Published online Aug 15, 2023. doi: 10.4251/wjgo.v15.i8.1436
Peer-review started: March 25, 2023
First decision: May 22, 2023
Revised: June 3, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: August 15, 2023
Processing time: 138 Days and 4.6 Hours
Gallbladder mucinous adenocarcinoma (GBMAC) is a rare subtype of gallbladder adenocarcinoma (GBAC), with limited knowledge of its survival outcomes from small case series and single-center retrospective analysis.
To compare the clinicopathological characteristics of GBMAC with typical GBAC and its prognostic factors to gain insights into this field.
This study was conducted using data from the Surveillance, Epidemiology, and End Results database, including cases of GBMAC and typical GBAC diagnosed from 2010 to 2017. The Pearson chi-square test or Fisher exact test was used to examine the differences in clinicopathological features between these two cohorts. In addition, propensity score matching (PSM) analysis was performed to balance the selection biases. Univariate and multivariate Cox hazards regression analyses were performed to determine independent prognostic factors for cancer-specific survival (CSS) and overall survival (OS). The Kaplan–Meier curves and log-rank tests were used to assess the OS and CSS of GBMAC and typical GBAC patients.
The clinicopathological and demographic characteristics of GBMAC were different from typical GBAC. They included a larger proportion of patients with unmarried status, advanced American Joint Committee on Cancer (AJCC) stage, higher T stage, higher N1 stage rate and lower N0 and N2 stage rates (P < 0.05). Multivariate analyses demonstrated that surgery [OS: Hazard ratio (HR) = 2.27, P = 0.0037; CSS: HR = 2.05, P = 0.0151], chemotherapy (OS: HR = 6.41, P < 0.001; CSS: HR = 5.24, P < 0.001) and advanced AJCC stage (OS: Stage IV: HR = 28.99, P = 0.0046; CSS: Stage III: HR = 12.31, P = 0.015; stage IV: HR = 32.69, P = 0.0015) were independent prognostic indicators for OS and CSS of GBMAC patients. Furthermore, after PSM analysis, there was no significant difference between GBMAC and matched typical GBAC patients regarding OS (P = 0.82) and CSS (P = 0.69).
The biological behaviors of GBMAC are aggressive and significantly different from that of typical GBAC. However, they show similar survival prognoses. Surgery, chemotherapy, and lower AJCC stage were associated with better survival outcomes. Further research is needed in the future to verify these results.
Core Tip: Gallbladder mucinous adenocarcinoma (GBMAC) is a rare subtype of gallbladder adenocarcinoma (GBAC), with limited knowledge of its survival outcomes. Based on a large database, we compared the clinicopathological characteristics of GBMAC with typical GBAC and identified prognostic factors for GBMAC. The results showed that the biological behaviors of GBMAC are significantly different from typical GBAC, while survival outcomes for GBMAC patients were not worse than those for typical GBAC patients. Surgery, chemotherapy, and lower American Joint Committee on Cancer stage were associated with better survival outcomes.
- Citation: Yang WW, Fang YT, Niu YR, Sun YK. Comparison of clinicopathological characteristics and survival outcomes between gallbladder mucinous adenocarcinoma and gallbladder adenocarcinoma: A propensity score-matched study. World J Gastrointest Oncol 2023; 15(8): 1436-1450
- URL: https://www.wjgnet.com/1948-5204/full/v15/i8/1436.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i8.1436
Although gallbladder carcinoma (GBC) is rare, only accounting for 0.6% of all cancers worldwide in 2020[1], it is the most common biliary tract cancer (BTC) worldwide and the sixth most common gastrointestinal tumor[2]. GBC is widely regarded as a highly aggressive malignancy with a poor overall 5-year survival rate of less than 5% and median overall survival (OS) of only six months[3,4]. Histologically, the majority of GBCs are adenocarcinomas, accounting for approximately 85%-90% of all cases, and other unusual subtypes include squamous, adenosquamous, adenoacanthomas, and undifferentiated carcinomas[5].
Lack of specific symptoms and less frequent clinical suspicion leads to difficulty in improving the outcomes of GBC. Surgical resection is the only potentially curative treatment for GBC. However, most GBCs are diagnosed at an advanced stage, rendering them ineligible for surgery. Even after surgery, the prognosis of GBC patients remains dismal due to a significant recurrence rate[6,7]. Although several novel prognostic factors have been identified in recent years, the prognosis of patients with gallbladder cancer is still poor[8,9]. Furthermore, the biological features, clinical manifestations, and prognoses are obviously different in various histological subtypes of GBC.
Gallbladder mucinous adenocarcinoma (MAC) (GBMAC) is an uncommon subtype of gallbladder adenocarcinoma (GBAC), accounting for 5%-10.8% of reported series[10,11]. GBMAC is histologically defined as adenocarcinoma with a volume of extracellular mucin accumulation above 50% of the tumor[12]. The abnormal amounts of mucin expression, caused by the deregulation of mucin core protein expression in adenocarcinoma, disrupts cell-cell interactions, thereby promoting cell plasticity and anchorage-independent growth, which contributes to tumor invasion and invasion metastasis[13-15]. Therefore, GBMAC has unique clinicopathological characteristics and prognosis.
Due to the rarity of GBMAC, there are few large randomized clinical studies of GBMAC, and characterization of clinicopathological features, prognosis, and clinical risk factors have been limited to individual case reports or small retrospective series[16-18]. With limited understanding, clinical practices in typical GBAC are also applied to GBMAC. However, GBMAC has distinct histologic, clinical, and molecular features, thus making a differential approach necessary. The clinical prognosis of GBMAC compared to typical GBAC remains unknown. Several studies reported that GBMAC possesses a more aggressive behavior and worse prognosis than typical GBAC[18]. The Surveillance, Epidemiology, and End Results (SEER) database is the largest cancer dataset established by the National Cancer Institute, covering about one-third of the United States' total population, providing cancer incidence and survival data to explore rare tumors[19]. Therefore, in this study, we performed a retrospective analysis to investigate clinicopathologic characteristics, prognostic factors, and treatment outcomes for GBMAC by comparing GBMAC patients and typical GBAC patients using data from the SEER database.
This retrospective study was conducted using data from the SEER database, collected from 18 established cancer registries covering about a third of the United States population[20]. This database contains cancer patients' demographic, clinicopathological, and survival data. The database used in this research was named Incidence-SEER Research Plus Data, 18 Registries, November 2020 Sub (2000-2018). SEER*Stat Software (www.seer.cancer.gov, version 8.4.0) was used to extract data on gallbladder cancer patients from the SEER database. This study is in accordance with all relevant ethical standards, the 1964 Helsinki Declaration, and its later amendments. The SEER database is a large public-use database, and the data released by the SEER database do not require institutional approval or informed patient consent.
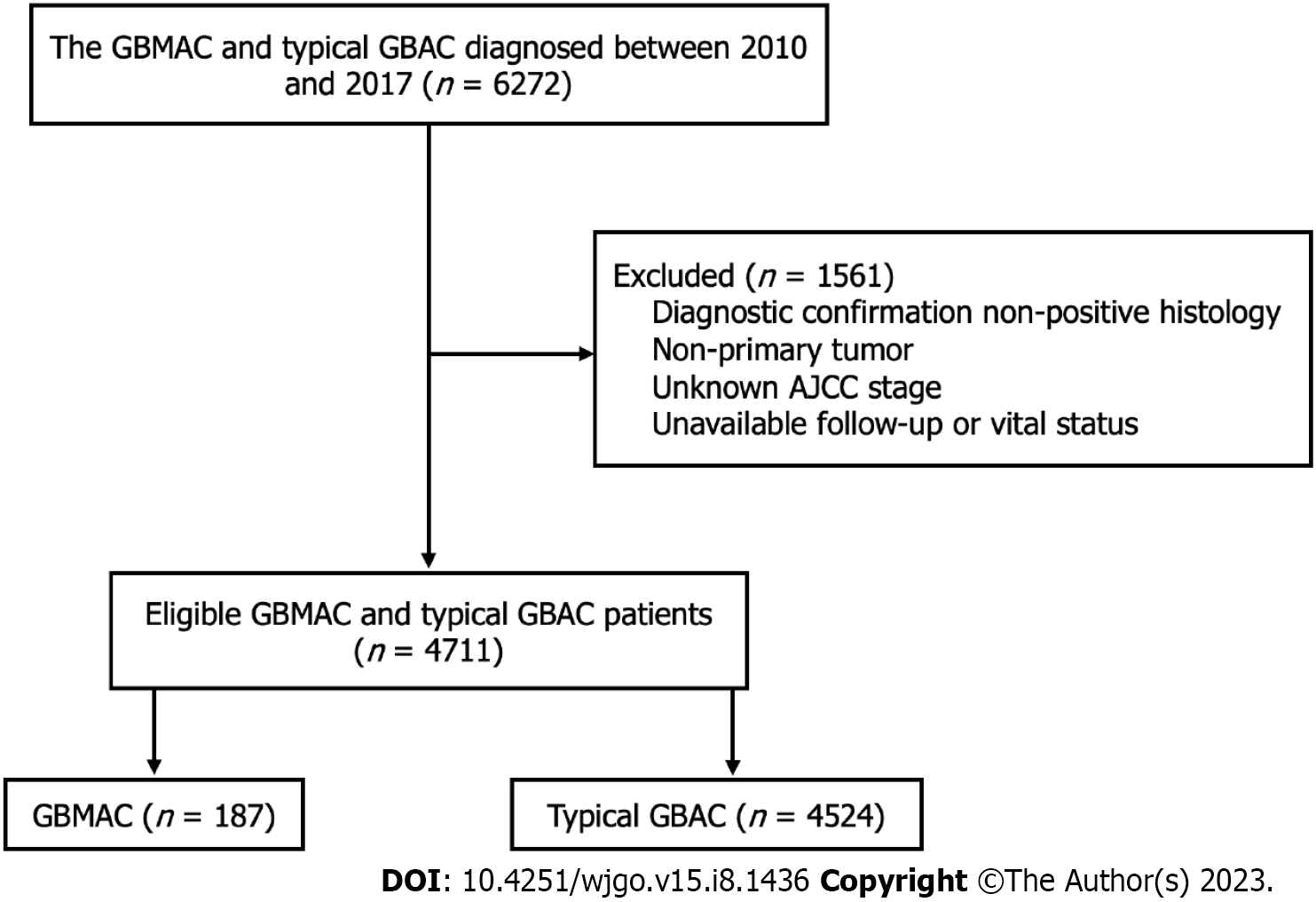
Cases of GBMAC and typical GBAC diagnosed between January 2010 and December 2017 were included in this study. The 3rd edition International Classification of Disease for Oncology (ICD-O-3) criteria was used to identify patients with GBMAC (ICD-O-3 codes: 8480/3 and 8481/3) and typical GBAC (ICD-O-3 code: 8140/3 and 8144/3). We carefully screened these patients for analysis. We only included the patients with these tumor sequence numbers labeled "one primary only" and histologic confirmation from biopsy or surgical pathology. Patients with no vital status information and unknown American Joint Committee on Cancer (AJCC) stage were excluded. Finally, 187 GBMAC patients and 4524 typical GBAC patients were included in the study. The flowchart of the patient selection process is summarized in Figure 1.
The following variables were extracted: Age at diagnosis, sex, race, marital status, histological grade, surgery status, chemotherapy status, radiotherapy status, AJCC stage, T stage, N stage, M stage, survival months, cause of death, and vital status. The 7th edition AJCC staging system was applied. The variable age was dichotomized as < 60 and ≥ 60.
As mentioned above, to analyze the prognostic factors of GBMAC, the clinicopathological features and OS of these GBMAC patients were compared with a large cohort of typical GBAC patients. The baseline characteristics of the GBMAC and typical GBAC cohort were displayed by the number and the percentage (n, %). The Pearson chi-square test or Fisher exact test was used to examine the differences between these two cohorts. In addition, a propensity score matching (PSM) analysis was performed to balance the differences and biases between the GBMAC and typical GBAC groups. The PSM model was based on race, marital status, radiotherapy status, and AJCC stage. The baseline characteristics of the GBMAC and typical GBAC were also determined in the matched data. Cancer-specific survival (CSS) was defined as the duration from the date of diagnosis until death due to cancer. Cox proportional hazards models were used to analyze associations of different variables with OS and CSS. Only variables significantly associated with survival in the univariate Cox analysis were included in the multivariate Cox analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and the univariate and multivariate Cox analyses were applied to the whole and matched data set. Moreover, the Kaplan-Meier method was used to establish the survival curves, and the log-rank test was used to assess any significant differences in OS and CSS stratified by histology before and after PSM. The Kaplan-Meier method and log-rank test were also applied in the univariable survival analysis to identify statistically significant covariates associated with CSS. Statistical analyses were performed using the R software (version 4.1.2). A two-sided P value less than 0.05 was considered statistically significant.
A total of 4711 patients were identified from the SEER database based on the inclusion and exclusion criteria mentioned. Among these included patients, 187 (3.97%) were GBMAC patients, and 4524 (96.03%) were typical GBAC patients. In our study, most of the basic demographic characteristics, including age, race, and sex, of GBMAC patients were not significantly different from those of typical GBAC patients. At the same time, marital status showed a notable difference between the two cohorts (P = 0.024). The GBMAC cohort included a larger proportion of patients with unmarried status. Compared with typical GBAC, the pathological subtype of GBMAC was significantly associated with advanced AJCC stage (P < 0.05), higher T stage (P < 0.05), and higher N1 stage rate and lower N0 and N2 stage rates (P < 0.05). Patients with AJCC stage III and IV tumors accounted for a larger proportion of GBMAC patients than typical GBAC patients (84% vs 73.5%, P < 0.05). T3-4 stage accounted for 56.1% of the GBMAC cohort while only making up 45.3% of the typical GBAC cohort. Moreover, N1 stage was found in 34.8% of the GBMAC patients, while N1 stage accounted for only 25.2% of patients in the typical GBAC cohort.
After PSM analysis, there were 374 patients in the propensity score-matched cohort, with no significant differences in the characteristics between these two groups. The detailed demographic and clinical characteristics of the two cohorts within the whole dataset and PSM dataset are presented in Table 1.
| Characteristic | Unmatched cohort (n = 4711) | Propensity score-matched cohort (n = 374) | ||||
| GBMAC (n = 187) | Typical GBAC (n = 4524) | P value | GBMAC (n = 187) | Typical GBAC (n = 187) | P value | |
| Age (yr) | 0.955 | 0.373 | ||||
| < 60 | 43 (23.0) | 1061 (23.5) | 43 (23.0) | 35 (18.7) | ||
| ≥ 60 | 144 (77.0) | 3463 (76.5) | 144 (77.0) | 152 (81.3) | ||
| Sex | 0.638 | 0.064 | ||||
| Female | 127 (67.9) | 3158 (69.8) | 127 (67.9) | 144 (77.0) | ||
| Male | 60 (32.1) | 1366 (30.2) | 60 (32.1) | 43 (23.0) | ||
| Race | 0.257 | 0.467 | ||||
| White | 133 (71.1) | 3373 (74.6) | 133 (71.1) | 128 (68.4) | ||
| Black | 34 (18.2) | 610 (13.5) | 34 (18.2) | 39 (20.9) | ||
| Others | 20 (10.7) | 523 (11.6) | 20 (10.7) | 18 (9.6) | ||
| Unknown | 0 (0.0) | 18 (0.4) | 0 (0.0) | 2 (1.1) | ||
| Marital status | 0.024 | 0.758 | ||||
| Married | 78 (41.7) | 2239 (49.5) | 78 (41.7) | 71 (38.0) | ||
| Unmarried | 105 (56.1) | 2101 (46.4) | 105 (56.1) | 112 (59.9) | ||
| Unknown | 4 (2.1) | 184 (4.1) | 4 (2.1) | 4 (2.1) | ||
| Grade | 0.567 | 0.613 | ||||
| Well differentiated | 24 (12.8) | 462 (10.2) | 24 (12.8) | 18 (9.6) | ||
| Moderately differentiated | 60 (32.1) | 1465 (32.4) | 60 (32.1) | 56 (29.9) | ||
| Poorly differentiated | 48 (25.7) | 1285 (28.4) | 48 (25.7) | 48 (25.7) | ||
| Undifferentiated | 0 (0.0) | 29 (0.6) | 0 (0.0) | 1 (0.5) | ||
| Unknown | 55 (29.4) | 1283 (28.4) | 55 (29.4) | 64 (34.2) | ||
| Surgery | 0.885 | 0.107 | ||||
| Yes | 127 (67.9) | 3000 (66.3) | 127 (67.9) | 111 (59.4) | ||
| No | 60 (32.1) | 1523 (33.7) | 60 (32.1) | 76 (40.6) | ||
| Unknown | 0 (0.0) | 1 (0.0) | ||||
| Chemotherapy | 0.369 | 1 | ||||
| Yes | 90 (48.1) | 2014 (44.5) | 90 (48.1) | 90 (48.1) | ||
| No/Unknown | 97 (51.9) | 2510 (55.5) | 97 (51.9) | 97 (51.9) | ||
| Radiotherapy | 0.211 | 1 | ||||
| Yes | 32 (17.1) | 616 (13.6) | 32 (17.1) | 32 (17.1) | ||
| No/Unknown | 155 (82.9) | 3908 (86.4) | 155 (82.9) | 155 (82.9) | ||
| AJCC | 0.005 | 0.226 | ||||
| I | 4 (2.1) | 335 (7.4) | 4 (2.1) | 11 (5.9) | ||
| II | 26 (13.9) | 867 (19.2) | 26 (13.9) | 19 (10.2) | ||
| III | 62 (33.2) | 1215 (26.9) | 62 (33.2) | 62 (33.2) | ||
| IV | 95 (50.8) | 2107 (46.6) | 95 (50.8) | 95 (50.8) | ||
| T stage | 0.006 | 0.435 | ||||
| T1 | 10 (5.3) | 507 (11.2) | 10 (5.3) | 19 (10.2) | ||
| T2 | 50 (26.7) | 1394 (30.8) | 50 (26.7) | 42 (22.5) | ||
| T3 | 87 (46.5) | 1808 (40.0) | 87 (46.5) | 88 (47.1) | ||
| T4 | 18 (9.6) | 242 (5.3) | 18 (9.6) | 15 (8.0) | ||
| Unknown | 22 (11.8) | 573 (12.7) | 22 (11.8) | 23 (12.3) | ||
| N stage | 0.013 | 0.312 | ||||
| N0 | 92 (49.2) | 2673 (59.1) | 92 (49.2) | 107 (57.2) | ||
| N1 | 65 (34.8) | 1138 (25.2) | 65 (34.8) | 49 (26.2) | ||
| N2 | 11 (5.9) | 336 (7.4) | 11 (5.9) | 13 (7.0) | ||
| Unknown | 19 (10.2) | 377 (8.3) | 19 (10.2) | 18 (9.6) | ||
| M stage | 0.668 | 1 | ||||
| M0 | 102 (54.5) | 2613 (57.8) | 102 (54.5) | 102 (54.5) | ||
| M1 | 85 (45.5) | 1910 (42.2) | 85 (45.5) | 85 (45.5) | ||
| Unknown | 0 (0.0) | 1 (0.0) | ||||
Univariate analyses for OS of GBMAC indicated that age at diagnosis, sex, race, and marital status were not significantly correlated with poor prognosis in GBMAC. However, poorly differentiated tumor (HR = 1.84, 95%CI: 1.03-3.27, P = 0.039), AJCC stage IV (HR = 9.94, 95%CI: 1.38-71.58, P = 0.023), T4 stage (HR = 2.59, 95%CI: 1.08-6.23, P = 0.033), and N2 stage (HR = 2.17, 95%CI: 1.14-4.12, P = 0.018) were associated with a poor prognosis in GBMAC. The results also showed that surgery, chemotherapy, and radiation could help GBMAC patients obtain a better prognosis in terms of OS (all P < 0.05). In multivariate analysis, only surgery (HR = 2.27, 95%CI: 1.31-3.96, P = 0.0037), chemotherapy (HR = 6.41, 95%CI: 4.07-10.09, P < 0.001), and AJCC stage IV (HR = 28.99, 95%CI: 2.83-297.29, P = 0.0046) were independent risk factors of OS.
Similar results were observed in multivariate analysis of CSS. In the univariate analyses of CSS, surgery, chemo
| Characteristic | Overall survival | Cancer-specific survival | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | ||||||||
| < 60 | Reference | Reference | Reference | Reference | ||||
| ≥ 60 | 1.4 (0.96-2.03) | 0.08 | NA | NA | 1.46 (0.98-2.18) | 0.063 | NA | NA |
| Sex | ||||||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 0.92 (0.66-1.29) | 0.632 | NA | NA | 1.01 (0.71-1.44) | 0.943 | NA | NA |
| Race | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Black | 1.18 (0.79-1.75) | 0.423 | NA | NA | 1.23 (0.81-1.87) | 0.322 | NA | NA |
| Others | 1.24 (0.74-2.07) | 0.41 | NA | NA | 1.22 (0.71-2.11) | 0.468 | NA | NA |
| Marital status | ||||||||
| Married | Reference | Reference | Reference | Reference | ||||
| Unmarried | 1.02 (0.74-1.4) | 0.914 | NA | NA | 1 (0.71-1.4) | 0.998 | NA | NA |
| Unknown | 1.16 (0.42-3.18) | 0.78 | NA | NA | 1.25 (0.45-3.44) | 0.671 | NA | NA |
| Histological grade | ||||||||
| Well differentiated | Reference | Reference | Reference | Reference | ||||
| Moderately differentiated | 1.51 (0.86-2.64) | 0.152 | 1.27 (0.7-2.3) | 0.4291 | 1.26 (0.71-2.23) | 0.434 | 1.01 (0.56-1.84) | 0.9612 |
| Poorly differentiated | 1.84 (1.03-3.27) | 0.039 | 1.45 (0.78-2.68) | 0.2354 | 1.64 (0.91-2.95) | 0.097 | 1.18 (0.64-2.18) | 0.5956 |
| Unknown | 2.14 (1.21-3.76) | 0.008 | 1.09 (0.54-2.21) | 0.8144 | 1.93 (1.09-3.41) | 0.025 | 0.61 (0.3-1.22) | 0.1601 |
| Surgery | ||||||||
| Yes | Reference | Reference | Reference | Reference | ||||
| No | 2.37 (1.7-3.32) | < 0.001 | 2.27 (1.31-3.96) | 0.0037 | 2.43 (1.71-3.45) | < 0.001 | 2.05 (1.15-3.66) | 0.0151 |
| Chemotherapy | ||||||||
| Yes | Reference | Reference | Reference | Reference | ||||
| No/Unknown | 1.94 (1.42-2.65) | < 0.001 | 6.41 (4.07-10.09) | < 0.001 | 1.84 (1.33-2.57) | < 0.001 | 5.24 (3.44-8) | < 0.001 |
| Radiotherapy | ||||||||
| Yes | Reference | Reference | Reference | Reference | ||||
| No/Unknown | 1.57 (1.04-2.38) | 0.033 | 0.72 (0.42-1.22) | 0.2204 | 1.35 (0.89-2.06) | 0.162 | NA | NA |
| AJCC | ||||||||
| I | Reference | Reference | Reference | Reference | ||||
| II | 2.85 (0.38-21.47) | 0.311 | 2.67 (0.27-26.23) | 0.3996 | 1.91 (0.25-14.82) | 0.535 | 2.73 (0.35-21.46) | 0.3393 |
| III | 5.28 (0.73-38.24) | 0.1 | 7.51 (0.84-66.94) | 0.0707 | 4.61 (0.64-33.5) | 0.131 | 12.31 (1.63-93.01) | 0.015 |
| IV | 9.94 (1.38-71.58) | 0.023 | 28.99 (2.83-297.29) | 0.0046 | 8.8 (1.22-63.37) | 0.031 | 32.69 (3.81-280.34) | 0.0015 |
| T stage | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 0.87 (0.38-1.95) | 0.727 | 1.32 (0.47-3.72) | 0.5965 | 0.65 (0.28-1.5) | 0.315 | NA | NA |
| T3 | 2.16 (0.99-4.69) | 0.052 | 1.9 (0.76-4.75) | 0.1676 | 1.88 (0.86-4.1) | 0.112 | NA | NA |
| T4 | 2.59 (1.08-6.23) | 0.033 | 1.05 (0.37-2.95) | 0.9314 | 2.34 (0.97-5.66) | 0.06 | NA | NA |
| Unknown | 2.08 (0.87-4.96) | 0.097 | 0.6 (0.22-1.65) | 0.3196 | 2.05 (0.86-4.87) | 0.106 | NA | NA |
| N stage | ||||||||
| N0 | Reference | Reference | Reference | Reference | ||||
| N1 | 1.08 (0.76-1.52) | 0.679 | 1.1 (0.73-1.68) | 0.6426 | 1.14 (0.79-1.64) | 0.494 | 1.09 (0.72-1.64) | 0.6778 |
| N2 | 2.17 (1.14-4.12) | 0.018 | 0.72 (0.34-1.51) | 0.3848 | 2.19 (1.11-4.3) | 0.023 | 0.82 (0.39-1.7) | 0.5865 |
| Unknown | 1.79 (1.06-3.05) | 0.031 | 1.43 (0.74-2.76) | 0.2833 | 2.08 (1.21-3.56) | 0.008 | 1.18 (0.63-2.19) | 0.6084 |
| M stage | ||||||||
| M0 | Reference | Reference | Reference | Reference | ||||
| M1 | 2.17 (1.58-2.98) | < 0.001 | 0.85 (0.37-1.95) | 0.705 | 2.25 (1.61-3.14) | < 0.001 | 0.94 (0.46-1.91) | 0.8572 |
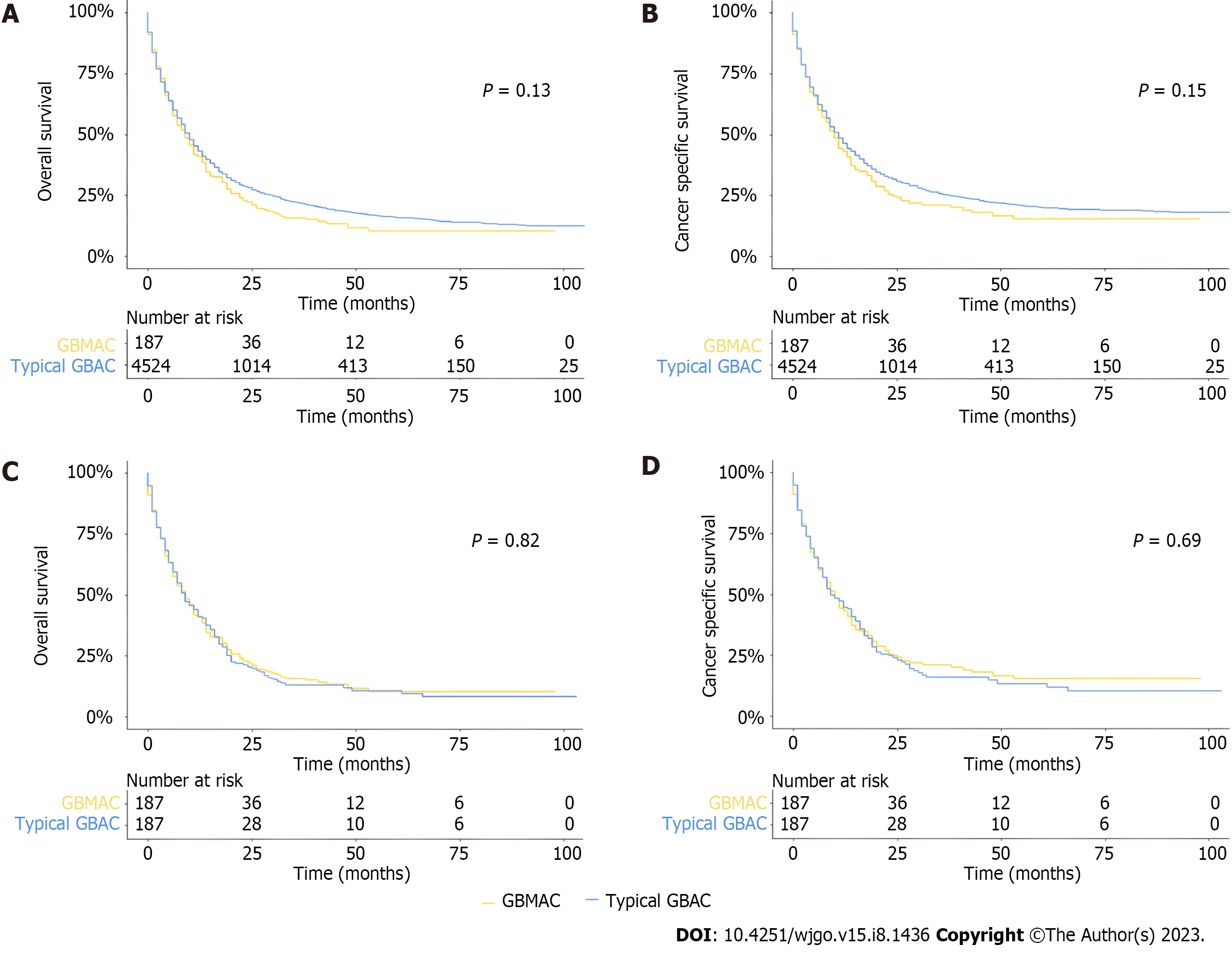
Kaplan-Meier curves and log-rank analysis were established to show and compare the survival prognoses of different groups in the whole cohort. As for OS, there was no significant difference between GBMAC and typical GBAC patients in the whole cohort, and there was no significant difference between the two cohorts in terms of CSS (Figure 2A and B). In the PSM cohort, the log-rank test revealed that patients with GBMAC also had similar OS (P = 0.82) and CSS (P = 0.69) to matched typical GBAC patients (Figure 2C and D). In the PSM analysis, several baseline factors were matched except for age, sex, surgery, chemotherapy, histological grade, T stage, N stage, and M stage. Therefore, we made some extra adjustments to analyze these baseline factors. After different adjustments, the GBMAC group did not show significantly higher risks of overall mortality and cancer-specific mortality than the typical GBAC group (Table 3).
| Outcomes | GBMAC, HR (95%CI) | P value |
| Cancer-specific mortality | ||
| Non-adjusted | 1.13 (0.96-1.34) | 0.15 |
| PSM non-adjusted | 0.96 (0.76-1.2) | 0.7 |
| PSM adjusted | 1.00 (0.79-1.28) | 0.98 |
| Adjust I | 1.11 (0.94-1.31) | 0.23 |
| Adjust II | 0.93 (0.78-1.10) | 0.39 |
| Adjust III | 1.14 (0.97-1.35) | 0.12 |
| Adjust IV | 1.01 (0.85-1.20) | 0.89 |
| Overall mortality | ||
| Non-adjusted | 1.13 (0.96-1.33) | 0.13 |
| PSM non-adjusted | 0.98 (0.78-1.22) | 0.83 |
| PSM adjusted | 1.02 (0.80-1.28) | 0.9 |
| Adjust I | 1.10 (0.94-1.30) | 0.22 |
| Adjust II | 0.94 (0.80-1.10) | 0.44 |
| Adjust III | 1.15 (0.98-1.35) | 0.08 |
| Adjust IV | 1.02 (0.87-1.20) | 0.78 |
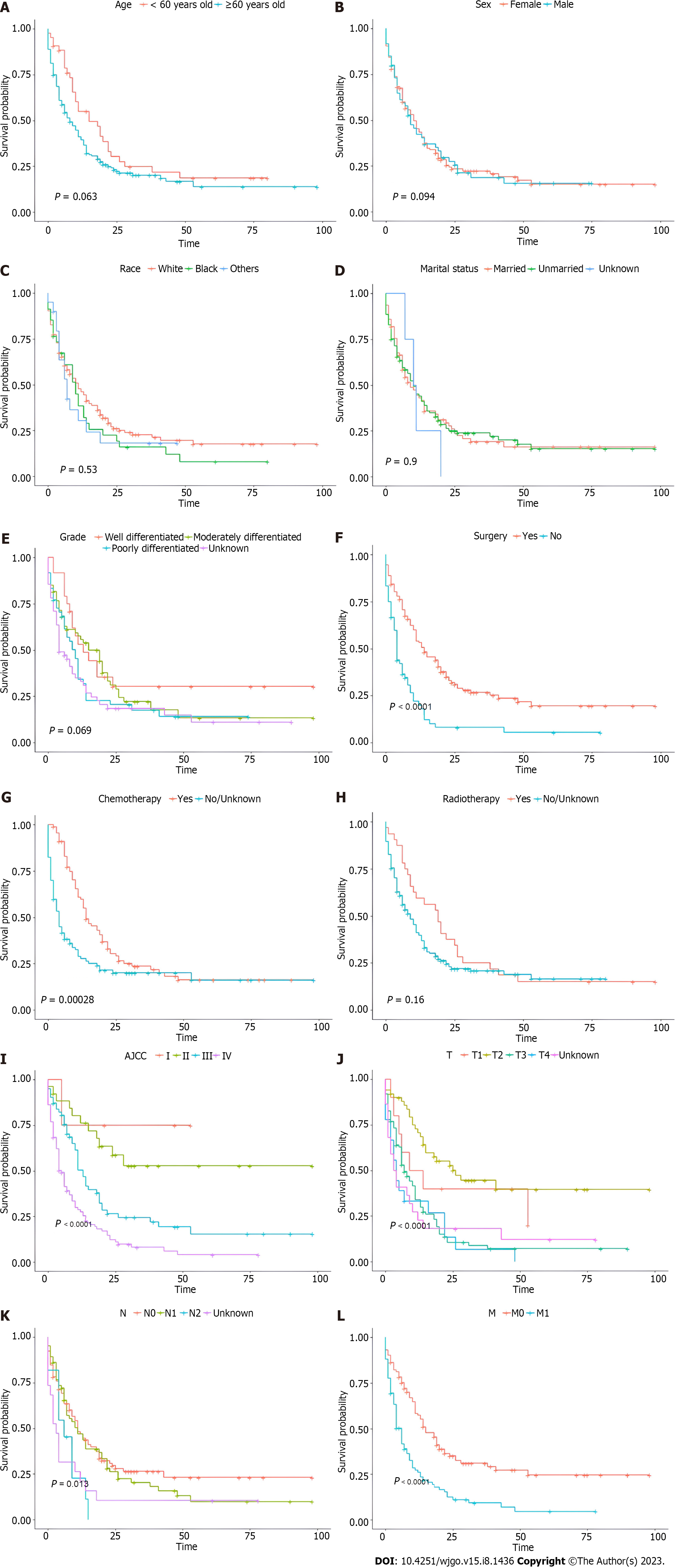
Among the GBMAC cohort, the Kaplan-Meier survival curves and log-rank test were also used to show how CSS changed with age at diagnosis (Figure 3A), sex (Figure 3B), race (Figure 3C), marital status (Figure 3D), histological grade (Figure 3E), surgery (Figure 3F), chemotherapy (Figure 3G), radiotherapy (Figure 3H), AJCC stage (Figure 3I), T stage (Figure 3J), N stage (Figure 3K), and M stage (Figure 3L). As for treatment, GBMAC patients who underwent surgery and chemotherapy achieved superior prognosis in terms of CSS (P < 0.05), while radiotherapy status did not predict the prognosis of these patients (Figure 3F-H). According to Figure 3I-L, AJCC stage, T stage, N stage, and M stage, the most traditional predictive prognosis model for gallbladder tumors, could reasonably predict the CSS (P < 0.05). A significantly poorer prognosis was detected in GBMAC patients with higher AJCC, T, N, and M stages.
MAC is extremely rare, accounting for only 1% of all adenocarcinomas, and its prognostic value is still uncertain[21]. Our understanding of MAC is mainly based on colorectal MAC and breast MAC, the two most common sites for MAC. According to several previous studies, colon MAC did not show a prognostic difference compared to conventional adenocarcinomas in colon cancer[21-24]. However, MAC of the rectum has a significantly worse prognosis than conventional rectal adenocarcinoma[25]. On the contrary, mucinous breast cancer is associated with an improved survival outcome compared with other subtypes of breast cancer[26]. GBMAC is relatively uncommon in gallbladder cancer. Some published studies suggested that extracellular mucin could activate or suppress cellular pathways conferring specific biologic properties to these tumor cells, and consequently, GBMAC exhibits aggressive clinical behaviors[18,27].
Due to the infrequency of GBMAC, only a few studies explored its clinical characteristics and prognoses. As mentioned before, the SEER database, as a large database, could provide a comprehensive and large sample size cohort of cancer patients. To the best of our knowledge, there is still no study systematically analyzing the clinical characteristics, survival outcomes, and prognostic predictors of GBMAC based on the SEER database. Only two specific analyses of GBMAC exist with an extremely small number of recruiting patients[16,18]. Our research took advantage of the large data set from the SEER database to explore the clinicopathological characteristics and prognostic factors for GBMAC, representing the first and the largest comparative analysis of GBMAC to date.
In the present study, we compared the clinicopathological characteristics of GBMAC with typical GBAC. Patients with GBMAC tend to present with unmarried status, advanced clinical stage, higher T stage, higher N1 stage rate, and lower N0 and N2 stage rates. Consistently, the same tendency was reported in previous studies. A study published in 2012 showed that GBMACs were significantly correlated with advanced stage and a larger size at the time of diagnosis[18]. Another study by Zou et al[16] indicated that GBMACs were associated with poor histological differentiation, higher CA19-9 level, larger tumor size, and higher lymphovascular and perineural invasion frequency, which directly affect the clinical stage of GBMACs[16]. Based on these investigations, we surmised that this phenomenon might be related to the aggressive biological features of MACs. Based on our study, unmarried marital status is also a significant determinant of GBMAC, and the majority of unmarried patients were female. We also hypothesize that there may be a correlation between GBMAC and estrogen exposure. However, no study has explored the relationship between GBMAC and estrogen exposure. Moreover, according to the SEER database, we cannot know if those unmarried women were nulliparous. Therefore, more research is needed to verify this speculation.
Like other subtypes of gallbladder cancer, patients with GBMAC had a poor prognosis. Therefore, the prognostic factors for survival should be seriously considered in making optimal treatment decisions. Based on the high-quality and large-sample data from the SEER database, we identified three independent prognostic predictors for GBMAC, including surgery status, chemotherapy status, and AJCC stage in terms of OS. Regarding CSS, the same prognostic factors for GBMAC were identified.
Currently, in terms of treatment for GBMAC, there is no guideline for the treatment of GBMAC due to the rarity of the histological type of tumor and lack of clinical trials. The therapy strategies for GBMAC referred to most gallbladder cancers, including surgery, radiotherapy, chemotherapy, targeted therapy, and even immunotherapy. Surgical resection remains the mainstay of treatment for GBCs, and radical resection with an R0 margin is considered the only curative treatment for GBCs[28]. In our study, as expected, surgery was the most commonly used therapy for patients with GBMAC, and Kaplan-Meier curves and log-rank tests indicated that surgery significantly increased CSS. In addition, from the univariate and multivariate Cox proportional hazards analyses, our study demonstrated that receiving surgery was an independent predictor of better prognosis in terms of CSS and OS. Therefore, surgical intervention should be taken if conditions permit in patients with GBMAC.
According to the National Comprehensive Cancer Network (NCCN) guideline, adjuvant chemotherapy is also a mainstay in treating BTCs. The BILCAP study, a multicenter phase Ⅲ study, randomized 447 patients with all types of biliary tract malignancies to receive adjuvant therapy or observation, demonstrating improved OS for patients who received adjuvant therapy[29]. This randomized clinical trial included 79 gallbladder cancer patients. A retrospective study by Akce et al[30] also found that adjuvant systemic therapy was associated with improved OS in adenosquamous carcinomas and adenocarcinomas of the gallbladder[30]. Based on several clinical trials, chemotherapy has also been the standard first-line treatment for advanced BTCs. However, it is still unclear if chemotherapy could provide survival benefits to patients with GBMAC due to the absence of any gallbladder cancer-specific clinical trials. Our study suggested that chemotherapy was another treatment-related prognostic predictor. The log-rank test results and univariate and multivariate Cox proportional hazards analyses all indicated that chemotherapy could bring survival benefits for GBMAC patients regarding OS and CSS. Therefore, an optimal chemotherapy regimen should be administered in patients with GBMAC to increase their chances of survival.
Adjuvant chemoradiotherapy has been proven to improve the survival prognosis significantly and gradually become the mainstay of GBC treatment according to NCCN guidelines[31,32]. Only a retrospective study of patients with gallbladder cancer demonstrated that adjuvant chemoradiation therapy could prolong survival in patients with adenosquamous carcinomas[33]. It is still unclear if adjuvant chemoradiation was used in patients with GBMAC due to the lack of systematic analysis in this group of patients. In the current study, from the baseline characteristics, we found that the percentage of patients treated with radiation therapy was relatively low compared with that of patients with surgery and chemotherapy. Log-rank tests and univariate and multivariate Cox regression analyses of survival time in GBMAC patients stratified by treatment status suggested that the patients treated with radiotherapy had similar OS and CSS to those who did not receive radiotherapy. The association between radiotherapy and prognosis needs to be further evaluated.
Moreover, our results indicated that GBMAC patients with advanced AJCC stage also exhibited worse survival outcomes. The AJCC staging system is the most common model for predicting the prognosis of cancer patients, including three parameters: T stage, N stage, and M stage[34]. The T stage reflects the tumor size; the N stage reflects the lymph node metastasis status; the M stage reflects the distant metastasis status. Our study confirmed the AJCC staging system's value in predicting GBMAC patients' survival outcomes, comparable to a previous study. Zou et al[16] found that lymph node metastasis was independently correlated with shorter OS for GBMAC patients after curative-intent resection[16]. Consequently, regularly screening and diagnosing GBMAC at an early stage is essential.
These prognostic variables can help clinicians determine individualized treatment options, design clinical studies, and adjust the follow-up treatments, which contributes to optimizing GBC therapy toward personalized medicine.
As mentioned, the survival outcomes of MACs largely depend on tumor locations. Currently. With the lack of research on GBMAC, it is still controversial regarding the clinical outcomes of this rare subtype of GBC. The only two published studies on GBMAC considered GBMAC to be aggressive cancer with an associated poor outcome compared to typical GBAC[16,18]. However, these two retrospective analyses contained a very small number of GBMAC cases (15 patients and 54 patients, respectively). Another study summarizing the overall demographical and histopathological features of MACs showed that survival time between the two cohorts was essentially the same[21]. Therefore, the prognosis of GBMAC should be evaluated in more extensive and comprehensive studies. In our study, the log-rank analysis of the whole cohort, including 187 patients diagnosed with GBMAC and 4524 patients with typical GBAC, revealed that GBMAC patients experienced similar OS and CSS compared with those with typical GBAC. Although we performed a PSM analysis to account for the differences in race, marital status, clinical stage, and therapies applied, the two groups showed no significant difference in OS and CSS. In other words, a prognostic difference has not been established between MACs and typical adenocarcinomas in gallbladder cancers. This contradicts the results of the two previous studies regarding the prognosis of GBMAC and some reasonable inferences based on in vitro studies.
There are several possible explanations. Firstly, similar to rectal cancers, the introduction of preoperative radiotherapy and chemotherapy combined with advancing surgical modalities might narrow the gap in prognosis between GBMAC and GBAC[24]. Secondly, the prognoses of gallbladder cancers were poor, and no noticeable difference in OS or CSS existed between various histological subtypes. Furthermore, patients with GBMAC account for a very small group of cancer patients without clinical trials suitable for them, and consequently, no specific drugs have been approved for GBMAC. Given the disturbing status in this area, these patients are more willing to receive surgery the first time when diagnosed, which was the only known way of managing the disease until now.
There are still several limitations of our study that should be considered. Firstly, this study is retrospective research. Thus, significant differences in the sizes between the two cohorts and intrinsic selection biases exist in this study. Even though PSM analysis could minimize these biases, this process would also eliminate a large number of cases causing a sampling bias. Furthermore, the availability of some crucial clinical information was limited, such as information on molecular pathology, genetic profiles, details of treatment regimens, comorbidities, and tumor progression. The molecular-genetic features are prognoses' predictive factors, and molecular pathological and genetic investigations have been widely applied in clinical practice. Although the SEER database includes information regarding the therapy of GBMAC, it lacks the details of therapies (i.e., surgical margins, radiation dose, chemotherapy regimen, therapeutic response, and recurrence rate). A further prospective study with large sample size and more comprehensive prognostic information is desired to verify our findings.
In conclusion, we systematically compared the clinicopathological characteristics of GBMAC with typical GBAC. Compared with typical GBAC, GBMAC showed different demographical and clinicopathological features with aggressive biological behaviors. The OS and CSS were not worse for patients with GBMAC than those with typical GBAC. Furthermore, we explored the correlation between various variables and the survival time of GBMAC patients. Finally, three prognostic predictors for GBMAC patients were identified: Surgery, chemotherapy, and AJCC stage. This study provides clinicians with deeper insights into this rare subtype of GBC, and more research is needed to verify our results.
Gallbladder carcinoma (GBC) is the most common biliary tract cancer worldwide and the sixth most common gastrointestinal tumor. GBC is widely regarded as a highly aggressive malignancy with a poor overall 5-year survival rate of less than 5% and median overall survival (OS) of only six months. The biological features, clinical manifestations, and prognoses are obviously different in various histological subtypes of GBC. Gallbladder mucinous adenocarcinoma (MAC) (GBMAC) is an uncommon subtype of gallbladder adenocarcinoma (GBAC) and has unique clinicopathological characteristics and prognosis.
Due to the rarity of GBMAC, there are few large randomized clinical studies of GBMAC, and characterization of clinicopathological features, prognosis, and clinical risk factors have been limited to individual case reports or small retrospective series. With limited understanding, clinical practices in typical GBAC are also applied to GBMAC. However, GBMAC has distinct histologic, clinical, and molecular features, thus making a differential approach necessary. The clinical prognosis of GBMAC compared to typical GBAC remains unknown.
We performed a retrospective analysis to investigate clinicopathologic characteristics, prognostic factors, and treatment outcomes for GBMAC by comparing GBMAC patients and typical GBAC patients using data from the Surveillance, Epidemiology, and End Results (SEER) database.
This retrospective study was conducted using data from the SEER database. Cases of GBMAC and typical GBAC diagnosed between January 2010 and December 2017 were included in this study. Finally, 187 GBMAC patients and 4524 typical GBAC were included in the study. To analyze the prognostic factors of GBMAC, the clinicopathological features and OS of these GBMAC patients were compared with a large cohort of typical GBAC patients. The Pearson chi-square test or Fisher exact test was used to examine the differences between these two cohorts. In addition, a propensity score matching (PSM) analysis was performed to balance the differences and biases between the GBMAC and typical GBAC groups. The PSM model was based on race, marital status, radiotherapy status, and American Joint Committee on Cancer (AJCC) stage. The baseline characteristics of the GBMAC and typical GBAC were also determined in the matched data. Cox proportional hazards models were used to analyze associations of different variables with OS and cancer-specific survival (CSS). Only variables significantly associated with survival in the univariate Cox analysis were included in the multivariate Cox analysis. Hazard ratios and 95% confidence intervals were calculated, and the univariate and multivariate Cox analyses were applied to the whole and matched data set. Moreover, the Kaplan-Meier method was used to establish the survival curves, and the log-rank test was used to assess any significant differences in OS and CSS stratified by histology before and after PSM. The univariable survival analysis applied the Kaplan-Meier method and log-rank test to identify statistically significant covariates associated with CSS. Statistical analyses were performed using the R software (version 4.1.2).
In our study, compared with typical GBAC, GBMAC was significantly associated with unmarried status, advanced AJCC stage, higher T stage, higher N1 stage rate, and lower N0 and N2 stage rates. After PSM analysis, there were no significant differences in the characteristics between these two groups. After univariate and multivariate analyses, only surgery, chemotherapy, and AJCC stage IV were independent risk factors for OS of GBMAC patients. Similar results were observed in multivariate analysis of CSS. Surgery, chemotherapy, and AJCC stage III and IV were independent prognostic indicators for CSS of GBMAC patients. As for OS and CSS, there was no significant difference between GBMAC and typical GBAC patients in the whole cohort. In the PSM cohort, patients with GBMAC also had similar OS and CSS to matched typical GBAC patients.
Compared with typical GBAC, GBMAC showed different demographical and clinicopathological features with aggressive biological behaviors. The OS and CSS were not worse for patients with GBMAC than those with typical GBAC. Furthermore, we explored the correlation between various variables and the survival time of GBMAC patients. Finally, three prognostic predictors for GBMAC patients were identified, including surgery status, chemotherapy status, and AJCC stage.
A further prospective study with large sample size and more comprehensive prognostic information is desired to verify our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karaca CA, Turkey; Zamani M, Iran S-Editor: Fan JR L-Editor: Webster JR P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63308] [Article Influence: 15827.0] [Reference Citation Analysis (174)] |
| 2. | Lendoire JC, Gil L, Duek F, Quarin C, Garay V, Raffin G, Rivaldi M, Alejandra O, Imventarza O. Relevance of residual disease after liver resection for incidental gallbladder cancer. HPB (Oxford). 2012;14:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Lai CH, Lau WY. Gallbladder cancer--a comprehensive review. Surgeon. 2008;6:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Levy AD, Murakata LA, Rohrmann CA Jr. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295-314; questionnaire, 549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 201] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Margonis GA, Gani F, Buettner S, Amini N, Sasaki K, Andreatos N, Ethun CG, Poultsides G, Tran T, Idrees K, Isom CA, Fields RC, Krasnick B, Weber SM, Salem A, Martin RC, Scoggins C, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Maithel SK, Pawlik TM. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford). 2016;18:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Ito H, Matros E, Brooks DC, Osteen RT, Zinner MJ, Swanson RS, Ashley SW, Whang EE. Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg. 2004;8:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Li YC, Li KS, Liu ZL, Tang YC, Hu XQ, Li XY, Shi AD, Zhao LM, Shu LZ, Lian S, Yan ZD, Huang SH, Sheng GL, Song Y, Liu YJ, Huan F, Zhang MH, Zhang ZL. Research progress of bile biomarkers and their immunoregulatory role in biliary tract cancers. Front Immunol. 2022;13:1049812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Priya R, Jain V, Akhtar J, Chauhan G, Sakhuja P, Goyal S, Agarwal AK, Javed A, Jain AP, Polisetty RV, Sirdeshmukh R, Kar S, Gautam P. Plasma-derived candidate biomarkers for detection of gallbladder carcinoma. Sci Rep. 2021;11:23554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Hart J, Modan B, Hashomer T. Factors affecting survival of patients with gallbladder neoplasms. Arch Intern Med. 1972;129:931-934. [PubMed] |
| 11. | Laitio M. Histogenesis of epithelial neoplasms of human gallbladder II. Classification of carcinoma on the basis of morphological features. Pathol Res Pract. 1983;178:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Hugen N, Brown G, Glynne-Jones R, de Wilt JH, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol. 2016;13:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Lee NK, Kim S, Kim HS, Jeon TY, Kim GH, Kim DU, Park DY, Kim TU, Kang DH. Spectrum of mucin-producing neoplastic conditions of the abdomen and pelvis: cross-sectional imaging evaluation. World J Gastroenterol. 2011;17:4757-4771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 14. | Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1106] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 15. | Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1353] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 16. | Zou RQ, Hu HJ, Liu F, Lv TR, Wang JK, Regmi P, Li FY. Comparison of clinicopathological characteristics of mucinous adenocarcinoma and conventional adenocarcinoma of gallbladder. Asian J Surg. 2023;46:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Vallonthaiel AG, Yadav R, Jain D, Mathur SR, Iyer VK. Mucinous adenocarcinoma of gallbladder: Subcategorisation on fine-needle aspiration cytology. Diagn Cytopathol. 2019;47:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Dursun N, Escalona OT, Roa JC, Basturk O, Bagci P, Cakir A, Cheng J, Sarmiento J, Losada H, Kong SY, Ducato L, Goodman M, Adsay NV. Mucinous carcinomas of the gallbladder: clinicopathologic analysis of 15 cases identified in 606 carcinomas. Arch Pathol Lab Med. 2012;136:1347-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer. 2012;36:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 727] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 21. | Benesch MGK, Mathieson A. Epidemiology of Mucinous Adenocarcinomas. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Warschkow R, Tarantino I, Huttner FJ, Schmied BM, Guller U, Diener MK, Ulrich A. Predictive value of mucinous histology in colon cancer: a population-based, propensity score matched analysis. Br J Cancer. 2016;114:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 25. | Hugen N, Verhoeven RH, Radema SA, de Hingh IH, Pruijt JF, Nagtegaal ID, Lemmens VE, de Wilt JH. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol. 2013;24:2819-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, Geyer FC, Weigelt B, Ashworth A, Reis-Filho JS. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010;222:282-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Adsay NV, Klimstra DS. Not all "mucinous carcinomas" are equal: time to redefine and reinvestigate the biologic significance of mucin types and patterns in the GI tract. Virchows Arch. 2005;447:111-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Cong LL, Cai ZQ, Guo P, Chen C, Liu DC, Li WZ, Wang L, Zhao Y, Si SB, Geng ZM. Decision of surgical approach for advanced gallbladder adenocarcinoma based on a Bayesian network. J Surg Oncol. 2017;116:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 804] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 30. | Akce M, Zakka K, Penley M, Jiang R, Alese OB, Shaib WL, Wu C, Behera M, El-Rayes BF. Clinicopathological features and survival outcomes of rare histologic variants of gallbladder cancer. J Surg Oncol. 2020;121:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Geng ZM, Cai ZQ, Zhang Z, Tang ZH, Xue F, Chen C, Zhang D, Li Q, Zhang R, Li WZ, Wang L, Si SB. Estimating survival benefit of adjuvant therapy based on a Bayesian network prediction model in curatively resected advanced gallbladder adenocarcinoma. World J Gastroenterol. 2019;25:5655-5666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 551] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 33. | Murimwa G, Hester C, Mansour JC, Polanco PM, Porembka MR, Wang SC, Zeh HJ Jr, Yopp AC. Comparative Outcomes of Adenosquamous Carcinoma of the Gallbladder: an Analysis of the National Cancer Database. J Gastrointest Surg. 2021;25:1815-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Giannis D, Cerullo M, Moris D, Shah KN, Herbert G, Zani S, Blazer DG 3rd, Allen PJ, Lidsky ME. Validation of the 8th Edition American Joint Commission on Cancer (AJCC) Gallbladder Cancer Staging System: Prognostic Discrimination and Identification of Key Predictive Factors. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |