underlying mechanisms.
Other demographic characteristics like age and race were also associated with patient survival. Older age at diagnosis portended poor prognosis. During the univariate Cox analysis, advanced tumor grade and disease stage indicated inferior survival but were not significantly associated with OS in multivariate Cox analysis. Surgery as a protective factor for MC patients was the most valuable factor in the nomogram, underscoring the importance of surgical treatment for patients with mucinous CRC.
There were still some limitations in our study. First, this is a retrospective study having inherent selection bias. Second, although the SEER program covers a large population, some potential prognostic factors, such as Ki-67, are not included in the database. Another limitation pertains to the accuracy of data related to appendiceal MC because pathologic classification and grading systems for AMNs have undergone significant changes over time. Finally, the lack of an external validation cohort limits the generalizability and application of this nomogram. Future research studying the underlying mechanisms of those prognostic risk factors is warranted.
In this population-based study, we explored the epidemiologic characteristics of mucinous CRC and found that the incidence of mucinous CRC decreased in both young and older patients. When stratified by anatomic location, incidence rates declined in almost all anatomic subgroups except for MCs in the appendix. In addition, we predicted the 1-year, 3-year, and 5-year OS of patients with MCs by developing a nomogram that could integrate T, N, and M stage with other fundamental characteristics, such as age, race, and primary tumor site. The nomogram model was internally validated and showed good performance.
Mucinous adenocarcinoma (MC) is a distinct histologic subtype of colorectal cancer (CRC), which differs from nonmucinous CRC in terms of clinicopathological characteristics and genetic features.
There are limited data about epidemiologic characteristics of MC, and little is known about the risk factors associated with the survival rates.
In this study, we aimed to determine the epidemiologic and clinicopathological characteristics of mucinous CRC.
We conducted a population-based analysis of the epidemiologic and clinicopathological characteristics of mucinous CRC on the basis of data extracted from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program.
Rates of mucinous CRC decreased from 4.50/100000 in 2000 to 1.54/100000 in 2018. Rates of mucinous CRCs in patients aged ≤ 50 years decreased 2.27%/year during 2000-2018, in sharp contrast to the upward trend in early-onset CRC. Incidence of appendiceal MCs increased from 0.14/100000 in 2000 to 0.24/100000 in 2018. On multivariable Cox analyses, age, race, tumor site, T stage, N stage, M stage, surgery, and chemotherapy were associated with overall survival. A nomogram was developed based on these factors, and the area under the curve for 1-year, 3-year, and 5-year overall survival in the training cohort was 0.778, 0.778 and 0.768, respectively, and was 0.805, 0.779 and 0.760 in the validation cohort, respectively.
We found that the incidence of mucinous CRC decreased both in young and older patients. However, the incidence of appendiceal MCs continued to increase. During the survival analysis, in addition to the TNM tumor stages, we found that age, race, tumor site, surgery, and chemotherapy were also significantly associated with survival. A nomogram based on these factors as well as the T, N, and M stages showed a good predictive performance.
We laid a foundation for future research exploring the prognostic implications of mucinous colorectal adenocarcinoma.
The authors of this study have no contribution to SEER data collection. We would like to thank the SEER database for its open access.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jena MK, India; Sahin TT, Turkey S-Editor: Li L L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55854] [Article Influence: 7979.1] [Reference Citation Analysis (132)] |
| 2. | Bosman FT, Carneiro F, Hruban RH and Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer, 2010.. |
| 3. | Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 5. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 389] [Article Influence: 129.7] [Reference Citation Analysis (6)] |
| 6. | Greene FL, Sobin LH. The staging of cancer: a retrospective and prospective appraisal. CA Cancer J Clin. 2008;58:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, Luo F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw Open. 2021;4:e2124750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 8. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2397] [Article Influence: 239.7] [Reference Citation Analysis (0)] |
| 9. | Du W, Mah JT, Lee J, Sankila R, Sankaranarayanan R, Chia KS. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum. 2004;47:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 906] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 11. | Li J, Yang L, Bai F, Cai Y, Zhang J, Wu Z, Fu Y, Deng Y. Clinicopathological, molecular features and prognosis of colorectal cancer with mucinous component. Future Oncol. 2021;17:1351-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Montminy EM, Zhou M, Maniscalco L, Abualkhair W, Kim MK, Siegel RL, Wu XC, Itzkowitz SH, Karlitz JJ. Contributions of Adenocarcinoma and Carcinoid Tumors to Early-Onset Colorectal Cancer Incidence Rates in the United States. Ann Intern Med. 2021;174:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Xie GD, Liu YR, Jiang YZ, Shao ZM. Epidemiology and survival outcomes of mucinous adenocarcinomas: A SEER population-based study. Sci Rep. 2018;8:6117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Shaib WL, Goodman M, Chen Z, Kim S, Brutcher E, Bekaii-Saab T, El-Rayes BF. Incidence and Survival of Appendiceal Mucinous Neoplasms: A SEER Analysis. Am J Clin Oncol. 2017;40:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Reynolds IS, Furney SJ, Kay EW, McNamara DA, Prehn JHM, Burke JP. Meta-analysis of the molecular associations of mucinous colorectal cancer. Br J Surg. 2019;106:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 17. | Zong Z, Luo Y, Ying H, Wang A, Li H, Yi C. Trends of incidence and survival in patients with gastrointestinal mucinous adenocarcinoma. Oncol Lett. 2018;16:5791-5798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Benesch MGK, Mathieson A. Epidemiology of Mucinous Adenocarcinomas. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Li ZP, Liu XY, Kao XM, Chen YT, Han SQ, Huang MX, Liu C, Tang XY, Chen YY, Xiang D, Huang YD, Lei ZJ, Chu XY. Clinicopathological characteristics and prognosis of colorectal mucinous adenocarcinoma and nonmucinous adenocarcinoma: a surveillance, epidemiology, and end results (SEER) population-based study. Ann Transl Med. 2020;8:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Warschkow R, Tarantino I, Huttner FJ, Schmied BM, Guller U, Diener MK, Ulrich A. Predictive value of mucinous histology in colon cancer: a population-based, propensity score matched analysis. Br J Cancer. 2016;114:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Lan YT, Chang SC, Lin PC, Lin CC, Lin HH, Huang SC, Lin CH, Liang WY, Chen WS, Jiang JK, Lin JK, Yang SH. Clinicopathological and Molecular Features of Colorectal Cancer Patients With Mucinous and Non-Mucinous Adenocarcinoma. Front Oncol. 2021;11:620146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Hugen N, Verhoeven RH, Radema SA, de Hingh IH, Pruijt JF, Nagtegaal ID, Lemmens VE, de Wilt JH. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol. 2013;24:2819-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Patel C, Behring M, Al Diffalha S, Dhall D, Lee G, Shanmugam C, Grizzle WE, Manne U. Immunophenotypic profiles and prognosis for colorectal mucinous adenocarcinomas are dependent on anatomic location. Cancer Med. 2023;12:9637-9643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ahadi M, Sokolova A, Brown I, Chou A, Gill AJ. The 2019 World Health Organization Classification of appendiceal, colorectal and anal canal tumours: an update and critical assessment. Pathology. 2021;53:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 26. | Shaib WL, Assi R, Shamseddine A, Alese OB, Staley C 3rd, Memis B, Adsay V, Bekaii-Saab T, El-Rayes BF. Appendiceal Mucinous Neoplasms: Diagnosis and Management. Oncologist. 2017;22:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (3)] |
| 27. | Ott C, Gerken M, Hirsch D, Fest P, Fichtner-Feigl S, Munker S, Schnoy E, Stroszczynski C, Vogelhuber M, Herr W, Evert M, Reng M, Schlitt HJ, Klinkhammer-Schalke M, Teufel A. Advanced Mucinous Colorectal Cancer: Epidemiology, Prognosis and Efficacy of Chemotherapeutic Treatment. Digestion. 2018;98:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Wu Q, Zhang S, Wang H, Zeng Y, Yang W, Pan W, Hong G, Gao W. A prognostic nomogram for predicting overall survival in colorectal mucinous adenocarcinoma patients based on the SEER database. Biomol Biomed. 2023;23:517-526. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, Roth AD, Klingbiel D, Bosman FT, Delorenzi M, Tejpar S. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 494] [Article Influence: 44.9] [Reference Citation Analysis (0)] |