Published online Jul 15, 2023. doi: 10.4251/wjgo.v15.i7.1262
Peer-review started: March 28, 2023
First decision: April 10, 2023
Revised: May 2, 2023
Accepted: June 13, 2023
Article in press: June 13, 2023
Published online: July 15, 2023
Processing time: 106 Days and 1.6 Hours
Although the current conventional treatment strategies for esophageal carcinoma (EC) have been proven effective, they are often accompanied by serious adverse events. Therefore, it is still necessary to continue to explore new therapeutic strategies for EC to improve the clinical outcome of patients.
To elucidate the clinical efficacy of concurrent chemoradiotherapy (CCRT) with thalidomide (THAL) and S-1 (tegafur, gimeracil, and oteracil potassium capsules) in the treatment of EC as well as its influence on serum tumor markers (STMs).
First, 62 patients with EC treated at the Zibo 148 Hospital between November 2019 and November 2022 were selected and grouped according to the received treatment. Among these, 30 patients undergoing CCRT with cis-platinum and 5-fluorouracil were assigned to the control group (Con), and 32 patients receiving CCRT with THAL and S-1 were assigned to the research group (Res). Second, inter-group comparisons were carried out with respect to curative efficacy, incidence of drug toxicities, STMs [carbohydrate antigen 125 (CA125) and macrophage inflammatory protein-3α (MIP-3α)], angiogenesis-related indicators [vascular endothelial growth factor (VEGF); VEGF receptor-1 (VEGFR-1); basic fibroblast growth factor (bFGF); angiogenin-2 (Ang-2)], and quality of life (QoL) [QoL core 30 (QLQ-C30)] after one month of treatment.
The analysis showed no statistical difference in the overall response rate and disease control rate between the two patient cohorts; however, the incidences of grade I–II myelosuppression and gastrointestinal reactions were significantly lower in the Res than in the Con. Besides, the post-treatment CA125, MIP-3α, VEGF, VEGFR-1, bFGF, and Ang-2 Levels in the Res were markedly lower compared with the pre-treatment levels and the corresponding post-treatment levels in the Con. Furthermore, more evident improvements in QLQ-C30 scores from the dimensions of physical, role, emotional, and social functions were determined in the Res.
The above results demonstrate the effectiveness of THAL + S-1 CCRT for EC, which contributes to mild side effects and significant reduction of CA125, MIP-3α, VEGF, VEGFR-1, bFGF, and Ang-2 Levels, thus inhibiting tumors from malignant progression and enhancing patients’ QoL.
Core Tip: Esophageal carcinoma (EC) is a common and fatal digestive tract tumor, and the current therapeutic methods such as surgical resection, radiotherapy and chemotherapy have limited effects and are accompanied by relatively serious adverse events. Therefore, clinical exploration of new treatment strategies for esophageal cancer should be continued to optimize the management of EC patients.
- Citation: Zhang TW, Zhang P, Nie D, Che XY, Fu TT, Zhang Y. Efficacy of concurrent chemoradiotherapy with thalidomide and S-1 for esophageal carcinoma and its influence on serum tumor markers. World J Gastrointest Oncol 2023; 15(7): 1262-1270
- URL: https://www.wjgnet.com/1948-5204/full/v15/i7/1262.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i7.1262
Esophageal carcinoma (EC) is a common fatal gastrointestinal (GI) tumor with a five-year survival rate of only 15%–25%. It is characterized by high malignancy, invasiveness, and easy metastasis[1]. According to global statistics, EC is broadly pathologically classified into esophageal squamous cell carcinoma and esophageal adenocarcinoma; the former is primarily distributed in Southeast Asia and Africa and the latter in Europe and North America[2,3]. Onco-pathologically, EC is shown to be associated with the abnormal proliferation of esophageal epithelial cells that induces invasive cancer or invasive adenocarcinoma; meanwhile, the etiology is related to factors such as esophageal mucosa contact with carcinogens and mechanical damage[4,5]. Moreover, the disease is mainly presented clinically as dysphagia and unexpected weight loss but usually with no specific early symptoms[6]. At the present stage, EC is mainly treated with surgical resection, radiotherapy, and chemotherapy. Despite the confirmed effectiveness of these conventional treatments, they are accompanied by serious adverse events, resulting in unsatisfactory clinical outcomes[7]. Therefore, there is an urgent need to explore new strategies for treating EC and optimizing the treatment options for patients with EC; this can be of great value for improving treatment efficacy as well as patient prognosis and symptoms.
