Published online Jul 15, 2023. doi: 10.4251/wjgo.v15.i7.1271
Peer-review started: April 1, 2023
First decision: April 19, 2023
Revised: April 27, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: July 15, 2023
Processing time: 101 Days and 22.1 Hours
No single endoscopic feature can reliably predict the pathological nature of colorectal tumors (CRTs).
To establish and validate a simple online calculator to predict the pathological nature of CRTs based on white-light endoscopy.
This was a single-center study. During the identification stage, 530 consecutive patients with CRTs were enrolled from January 2015 to December 2021 as the derivation group. Logistic regression analysis was performed. A novel online calculator to predict the pathological nature of CRTs based on white-light images was established and verified internally. During the validation stage, two series of 110 images obtained using white-light endoscopy were distributed to 10 endoscopists [five highly experienced endoscopists and five less experienced endoscopists (LEEs)] for external validation before and after systematic training.
A total of 750 patients were included, with an average age of 63.6 ± 10.4 years. Early colorectal cancer (ECRC) was detected in 351 (46.8%) patients. Tumor size, left semicolon site, rectal site, acanthosis, depression and an uneven surface were independent risk factors for ECRC. The C-index of the ECRC calculator prediction model was 0.906 (P = 0.225, Hosmer–Lemeshow test). For the LEEs, significant improvement was made in the sensitivity, specificity and accuracy (57.6% vs 75.5%; 72.3% vs 82.4%; 64.2% vs 80.2%; P < 0.05), respectively, after training with the ECRC online calculator prediction model.
A novel online calculator including tumor size, location, acanthosis, depression, and uneven surface can accurately predict the pathological nature of ECRC.
Core Tip: White light endoscopy remains the most basic and indispensable tool in diagnosing colorectal tumors (CRTs). No single endoscopic feature can reliably predict the pathological nature of CRTs. Here, we investigated the endoscopic findings of CRTs, including lobulation, erosion, expansion, depression, acanthosis, lifting sign, stiffness, nodules larger than 10 mm, and so on. A logistic regression analysis was performed, and a novel online calculator for predicting the pathological nature of CRTs based on white-light imaging was established and verified.
- Citation: Wang YD, Wu J, Huang BY, Guo CM, Wang CH, Su H, Liu H, Wang MM, Wang J, Li L, Ding PP, Meng MM. Development and validation of an online calculator to predict the pathological nature of colorectal tumors. World J Gastrointest Oncol 2023; 15(7): 1271-1282
- URL: https://www.wjgnet.com/1948-5204/full/v15/i7/1271.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i7.1271
According to the latest global cancer statistics[1], there were more than 1.9 million new cases of colorectal cancer (CRC) and more than 930000 deaths worldwide in 2020. CRC ranks third in terms of incidence and second in terms of mortality among all malignancies. In China, the incidence of CRC has been increasing yearly, partially attributed to lifestyle changes and a westernized dietary pattern[2]. CRC is generally defined as malignant progression from adenomas through an adenoma–carcinoma sequence[3,4]. Early detection and removal of adenomas will provide an opportunity for screening and preventing the development of early CRC (ECRC). This strategy can substantially reduce the incidence and mortality of CRC[5,6].
Colorectal adenoma (CRA) and ECRC (including high-grade intraepithelial neoplasia and intramucosal cancer) are absolute indications for endoscopic treatment. superficial Submucosal invasion (SMI) corresponding to submucosal invasion < 1000 µm) stage CRC with superficial infiltration is a relative indication for endoscopic treatment. This approach requires strict pathological evaluation of resected specimens to determine the presence of lymphatic and vascular infiltration and the necessity for extensive surgery[7]. Therefore, it is important to differentiate colorectal neoplastic from nonneoplastic lesions and to determine the depth of invasion of colorectal neoplastic lesions based on endoscopic features. At present, Kudo’s pit, capillary, and surface vascular patterns are widely applied to assess the risk of CRC[8-10]. However, these staging systems require staining endoscopy, magnification endoscopy (ME), narrow-band imaging (NBI), and experienced endoscopists who can perform NBI and ME. However, ordinary hospitals lack experienced endoscopists and top-tier endoscopic equipment, so the above staging systems are not applicable. In this study, we aimed to establish a simple, practical and stable online calculator to predict the nature of colorectal tumors (CRTs) based on white-light imaging (WLI). This calculator can assist endoscopists in diagnosing ECRC, improving the detection rate, and selecting treatment protocols.
We carefully reviewed two datasets: One for the development and internal validation of a calculator and another for the external validation of the calculator. Patients who met the following inclusion criteria were recruited: (1) ECRC or CRA detected by colonoscopy; (2) Accurate pathological diagnosis; and (3) High-quality endoscopic images. The histological diagnosis was based on the World Health Organization criteria. Exclusion criteria included the following: (1) ECRC or CRA not treated with endoscopy or surgery; (2) Patients with familial adenomatous polyposis, Lynch syndrome or Peutz–Jeghers syndrome, inflammatory bowel disease, intestinal tuberculosis; (3) Patients who underwent colectomy for other diseases; (4) Poor intestinal preparation; and (5) Patients with incomplete medical records. Patient demographics (age and sex) and clinicopathological characteristics (tumor location, size, differentiation, gross type, depth of invasion), as well as endoscopic features (redness, erosion, expansion, depression, uneven surface, lobulation, acanthosis, and nodules larger than 10 mm), were independently evaluated by three experienced endoscopists.
A total of 10 endoscopists with varying levels of experience participated in the present study. The endoscopists were divided into two groups: A group of less experienced endoscopists (LEEs) who had performed fewer than 1000 colonoscopies and a group of highly experienced endoscopists (HEEs) who had performed more than 3000 colonoscopies[11].
The present study consisted of two phases. During the identification phase, we retrospectively reviewed 530 patients who underwent surgery or endoscopic treatment for ECRC or CRA between January 2015 and December 2021 at Beijing Shijitan Hospital, Capital Medical University. Baseline information on demographic, clinicopathological and endoscopic characteristics of all patients was collected. Then, logistic regression analysis was performed, and a novel online calculator to predict the pathological nature of CRTs based on WLI was developed and verified internally.
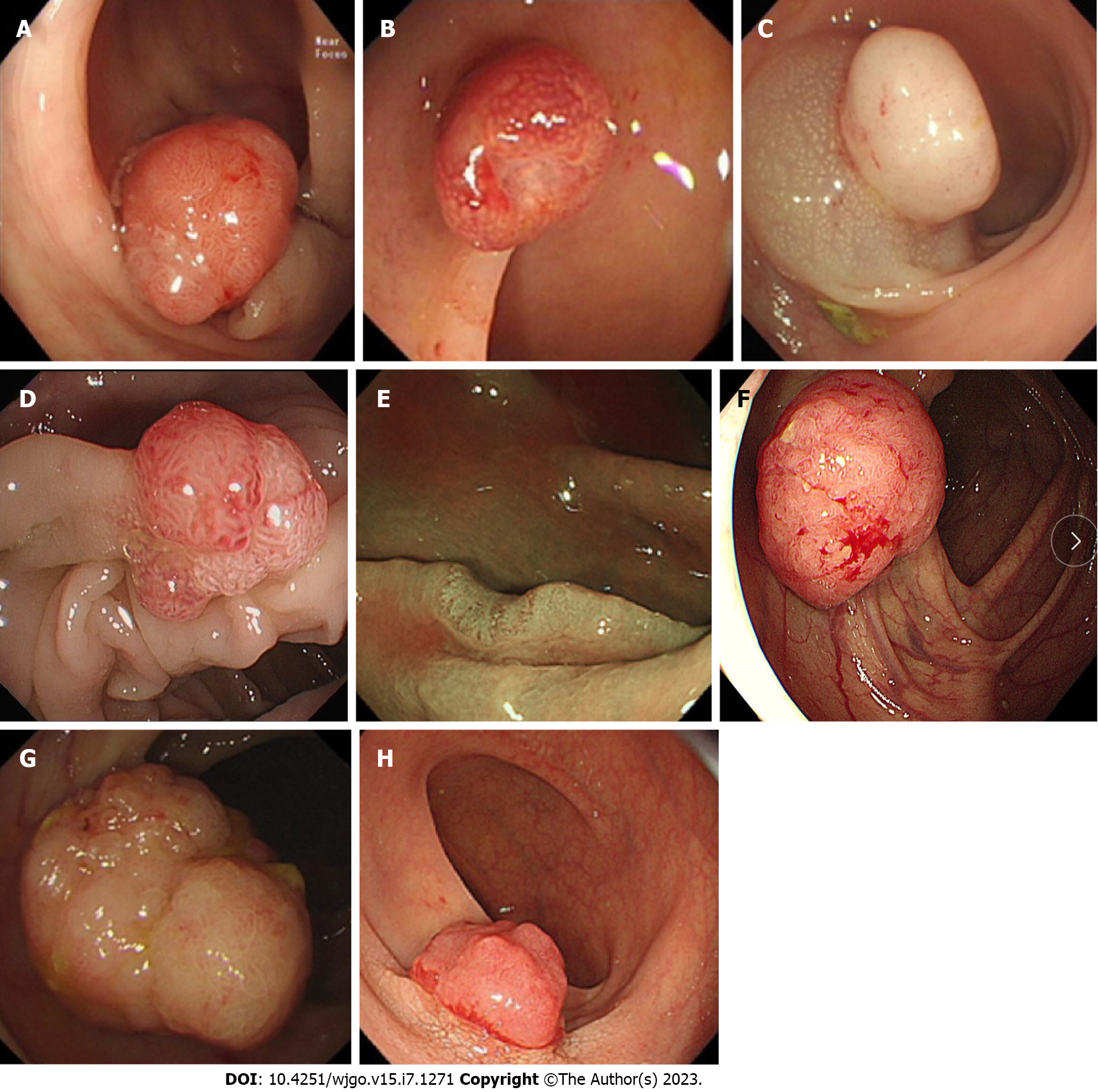
During the validation phase, external validation of the calculator was performed. Ten endoscopists were required to independently evaluate a series of 110 images of CRTs according to WLI. Then, a systematic training program on this online calculator was conducted. During the training process, a schematic representation of the calculator was posted on the wall of each endoscopic room, and the images (Figure 1) used to educate the participants were presented in PowerPoint (Microsoft Corp.) by the leading investigator who was not involved in this study. Afterward, the participants were immediately asked to score another series of 110 images of CRTs by using the calculator (posttest). These images were retrospectively collected from 220 CRT patients who had undergone colonoscopy between January 2015 and December 2021 by the leading investigator.
All endoscopic images were taken using an endoscope (PCF-H260, PCF-Q260, CF-H260, CF-HQ290 and PCF-H290; Olympus, Tokyo, Japan) during preoperative diagnosis based on WLI. Generally, NBI + ME, stained endoscopy and ultrasound endoscopic evaluation were performed when carcinoma or submucosal carcinoma was suspected. The location, diameter, color, substrate, surface and morphology of the tumors were noted. Tumor location was divided into the right colon (including the cecum, ascending colon, transverse colon, and splenic flexure), left colon (including the descending colon and sigmoid colon) and rectum. Lesion size was estimated using 7 mm diameter open-biopsy forceps. Paris staging[12] was used to classify and describe the morphology, while tumors larger than 10 mm growing superficially along the intestinal lumen were defined as the laterally spreading tumor (LST) type.
We investigated endoscopic findings of the CRTs, including lobulation, erosion, expansion, depression, acanthosis, lifting sign, stiffness, and nodules larger than 10 mm. The definitions of the eight endoscopic findings were as follows (Figure 1): (1) Hyperemia: Redness and hyperemia on the surface of a tumor; (2) Erosion: Erosion and hyperemia on the surface of a tumor; (3) Acanthosis: Chicken skin mucosa beside the tumor; (4) Lobulation: Multiple nodules on the surface of a tumor; (5) Depression: Depressed demarcation on the surface of a tumor; (6) Expansion: A bursting appearance due to the expansive growth of a tumor; (7) Large nodule: Nodules larger than 10 mm; and (8) Uneven surface: Surface with bulges and depressions.
This study was approved by the Ethics Committee of Beijing Shijitan Hospital, Capital Medical University, No. sjtkyll-lx-2020 (19). The requirement to obtain informed consent was waived owing to the retrospective-design portion of the study. In the prospective-design portion of the study, informed consent was obtained from patients before endoscopy.
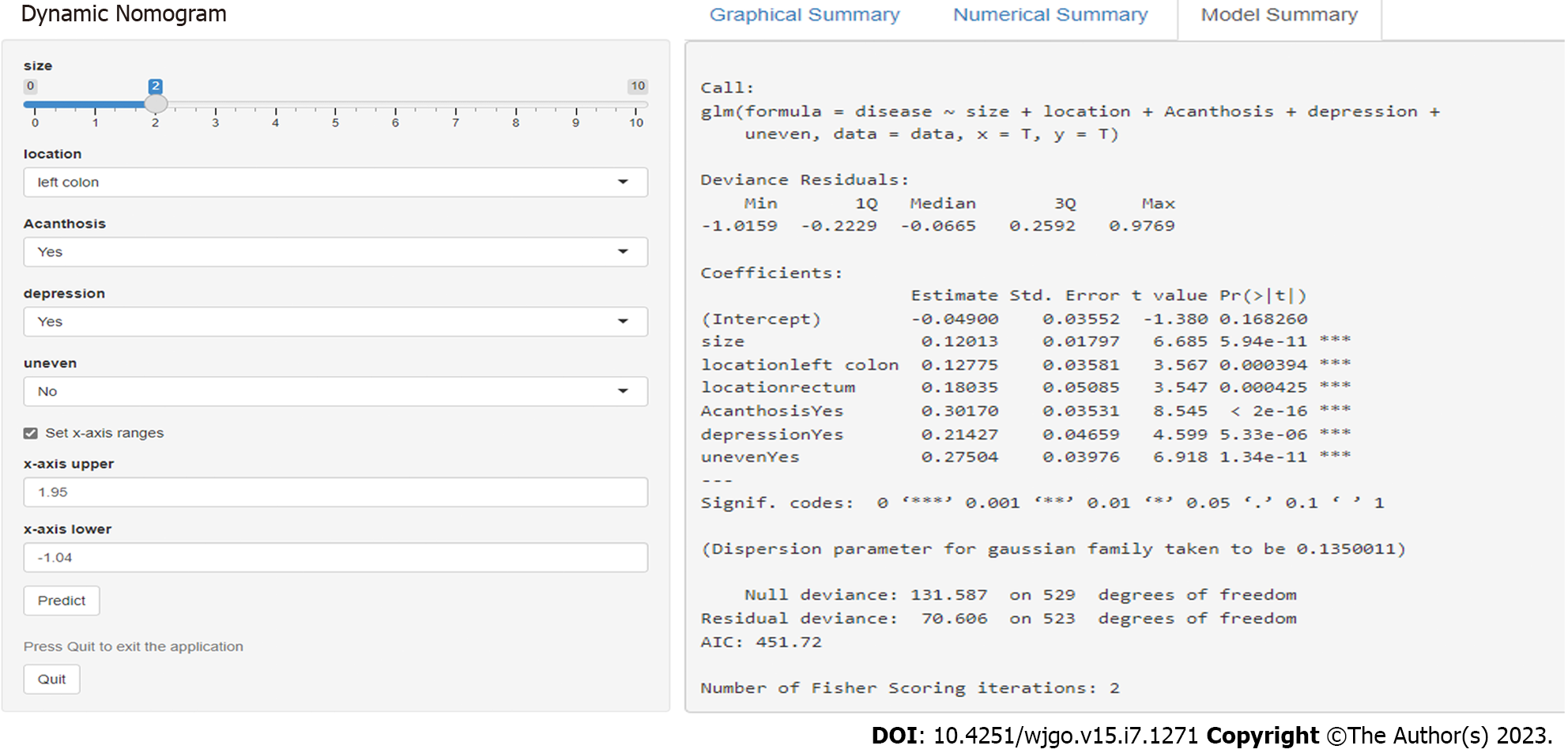
All statistical analyses were conducted using SPSS version 21.0 for Windows (SPSS, Chicago, IL, United States). Baseline information on demographic, endoscopic and clinicopathological characteristics was collected as candidate risk variables. The nature of CRTs was defined as a dependent variable. Continuous variables are presented as the mean ± SD, whereas categorical variables are presented as percentages. For comparisons of categorical and continuous variables, chi-square tests or individual sample t tests were applied, as appropriate. Univariate logistic regression analyses were performed on the derivation dataset to identify risk factors for ECRC. Multivariable logistic regression analyses were conducted on variables with a P < 0.05 for univariate analysis. The multivariate logistic regression model was built from the set of candidate variables by removing predictors based on P values in a stepwise manner. Model discrimination was assessed by calculating the area under the receiver operator characteristic (AUROC) curve (or C-index), whereas model calibration was determined by the Hosmer–Lemeshow (H-L) test. The nomogram was formulated based on multivariate analysis by using the RMS package (R software 4.1.3). The performance of the nomogram model was examined by calibration (calibration curves), discrimination (AUC) and clinical usefulness (decision curves), which was validated in the validation cohort. The “shiny: Web Application Framework for R” package was used to develop an online tool (https://nomogram7474.shinyapps.io/DynNomapp/).
The performance of the calculator in the histological prediction of CRTs included sensitivity, specificity, predictive values, and accuracy. All these indicators were calculated in 2 phases, with the histopathological diagnosis as the gold standard. Estimation of diagnostic accuracy was based on average values and 95%CI. Sensitivity, specificity, and accuracy were compared between the two phases and between the two groups by using the paired-samples Student’s t-test and independent-samples Student’s-test, respectively. A P < 0.05 was considered statistically significant.
A total of 750 patients were enrolled in this study, with 530 cases in the derivation group and 220 cases in the validation group. The mean age was 63.6 ± 10.4 years, and 499 patients (66.5%) were male. ECRC was detected in 351 (46.8%) patients, including 243 (45.8%; 243/530) in the derivation group and 108 (49.1%; 108/220) in the validation group. The incidence of ECRC was not significantly different between the derivation group and the validation group (P > 0.05). The mean size of the lesion was 15.32 ± 9.68 mm. Lesions were located in the right semicolon (n = 358), left semicolon (n = 266), and rectum (n = 126). The size and location of the lesions were not significantly different between the derivation group and the validation group (P > 0.05).
In univariate models, location, size, hyperemia, erosion, acanthosis, lobulation, depression, expansive appearance, a large nodule and an uneven surface were associated with the development of ECRC (P < 0.05) (Table 1).
| Variables | CRA (n = 287) | ECRC (n = 243) | P value |
| Sex | 0.296 | ||
| Male (n, %) | 191 (66.6) | 172 (70.8) | |
| Female (n, %) | 96 (33.4) | 71 (29.2) | |
| Age (yr) (mean ± SD) | 60.86 ± 10.37 | 64.65 ± 9.91 | 0.146 |
| Type | 0.086 | ||
| 0-I (n, %) | 210 (73.2) | 180 (74.1) | |
| 0-II (n, %) | 45 (15.7) | 25 (10.3) | |
| LST (n, %) | 32 (11.1) | 38 (15.6) | |
| Location | 0 | ||
| Rectum (n, %) | 26 (9.1) | 54 (22.2) | |
| Left semicolon (n, %) | 140 (48.8) | 136 (56.0) | |
| Right semicolon (n, %) | 121 (42.2) | 53 (21.8) | |
| Size (mm, mean ± SD) | 10.36 ± 5.61 | 19.28 ± 11.36 | 0 |
| Size (mm) | 0 | ||
| < 10 (n, %) | 147 (51.2) | 25 (10.3) | |
| ≥ 10 (n, %) | 140 (48.8) | 218 (89.7) | |
| Size (mm) | 0 | ||
| ≤ 20 (n, %) | 257 (89.5) | 129 (53.1) | |
| > 20 (n, %) | 30 (10.5) | 114 (46.9) | |
| White-light endoscopy | |||
| Hyperemia (n, %) | 76 (26.5) | 160 (65.8) | 0 |
| Erosion (n, %) | 3 (1.0) | 38 (15.6) | 0 |
| Acanthosis (n, %) | 57 (19.9) | 166 (68.3) | 0 |
| Lobulation (n, %) | 66 (23.0) | 100 (41.2) | 0 |
| Depression (n, %) | 12 (4.2) | 74 (30.5) | 0 |
| Expansive appearance (n, %) | 37 (12.9) | 63 (25.9) | 0 |
| Large nodule (n, %) | 15 (5.2) | 44 (18.1) | 0 |
| Uneven surface (n, %) | 24 (8.4) | 125 (51.4) | 0 |
In multivariate models, size [odds ratio (OR), 5.233; 95%CI, 2.008-13.636], left semicolon site (OR, 2.338; 95%CI, 1.329-4.111), rectal site (OR, 3.715; 95%CI, 1.692-8.160), acanthosis (OR, 5.199; 95%CI, 3.057-8.842), depression (OR, 5.162; 95%CI, 2.216-12.021) and an uneven surface (OR, 5.583; 95%CI, 3.030-10.286) were independent risk factors for ECRC (Table 2).
| Variable | Univariate Model | Multivariate Model | ||
| P value | OR (95%CI) | β | P value | |
| Size | < 0.001 | 5.233 (2.008-13.636) | 1.655 | 0.001 |
| Left semicolon | < 0.001 | 2.338 (1.329-4.111) | 0.849 | 0.003 |
| Rectum | < 0.001 | 3.715 (1.692-8.160) | 1.312 | 0.001 |
| Hyperemia | < 0.001 | 1.305 (0.756-2.251) | 0.266 | 0.339 |
| Erosion | < 0.001 | 3.848 (0.820-18.052) | 1.348 | 0.088 |
| Acanthosis | < 0.001 | 5.199 (3.057-8.842) | 1.648 | 0 |
| Lobulation | < 0.001 | 1.276 (0.729-2.233) | 0.243 | 0.394 |
| Depression | < 0.001 | 5.162 (2.216-12.021) | 1.641 | 0 |
| Expansive appearance | < 0.001 | 0.910 (0.471-1.756) | -0.095 | 0.778 |
| Large nodule | < 0.001 | 1.146 (0.480-2.732) | 0.136 | 0.759 |
| Uneven surface | < 0.001 | 5.583 (3.030-10.286) | 1.72 | 0 |
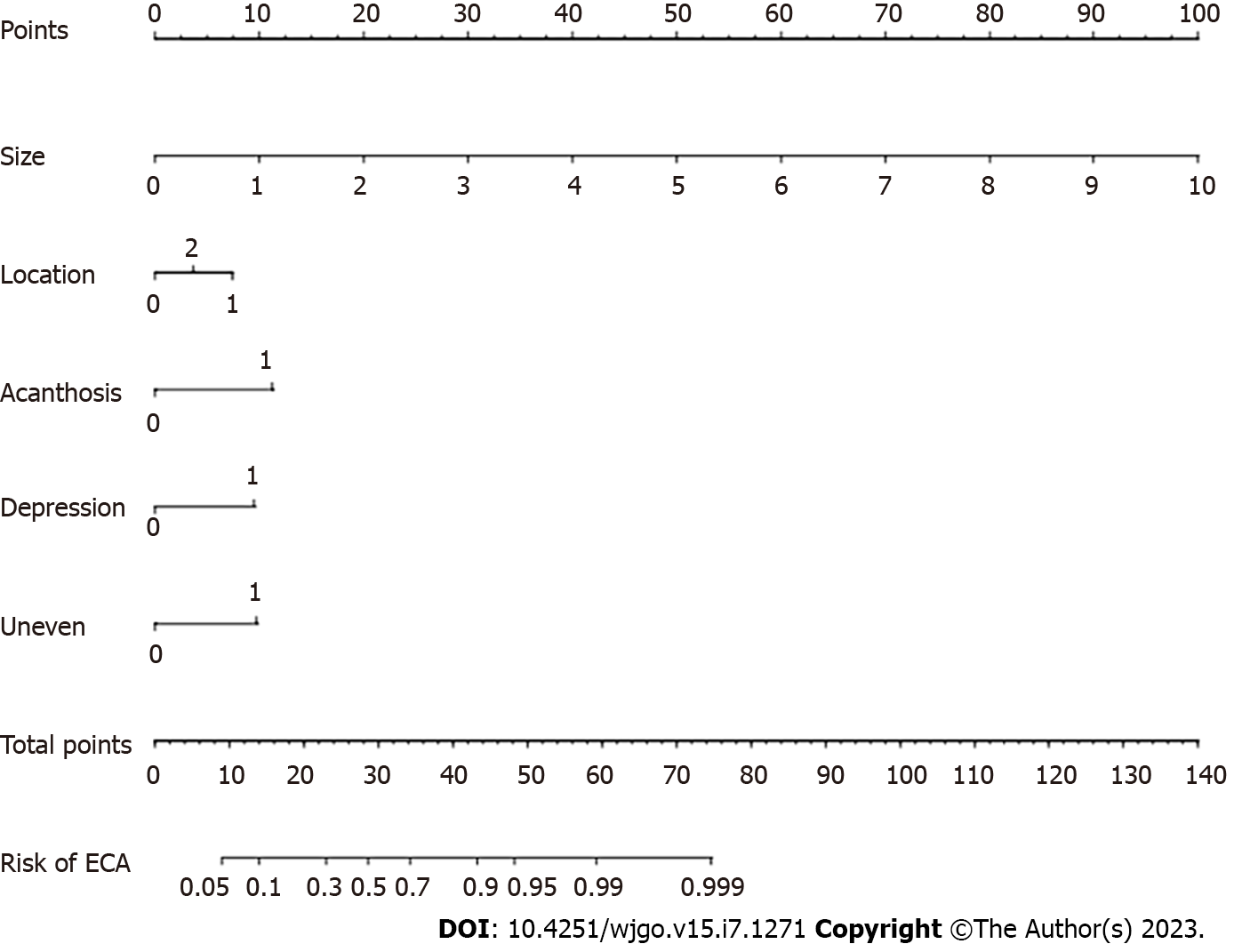
An online calculator to predict the pathological nature of CRTs was established according to the above six independent risk factors (size, left semicolon site, rectal site, acanthosis, depression and uneven surface). A nomogram was constructed with point scales of these variables (Figure 2). The sum of each variable point was plotted on the total point axis. The probability rate of ECRC was obtained by drawing a vertical line from the plotted total point axis straight down to the outcome axis. Based on these nomogram models, online web-based calculators were developed to assess the probability of ECRC among patients with CRTs. The calculator is available at https://nomogram7474.shinyapps.io/DynNomapp/. When users simply input the requested information, the probability of ECRC can be derived (Figure 3).
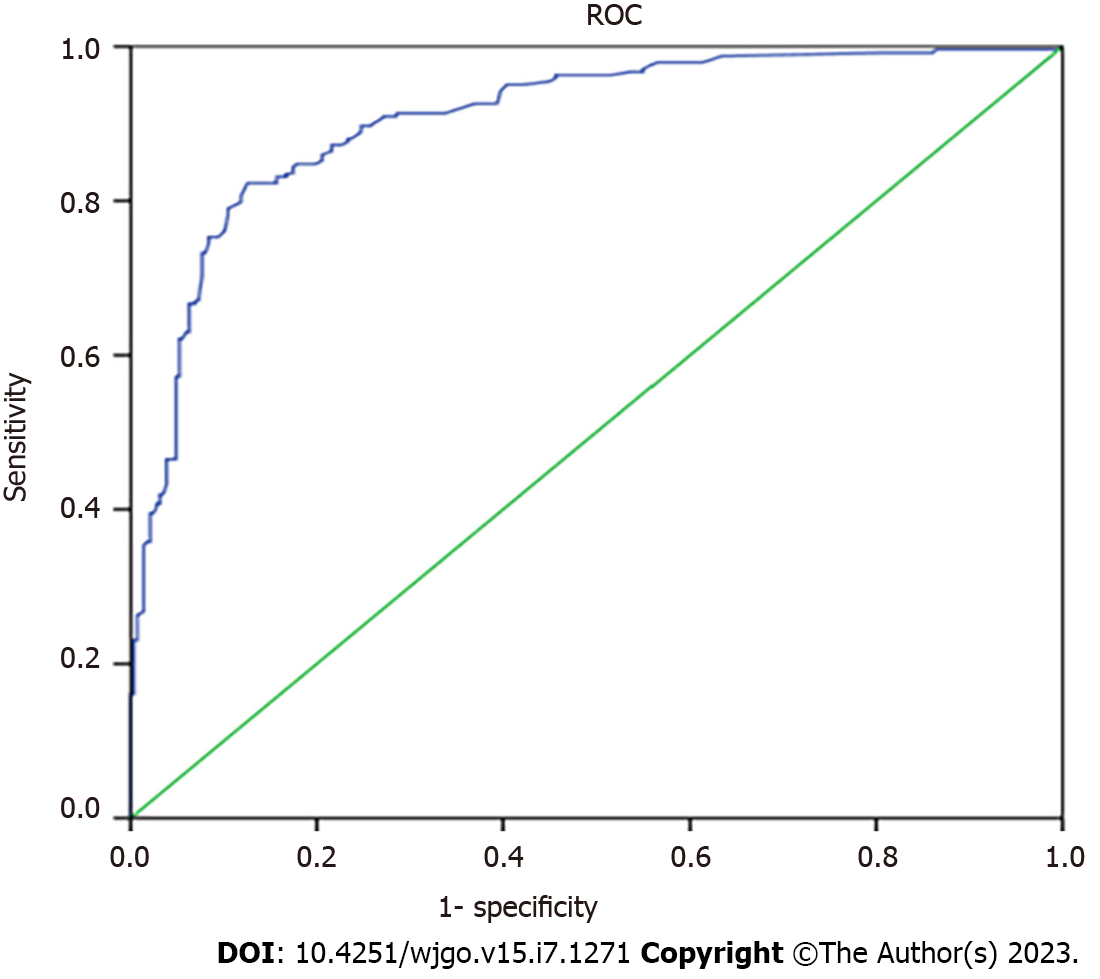
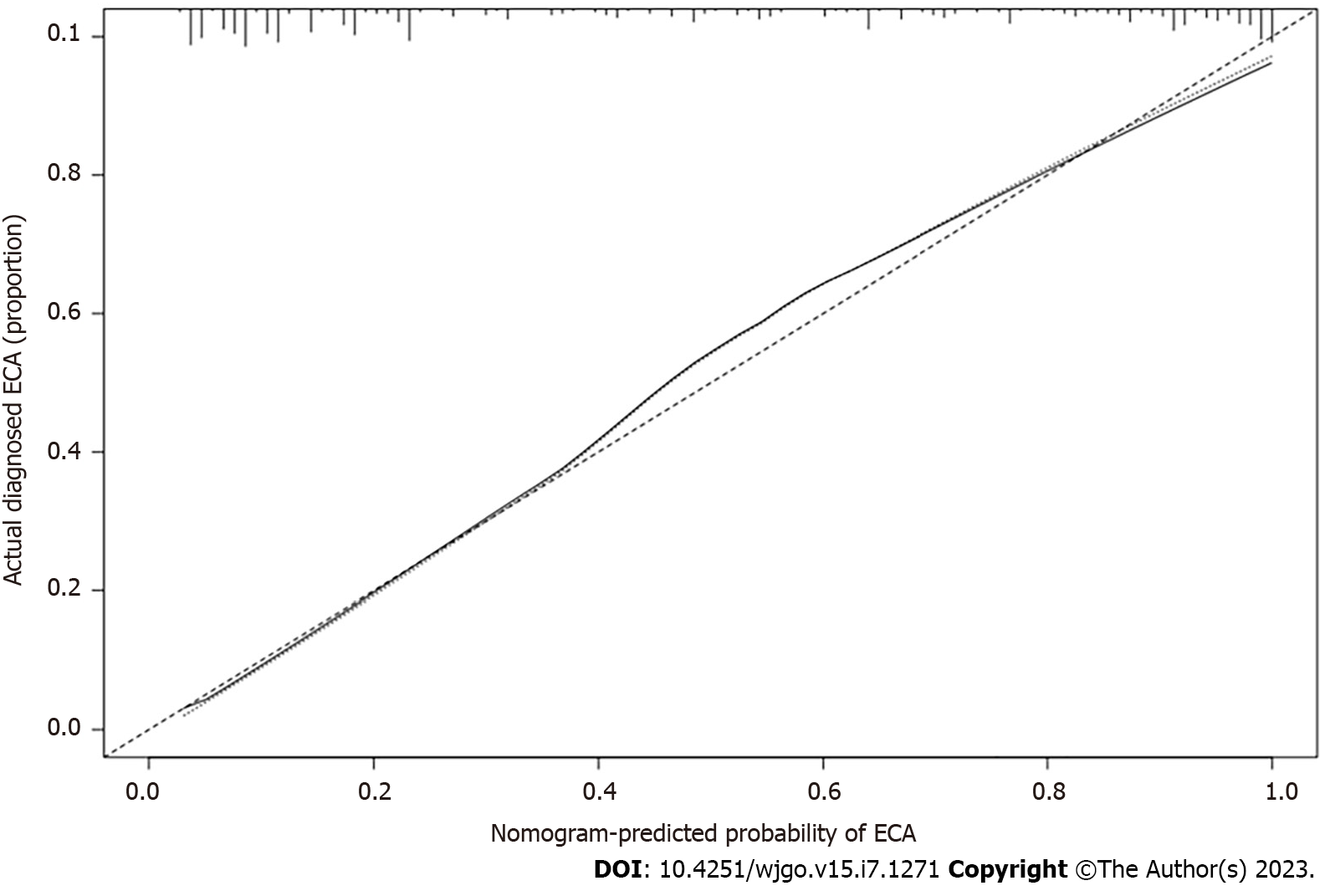
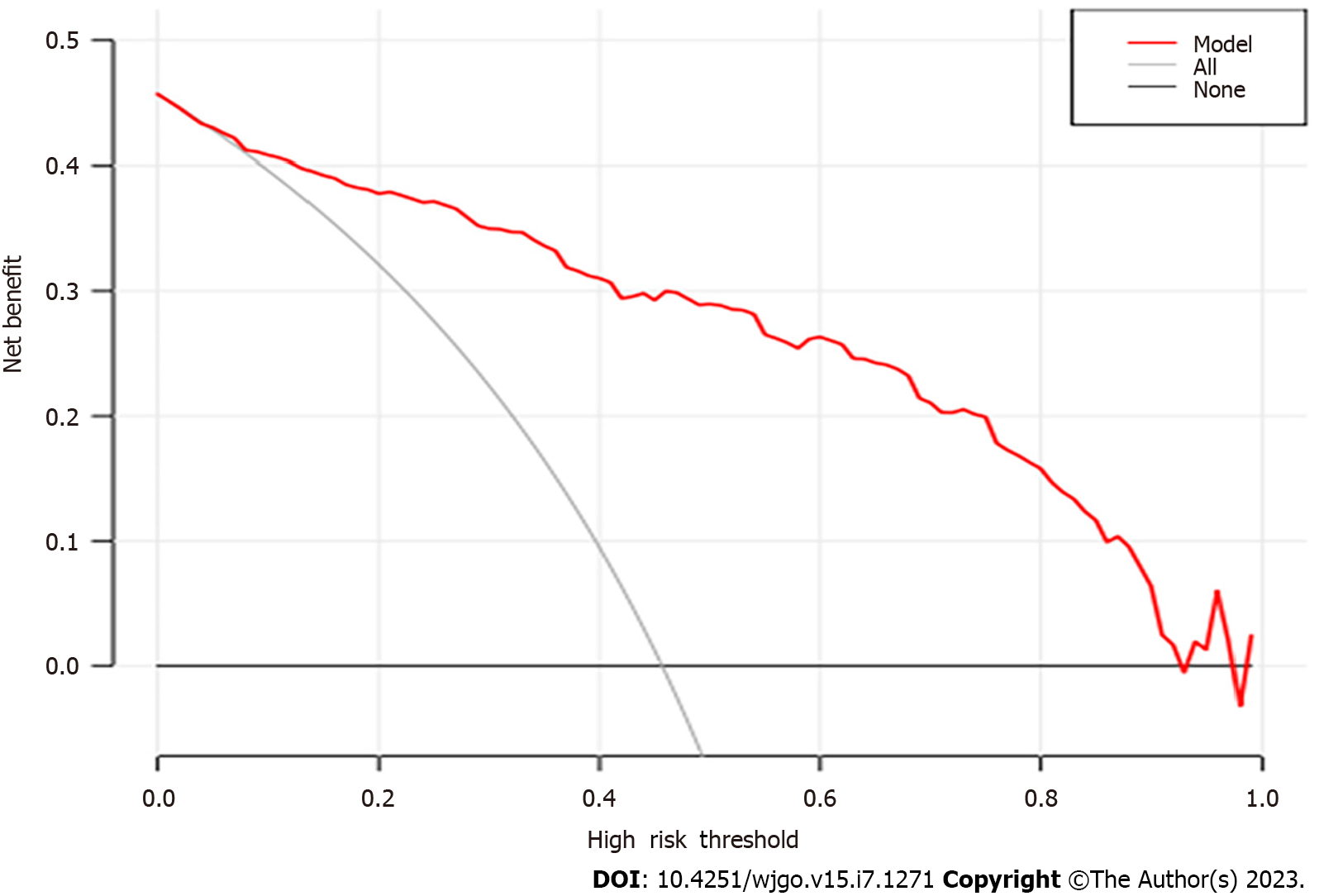
After performing the internal validation by generating 1000 bootstrap replications, the calculator remained highly accurate, with a resulting AUROC (C-index) of 0.906 (95%CI, 0.880-0.932) (Figure 4). Moreover, the calibration plot of the internal validation demonstrated good calibration (χ2 = 10.614; P = 0.225) with the Hosmer-Lemeshow (H–L) test (Figure 5). Decision Curve Analysis was performed to ascertain its clinical usefulness (Figure 6). These results indicated good clinical applicability of the calculator in predicting the pathological nature of CRT according to good net benefit with wide and practical ranges of threshold probabilities.
To explore whether the calculator would be applicable to endoscopists, we conducted an external validation study among 10 endoscopists with varying levels of experience. Comparisons of performance for diagnosing CRT histology between the pretraining test and posttraining test (Table 3) were as follows: LEEs made significant improvements in the sensitivity, specificity and accuracy in the posttraining test compared with the pretraining test. The LEEs’ performance characteristics in the pretraining test vs posttraining test were as follows: Sensitivity 57.6% vs 75.5% (P = 0.004), specificity 72.3% vs 82.4% (P = 0.023), and accuracy 64.2% vs 80.2% (P < 0.001). The κ-values of the LEEs in the pretraining test and posttraining test were 0.72 and 0.83, respectively, indicating good (> 0.60) to excellent (> 0.80) agreement. The HEEs made significant improvement in sensitivity in the posttraining test compared with the pretraining test but not in specificity or accuracy. The HEEs’ performance in the pretraining test vs posttraining test were as follows: Sensitivity 71.2% vs 80.4% (P = 0.043), specificity 82.1% vs 88.2% (P = 0.223), and accuracy 76.5% vs 86.0% (P = 0.071). The κ-values of the HEEs in the pretraining test and posttraining test were 0.81 and 0.89, respectively. The κ-values were improved in both groups, especially in the LEEs, suggesting that the LEEs benefited more from this predicting calculator.
| Group | Pretraining test (95%CI) | Posttraining test (95%CI) | P value |
| LEE | |||
| Sensitivity | 57.6 (48.5-66.4) | 75.5 (67.0-82.4) | 0.004 |
| Specificity | 72.3 (61.8-80.5) | 82.4 (74.2-88.5) | 0.032 |
| Accuracy | 64.2 (56.4-70.3) | 80.2 (71.3-87.6) | < 0.001 |
| HEE | |||
| Sensitivity | 71.2 (60.4-80.1) | 80.4 (71.6-88.1) | 0.043 |
| Specificity | 82.1 (73.8-88.2) | 88.2 (81.5-95.3) | 0.223 |
| Accuracy | 76.5 (69.3-83.6) | 86.0 (80.2-91.7) | 0.071 |
CRA is a precancerous lesion of CRC, since most CRCs develop from CRAs through the adenoma-carcinoma pathway. Without timely intervention, precancerous lesions will progress to CRC within 10 to 15 years[13]. Notably, if the lesions are detected in the early stages of CRC and treated in a timely manner, the 5-year survival rate of these patients can reach as high as 90%[14]. In contrast, if the lesions are detected in the late stages of CRC, the 5-year survival rate will be reduced to less than 10%. Colonic endoscopy can be used for direct observation of intestinal lesions, which is irreplaceable in the examination of intestinal diseases, especially CRC. To improve the detection rate of precancerous lesions and early-stage CRC, assistive techniques, such as chromoendoscopy, magnifying endoscopy, fluorescence endoscopy, confocal laser endoscopy, and electronic staining endoscopy, have been introduced into clinical practice. However, the process of chromoendoscopy is complicated, time-consuming, labor-intensive, and requires magnification endoscopes. In addition, fluorescence endoscopy and confocal laser endoscopy are expensive. These disadvantages limit the application of the above techniques. Moreover, in clinical practice, the experience and the degree of image interpretation can vary greatly between endoscopists, which results in different judgments being made for the same lesion and thus a decrease in the accuracy of colonoscopy. To solve this problem, many endoscopists with extensive experience have defined and standardized the characteristics of CRTs, and several staging systems have been established and promoted in an attempt to improve the diagnostic accuracy and to reduce the possibility of missed diagnoses. With the advent of magnifying endoscopy, the resolution of imaging has substantially improved. Currently, endoscopists can clearly observe the morphology of glandular duct openings and microvasculature on the mucosal surface of CRTs. Kudo’s pit pattern classification[15] under magnifying chromoendoscopy was proposed in 1994. Later, a microvessel pattern under magnifying NBI was proposed by Sano et al[16] in 2006. These staging systems have been highly effective in predicting the histology of CRTs. Subsequently, JNET typing[17], Hiroshima typing[18], and Jikei typing[19] emerged based on mucosal microvascular morphology and surface structure. These typing systems have better performance in differentiating colorectal neoplastic and nonneoplastic lesions by combining the endoscopic features of lesions. Accordingly, the accuracy of differentiating benign from malignant lesions can be improved by providing appropriate training to primary endoscopists. However, the above typing systems require time-consuming, labor-intensive, and magnification endoscopes. Unfortunately, magnification endoscopes are not widely applied in the majority of primary-level hospitals, and LEE lacks experience in NBI + ME. These conditions limit the promotion of staging systems such as Kudo’ pit pattern classification, NICE, and JNET in primary-level hospitals. Therefore, in this study, we established an online calculator to predict the pathological nature of CRTs based on white-light endoscopy. The model consists of five variables: Location of the lesion, size of the lesion, acanthosis, depression, and an uneven surface. The AUC of the scoring system in our modeling cohort was 0.906 (> 0.80), indicating a good degree of differentiation. Based on the Hosmer-Lemeshow goodness-of-fit test (P = 0.225, > 0.1), our prediction model has value for risk stratification among patients with CRTs of unknown nature, which can provide a preliminary basis for the differential diagnosis of CRT. External verification identified significant improvement in the sensitivity and specificity in the posttraining test compared with the pretraining test, especially in the LEEs. Thus, this calculator may be applicable in primary-level hospitals. Our model and its scoring system may have good clinical credibility. First, the methods used for establishing and verifying the models are widely accepted, with external validation among endoscopists with different levels of experience. Second, all of the potential predictors were included, and there were no obvious missing items. Third, five variables (location, size, acanthosis, depression, and uneven surface) associated with CRC were obtained by logistic regression models.
The incidence of left-sided CRC (LCRC) is higher than that of right-sided CRC (RCRC). The American Cancer Society confirms a higher proportion of LCRC (51%) than RCRC (42%) in the United States[20]. Patients with RCRC present with more advanced tumor stages than those with LCRC[21]. Furthermore, higher TNM stages, larger tumors, increased frequency of vascular invasion, mucinous type, high grades and invasive tumor borders were more common in RCRC, whereas annular and polypoid tumors were more common in LCRC[21,22]. In our study, more patients were diagnosed with LCRC than RCRC, which was similar to previous studies.
CRC originates from a CRA, which slowly increases in size, followed by dysplasia and malignant transformation[23]. The size of a CRA is predictive for CRC diagnosis, which underscores the significance of this factor, especially considering its association with a less favorable histology and increased long-term risk of CRC[24]. The 10 mm cutoff represents a critical factor, since a small percentage of larger polyps contain cancerous cells[25,26]. Of the 530 Lesions with CRTs, 243 were diagnosed as ECRC. The mean size of the lesions was 19.28 ± 11.36 mm, of which 89.7% were ≥ 10 mm, consistent with previous studies.
It was reported that demarcated depression, fullness, and stalk swelling were typical findings of ECRC. Notably, 2.0% of the tumors were carcinoma, especially depressed tumors, which had a significantly higher frequency of carcinoma and submucosal invasion regardless of tumor size[27]. The Japanese Guideline for CRC has listed the following endoscopic findings as diagnostic indicators of SM-Ca: Expansive appearance, erosion/ulceration, fold convergence, and deformation/stiffness[28]. In univariate models, most lesions of ECRC had the following characteristics based on WLI: Hyperemia, erosion, acanthosis, lobulation, depression, expansive (sun-burst) appearance, larger nodules, and an uneven surface. In multivariate models, five independent risk factors, size, location, acanthosis, depression, and an uneven surface, were predictive indicators of ECRC. Thus, a simple online calculator to predict the pathological nature of CRTs based on the WLI was established, with an AUC value of 90.6% and high diagnostic specificity and accuracy. Internal and external validation of this model indicated good consistency of CRC risk with postoperative pathology and good agreement in application between endoscopists with various levels of experience.
In conclusion, we present a novel online calculator to predict the pathological nature of CRTs. This calculator may play a practical and important role in reducing the cost and duration of colonoscopy. However, this was a single-center study, and further high-quality, multicenter clinical studies should be conducted to assess the stability and generalizability of this scoring system.
No single endoscopic feature can reliably predict the pathological nature of colorectal tumors (CRTs).
Kudo’s pit, capillary, and surface vascular patterns that can predict the pathological nature of CRTs require experienced endoscopists who can perform narrow-band imaging and magnification endoscopy. This is difficult for endoscopists who lack experience and for endoscopists in ordinary hospitals.
This study aimed to establish and validate a simple online calculator to predict the pathological nature of CRTs based on white-light endoscopy.
This was a single-center study. The endoscopic features of 530 cases of CRTs were analyzed, and logistic regression analysis was performed to establish a novel online calculator that can predict the pathological nature of CRTs. We also conducted internal and external validation on the modified model.
A novel online calculator including size, location, acanthosis, depression and unevenness to predict the pathological nature of CRTs based on white-light imaging was established. Internal and external validation of this model indicated good consistency of colorectal cancer risk with postoperative pathology.
We present a novel online calculator to predict the pathological nature of CRTs. This calculator may be instrumental in reducing the cost and duration of colonoscopy.
This calculator may play a practical and important role in primary-level hospitals and may significantly improve the diagnostic accuracy of primary physicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maher CA, United States; Rumpold H, Austria S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 4. | Allen JI. Molecular biology of colon polyps and colon cancer. Semin Surg Oncol. 1995;11:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Kahi CJ, Rex DK, Imperiale TF. Screening, surveillance, and primary prevention for colorectal cancer: a review of the recent literature. Gastroenterology. 2008;135:380-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2286] [Article Influence: 175.8] [Reference Citation Analysis (2)] |
| 7. | Tanaka S, Saitoh Y, Matsuda T, Igarashi M, Matsumoto T, Iwao Y, Suzuki Y, Nozaki R, Sugai T, Oka S, Itabashi M, Sugihara KI, Tsuruta O, Hirata I, Nishida H, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2021;56:323-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Togashi K, Osawa H, Koinuma K, Hayashi Y, Miyata T, Sunada K, Nokubi M, Horie H, Yamamoto H. A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc. 2009;69:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Rastogi A, Keighley J, Singh V, Callahan P, Bansal A, Wani S, Sharma P. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol. 2009;104:2422-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Macken E, Moreels T, Vannoote J, Siersema PD, Van Cutsem E. Quality assurance in colonoscopy for colorectal cancer diagnosis. Eur J Surg Oncol. 2011;37:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 646] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 13. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1586] [Article Influence: 264.3] [Reference Citation Analysis (2)] |
| 14. | Courtney RJ, Paul CL, Carey ML, Sanson-Fisher RW, Macrae FA, D'Este C, Hill D, Barker D, Simmons J. A population-based cross-sectional study of colorectal cancer screening practices of first-degree relatives of colorectal cancer patients. BMC Cancer. 2013;13:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 473] [Article Influence: 15.3] [Reference Citation Analysis (4)] |
| 16. | Sano Y, Horimatsu T, Fu KI, Katagiri A, Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesions using a narrow-band imaging system. Dig. Endosc. 2006;18:544-551. [RCA] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Iwatate M, Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Ishikawa H, Murakami Y, Yoshida S, Saito Y; Japan NBI Expert Team (JNET). Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Oka S, Tanaka S, Takata S, Kanao H, Chayama K. Clinical usefulness of narrow band imaging magnifying classification for colorectal tumors based on both surface pattern and microvessel features. Dig Endosc. 2011;23 Suppl 1:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Wada Y, Kudo SE, Misawa M, Ikehara N, Hamatani S. Vascular pattern classification of colorectal lesions with narrow band imaging magnifying endoscopy. Dig Endosc. 2011;23 Suppl 1: 106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Meza R, Jeon J, Renehan AG, Luebeck EG. Colorectal cancer incidence trends in the United States and United kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res. 2010;70:5419-5429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992-2001. Cancer. 2006;107:1142-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Gupta S, Balasubramanian BA, Fu T, Genta RM, Rockey DC, Lash R. Polyps with advanced neoplasia are smaller in the right than in the left colon: implications for colorectal cancer screening. Clin Gastroenterol Hepatol. 2012;10:1395-1401.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Qaseem A, Denberg TD, Hopkins RH Jr, Humphrey LL, Levine J, Sweet DE, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 24. | Pickhardt PJ, Pooler BD, Kim DH, Hassan C, Matkowskyj KA, Halberg RB. The Natural History of Colorectal Polyps: Overview of Predictive Static and Dynamic Features. Gastroenterol Clin North Am. 2018;47:515-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Parsa N, Ponugoti P, Broadley H, Garcia J, Rex DK. Risk of cancer in 10 - 19 mm endoscopically detected colorectal lesions. Endoscopy. 2019;51:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Halfter K, Bauerfeind L, Schlesinger-Raab A, Schmidt M, Schubert-Fritschle G, Hölzel D, Engel J. Colonoscopy and polypectomy: beside age, size of polyps main factor for long-term risk of colorectal cancer in a screening population. J Cancer Res Clin Oncol. 2021;147:2645-2658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Zhang YL, Zhang ZS, Wu BP, Zhou DY. Early diagnosis for colorectal cancer in China. World J Gastroenterol. 2002;8:21-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 490] [Article Influence: 49.0] [Reference Citation Analysis (0)] |