Thalidomide (THAL), which was originally used as a sedative for the relief of vomiting and nausea during pregnancy, has been used to treat solid tumors because of its anti-angiogenesis effects[8]. Its anti-tumor mechanism is reported to be linked to the regulation of the secretion of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)[9]. Another molecular study shows that THAL exerts immunomodulatory actions by altering the expression of tumor necrosis factor receptor superfamilies in T cell subsets[10]. The fluorouracil drug S-1 consists of tegafur, gimeracil, and oteracil potassium. It can be used to treat gastric, pancreatic, gallbladder, colorectal, and esophageal cancers[11,12] and has been shown to not only exert better anticancer performance than 5-fluorouracil (5-FU) but also reduce cancer cells' resistance to chemotherapy[13]. In the analysis by Wang et al[14], definitive concurrent chemoradiotherapy (CCRT) with S-1 and cisplatin significantly improved the survival outcomes of older adults (≥ 60 years old) with EC.
The present study attempts to analyze the effectiveness of CCRT with THAL + S-1 for EC and its influence on serum tumor markers (STMs) in order to provide a new feasible scheme for improving the survival outcome of patients with EC.
The study population comprised 62 patients with EC treated at the Zibo 148 Hospital between November 2019 and November 2022. The patients were grouped according to the received treatment. A total of 30 patients undergoing CCRT with cis-platinum (DDP) and 5-FU were assigned to the control group (Con), and 32 patients receiving CCRT with THAL and S-1 were assigned to the research group (Res). The Con consisted of 17 males and 13 females with a mean age of 61.03 ± 6.91 years, and the Res consisted of 19 males and 13 females with a mean age of 61.44 ± 10.61 years. The present study is retrospective.
Inclusion criteria: (1) Patients gastroscopically and pathologically diagnosed with EC and meeting the American Joint Committee on Cancer clinical staging criteria for EC; (2) patients with a life expectancy of three or more months; (3) patients with a Karnovsky Performance Scale score of ≥70 points; (4) patients capable of eating semi-liquid food; and (5) patients with normal hepatorenal function and blood routines.
Exclusion criteria: (1) Patients with an intolerance to the treatment scheme of the present study; (2) patients with other malignancies; (3) patients with hepatic fibrosis, (4) patients with renal fibrosis and other systemic fibrosis; (5) patients undergoing chemotherapy or other adjuvant treatment programs; and (6) patients with distant metastasis of the tumor.
All patients received three-dimensional intensity modulated radiation therapy. Gross tumor volume and organs at risk were delineated according to the pathology and imaging findings, and treatment schemes were specified based on patients' conditions. The prescribed dose was 56–70 Gy, with a single dose of 2 Gy administered five times a week for four weeks. Of these, 95% of the planned target area was irradiated with a prescription dose of at least 100% for the whole lung, no more than 25%–30% for V20, and no more than 18% for V30; the upper limits were set at 30 Gy and 45 Gy for the heart and the spinal cord, respectively.
The Con was treated with CCRT using DDP + FU. Cisplatin 20 mg/(m²·d) and 5-FU 500 mg/(m²·d) were given intravenously for 5 consecutive days; patients received two cycles of CCRT, which were performed at week 1 and week 4 of radiotherapy, respectively.
The Res received CCRT using THAL + S-1. Patients were given S-1 capsules, 40 mg/time, twice a day from day 1 to day 14; this was repeated every 21 days simultaneously with radiotherapy. The THAL was administered at a dose of 100 mg/d before bedtime in the first week and gradually increased to 200 mg/d in the second week until the end of radiotherapy. Patients in both groups were given symptomatic treatments, such as antiemesis, stomach protection, and nutritional support during chemotherapy, with their blood routines and hepatorenal function monitored weekly and biweekly, respectively.
Short-term efficacy: The clinical efficacy, which was evaluated with reference to the Response Evaluation Criteria in Solid Tumors, was determined as complete response (CR; the tumor disappeared completely, with smooth margins shown by the barium meal test, smooth passage of the barium agent, slightly rigid tube wall, no narrowing or slight narrowing of the lumen, and basically recovered or thickened mucosa), partial response (PR; most of the lesions disappeared without obvious distortion, angulation, or extraluminal ulcer; the barium passed smoothly, but the edges were not smooth, with small filling defects or niches, or the lumen was obviously narrowed, although the edges were smooth), stable disease (SD; the lesion had residual or no obvious improvement at the end of radiotherapy, with obvious filling defects or niches), or progressive disease (worsened niche or stenosis). The overall response rate (ORR) is the sum of patients with CR and patients with PR as a percentage of the total number of cases. The disease control rate (DCR) is the sum of the percentages of CR, PR, and SD. The short-term efficacy was evaluated after radiotherapy in both groups.
Incidence of drug toxicities: The adverse reactions of anticancer drugs were classified into grades I–II or III–IV according to the WHO classification of adverse drug reactions, and the number of cases of myelosuppression (MS), GI reactions, and radiation esophagitis (RE) as well as the percentages of the corresponding side effect grades were recorded.
Serum tumor markers and angiogenesis-related indicators: Before and after treatment, 5 mL of venous blood was collected on an empty stomach during the morning hours and sent to the laboratory for examination after centrifugation. The levels of carbohydrate antigen 125 (CA125), macrophage inflammatory protein-3α (MIP-3α), VEGF, VEGF receptor-1 (VEGFR-1), bFGF, and angiogenin-2 (Ang-2) were determined by the enzyme-linked immunosorbent assay (ELISA).
Quality of life (QoL): Patients’ QoL was assessed and compared at one month after treatment, using the QoL Questionnaire core 30. The scale includes five functional dimensions: body, role, emotion, cognition, and social function. A higher score suggests a better QoL.
In the present study, the SPSS 22.0 software was used for data analysis, and GraphPad Prism 7.0 was used for image rendering and export. The significance threshold was P < 0.05. mean ± SEM was used for statistical description of continuous variables (e.g., age, tumor diameter, and CA125 expression), and the t-test and paired t-test were used for inter-group and intra-group comparisons (before and after treatment), respectively. Categorical variables (e.g., sex, clinical staging, and history of alcoholism) were described by frequencies (percentages), and the comparison between groups was made using the χ2 test.
As indicated by Table 1, the two patient cohorts have no evident differences in age, sex, clinical staging, tumor diameter, alcoholism history, smoking history, and family history (P > 0.05).
| Indicators | Control group (n = 30) | Research group (n = 32) | χ2/t | P value |
| Age (years old) | 61.03 ± 6.91 | 61.44 ± 10.61 | 0.179 | 0.859 |
| Gender (male/female) | 17/13 | 19/13 | 0.047 | 0.829 |
| Clinical staging (II/III) | 18/12 | 16/16 | 0.625 | 0.429 |
| Tumor diameter (cm) | 5.68 ± 1.29 | 5.80 ± 1.50 | 0.337 | 0.738 |
| History of alcoholism (with/without) | 11/19 | 8/24 | 0.992 | 0.319 |
| History of smoking (with/without) | 8/22 | 7/25 | 0.194 | 0.660 |
| Family medical history (yes/no) | 5/25 | 9/23 | 1.163 | 0.281 |
The short-term curative effects between the Res and the Con at one month after treatment were analyzed and compared (Table 2). The ORRs of the Res and the Con were determined as 62.50% and 60.00%, respectively, while the DCRs were determined as 87.50% and 83.33%, respectively, showing no significant inter-group differences in both indicators (P > 0.05).
| Indicators | Control group (n = 30) | Research group (n = 32) | χ2 | P value |
| Complete response | 6 (20.00) | 7 (21.88) | - | - |
| Partial response | 12 (40.00) | 13 (40.63) | - | - |
| Stable disease | 7 (23.33) | 8 (25.00) | - | - |
| Progressive disease | 5 (16.67) | 4 (12.50) | - | - |
| Overall response rate | 18 (60.00) | 20 (62.50) | 0.041 | 0.840 |
| Disease control rate | 25 (83.33) | 28 (87.50) | 0.217 | 0.642 |
The main drug toxicities were MS, GI reactions, and RE, with the incidence of grade I–II MS and grade I–II GI reactions markedly lower in the Res compared with the Con (P < 0.05; Table 3).
| Indicators | Control group (n = 30) | Research group (n = 32) | χ2 | P value |
| Myelosuppression | ||||
| I-II | 13 (43.33) | 6 (18.75) | 4.403 | 0.036 |
| III-IV | 6 (20.00) | 2 (6.25) | 2.605 | 0.107 |
| Gastrointestinal reactions | ||||
| I-II | 15 (50.00) | 8 (25.00) | 4.147 | 0.042 |
| III-IV | 7 (23.33) | 3 (9.38) | 2.230 | 0.135 |
| Radiation esophagitis | ||||
| I-II | 14 (46.67) | 13 (40.63) | 0.230 | 0.632 |
| III-IV | 7 (23.33) | 6 (18.75) | 0.196 | 0.658 |
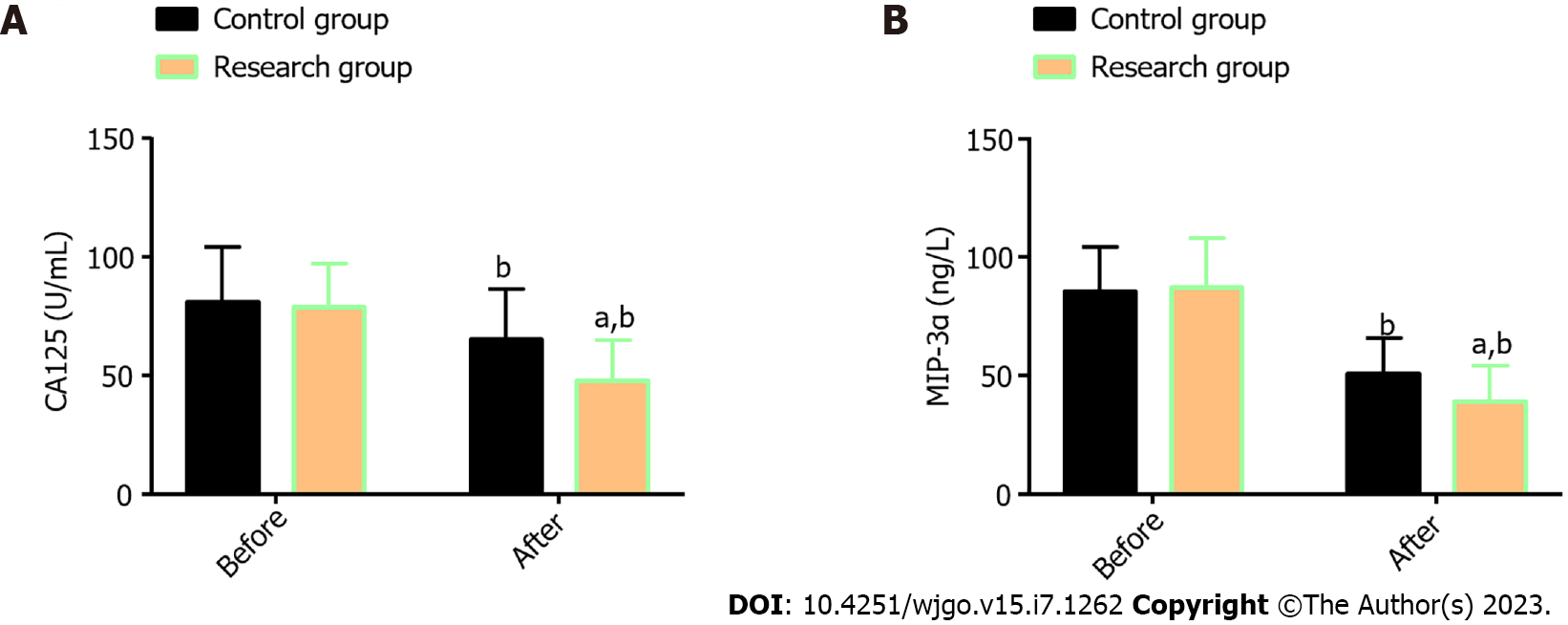
Two STMSs, CA125 and MIP-3α, were measured; no statistical differences were found in the corresponding pre-treatment levels between the Res and the Con (P > 0.05); a marked reduction in both indexes was determined in the two patient cohorts, especially in the Res (P < 0.05; Figure 1).
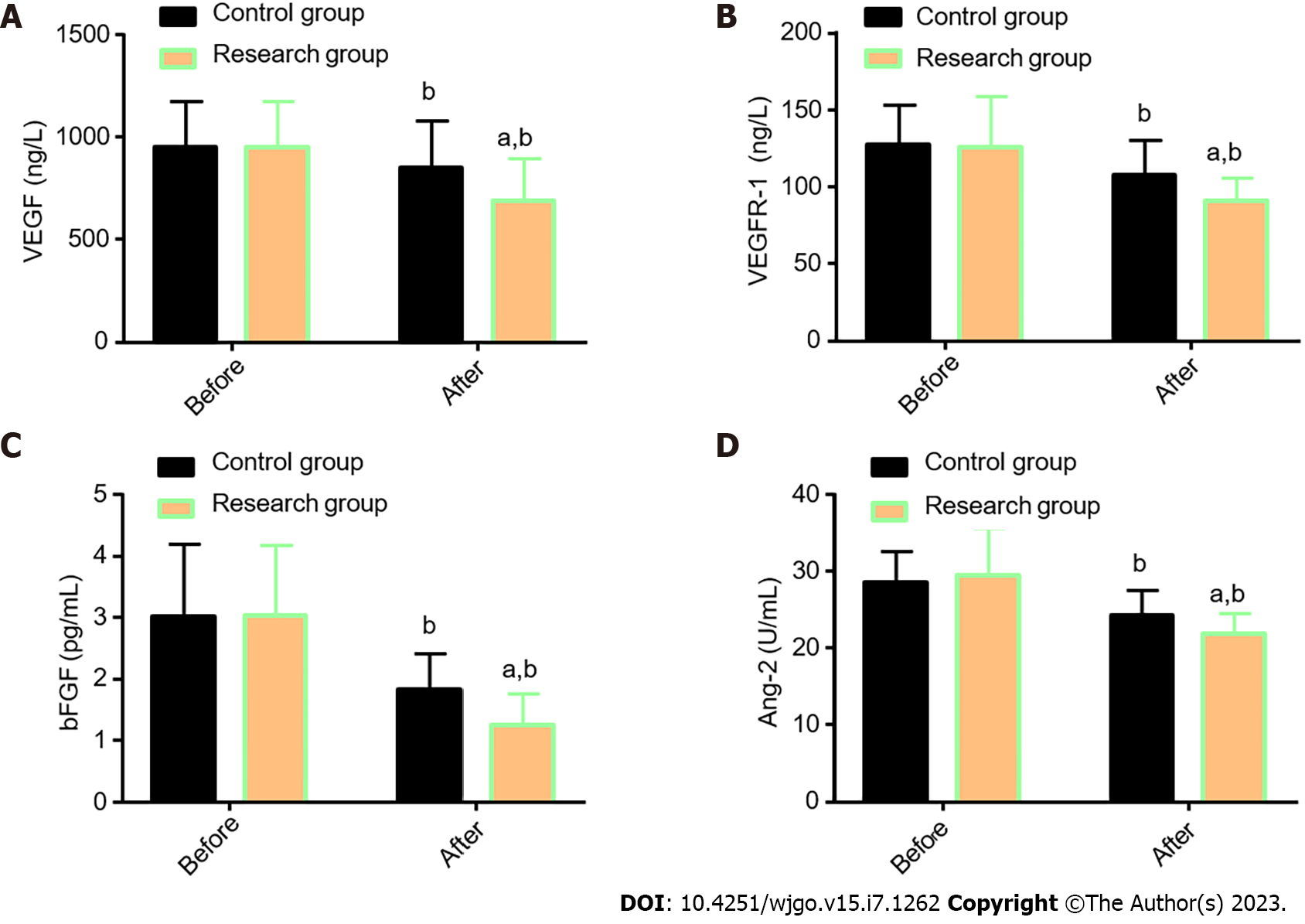
The angiogenesis-related indexes VEGF, VEGFR-1, bFGF, and Ang-2, were determined in both groups for comparative analysis (Figure 2). The VEGF, VEGFR-1, bFGF, and Ang-2 Levels were similar in the two cohorts before treatment (P > 0.05), but their levels reduced significantly after treatment (P < 0.05), with even lower levels in the Res (P < 0.05).
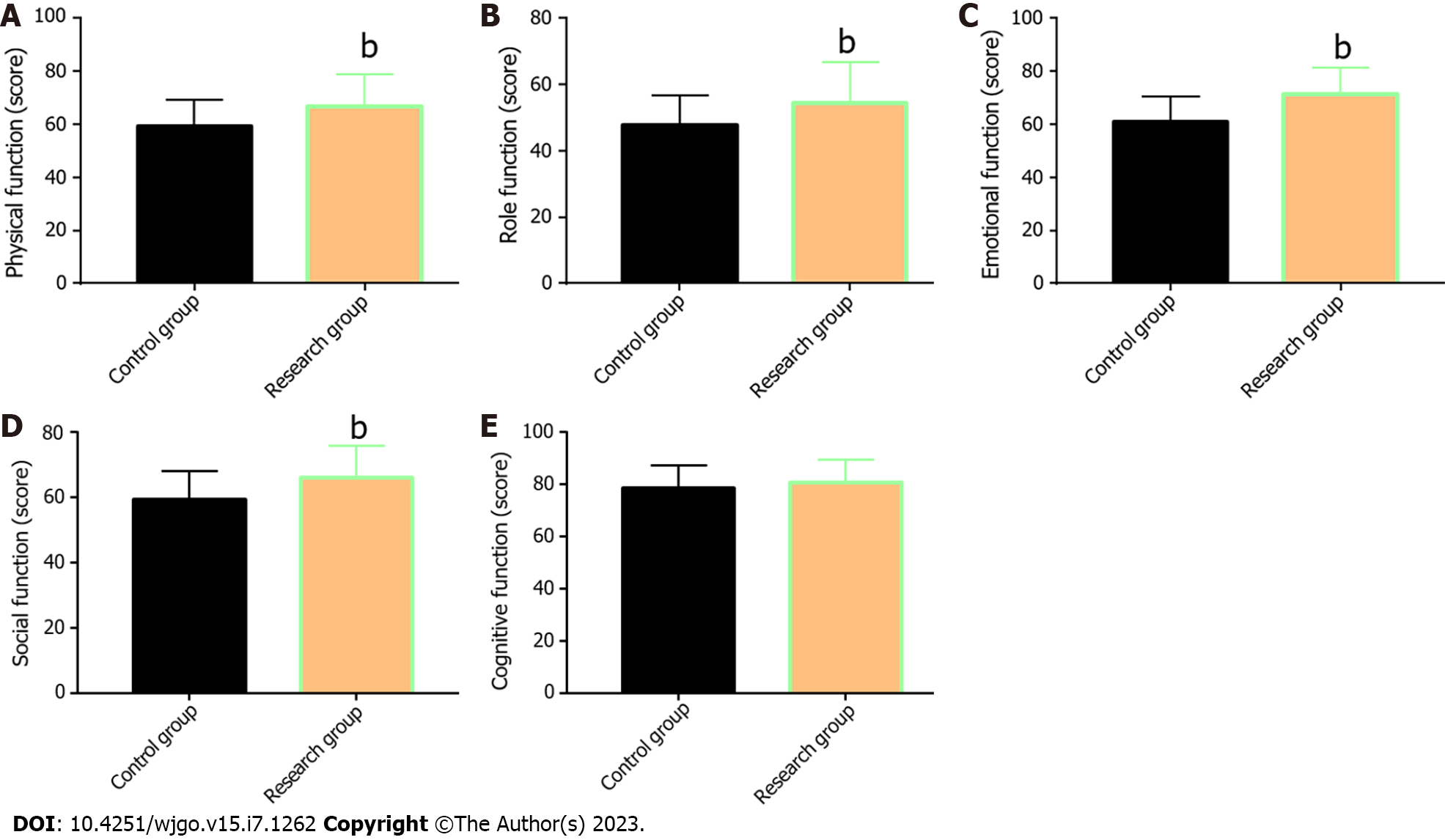
The QoL of the two groups was compared and evaluated from five aspects: physical, role, emotional, social, and cognitive function (Figure 3). The data showed that with the exception of cognitive function, the aspect scores in the two groups increased significantly after treatment (P < 0.05), with more marked increases in the Res when compared with the Con (P < 0.05).
The present study focuses on the efficacy of CCRT with THAL + S-1 for EC and its influence on patient STMs. Given the current scanty of research in this field, the present analysis is helpful in gaining a new understanding of the effectiveness of this CCRT protocol for patients with EC.
Many researchers have provided clinical references for EC treatment by analyzing relevant treatment strategies. For example, Song et al[15] showed that CCRT with S-1 effectively enhanced the curative effect and survival of elderly patients with non-metastatic esophageal squamous cell carcinoma compared with radiotherapy alone without increasing acute adverse reactions. As reported by McDowell et al[16], intensity-modulated radiotherapy combined with chemotherapy is conducive to improving the prognosis of patients with cervical EC. Ma et al[17] also pointed out that three-dimensional conformal radiotherapy was helpful in controlling mediastinal lymph node metastasis and recurrence after EC surgery, in addition to significantly improving the local tumor control rate and long-term survival rate. In the present study, the effectiveness of DDP + 5-FU vs THAL + S-1 were analyzed. The standard CCRT scheme for EC (DDP + 5-FU) still causes local failures in 46% of patients and fatal threats in 20%[18]. Hence, introducing new CCRT schemes is critical. Thalidomide can exert anti-inflammatory and immunosuppressive actions by inhibiting inflammatory factors and regulating key immunoregulatory molecules, thus exerting anti-tumor activity[19]. Among the S-1 components, tegafur is a prodrug of 5-Fu, and gimeracil can prolong the effective drug properties of 5-Fu in the blood by reducing dihydropyrimidine dehydrogenase[20].
In the present study, the Res was administered with THAL + S-1 and the Con was administered with DDP + 5-FU. The ORR and DCR of the Res were determined as 62.50% and 87.50%, respectively (slightly higher than but not significantly different from those in the Con); these results suggest equivalent curative efficacy of THAL + S-1 to that of DDP + 5-FU. Furthermore, according to the investigation of drug toxicities, the incidences of grade I–II MS and grade I–II GI reactions were identified as notably lower in the Res than in the Con, while the incidence of RE was similar, indicating the safety profile of THAL + S-1. This may be related to the inhibition of FU-related GI toxicity by oteracil potassium, one of the S-1 components[20].
Previous literature has shown a correlation of CA125 with lymph node metastasis and blood-borne metastasis of EC, as well as an association between MIP-3α (also known as CCL20) and the occurrence, development, and metastasis of EC[21,22]. While VEGF and VEGFR-1 are both significantly related to the poor prognosis of patients with EC[23], previous studies have also shown that abnormal overexpression of bFGF in advanced esophageal squamous cell carcinoma is closely associated with the development of invasive carcinoma. As a key regulator of tumor angiogenesis, Ang-2 also mediates the malignant procession of esophageal squamous cell carcinoma[24,25].
By further quantifying STMs (CA125 and MIP-3α) and angiogenesis-related indicators (VEGF, VEGFR-1, bFGF, and Ang-2) using ELISA, it was found that the post-treatment CA125, MIP-3α, VEGF, VEGFR-1, bFGF, and Ang-2 Levels in the Res were evidently lower than those before treatment and the Con levels. This suggests that CCRT with THAL + S-1 has a significant inhibitory effect on STMs and angiogenesis-related indicators in patients with EC.
In the research of Wang et al[26], THAL validly suppressed the increase of the serum VEGF level in patients with EC during treatment; this is similar to our research results. Tsuji et al[27] also reported the inhibitory action of S-1 against VEGF levels in patients with metastatic breast cancer, indicating certain anti-angiogenesis activity of S-1. Finally, the QoL of the two groups after treatment was evaluated and compared regarding five aspects: physical, role, emotional, social, and cognitive function. The Res was found to have higher scores in all the other four dimensions after treatment except the cognitive function, suggesting that THAL + S-1 is more effective than DDP + 5-FU in enhancing QoL in patients with EC.
Taken together, CCRT with THAL + S-1 is effective in the treatment of EC, with certain safety. This CCRT protocol has a significant inhibitory effect on STMs (CA125 and MIP-3α) and angiogenesis-related indicators (VEGF, VEGFR-1, bFGF, and Ang-2) and is conducive to improving patients’ QoL, providing a new choice for clinical treatment of patients with EC. In addition, since the sample size included in the present study is limited, increasing the sample size in the future will be conducive to enhancing the credibility of the experimental results.
Treatment strategies for esophageal carcinoma (EC) still need to be explored and optimized to improve patient symptoms, efficacy and prognosis.
This study provided a novel and feasible scheme to improve the survival outcome of EC patients.
This research intends to elucidate the clinical efficacy of concurrent chemoradiotherapy (CCRT) with thalidomide (THAL) and S-1 (tegafur, gimeracil and oteracil potassium capsules) for EC and its influence on serum tumor markers (STMs).
Thirty cases of EC undergoing CCRT with cis-platinum and 5-fluorouracil were assigned to the control group (Con) and 32 cases receiving CCRT with THAL and S-1 were included in the research group (Res). The efficacy, incidence of drug toxicities, STMs [carbohydrate antigen 125 (CA125), macrophage inflammatory protein-3α (MIP-3α)], angiogenesis-related indicators [vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor-1 (VEGFR-1), basic fibroblast growth factor, (bFGF), angiogenin-2 (Ang-2)], and quality of life [Quality of Life Questionnaire core 30, (QLQ-C30)] of the two groups were collected for comparative analysis.
The overall response rate and disease control rate were not statistically different between the two patient cohorts, but the incidence of grade I-II myelosuppression and gastrointestinal reactions was significantly lower in the Res. In addition, the Res showed markedly reduced CA125, MIP-3α, VEGF, VEGFR-1, bFGF, and Ang-2 Levels after treatment, lower than those in the Con. Moreover, a better quality of life was determined in the Res, which was supported by more significant improvements in QLQ-C30 scores from physical, role, emotional and social function dimensions.
CCRT with THAL and S-1 has a definite efficacy in the treatment of EC, which can significantly reduce CA125, MIP-3α, VEGF, VEGFR-1, bFGF and Ang-2 Levels while contributing to mild toxicities, thereby inhibiting tumor malignant progression and helping to improve the quality of life of patients.
Although this study provides a new choice for the clinical treatment of EC patients by demonstrating the clinical efficacy and safety of CCRT with THAL and S-1, the credibility of our findings needs to be validated by increasing the sample size in the future due to the limited cases included in this research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brethauer SA, United States; Terashima M, Japan S-Editor: Wang JL L-Editor: A P-Editor: Yu HG
| 1. | Yu G, Yu W, Xu X, Ye B, Yao L. Neoadjuvant immunotherapy for resectable esophageal cancer: A protocol of meta-analysis. PLoS One. 2021;16:e0252829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020;5:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 3. | Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, Imamura Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 4. | Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 737] [Article Influence: 92.1] [Reference Citation Analysis (2)] |
| 5. | Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 435] [Article Influence: 87.0] [Reference Citation Analysis (1)] |
| 6. | Short MW, Burgers KG, Fry VT. Esophageal Cancer. Am Fam Physician. 2017;95:22-28. [PubMed] |
| 7. | Bollschweiler E, Plum P, Mönig SP, Hölscher AH. Current and future treatment options for esophageal cancer in the elderly. Expert Opin Pharmacother. 2017;18:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Ito T, Handa H. Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. Int J Hematol. 2016;104:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Medinger M, Halter J, Heim D, Buser A, Gerull S, Stern M, Passweg J. Angiogenic markers in plasma cell myeloma patients treated with novel agents. Anticancer Res. 2015;35:1085-1090. [PubMed] |
| 10. | Kim BS, Kim JY, Kim EJ, Lee JG, Joo DJ, Huh KH, Kim MS, Kim YS. Role of Thalidomide on the Expression of OX40, 4-1BB, and GITR in T Cell Subsets. Transplant Proc. 2016;48:1270-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Yasui H, Kawakami T, Kashiwagi H, Mori K, Omae K, Kasai J, Yoshisue K, Kawahira M, Tsushima T, Machida N, Fukutomi A, Yamaguchi K. Pharmacokinetics of S-1 monotherapy in plasma and in tears for gastric cancer patients. Int J Clin Oncol. 2019;24:660-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Hiratsuka T, Etoh T, Hara T, Akagi T, Tahara K, Matsumoto T, Ogawa T, Fujii K, Shiromizu A, Shiroshita H, Inomata M. Long-term outcomes of neoadjuvant-synchronous S-1 plus radiotherapy for locally advanced rectal cancer: a multi-institutional prospective phase II study. J Anus Rectum Colon. 2018;2:168-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Fukushima M, Sakamoto K, Sakata M, Nakagawa F, Saito H, Sakata Y. Gimeracil, a component of S-1, may enhance the antitumor activity of X-ray irradiation in human cancer xenograft models in vivo. Oncol Rep. 2010;24:1307-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Wang H, Li G, Chen L, Duan Y, Zou C, Hu C. Definitive concurrent chemoradiotherapy with S-1 and cisplatin in elderly esophageal squamous cell carcinoma patients. J Thorac Dis. 2017;9:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Song GM, Tian X, Liu XL, Chen H, Zhou JG, Bian W, Chen WQ. Concurrent chemo-radiotherapy with S-1 as an alternative therapy for elderly Chinese patients with non-metastatic esophageal squamous cancer: evidence based on a systematic review and meta-analysis. Oncotarget. 2017;8:37963-37973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | McDowell LJ, Huang SH, Xu W, Che J, Wong RKS, Brierley J, Kim J, Cummings B, Waldron J, Bayley A, Hansen A, Witterick I, Ringash J. Effect of Intensity Modulated Radiation Therapy With Concurrent Chemotherapy on Survival for Patients With Cervical Esophageal Carcinoma. Int J Radiat Oncol Biol Phys. 2017;98:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Ma DY, Tan BX, Liu M, Li XF, Zhou YQ, Lu Y. Concurrent three-dimensional conformal radiotherapy and chemotherapy for postoperative recurrence of mediastinal lymph node metastases in patients with esophageal squamous cell carcinoma: a phase 2 single-institution study. Radiat Oncol. 2014;9:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Yoshii T, Hara H, Asayama M, Kumekawa Y, Miyazawa S, Takahashi N, Matsushima T, Shimizu S, Saito Y. Chemoradiotherapy with FOLFOX for esophageal squamous cell cancer with synchronous rectal cancer: Four case reports and a literature review. Mol Clin Oncol. 2020;12:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kim EJ, Lee JG, Kim JY, Song SH, Joo DJ, Huh KH, Kim MS, Kim BS, Kim YS. Enhanced immune-modulatory effects of thalidomide and dexamethasone co-treatment on T cell subsets. Immunology. 2017;152:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Zhao J, Lei J, Yu J, Zhang C, Song X, Zhang N, Wang Y, Zhang S. Clinical efficacy and safety of apatinib combined with S-1 in advanced esophageal squamous cell carcinoma. Invest New Drugs. 2020;38:500-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Zhao H, Chen W, Wu J, Wang L, Mao W. Clinical significance of preoperative serum tumor markers in esophageal squamous cell carcinoma. J Cancer Res Ther. 2014;10 Suppl:C179-C185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Li Z, Qian J, Li J, Zhu C. Clinical Significance of Serum Chemokines in Esophageal Cancer. Med Sci Monit. 2019;25:5850-5855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kilic E, Schild SE, Thorns C, Bajrovic A, Rades D. Prognostic role of vascular endothelial growth factor and its receptor-1 in patients with esophageal cancer. Anticancer Res. 2014;34:5221-5226. [PubMed] |
| 24. | Kitadai Y, Onogawa S, Kuwai T, Matsumura S, Hamada H, Ito M, Tanaka S, Yoshihara M, Chayama K. Angiogenic switch occurs during the precancerous stage of human esophageal squamous cell carcinoma. Oncol Rep. 2004;11:315-319. [PubMed] |
| 25. | Zhou YZ, Fang XQ, Li H, Diao YT, Yang YF, Zhao DL, Wu K, Li HQ. Role of serum angiopoietin-2 level in screening for esophageal squamous cell cancer and its precursors. Chin Med J (Engl). 2007;120:1216-1219. [PubMed] |
| 26. | Wang J, Yu J, Wang J, Ni X, Sun Z, Sun W, Sun S, Lu Y. Thalidomide combined with chemo-radiotherapy for treating esophageal cancer: A randomized controlled study. Oncol Lett. 2019;18:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Tsuji W, Ishiguro H, Tanaka S, Takeuchi M, Ueno T, Toi M. Orally administered S-1 suppresses circulating endothelial cell counts in metastatic breast cancer patients. Int J Clin Oncol. 2014;19:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |